Essential Oil Molecules Can Break the Loop of Oxidative Stress in Neurodegenerative Diseases
Abstract
:Simple Summary
Abstract
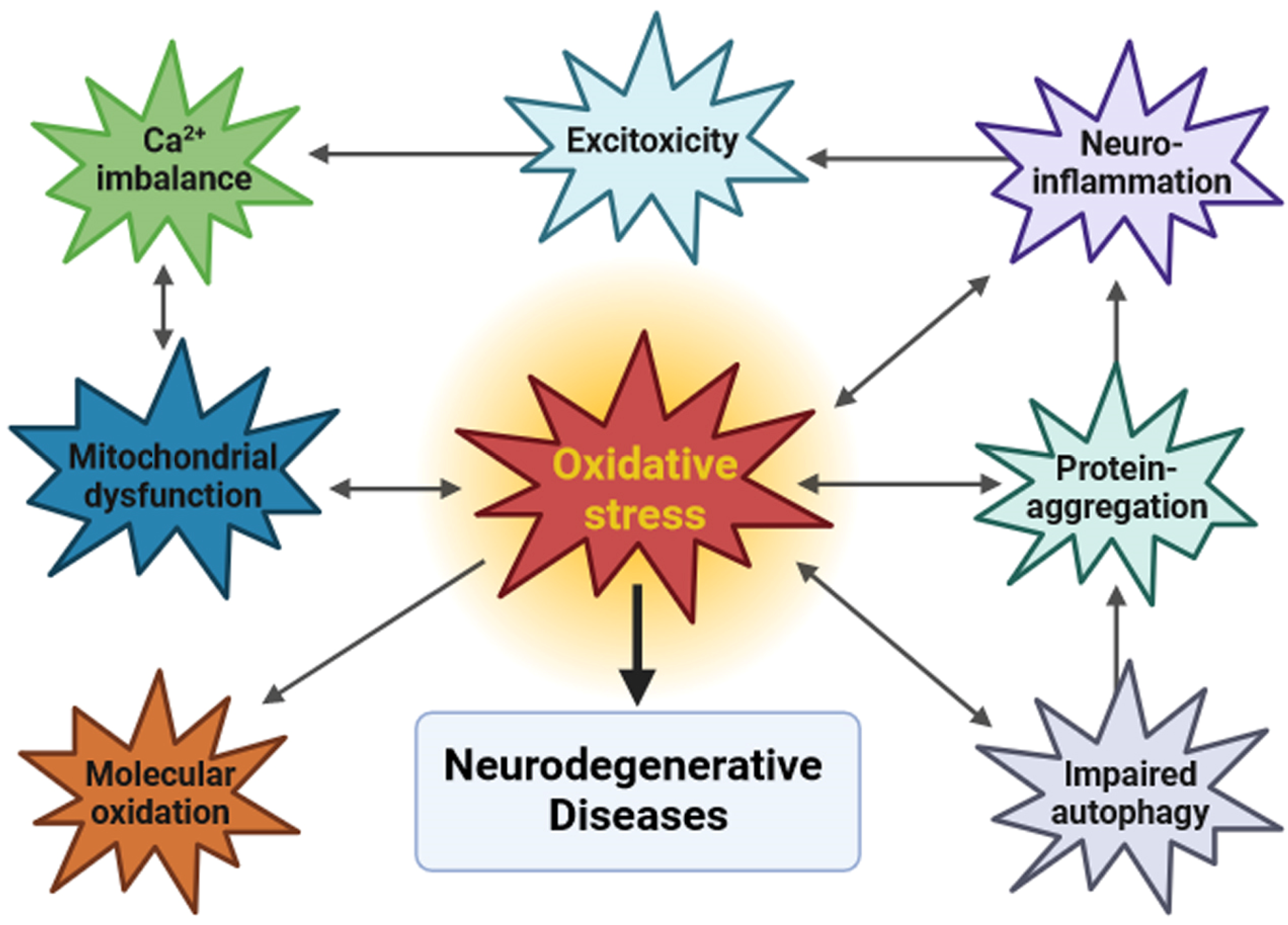
1. Neurodegeneration and Oxidative Stress
2. Current Therapeutic Approaches to NDs
3. Essential Oils and Neurodegenerative Diseases
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Komleva, Y.; Chernykh, A.; Lopatina, O.; Gorina, Y.; Lokteva, I.; Salmina, A.; Gollasch, M. Inflamm-Aging and Brain Insulin Resistance: New Insights and Role of Life-Style Strategies on Cognitive and Social Determinants in Aging and Neurodegeneration. Front. Neurosci. 2021, 14, 618395. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Doneddu, P.E.; Riva, N.; Nobile-Orazio, E.; Quattrini, A. Diet, Microbiota and Brain Health: Unraveling the Network Intersecting Metabolism and Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 7471. [Google Scholar] [CrossRef] [PubMed]
- Espinós, C.; Galindo, M.I.; García-Gimeno, M.A.; Ibáñez-Cabellos, J.S.; Martínez-Rubio, D.; Millán, J.M.; Rodrigo, R.; Sanz, P.; Seco-Cervera, M.; Sevilla, T.; et al. Oxidative Stress, a Crossroad between Rare Diseases and Neurodegeneration. Antioxidants 2020, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Bordoni, L.; Gabbianelli, R. Mitochondrial DNA and Neurodegeneration: Any Role for Dietary Antioxidants? Antioxidants 2020, 9, 764. [Google Scholar] [CrossRef]
- Dominiak, K.; Jarmuszkiewicz, W. The Relationship between Mitochondrial Reactive Oxygen Species Production and Mitochondrial Energetics in Rat Tissues with Different Contents of Reduced Coenzyme Q. Antioxidants 2021, 10, 533. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Behl, T.; Aleya, L.; Rahman, M.H.; Kumar, A.; Arora, S.; Akter, R. Role of Metallic Pollutants in Neurodegeneration: Effects of Aluminum, Lead, Mercury, and Arsenic in Mediating Brain Impairment Events and Autism Spectrum Disorder. Environ. Sci. Pollut. Res. 2021, 28, 8989–9001. [Google Scholar] [CrossRef]
- Shichiri, M. The Role of Lipid Peroxidation in Neurological Disorders. J. Clin. Biochem. Nutr. 2014, 54, 151–160. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Liu, J.; Xie, F.; Su, B.; Wang, X. Abnormalities of Mitochondrial Dynamics in Neurodegenerative Diseases. Antioxidants 2017, 6, 25. [Google Scholar] [CrossRef]
