Culturable Bacterial Endophytes of Wild White Poplar (Populus alba L.) Roots: A First Insight into Their Plant Growth-Stimulating and Bioaugmentation Potential
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Isolation of Culturable Bacterial Endophytes from P. alba Roots
2.2. Sex Determination of Plants from Which Bacterial Endophytes Were Obtained
2.3. Testing the Ability of Bacterial Strains to Grow on Nutrient Media without Nitrogen Sources
2.4. DNA Isolation, Library Preparation, and Whole-Genome Sequencing
2.5. Endophyte Genome Assemblies and Annotation
2.6. Determining Taxonomic Affiliation
3. Results
3.1. Overall Characteristics of Sequenced Genomes of Endophytic Bacteria
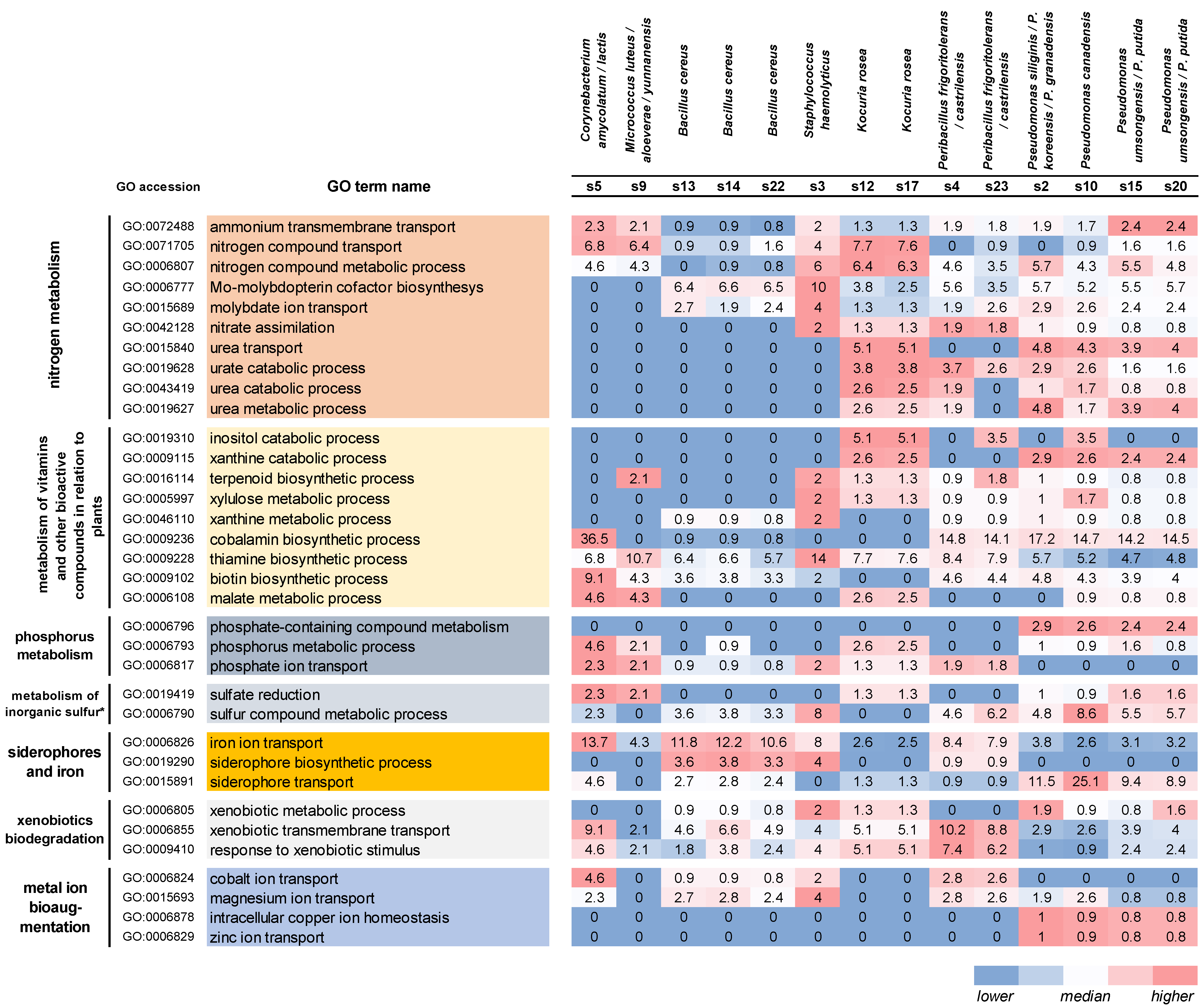
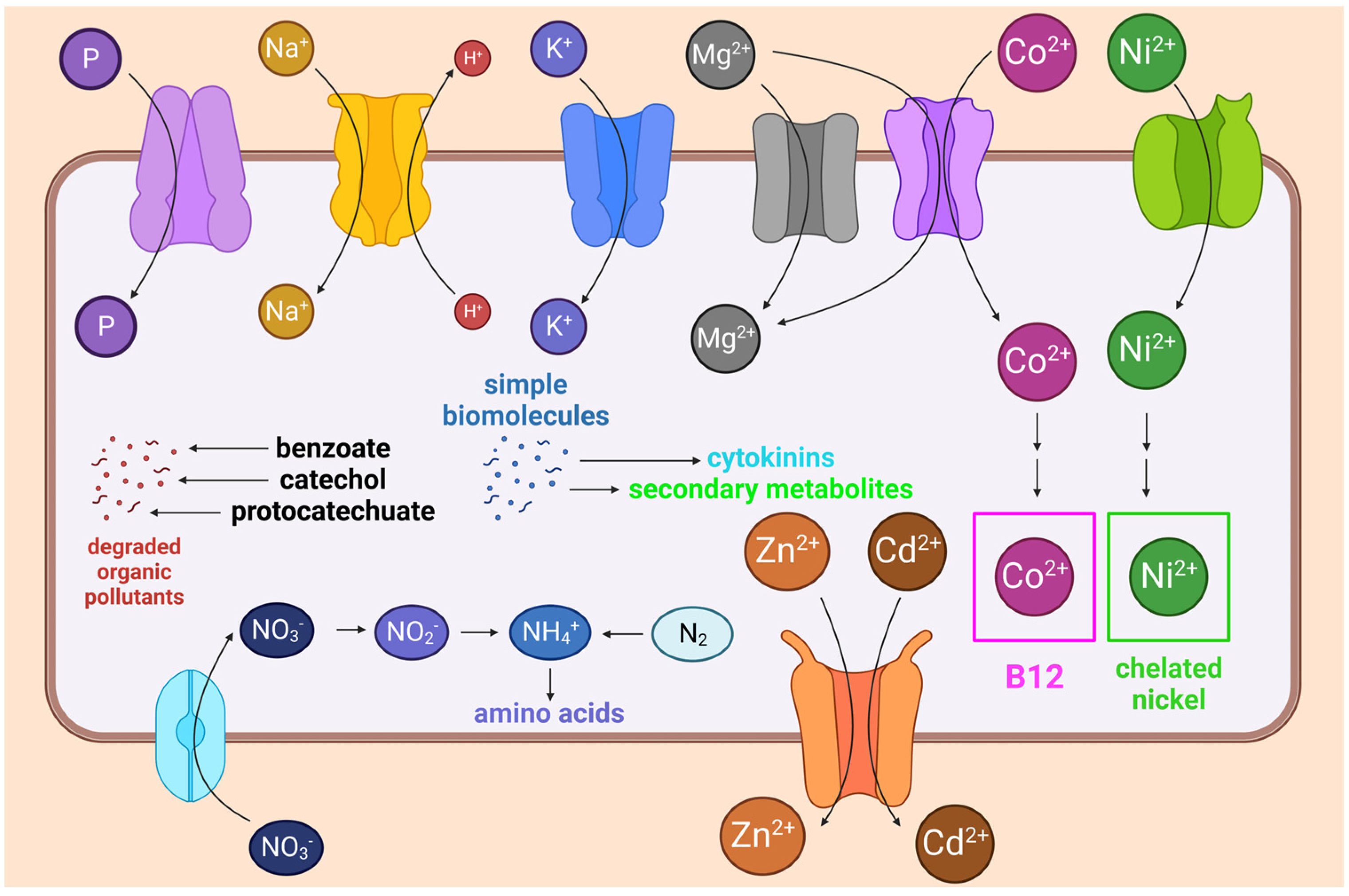
3.2. Genes and Biochemical Pathways That Promote Plant Growth and Soil Bioaugmentation
3.3. Ability of Poplar Endophytes to Grow on Nutrient Medium without a Nitrogen Source
3.4. Secondary Metabolite Biosynthesis Clusters
3.5. Antiviral Defense Systems of Poplar Endophytic Bacteria
3.6. Pathogenic Potential and Antibiotic Resistance of Poplar Endophytic Bacteria
3.6.1. Pathogenicity of Poplar Endophytes to Humans
3.6.2. AMR Genes
4. Discussion
4.1. Ecology of the Studied Bacteria: Representation in Soils, Rhizosphere, and Endophytes of Other Plants
4.2. Metabolism of Nitrogen, Phosphorus, and Metals and Tolerance to Heavy Metals
4.3. Biodegradation of Organic Pollutants
4.4. Ability to Biosynthesize Secondary Metabolites
4.5. Antiviral Defense Systems
4.6. Safety Issues of White Poplar Endophytic Bacteria
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.; Chen, L.; Gleisner, R.; Zhu, J.Y. Co-production of bioethanol and furfural from poplar wood via low temperature (≤90 °C) acid hydrotropic fractionation (AHF). Fuel 2019, 254, 115572. [Google Scholar] [CrossRef]
- Jiménez-López, L.; Martín-Sampedro, R.; Eugenio, M.E.; Santos, J.I.; Sixto, H.; Cañellas, I.; Ibarra, D. Co-production of soluble sugars and lignin from short rotation white poplar and black locust crops. Wood Sci. Technol. 2020, 54, 1617–1643. [Google Scholar] [CrossRef]
- Di Lonardo, S.; Capuana, M.; Arnetoli, M.; Gabbrielli, R.; Gonnelli, C. Exploring the metal phytoremediation potential of three Populus alba L. clones using an in vitro screening. Environ. Sci. Pollut. Res. Int. 2011, 18, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kostic, O.; Gajic, G.; Jaric, S.; Vukov, T.; Matic, M.; Mitrovic, M.; Pavlovic, P. An Assessment of the Phytoremediation Potential of Planted and Spontaneously Colonized Woody Plant Species on Chronosequence Fly Ash Disposal Sites in Serbia-Case Study. Plants 2021, 11, 110. [Google Scholar] [CrossRef]
- Ciadamidaro, L.; Madejón, P.; Madejón, E. Soil chemical and biochemical properties under Populus alba growing: Three years study in trace element contaminated soils. Appl. Soil Ecol. 2014, 73, 26–33. [Google Scholar] [CrossRef]
- Vannucchi, F.; Imperato, V.; Saran, A.; Staykov, S.; D’Haen, J.; Sebastiani, L.; Vangronsveld, J.; Thijs, S. Inoculated Seed Endophytes Modify the Poplar Responses to Trace Elements in Polluted Soil. Agronomy 2021, 11, 1987. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z.; Meng, S.; Wu, F. Soil properties and microbial community in the rhizosphere of Populus alba var. pyramidalis along a chronosequence. Microbiol. Res. 2021, 250, 126812. [Google Scholar] [CrossRef] [PubMed]
- Simmer, R.; Mathieu, J.; da Silva, M.L.B.; Lashmit, P.; Gopishetty, S.; Alvarez, P.J.J.; Schnoor, J.L. Bioaugmenting the poplar rhizosphere to enhance treatment of 1,4-dioxane. Sci. Total Environ. 2020, 744, 140823. [Google Scholar] [CrossRef]
- van der Lelie, D.; Taghavi, S.; Monchy, S.; Schwender, J.; Miller, L.; Ferrieri, R.; Rogers, A.; Wu, X.; Zhu, W.; Weyens, N.; et al. Poplar and its bacterial endophytes: Coexistence and harmony. Crit. Rev. Plant Sci. 2009, 28, 346–358. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef]
- Berg, G.; Eberl, L.; Hartmann, A. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 2005, 7, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- DeMers, M. Alternaria alternata as endophyte and pathogen. Microbiology 2022, 168, 001153. [Google Scholar] [CrossRef]
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.J.; Sessitsch, A. Ecology and Genomic Insights into Plant-Pathogenic and Plant-Nonpathogenic Endophytes. Annu. Rev. Phytopathol. 2017, 55, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, G.; Fraser, S.J.; Ridout, M.; Busby, P.E. Leaf Endophytes of Populus trichocarpa Act as Pathogens of Neighboring Plant Species. Front. Microbiol. 2020, 11, 573056. [Google Scholar] [CrossRef] [PubMed]
- Hagaggi, N.S.A.; Mohamed, A.A.A. Plant-bacterial endophyte secondary metabolite matching: A case study. Arch. Microbiol. 2020, 202, 2679–2687. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Fan, X.; Wang, Y.; Kusstatscher, P.; Duan, J.; Wu, S.; Chen, S.; Qiao, K.; Wang, Y.; Ma, B.; et al. Bacterial seed endophyte shapes disease resistance in rice. Nat. Plants 2021, 7, 60–72. [Google Scholar] [CrossRef]
- Lacerda, I.; Polonio, J.C.; Golias, H.C. Endophytic Fungi as a Source of Antiviral Compounds—A Review. Chem. Biodivers. 2022, 19, e202100971. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Klimova, E.; Rodriguez-Pena, K.; Sanchez, S. Endophytes as sources of antibiotics. Biochem. Pharmacol. 2017, 134, 1–17. [Google Scholar] [CrossRef]
- Burragoni, S.G.; Jeon, J. Applications of endophytic microbes in agriculture, biotechnology, medicine, and beyond. Microbiol. Res. 2021, 245, 126691. [Google Scholar] [CrossRef]
- Doty, S.L. Enhancing phytoremediation through the use of transgenics and endophytes. New Phytol. 2008, 179, 318–333. [Google Scholar] [CrossRef]
- Kumar, A.; Tripti; Voropaeva, O.; Maleva, M.; Panikovskaya, K.; Borisova, G.; Rajkumar, M.; Bruno, L.B. Bioaugmentation with copper tolerant endophyte Pseudomonas lurida strain EOO26 for improved plant growth and copper phytoremediation by Helianthus annuus. Chemosphere 2021, 266, 128983. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, C.; Oliveira, R.S.; Freitas, H.; Luo, Y. Bioaugmentation with Endophytic Bacterium E6S Homologous to Achromobacter piechaudii Enhances Metal Rhizoaccumulation in Host Sedum plumbizincicola. Front. Plant Sci. 2016, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Doty, S.L.; Sher, A.W.; Fleck, N.D.; Khorasani, M.; Bumgarner, R.E.; Khan, Z.; Ko, A.W.; Kim, S.H.; DeLuca, T.H. Variable Nitrogen Fixation in Wild Populus. PLoS ONE 2016, 11, e0155979. [Google Scholar] [CrossRef] [PubMed]
- Utturkar, S.M.; Cude, W.N.; Robeson, M.S., Jr.; Yang, Z.K.; Klingeman, D.M.; Land, M.L.; Allman, S.L.; Lu, T.Y.; Brown, S.D.; Schadt, C.W.; et al. Enrichment of Root Endophytic Bacteria from Populus deltoides and Single-Cell-Genomics Analysis. Appl. Environ. Microbiol. 2016, 82, 5698–5708. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Cheng, Y.; Rahman, E. Diversity analysis of Populus euphratica endophytic bacteria in Tarim River Basin, China. PeerJ 2023, 11, e15934. [Google Scholar] [CrossRef] [PubMed]
- Albrectsen, B.R.; Siddique, A.B.; Decker, V.H.G.; Unterseher, M.; Robinson, K.M. Both plant genotype and herbivory shape aspen endophyte communities. Oecologia 2018, 187, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, G.; Han, S.; Yang, J.; He, X.; Li, H. Differentiation and Identification of Endophytic Bacteria from Populus Based on Mass Fingerprints and Gene Sequences. Int. J. Mol. Sci. 2023, 24, 13449. [Google Scholar] [CrossRef] [PubMed]
- Hanak, A.M.; Nagler, M.; Weinmaier, T.; Sun, X.; Fragner, L.; Schwab, C.; Rattei, T.; Ulrich, K.; Ewald, D.; Engel, M.; et al. Draft Genome Sequence of the Growth-Promoting Endophyte Paenibacillus sp. P22, Isolated from Populus. Genome Announc. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Gkorezis, P.; Van Hamme, J.D.; Bottos, E.M.; Thijs, S.; Balseiro-Romero, M.; Monterroso, C.; Kidd, P.S.; Rineau, F.; Weyens, N.; Vangronsveld, J. Draft Genome Sequence of Pantoea ananatis GB1, a Plant-Growth-Promoting Hydrocarbonoclastic Root Endophyte, Isolated at a Diesel Fuel Phytoremediation Site Planted with Populus. Genome Announc. 2016, 4, 10-1128. [Google Scholar] [CrossRef]
- Li, Y.; Bian, D.R.; Chang, J.P.; Guo, L.M.; Yang, X.Q. Sphingomonas populi sp. nov., isolated from bark of Populus × euramericana. Int. J. Syst. Evol. Microbiol. 2020, 70, 897–901. [Google Scholar] [CrossRef]
- Kaldorf, M.; Koch, B.; Rexer, K.H.; Kost, G.; Varma, A. Patterns of interaction between Populus Esch5 and Piriformospora indica: A transition from mutualism to antagonism. Plant Biol. 2005, 7, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Beckers, B.; Op De Beeck, M.; Weyens, N.; Boerjan, W.; Vangronsveld, J. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees. Microbiome 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Leite Montalvao, A.P.; Kersten, B.; Fladung, M.; Müller, N. The genetic basis of sex determination in Populus provides molecular markers across the genus and indicates convergent evolution. Silvae Genet. 2021, 70, 145–155. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014, 15, R46. [Google Scholar] [CrossRef]