- Peggion, C.; Scalcon, V.; Massimino, M.L.; Nies, K.; Lopreiato, R.; Rigobello, M.P.; Bertoli, A. SOD1 in ALS: Taking Stock in Pathogenic Mechanisms and the Role of Glial and Muscle Cells. Antioxidants 2022, 11, 614. [Google Scholar] [CrossRef]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, Calcium and Mitochondria: A Triad in Synaptic Neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shih, H.-P.; Vigont, V.; Hrdlicka, L.; Diggins, L.; Singh, C.; Mahoney, M.; Chesworth, R.; Shapiro, G.; Zimina, O.; et al. Neuronal Store-Operated Calcium Entry Pathway as a Novel Therapeutic Target for Huntington’s Disease Treatment. Chem. Biol. 2011, 18, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Egorova, P.; Popugaeva, E.; Bezprozvanny, I. Disturbed Calcium Signaling in Spinocerebellar Ataxias and Alzheimer’s Disease. Semin. Cell Dev. Biol. 2015, 40, 127–133. [Google Scholar] [CrossRef]
- Hisatsune, C.; Hamada, K.; Mikoshiba, K. Ca2+ Signaling and Spinocerebellar Ataxia. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1733–1744. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.; Shin, C.; Lee, S. Intersection between Redox Homeostasis and Autophagy: Valuable Insights into Neurodegeneration. Antioxidants 2021, 10, 694. [Google Scholar] [CrossRef] [PubMed]
- Koopman, M.B.; Ferrari, L.; Rüdiger, S.G.D. How Do Protein Aggregates Escape Quality Control in Neurodegeneration? Trends Neurosci. 2022, 45, 257–271. [Google Scholar] [CrossRef]
- Maurya, S.K.; Bhattacharya, N.; Mishra, S.; Bhattacharya, A.; Banerjee, P.; Senapati, S.; Mishra, R. Microglia Specific Drug Targeting Using Natural Products for the Regulation of Redox Imbalance in Neurodegeneration. Front. Pharmacol. 2021, 13, 654489. [Google Scholar] [CrossRef]
- Sood, A.; Preeti, K.; Fernandes, V.; Khatri, D.K.; Singh, S.B. Glia: A Major Player in Glutamate–GABA Dysregulation-mediated Neurodegeneration. J. Neurosci. Res. 2021, 99, 3148–3189. [Google Scholar] [CrossRef]
- Se Thoe, E.; Fauzi, A.; Tang, Y.Q.; Chamyuang, S.; Chia, A.Y.Y. A Review on Advances of Treatment Modalities for Alzheimer’s Disease. Life Sci. 2021, 276, 119129. [Google Scholar] [CrossRef]
- Kimura, S.; Harashima, H. Current Status and Challenges Associated with CNS-Targeted Gene Delivery across the BBB. Pharmaceutics 2020, 12, 1216. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global Estimates on the Number of Persons across the Alzheimer’s Disease Continuum. Alzheimer’s Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef]