- Tanizawa, Y.; Fujisawa, T.; Nakamura, Y. DFAST: A flexible prokaryotic genome annotation pipeline for faster genome publication. Bioinformatics 2018, 34, 1037–1039. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Simao, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.J.; Meaden, S.; Mestre, M.R.; Palmer, C.; Toro, N.; Fineran, P.C.; Jackson, S.A. PADLOC: A web server for the identification of antiviral defence systems in microbial genomes. Nucleic Acids Res. 2022, 50, W541–W550. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.D.; Assaf, R.; Brettin, T.; Conrad, N.; Cucinell, C.; Davis, J.J.; Dempsey, D.M.; Dickerman, A.; Dietrich, E.M.; Kenyon, R.W.; et al. Introducing the Bacterial and Viral Bioinformatics Resource Center (BV-BRC): A resource combining PATRIC, IRD and ViPR. Nucleic Acids Res. 2023, 51, D678–D689. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef] [PubMed]
- Wattam, A.R.; Davis, J.J.; Assaf, R.; Boisvert, S.; Brettin, T.; Bun, C.; Conrad, N.; Dietrich, E.M.; Disz, T.; Gabbard, J.L.; et al. Improvements to PATRIC, the all-bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res. 2017, 45, D535–D542. [Google Scholar] [CrossRef]
- Feldgarden, M.; Brover, V.; Gonzalez-Escalona, N.; Frye, J.G.; Haendiges, J.; Haft, D.H.; Hoffmann, M.; Pettengill, J.B.; Prasad, A.B.; Tillman, G.E.; et al. AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci. Rep. 2021, 11, 12728. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Zhu, F.; Han, B.; Kumar, P.; Liu, X.; Ma, X.; Wei, X.; Huang, L.; Guo, Y.; Han, L.; Zheng, C.; et al. Update of TTD: Therapeutic Target Database. Nucleic Acids Res. 2010, 38, D787–D791. [Google Scholar] [CrossRef]
- Saier, M.H.; Reddy, V.S.; Moreno-Hagelsieb, G.; Hendargo, K.J.; Zhang, Y.; Iddamsetty, V.; Lam, K.J.K.; Tian, N.; Russum, S.; Wang, J.; et al. The Transporter Classification Database (TCDB): 2021 update. Nucleic Acids Res. 2021, 49, D461–D467. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef] [PubMed]
- Sayers, S.; Li, L.; Ong, E.; Deng, S.; Fu, G.; Lin, Y.; Yang, B.; Zhang, S.; Fa, Z.; Zhao, B.; et al. Victors: A web-based knowledge base of virulence factors in human and animal pathogens. Nucleic Acids Res. 2019, 47, D693–D700. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Chuvochina, M.; Rinke, C.; Mussig, A.J.; Chaumeil, P.A.; Hugenholtz, P. GTDB: An ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 2022, 50, D785–D794. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Carbasse, J.S.; Peinado-Olarte, R.L.; Goker, M. TYGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res. 2022, 50, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; He, Y.; Korpelainen, H.; Niinemets, U.; Li, C. Allelochemicals and soil microorganisms jointly mediate sex-specific belowground interactions in dioecious Populus cathayana. New Phytol. 2023, 240, 1519–1533. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, L.; Liu, J.; Korpelainen, H.; Li, C. Plant sex affects plant-microbiome assemblies of dioecious Populus cathayana trees under different soil nitrogen conditions. Microbiome 2022, 10, 191. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Ayangbenro, A.S.; Babalola, O.O. Genomic Analysis of Endophytic Bacillus cereus T4S and Its Plant Growth-Promoting Traits. Plants 2021, 10, 1776. [Google Scholar] [CrossRef]
- Balabanova, L.; Averianova, L.; Marchenok, M.; Son, O.; Tekutyeva, L. Microbial and Genetic Resources for Cobalamin (Vitamin B12) Biosynthesis: From Ecosystems to Industrial Biotechnology. Int. J. Mol. Sci. 2021, 22, 4522. [Google Scholar] [CrossRef]
- Eitinger, T.; Mandrand-Berthelot, M.A. Nickel transport systems in microorganisms. Arch. Microbiol. 2000, 173, 1–9. [Google Scholar] [CrossRef]
- Desguin, B.; Soumillion, P.; Hols, P.; Hausinger, R.P. Nickel-pincer cofactor biosynthesis involves LarB-catalyzed pyridinium carboxylation and LarE-dependent sacrificial sulfur insertion. Proc. Natl. Acad. Sci. USA 2016, 113, 5598–5603. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Wang, Y.; Xie, X.; Shi, Q. The Connection between Czc and Cad Systems Involved in Cadmium Resistance in Pseudomonas putida. Int. J. Mol. Sci. 2021, 22, 9697. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Diaz, J.; Escobar-Zepeda, A.; Adaya, L.; Rojas-Vargas, J.; Cuervo-Amaya, D.H.; Sanchez-Reyes, A.; Pardo-Lopez, L. Paenarthrobacter sp. GOM3 Is a Novel Marine Species With Monoaromatic Degradation Relevance. Front. Microbiol. 2021, 12, 713702. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Liao, T.H.; Wang, J.; Ye, Y.; Wei, Y.C.; Zhou, H.K.; Xiao, Y.; Zhi, X.Y.; Shao, Z.H.; Lyu, L.D.; et al. A recently evolved diflavin-containing monomeric nitrate reductase is responsible for highly efficient bacterial nitrate assimilation. J. Biol. Chem. 2020, 295, 5051–5066. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.F.; Liras, P. Molecular Mechanisms of Phosphate Sensing, Transport and Signalling in Streptomyces and Related Actinobacteria. Int. J. Mol. Sci. 2021, 22, 1129. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Vad, B.S.; Stenvang, M.; Otzen, D.E.; Meyer, R.L. The antimicrobial mechanism of action of epsilon-poly-l-lysine. Appl. Environ. Microbiol. 2014, 80, 7758–7770. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Cui, K.; Li, Q.; Cao, J.; Jiang, W. Epsilon-poly-l-lysine (epsilon-PL) exhibits multifaceted antifungal mechanisms of action that control postharvest Alternaria rot. Int. J. Food Microbiol. 2021, 348, 109224. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.; Morais, T.P.; Zaini, P.A.; Campos, C.S.; Almeida-Souza, H.O.; Dandekar, A.M.; Nascimento, R.; Goulart, L.R. Antimicrobial activity of Epsilon-Poly-L-lysine against phytopathogenic bacteria. Sci. Rep. 2020, 10, 11324. [Google Scholar] [CrossRef]
- Teta, R.; Marteinsson, V.T.; Longeon, A.; Klonowski, A.M.; Groben, R.; Bourguet-Kondracki, M.L.; Costantino, V.; Mangoni, A. Thermoactinoamide A, an Antibiotic Lipophilic Cyclopeptide from the Icelandic Thermophilic Bacterium Thermoactinomyces vulgaris. J. Nat. Prod. 2017, 80, 2530–2535. [Google Scholar] [CrossRef]
- Ponpandian, L.N.; Rim, S.O.; Shanmugam, G.; Jeon, J.; Park, Y.H.; Lee, S.K.; Bae, H. Phylogenetic characterization of bacterial endophytes from four Pinus species and their nematicidal activity against the pine wood nematode. Sci. Rep. 2019, 9, 12457. [Google Scholar] [CrossRef]
- Li, K.; Chen, W.H.; Bruner, S.D. Microbial siderophore-based iron assimilation and therapeutic applications. Biometals 2016, 29, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Marahiel, M.A. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef] [PubMed]
- Vodyanitskii, Y.N.; Shoba, S.A. Biogeochemistry of carbon, iron, and heavy metals in wetlands (Analytical review). Mosc. Univ. Soil. Sci. Bull. 2015, 70, 89–97. [Google Scholar] [CrossRef]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From Plants to Food and Feed Industries. Methods Mol. Biol. 2018, 1852, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Stewart, J.J.; Lopez-Pozo, M.; Polutchko, S.K.; Adams, W.W., 3rd. Zeaxanthin, a Molecule for Photoprotection in Many Different Environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and Photosynthesis. Subcell. Biochem. 2016, 79, 111–139. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Niu, W.; Pang, L.; Bian, X.; Zhang, Y.; Zhong, G. Unusual Post-Translational Modifications in the Biosynthesis of Lasso Peptides. Int. J. Mol. Sci. 2022, 23, 7231. [Google Scholar] [CrossRef] [PubMed]
- Semenzato, G.; Alonso-Vasquez, T.; Del Duca, S.; Vassallo, A.; Riccardi, C.; Zaccaroni, M.; Mucci, N.; Padula, A.; Emiliani, G.; Palumbo Piccionello, A.; et al. Genomic Analysis of Endophytic Bacillus-Related Strains Isolated from the Medicinal Plant Origanum vulgare L. Revealed the Presence of Metabolic Pathways Involved in the Biosynthesis of Bioactive Compounds. Microorganisms 2022, 10, 919. [Google Scholar] [CrossRef]
- Hegemann, J.D.; Zimmermann, M.; Xie, X.; Marahiel, M.A. Lasso peptides: An intriguing class of bacterial natural products. Acc. Chem. Res. 2015, 48, 1909–1919. [Google Scholar] [CrossRef]
- Lohans, C.T.; Huang, Z.; van Belkum, M.J.; Giroud, M.; Sit, C.S.; Steels, E.M.; Zheng, J.; Whittal, R.M.; McMullen, L.M.; Vederas, J.C. Structural characterization of the highly cyclized lantibiotic paenicidin A via a partial desulfurization/reduction strategy. J. Am. Chem. Soc. 2012, 134, 19540–19543. [Google Scholar] [CrossRef]
- Hampton, H.G.; Watson, B.N.J.; Fineran, P.C. The arms race between bacteria and their phage foes. Nature 2020, 577, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Safari, F.; Sharifi, M.; Farajnia, S.; Akbari, B.; Karimi Baba Ahmadi, M.; Negahdaripour, M.; Ghasemi, Y. The interaction of phages and bacteria: The co-evolutionary arms race. Crit. Rev. Biotechnol. 2020, 40, 119–137. [Google Scholar] [CrossRef]
- Di Felice, F.; Micheli, G.; Camilloni, G. Restriction enzymes and their use in molecular biology: An overview. J. Biosci. 2019, 44, 38. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Koblan, L.W.; Liu, D.R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020, 38, 824–844. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Melamed, S.; Ofir, G.; Leavitt, A.; Lopatina, A.; Keren, M.; Amitai, G.; Sorek, R. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 2018, 359, eaar4120. [Google Scholar] [CrossRef] [PubMed]
- Millman, A.; Melamed, S.; Leavitt, A.; Doron, S.; Bernheim, A.; Hor, J.; Garb, J.; Bechon, N.; Brandis, A.; Lopatina, A.; et al. An expanded arsenal of immune systems that protect bacteria from phages. Cell Host Microbe 2022, 30, 1556–1569.e1555. [Google Scholar] [CrossRef] [PubMed]
- Phillips, Z.N.; Husna, A.U.; Jennings, M.P.; Seib, K.L.; Atack, J.M. Phasevarions of bacterial pathogens—Phase-variable epigenetic regulators evolving from restriction-modification systems. Microbiology 2019, 165, 917–928. [Google Scholar] [CrossRef]