- Aguzzi, A.; O’Connor, T. Protein Aggregation Diseases: Pathogenicity and Therapeutic Perspectives. Nat. Rev. Drug Discov. 2010, 9, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; de Haro, M.; Al-Ramahi, I.; Garaicoechea, L.L.; Jeong, H.-H.; Sonn, J.Y.; Tadros, B.; Liu, Z.; Botas, J.; Zoghbi, H.Y. Evolutionarily Conserved Regulators of Tau Identify Targets for New Therapies. Neuron 2023, 111, 824–838.e7. [Google Scholar] [CrossRef] [PubMed]
- Puranik, N.; Yadav, D.; Song, M. Advancements in the Application of Nanomedicine in Alzheimer’s Disease: A Therapeutic Perspective. Int. J. Mol. Sci. 2023, 24, 14044. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Shusharina, N.; Yukhnenko, D.; Botman, S.; Sapunov, V.; Savinov, V.; Kamyshov, G.; Sayapin, D.; Voznyuk, I. Modern Methods of Diagnostics and Treatment of Neurodegenerative Diseases and Depression. Diagnostics 2023, 13, 573. [Google Scholar] [CrossRef]
- Weiss, D.; Volkmann, J.; Fasano, A.; Kühn, A.; Krack, P.; Deuschl, G. Changing Gears—DBS For Dopaminergic Desensitization in Parkinson’s Disease? Ann. Neurol. 2021, 90, 699–710. [Google Scholar] [CrossRef]
- Swinnen, B.; Robberecht, W. The Phenotypic Variability of Amyotrophic Lateral Sclerosis. Nat. Rev. Neurol. 2014, 10, 661–670. [Google Scholar] [CrossRef]
- Tzeplaeff, L.; Wilfling, S.; Requardt, M.V.; Herdick, M. Current State and Future Directions in the Therapy of ALS. Cells 2023, 12, 1523. [Google Scholar] [CrossRef]
- Petrocelli, G.; Farabegoli, F.; Valerii, M.C.; Giovannini, C.; Sardo, A.; Spisni, E. Molecules Present in Plant Essential Oils for Prevention and Treatment of Colorectal Cancer (CRC). Molecules 2021, 26, 885. [Google Scholar] [CrossRef]
- Saracino, I.M.; Foschi, C.; Pavoni, M.; Spigarelli, R.; Valerii, M.C.; Spisni, E. Antifungal Activity of Natural Compounds vs. Candida spp.: A Mixture of Cinnamaldehyde and Eugenol Shows Promising In Vitro Results. Antibiotics 2022, 11, 73. [Google Scholar] [CrossRef]
- Aruoma, O.I. Free Radicals, Oxidative Stress, and Antioxidants in Human Health and Disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Abd Rashed, A.; Abd Rahman, A.Z.; Rathi, D.N.G. Essential Oils as a Potential Neuroprotective Remedy for Age-Related Neurodegenerative Diseases: A Review. Molecules 2021, 26, 1107. [Google Scholar] [CrossRef]