- Shmakov, S.; Smargon, A.; Scott, D.; Cox, D.; Pyzocha, N.; Yan, W.; Abudayyeh, O.O.; Gootenberg, J.S.; Makarova, K.S.; Wolf, Y.I.; et al. Diversity and evolution of class 2 CRISPR-Cas systems. Nat. Rev. Microbiol. 2017, 15, 169–182. [Google Scholar] [CrossRef]
- Patel, P.H.; Maxwell, K.L. Prophages provide a rich source of antiphage defense systems. Curr. Opin. Microbiol. 2023, 73, 102321. [Google Scholar] [CrossRef]
- Gao, L.; Altae-Tran, H.; Bohning, F.; Makarova, K.S.; Segel, M.; Schmid-Burgk, J.L.; Koob, J.; Wolf, Y.I.; Koonin, E.V.; Zhang, F. Diverse enzymatic activities mediate antiviral immunity in prokaryotes. Science 2020, 369, 1077–1084. [Google Scholar] [CrossRef]
- Tal, N.; Millman, A.; Stokar-Avihail, A.; Fedorenko, T.; Leavitt, A.; Melamed, S.; Yirmiya, E.; Avraham, C.; Brandis, A.; Mehlman, T.; et al. Bacteria deplete deoxynucleotides to defend against bacteriophage infection. Nat. Microbiol. 2022, 7, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Millman, A.; Melamed, S.; Amitai, G.; Sorek, R. Diversity and classification of cyclic-oligonucleotide-based anti-phage signalling systems. Nat. Microbiol. 2020, 5, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Ofir, G.; Melamed, S.; Sberro, H.; Mukamel, Z.; Silverman, S.; Yaakov, G.; Doron, S.; Sorek, R. DISARM is a widespread bacterial defence system with broad anti-phage activities. Nat. Microbiol. 2018, 3, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, T.; Sberro, H.; Weinstock, E.; Cohen, O.; Doron, S.; Charpak-Amikam, Y.; Afik, S.; Ofir, G.; Sorek, R. BREX is a novel phage resistance system widespread in microbial genomes. EMBO J. 2015, 34, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Koopal, B.; Potocnik, A.; Mutte, S.K.; Aparicio-Maldonado, C.; Lindhoud, S.; Vervoort, J.J.M.; Brouns, S.J.J.; Swarts, D.C. Short prokaryotic Argonaute systems trigger cell death upon detection of invading DNA. Cell 2022, 185, 1471–1486.e1419. [Google Scholar] [CrossRef] [PubMed]
- Bidnenko, E.; Chopin, A.; Ehrlich, S.D.; Chopin, M.C. Activation of mRNA translation by phage protein and low temperature: The case of Lactococcus lactis abortive infection system AbiD1. BMC Mol. Biol. 2009, 10, 4. [Google Scholar] [CrossRef]
- Dy, R.L.; Przybilski, R.; Semeijn, K.; Salmond, G.P.; Fineran, P.C. A widespread bacteriophage abortive infection system functions through a Type IV toxin-antitoxin mechanism. Nucleic Acids Res. 2014, 42, 4590–4605. [Google Scholar] [CrossRef]
- Rye, P.T.; Delaney, J.C.; Netirojjanakul, C.; Sun, D.X.; Liu, J.Z.; Essigmann, J.M. Mismatch repair proteins collaborate with methyltransferases in the repair of O6-methylguanine. DNA Repair 2008, 7, 170–176. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus Group: Bacillus Species with Pathogenic Potential. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Enosi Tuipulotu, D.; Mathur, A.; Ngo, C.; Man, S.M. Bacillus cereus: Epidemiology, Virulence Factors, and Host-Pathogen Interactions. Trends Microbiol. 2021, 29, 458–471. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Fracchia, F.; Mangeot-Peter, L.; Jacquot, L.; Martin, F.; Veneault-Fourrey, C.; Deveau, A. Colonization of Naive Roots from Populus tremula × alba Involves Successive Waves of Fungi and Bacteria with Different Trophic Abilities. Appl. Environ. Microbiol. 2021, 87, e02541-20. [Google Scholar] [CrossRef] [PubMed]
- Veach, A.M.; Morris, R.; Yip, D.Z.; Yang, Z.K.; Engle, N.L.; Cregger, M.A.; Tschaplinski, T.J.; Schadt, C.W. Rhizosphere microbiomes diverge among Populus trichocarpa plant-host genotypes and chemotypes, but it depends on soil origin. Microbiome 2019, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Pleban, S.; Chernin, L.; Chet, I. Chitinolytic activity of an endophytic strain of Bacillus cereus. Lett. Appl. Microbiol. 1997, 25, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gao, Y.; Li, X.; Chen, S.; Yan, S.; Tian, X. Identification and Nematicidal Characterization of Proteases Secreted by Endophytic Bacteria Bacillus cereus BCM2. Phytopathology 2020, 110, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, S.; Nachiyar, C.V. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac. J. Trop. Biomed. 2012, 2, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Sun, Z.; Li, Y.; He, H.; Zhang, Y.; Yang, X.; Wang, D.; Dong, B.; Zhou, H.; et al. Whole-genome analysis revealed the growth-promoting mechanism of endophytic bacterial strain Q2H1 in potato plants. Front. Microbiol. 2022, 13, 1035901. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Reina, J.C.; Sampedro, I.; Llamas, I.; Martinez-Checa, F. Peribacillus castrilensis sp. nov.: A Plant-Growth-Promoting and Biocontrol Species Isolated From a River Otter in Castril, Granada, Southern Spain. Front. Plant Sci. 2022, 13, 896728. [Google Scholar] [CrossRef]
- Montecillo, J.A.V.; Bae, H. In Silico analysis of koranimine, a cyclic imine compound from Peribacillus frigoritolerans reveals potential nematicidal activity. Sci. Rep. 2022, 12, 18883. [Google Scholar] [CrossRef]
- Gan, H.Y.; Gan, H.M.; Savka, M.A.; Triassi, A.J.; Wheatley, M.S.; Smart, L.B.; Fabio, E.S.; Hudson, A.O. Whole-genome sequences of 13 endophytic bacteria isolated from shrub willow (Salix) grown in geneva, new york. Genome Announc. 2014, 2. [Google Scholar] [CrossRef]
- Upreti, R.; Thomas, P. Root-associated bacterial endophytes from Ralstonia solanacearum resistant and susceptible tomato cultivars and their pathogen antagonistic effects. Front. Microbiol. 2015, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ji, B.; Su, X.; Li, H.; Dong, C.; Chen, S.; Zhu, Y.; Feng, W. Isolation of endophytic bacteria from Rehmannia glutinosa Libosch and their potential to promote plant growth. J. Gen. Appl. Microbiol. 2020, 66, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Kukla, M.; Plociniczak, T.; Piotrowska-Seget, Z. Diversity of endophytic bacteria in Lolium perenne and their potential to degrade petroleum hydrocarbons and promote plant growth. Chemosphere 2014, 117, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Husseiny, S.; Dishisha, T.; Soliman, H.A.; Adeleke, R.; Raslan, M. Characterization of growth promoting bacterial endophytes isolated from Artemisia annua L. S. Afr. J. Bot. 2021, 143, 238–247. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Fischbach, M.A. What Lives On Our Skin: Ecology, Genomics and Therapeutic Opportunities Of the Skin Microbiome. Drug Discov. Today Dis. Mech. 2013, 10, e83–e89. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Swarna, G.K.; Roy, P.K.; Patil, P. Identification of culturable and originally non-culturable endophytic bacteria isolated from shoot tip cultures of banana cv. Grand Naine. Plant Cell Tissue Organ Cult. 2008, 93, 55–63. [Google Scholar] [CrossRef]
- Brown, S.D.; Utturkar, S.M.; Klingeman, D.M.; Johnson, C.M.; Martin, S.L.; Land, M.L.; Lu, T.Y.; Schadt, C.W.; Doktycz, M.J.; Pelletier, D.A. Twenty-one genome sequences from Pseudomonas species and 19 genome sequences from diverse bacteria isolated from the rhizosphere and endosphere of Populus deltoides. J. Bacteriol. 2012, 194, 5991–5993. [Google Scholar] [CrossRef] [PubMed]
- Tambong, J.T.; Xu, R.; Bromfield, E.S.P. Pseudomonas canadensis sp. nov., a biological control agent isolated from a field plot under long-term mineral fertilization. Int. J. Syst. Evol. Microbiol. 2017, 67, 889–895. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]

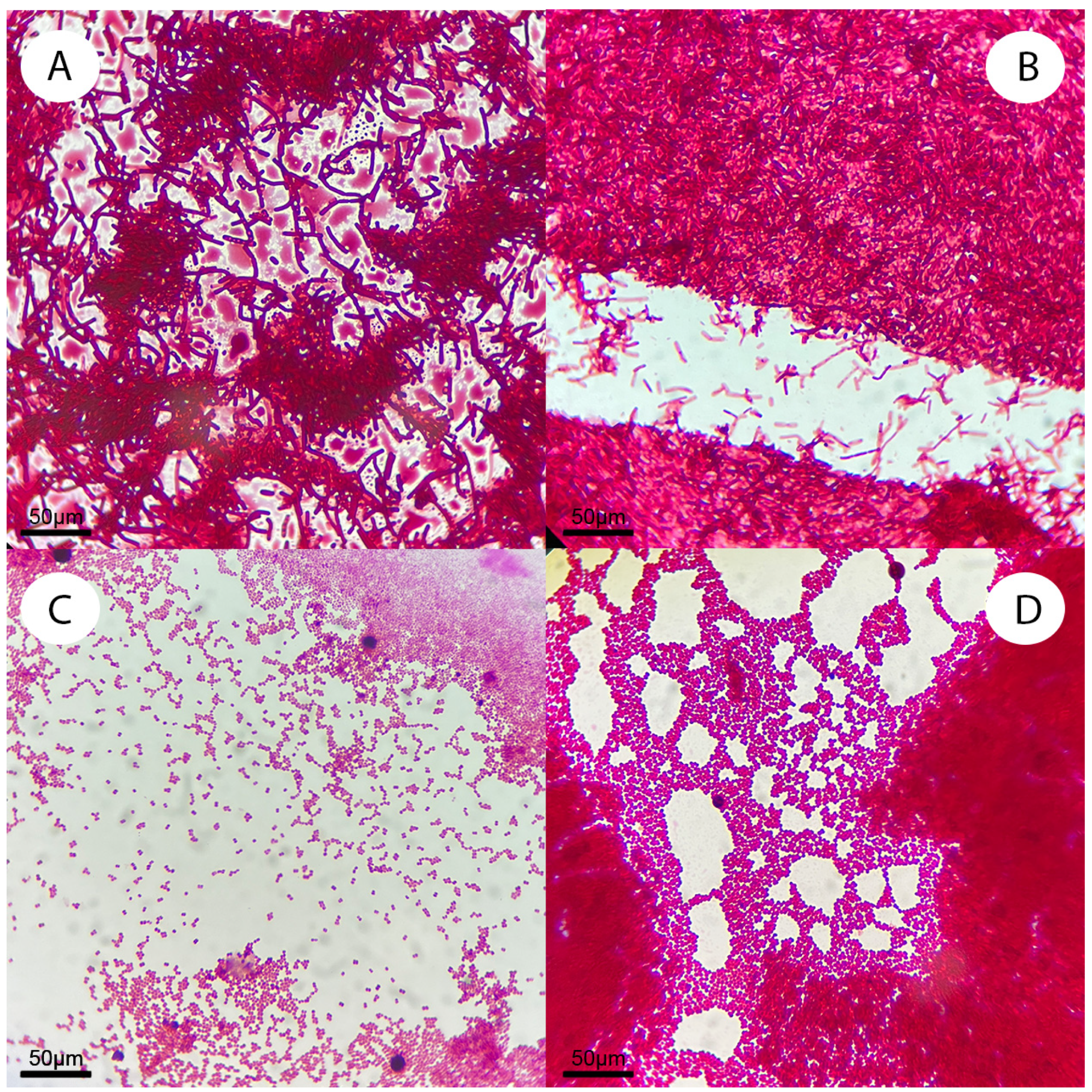
| Strain | Location |
|---|---|
| s2 | 56.349087, 44.059552 |
| s3 | 56.352357, 44.059899 |
| s4 | 44.027473, 43.06353 |
| s5 | 43.491915, 39.896625 |
| s9 | 41.8821, 48.534351 |
| s10 | 56.346653, 44.061317 |
| s12 | 43.954701, 42.765658 |
| s13 | 41.881878, 48.534617 |
| s14 | 41.880614, 48.528303 |
| s15 | 50.793133, 41.979933 |
| s17 | 43.954701, 42.765658 |
| s20 | 50.786221, 41.986017 |
| s22 | 50.789744, 41.988373 |
| s23 | 44.027603, 43.067865 |
| Strain | Number of Contigs (>1 Kb) | Largest Contig, Kb | Total Length, Mb | GC (%) | N50, Kb | auN, Kb | L50 | Reference Genome Length, Mb |
|---|---|---|---|---|---|---|---|---|
| s2 | 59 | 774.2 | 5.90 | 60.0 | 202.6 | 293.0 | 9 | 6.5 |
| s3 | 47 | 388.7 | 2.47 | 32.8 | 98.3 | 140.0 | 7 | 2.6 |
| s4 | 45 | 605.5 | 5.59 | 40.6 | 316.5 | 306.4 | 7 | 5.6 |
| s5 | 38 | 342.7 | 2.50 | 58.9 | 153.0 | 158.7 | 6 | 2.5 |
| s9 | 40 | 359.6 | 2.52 | 72.8 | 128.3 | 172.2 | 6 | 2.5 |
| s10 | 38 | 768.5 | 6.29 | 60.3 | 450.6 | 420.9 | 6 | 6.4 |
| s12 | 79 | 303.6 | 4.17 | 72.2 | 182.4 | 138.6 | 10 | 4 |
| s13 | 29 | 877.4 | 5.41 | 35.3 | 570.2 | 541.5 | 4 | 5.8 |
| s14 | 30 | 788.5 | 5.31 | 35.1 | 451.8 | 466.1 | 4 | 5.8 |
| s15 | 98 | 395.1 | 6.88 | 60.3 | 159.3 | 163.0 | 15 | 6.2 * |
| s17 | 197 | 122.0 | 4.19 | 72.2 | 33.3 | 44.1 | 35 | 4 |
| s20 | 144 | 269.2 | 6.72 | 60.4 | 99.7 | 100.6 | 25 | 6.2 * |
| s22 | 199 | 765.7 | 5.85 | 35.0 | 302.8 | 324.4 | 7 | 5.8 |
| s23 | 89 | 941.0 | 5.67 | 40.2 | 185.3 | 298.0 | 8 | 5.6 |
| Strain | Number of CDSs | Number of rRNA Genes | Number of tRNA Genes | Completeness, CheckM (DFAST) | Completeness, BUSCO | Contamination, CheckM (DFAST) | Contamination, BUSCO | Taxonomic Classification (GTDB (G), TYGS (T)) | Closest Type Strains (or Species Representatives), ANI |
|---|---|---|---|---|---|---|---|---|---|
| s2 | 5203 | 4 | 66 | 100.00% | 100.00% | 0.19% | 0.27% | Pseudomonas siliginis (G *, T *) | P. siliginis SWRI31 [96.46%] |