- Pérez-Rosés, R.; Risco, E.; Vila, E.; Peñalver, P.; Cañigueral, S. Biological and Nonbiological Antioxidant Activity of Some Essential Oils. J. Agric. Food Chem. 2016, 64, 4716–4724. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G.; Olupona, A.J.; Oyeleye, S.I.; Adewuni, T.M.; Nwanna, E.E. Comparative Study of Chemical Composition, In Vitro Inhibition of Cholinergic and Monoaminergic Enzymes, and Antioxidant Potentials of Essential Oil from Peels and Seeds of Sweet Orange (Citrus sinensis [L.] Osbeck) Fruits. J. Food Biochem. 2016, 40, 53–60. [Google Scholar] [CrossRef]
- Yen, P.-L.; Cheng, S.-S.; Wei, C.-C.; Lin, H.-Y.; Liao, V.H.-C.; Chang, S.-T. Antioxidant Activities and Reduced Amyloid-β Toxicity of 7-Hydroxycalamenene Isolated from the Essential Oil of Zelkova Serrata Heartwood. Nat. Prod. Commun. 2016, 11, 1357–1362. [Google Scholar] [CrossRef]
- Muhammad, F.; Liu, Y.; Wang, N.; Zhao, L.; Zhou, Y.; Yang, H.; Li, H. Rose Essential Oil Diminishes Dopaminergic Neuron Degenerations and Reduces A-synuclein Aggregation in Caenorhabditis elegans Models of Parkinson’s Disease. Phytother. Res. 2023, 37, 2877–2893. [Google Scholar] [CrossRef]
- Zhu, S.; Li, H.; Dong, J.; Yang, W.; Liu, T.; Wang, Y.; Wang, X.; Wang, M.; Zhi, D. Rose Essential Oil Delayed Alzheimer’s Disease-like Symptoms by SKN-1 Pathway in C. elegans. J. Agric. Food Chem. 2017, 65, 8855–8865. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, E.; Paudel, Y.N.; Piperi, C.; Mishra, A. Neuroprotective Potential of Cinnamon and Its Metabolites in Parkinson’s Disease: Mechanistic Insights, Limitations, and Novel Therapeutic Opportunities. J. Biochem. Mol. Toxicol. 2021, 35, e22720. [Google Scholar] [CrossRef] [PubMed]
- Sihoglu Tepe, A.; Ozaslan, M. Anti-Alzheimer, Anti-Diabetic, Skin-Whitening, and Antioxidant Activities of the Essential Oil of Cinnamomum zeylanicum. Ind. Crops Prod. 2020, 145, 112069. [Google Scholar] [CrossRef]
- Abuhamdah, S.; Abuhamdah, R.; Howes, M.-J.R.; Al-Olimat, S.; Ennaceur, A.; Chazot, P.L. Pharmacological and Neuroprotective Profile of an Essential Oil Derived from Leaves of Aloysia citrodora Palau. J. Pharm. Pharmacol. 2015, 67, 1306–1315. [Google Scholar] [CrossRef]
- Hritcu, L.; Bagci, E.; Aydin, E.; Mihasan, M. Antiamnesic and Antioxidants Effects of Ferulago Angulata Essential Oil Against Scopolamine-Induced Memory Impairment in Laboratory Rats. Neurochem. Res. 2015, 40, 1799–1809. [Google Scholar] [CrossRef]
- Sadiki, F.Z.; Idrissi, M.E.; Cioanca, O.; Trifan, A.; Hancianu, M.; Hritcu, L.; Postu, P.A. Tetraclinis Articulata Essential Oil Mitigates Cognitive Deficits and Brain Oxidative Stress in an Alzheimer’s Disease Amyloidosis Model. Phytomedicine 2019, 56, 57–63. [Google Scholar] [CrossRef]
- Issa, M.Y.; Ezzat, M.I.; Sayed, R.H.; Elbaz, E.M.; Omar, F.A.; Mohsen, E. Neuroprotective Effects of Pulicaria Undulata Essential Oil in Rotenone Model of Parkinson’s Disease in Rats: Insights into Its Anti-Inflammatory and Antioxidant Effects. S. Afr. J. Bot. 2020, 132, 289–298. [Google Scholar] [CrossRef]
- Nikolova, G.; Karamalakova, Y.; Kovacheva, N.; Stanev, S.; Zheleva, A.; Gadjeva, V. Protective Effect of Two Essential Oils Isolated from Rosa Damascena Mill. and Lavandula Angustifolia Mill, and Two Classic Antioxidants against L-Dopa Oxidative Toxicity Induced in Healthy Mice. Regul. Toxicol. Pharmacol. 2016, 81, 1–7. [Google Scholar] [CrossRef]
- Prediger, R.D.; Bortolanza, M.; de Castro Issy, A.C.; dos Santos, B.L.; Del Bel, E.; Raisman-Vozari, R. Dopaminergic Neurons in Parkinson’s Disease. In Handbook of Neurotoxicity; Springer: New York, NY, USA, 2014; pp. 753–788. [Google Scholar]
- Pavan, B.; Bianchi, A.; Botti, G.; Ferraro, L.; Valerii, M.C.; Spisni, E.; Dalpiaz, A. Pharmacokinetic and Permeation Studies in Rat Brain of Natural Compounds Led to Investigate Eugenol as Direct Activator of Dopamine Release in PC12 Cells. Int. J. Mol. Sci. 2023, 24, 1800. [Google Scholar] [CrossRef]
- Vora, U.; Vyas, V.K.; Wal, P.; Saxena, B. Effects of Eugenol on the Behavioral and Pathological Progression in the MPTP-Induced Parkinson’s Disease Mouse Model. Drug Discov. Ther. 2022, 16, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Kabuto, H.; Yamanushi, T.T. Effects of Zingerone [4-(4-Hydroxy-3-Methoxyphenyl)-2-Butanone] and Eugenol [2-Methoxy-4-(2-Propenyl)Phenol] on the Pathological Progress in the 6-Hydroxydopamine-Induced Parkinson’s Disease Mouse Model. Neurochem. Res. 2011, 36, 2244–2249. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-J.; Kim, N.; Jeon, S.H.; Gee, M.S.; Kim, J.-W.; Lee, J.K. Eugenol Relieves the Pathological Manifestations of Alzheimer’s Disease in 5×FAD Mice. Phytomedicine 2023, 118, 154930. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Mohammadi, S.; Askari, S.; Alani, B.; Moosavi, M.; Ghasemi, R. Cinnamaldehyde Regulates Insulin and Caspase-3 Signaling Pathways in the Sporadic Alzheimer’s Disease Model: Involvement of Hippocampal Function via IRS-1, Akt, and GSK-3β Phosphorylation. J. Mol. Neurosci. 2022, 72, 2273–2291. [Google Scholar] [CrossRef] [PubMed]