| s3 | 2481 | 4 | 49 | 99.80% | 99.67% | 0.21% | 0.00% | Staphylococcus haemolyticus (G, T) | S. haemolyticus NCTC 11042 [97.13%] |
| s4 | 5449 | 6 | 67 | 99.35% | 100.00% | 0.66% | 0.81% | Peribacillus frigoritolerans (G, T) | P. frigoritolerans FJAT-2396 [97.10%], DSM 8801 [96.81%]; P. castrilensis N3 LMG 32505 [98.28%] |
| s5 | 2198 | 3 | 53 | 99.49% | 98.29% | 0.41% | 0.00% | Corynebacterium amycolatum (G *, T *) | C. amycolatum “A” SK46 § [95.23%]; C. amycolatum ATCC 49368 [94.83%] |
| s9 | 2299 | 3 | 52 | 98.54% | 98.88% | 0.23% | 0.19% | Micrococcus luteus (G, T) | M. luteus ATCC 4698 [97.22%]; M. aloeverae DSM 27472 [98.26%]; M. yunnanensis DSM 21948 [98.16%] |
| s10 | 5718 | 4 | 59 | 100.00% | 100.00% | 0.35% | 0.55% | Pseudomonas canadensis (G, T) | P. canadensis 2–92 [97.70%] |
| s12 | 3808 | 3 | 55 | 99.54% | 99.07% | 4.87% | 1.69% | Kocuria rosea (G, T) | K. rosea ATCC 186 [99.37%] |
| s13 | 5448 | 8 | 56 | 99.22% | 100.00% | 0.59% | 4.03% | Bacillus cereus (G, T) | B. cereus ATCC 14579 [98.26%] |
| s14 | 5267 | 6 | 59 | 99.22% | 99.34% | 0.79% | 4.10% | Bacillus bombysepticus (G), B. cereus (T) | B. cereus ATCC 14579 [97.10%]; B. cereus FORC087 (reclassified into B. bombysepticus in GTDB) [98.52%] |
| s15 | 6159 | 3 | 55 | 81.04% | 99.74% | 12.99% | 1.64% | Pseudomonas sp. (G) ** | P. putida “G” ASAD §§ [93.13%]; P. umsongensis DSM 16611 [91.19%] |
| s17 | 3817 | 3 | 53 | 99.54% | 99.44% | 5.37% | 1.69% | Kocuria rosea (G, T) | K. rosea ATCC 186 [99.38%] |
| s20 | 6026 | 4 | 66 | 80.99% | 99.45% | 11.66% | 1.65% | Pseudomonas sp. (G) ** | P. putida “G” ASAD §§ [93.19%]; P. umsongensis DSM 16611 [91.10%] |
| s22 | 5629 | 5 | 70 | 99.22% | 99.67% | 2.54% | 4.07% | B. cereus (G, T) | B. cereus ATCC 14579 [98.18%] |
| s23 | 5352 | 6 | 71 | 99.35% | 100.00% | 3.58% | 3.23% | P. frigoritolerans (G, T) | P. frigoritolerans FJAT-2396 [98.75%]; DSM 8801 [97.18%]; P. castrilensis N3 (reclassified into P. frigoritolerans in GTDB) [97.27%] |
| Phylum | Class | Order | Family | Genus | Species | Strain |
|---|---|---|---|---|---|---|
| Bacillota | Bacilli | Bacillales | Bacillaceae | Bacillus | B. cereus | s13 |
| s22 | ||||||
| s14 | ||||||
| Peribacillus | P. frigoritolerans | s4 | ||||
| s23 | ||||||
| Staphylococcaceae | Staphylococcus | S. haemolyticus | s3 | |||
| Actinomycetota | Actinomycetes | Micrococcales | Micrococcaceae | Micrococcus | M. luteus. | s9 |
| Kocuria | K. rosea | s12 | ||||
| s17 | ||||||
| Mycobacteriales | Corynebacteriaceae | Corynebacterium | C. amycolatum | s5 | ||
| Pseudomonadota | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Pseudomonas | P. canadensis | s10 |
| P. siliginis | s2 | |||||
| Pseudomonas sp. | s15 | |||||
| s20 |
| Strain | Bacterial Species | Sex of the Host Tree |
|---|---|---|
| s2 | P. siliginis | Male |
| s3 | S. haemolyticus | Female |
| s4 | P. frigoritolerans | Male |
| s5 | C. amycolatum | Female |
| s9 | M. luteus | Male |
| s10 | P. canadensis | Male |
| s12 | K. rosea | Male |
| s13 | B. cereus | Male |
| s14 | B. cereus | Male |
| s15 | Pseudomonas sp. | Male |
| s17 | K. rosea | Male |
| s20 | Pseudomonas sp. | Male |
| s22 | B. cereus | Female |
| s23 | P. frigoritolerans | Female |
| Strain | Ashby’s Mannitol Medium | Ashby’s Medium | Dobereiner’s Medium Malic Acid + | Dobereiner’s Medium Malic Acid − |
|---|---|---|---|---|
| s2 | + | + | − | + |
| s3 | + | + | − | + |
| s4 | + | + | − | − |
| s5 | + | + | − | − |
| s9 | + | + | − | − |
| s10 | + | + | + | + |
| s12 | + | + | − | + |
| s13 | + | + | − | − |
| s14 | + | − | − | + |
| s15 | + | − | − | + |
| s17 | + | + | − | − |
| s20 | + | + | − | + |
| s22 | + | + | − | + |
| s23 | + | + | − | + |
| Strain | Bacterial Species | Region | From | To | Type | Most Similar Known Cluster | Similarity |
|---|---|---|---|---|---|---|---|
| s13 | B. cereus | Region 1.1 | 124,986 | 138,693 | NI-siderophore | petrobactin | 100% |
| Region 9.1 | 45,152 | 96,900 | NRP-metallophore | bacillibactin | 85% | ||
| s22 | B. cereus | Region 6.3 | 202,313 | 254,061 | NRP-metallophore | bacillibactin | 85% |
| Region 14.1 | 9815 | 23,522 | NI-siderophore | petrobactin | 100% | ||
| Region 134.1 | 1 | 1304 | NRPS | thermoactinoamide A | 100% | ||
| s14 | B. cereus | Region 7.1 | 41,536 | 93,284 | NRP-metallophore | bacillibactin | 85% |
| Region 14.1 | 9914 | 23,621 | NI-siderophore | petrobactin | 100% | ||
| s4 | P. frigoritolerans | Region 1.2 | 537,557 | 553,069 | NI-siderophore | schizokinen | 60% |
| Region 11.1 | 60,018 | 128,334 | NRPS | koranimine | 87% | ||
| s23 | P. frigoritolerans | Region 3.1 | 66,600 | 126,919 | NRPS | koranimine | 87% |
| Region 6.1 | 51,405 | 66,917 | NI-siderophore | schizokinen | 60% | ||
| Region 14.1 | 42,776 | 66,671 | lassopeptide | paeninodin | 100% | ||