- Do, J.; Kim, N.; Jeon, S.H.; Gee, M.S.; Ju, Y.-J.; Kim, J.-H.; Oh, M.S.; Lee, J.K. Trans-Cinnamaldehyde Alleviates Amyloid-Beta Pathogenesis via the SIRT1-PGC1α-PPARγ Pathway in 5×FAD Transgenic Mice. Int. J. Mol. Sci. 2020, 21, 4492. [Google Scholar] [CrossRef]
- Mustafa, H.N. Neuro-Amelioration of Cinnamaldehyde in Aluminum-Induced Alzheimer’s Disease Rat Model. J. Histotechnol. 2020, 43, 11–20. [Google Scholar] [CrossRef]
- Bae, W.-Y.; Choi, J.-S.; Jeong, J.-W. The Neuroprotective Effects of Cinnamic Aldehyde in an MPTP Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 551. [Google Scholar] [CrossRef]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Secondo, A.; Pannaccione, A. The Antioxidant Activity of Limonene Counteracts Neurotoxicity Triggered By Aβ1–42 Oligomers in Primary Cortical Neurons. Antioxidants 2021, 10, 937. [Google Scholar] [CrossRef]
- Shin, M.; Liu, Q.F.; Choi, B.; Shin, C.; Lee, B.; Yuan, C.; Song, Y.J.; Yun, H.S.; Lee, I.-S.; Koo, B.-S.; et al. Neuroprotective Effects of Limonene (+) against Aβ42-Induced Neurotoxicity in a Drosophila Model of Alzheimer’s Disease. Biol. Pharm. Bull. 2020, 43, 409–417. [Google Scholar] [CrossRef]
- Eddin, L.B.; Azimullah, S.; Jha, N.K.; Nagoor Meeran, M.F.; Beiram, R.; Ojha, S. Limonene, a Monoterpene, Mitigates Rotenone-Induced Dopaminergic Neurodegeneration by Modulating Neuroinflammation, Hippo Signaling and Apoptosis in Rats. Int. J. Mol. Sci. 2023, 24, 5222. [Google Scholar] [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a Phytocannabinoid Attenuates Oxidative Stress, Neuroinflammation, Glial Activation, and Salvages Dopaminergic Neurons in a Rat Model of Parkinson Disease. Mol. Cell. Biochem. 2016, 418, 59–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Q.; Wang, S.; Liao, Z.; Jin, H.; Huang, S.; Hong, X.; Liu, Y.; Pang, J.; Shen, Q.; et al. The Food Additive β-Caryophyllene Exerts Its Neuroprotective Effects Through the JAK2-STAT3-BACE1 Pathway. Front. Aging Neurosci. 2022, 14, 814432. [Google Scholar] [CrossRef]
- Askari, V.R.; Shafiee-Nick, R. The Protective Effects of β-Caryophyllene on LPS-Induced Primary Microglia M1/M2 Imbalance: A Mechanistic Evaluation. Life Sci. 2019, 219, 40–73. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Askari, V.R. A Mechanistic Review on Immunomodulatory Effects of Selective Type Two Cannabinoid Receptor β-Caryophyllene. Biofactors 2022, 48, 857–882. [Google Scholar] [CrossRef] [PubMed]