| s3 | S. haemolyticus | Region 8.1 | 70,705 | 85,702 | NI-siderophore | staphyloferrin A | 100% |
| s9 | M. luteus | Region 22.1 | 9041 | 23,211 | terpene | carotenoid | 66% |
| s12 | K. rosea | Region 11.1 | 89,770 | 123,837 | NAPAA | branched-chain fatty acid | 100% |
| s17 | K. rosea | Region 52.1 | 5871 | 26,881 | NAPAA | ε-poly-L-lysine | 100% |
| Region 54.1 | 649 | 26,385 | NAPAA | branched-chain fatty acid | 66% |
| Strain | Bacterial Species | Count | Systems |
|---|---|---|---|
| s13 | B. cereus | 7 | DMS, Mokosh TypeII, PD-T4-6 (×2), RM type IV, argonaute solo, wadjet type III |
| s22 | B. cereus | 10 | DMS, Lamassu, Mokosh Type II, PD-T4-6 (×2), RM type IV, argonaute solo (×2), wadjet type III |
| s14 | B. cereus/bombysepticus | 7 | Lamassu, Mokosh Type II, PD-T4-6 (×2), Tiamat, argonaute solo, argonaute type III |
| s4 | P. frigoritolerans | 8 | PD-T4-6, PT DndFGH, RM type I, Uzume (×2), VSPR, cas type I-B1, septu type I |
| s23 | P. frigoritoleran | 5 | HEC-06, PD-T4-6, PT SspABCD, PT SspFGH, RM type IV |
| s3 | S. haemolyticus | 3 | DRT class I, RM type II (×2) |
| s9 | M. luteus | 16 | AbiD (×2), AbiE, DMS (×2), Mokosh Type II, RM type I (×2), RM type IIG, RM type IV, RosmerTA, SoFic, Uzume, cbass type I, dXTPase, wadjet type I |
| s12 | K. rosea | 7 | DMS (×2), PD-T4-6, cbass, dXTPase, mza, ppl |
| s17 | K. rosea | 7 | DMS (×2), PD-T4-6, cbass, dXTPase, mza, ppl |
| s5 | C. amycolatum | 9 | DMS other (×2), HEC-06, PD-T4-6, RM type II (×2), RM type IV, SoFic, dXTPase |
| s10 | P. canadensis | 8 | DMS, DRT type III, GAO 19, PD-T4-6, kiwa (×2), septu type I, tmn |
| s2 | P.siliginis | 11 | DMS (×3), DRT class I, DRT class II, PD-T4-6, PD-T4-7, SEFIR, SoFic, VSPR, septu type I |
| s15 | Pseudomonas sp. | 7 | AbiD (×2), Azaca, DMS, Dpd, SoFic (×2) |
| s20 | Pseudomonas sp. | 7 | AbiD (×2), DMS (×2), DRT class I, SoFic, gabija |
| Antibiotic Resistance | Drug Target | Transporter | Virulence Factor | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Bacterial Species | CARD | PATRIC | NDARO | DrugBank | TTD | TCDB | PATRIC_VF | VFDB | Victors |
| s13 | B. cereus | 7 | 49 | 4 | 28 | 47 | 6 | 11 | ||
| s22 | B. cereus | 6 | 54 | 4 | 28 | 52 | 1 | 12 | 12 | |
| s14 | B. cereus/bombysepticus | 6 | 51 | 4 | 27 | 47 | 9 | 12 | ||
| s4 | P. frigoritolerans | 1 | 42 | 13 | 1 | 14 | 2 | 1 | 3 | |
| s23 | P. frigoritolerans. | 1 | 45 | 14 | 2 | 16 | 3 | 4 | 5 | |
| s3 | S. haemolyticus | 15 | 40 | 8 | 26 | 10 | 45 | 1 | 18 | |
| s9 | M. luteus | 1 | 28 | 6 | 1 | 8 | 3 | 2 | 1 | |
| s12 | K. rosea | 1 | 42 | 2 | 3 | 1 | ||||
| s17 | K. rosea | 1 | 42 | 2 | 4 | 1 | ||||
| s5 | C. amycolatum | 1 | 23 | 3 | 1 | 5 | 4 | 2 | 3 | |
| s10 | P. canadensis | 3 | 72 | 27 | 6 | 73 | 1 | 27 | 25 | |
| s2 | P. siliginis | 5 | 66 | 28 | 8 | 69 | 24 | 28 | ||
| s15 | Pseudomonas sp. | 2 | 85 | 32 | 6 | 66 | 26 | 24 | ||
| s20 | Pseudomonas sp. | 3 | 85 | 1 | 37 | 7 | 68 | 25 | 25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gladysh, N.S.; Bogdanova, A.S.; Kovalev, M.A.; Krasnov, G.S.; Volodin, V.V.; Shuvalova, A.I.; Ivanov, N.V.; Popchenko, M.I.; Samoilova, A.D.; Polyakova, A.N.; et al. Culturable Bacterial Endophytes of Wild White Poplar (Populus alba L.) Roots: A First Insight into Their Plant Growth-Stimulating and Bioaugmentation Potential. Biology 2023, 12, 1519. https://doi.org/10.3390/biology12121519
Gladysh NS, Bogdanova AS, Kovalev MA, Krasnov GS, Volodin VV, Shuvalova AI, Ivanov NV, Popchenko MI, Samoilova AD, Polyakova AN, et al. Culturable Bacterial Endophytes of Wild White Poplar (Populus alba L.) Roots: A First Insight into Their Plant Growth-Stimulating and Bioaugmentation Potential. Biology. 2023; 12(12):1519. https://doi.org/10.3390/biology12121519
Chicago/Turabian StyleGladysh, Natalya S., Alina S. Bogdanova, Maxim A. Kovalev, George S. Krasnov, Vsevolod V. Volodin, Anastasia I. Shuvalova, Nikita V. Ivanov, Mikhail I. Popchenko, Aleksandra D. Samoilova, Aleksandra N. Polyakova, and et al. 2023. "Culturable Bacterial Endophytes of Wild White Poplar (Populus alba L.) Roots: A First Insight into Their Plant Growth-Stimulating and Bioaugmentation Potential" Biology 12, no. 12: 1519. https://doi.org/10.3390/biology12121519
APA StyleGladysh, N. S., Bogdanova, A. S., Kovalev, M. A., Krasnov, G. S., Volodin, V. V., Shuvalova, A. I., Ivanov, N. V., Popchenko, M. I., Samoilova, A. D., Polyakova, A. N., Dmitriev, A. A., Melnikova, N. V., Karpov, D. S., Bolsheva, N. L., Fedorova, M. S., & Kudryavtseva, A. V. (2023). Culturable Bacterial Endophytes of Wild White Poplar (Populus alba L.) Roots: A First Insight into Their Plant Growth-Stimulating and Bioaugmentation Potential. Biology, 12(12), 1519. https://doi.org/10.3390/biology12121519