- Manouchehrabadi, M.; Farhadi, M.; Azizi, Z.; Torkaman-Boutorabi, A. Carvacrol Protects Against 6-Hydroxydopamine-Induced Neurotoxicity in In Vivo and In Vitro Models of Parkinson’s Disease. Neurotox. Res. 2020, 37, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Nazzaro, F. Anti-Cholinesterase and Anti-α-Amylase Activities and Neuroprotective Effects of Carvacrol and p-Cymene and Their Effects on Hydrogen Peroxide Induced Stress in SH-SY5Y Cells. Int. J. Mol. Sci. 2023, 24, 6073. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.; Safari, S.; Komaki, S.; Karimi, S.A.; Golipoor, Z.; Komaki, A. The Effects of Carvacrol and P-Cymene on Aβ1–42 -Induced Long-Term Potentiation Deficit in Male Rats. CNS Neurosci. Ther. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Mohamed Fizur, N.M.; Jha, N.K.; Ashraf, G.M.; Ojha, S. Neuroprotective Potential and Underlying Pharmacological Mechanism of Carvacrol for Alzheimer’s and Parkinson’s Diseases. Curr. Neuropharmacol. 2023, 21, 1421–1432. [Google Scholar] [CrossRef]
- Munir, S.; Hafeez, R.; Younis, W.; Malik, M.N.H.; Munir, M.U.; Manzoor, W.; Razzaq, M.A.; Pessoa, L.B.; Lopes, K.S.; Lívero, F.A.D.R.; et al. The Protective Effect of Citronellol against Doxorubicin-Induced Cardiotoxicity in Rats. Biomedicines 2023, 11, 2820. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Parekh, K.A.; Ojha, S.K.; Beiram, R. Effect of citronellol on oxidative stress, neuroinflammation and autophagy pathways in an in vivo model of Parkinson’s disease. Heliyon 2022, 8, e11434. [Google Scholar] [CrossRef]
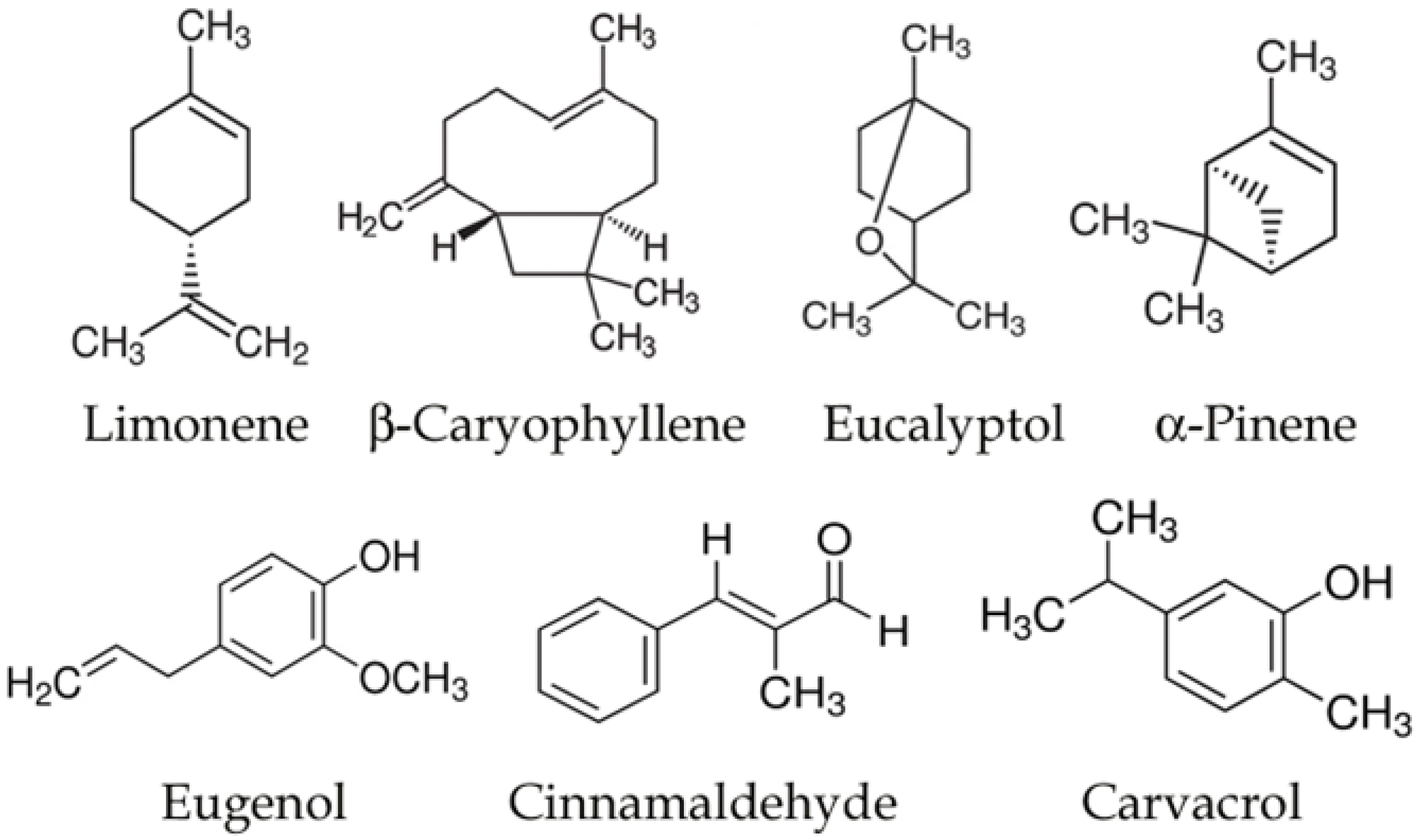
| Essential Oil/Eo Single Compound | Disease Model | Effects | References |
|---|---|---|---|
| cinnamaldehyde | PD: 6-OHDA induced apoptosis in PC12 cells | Decreased cyt-c Increased surviving Reduced p-p44/42/p44/42 levels Reduced cytotoxicity | [41] |
| cinnamaldehyde | PD: MPP+ induced neurotoxicity in human neuroblastoma BE(2)-M17 cell | Recovery of MPP+ induced cell death | [41] |
| Cinnamomum zeylanicum | PD and AD models: enzymatic assays | Monoamine oxidase (MAO A and MAO-B) inhibition | [42] |
| Aloysia citrodora | AD model: neuroblastoma cell line treated with hydrogen peroxide or β-amyloid | Antioxidant Radical-scavenging activity Protection against β-amyloid-induced neurotoxicity | [43] |
| eugenol | Neuronal cell model | Increased cell survival Enhanced dopamine secretion | [49] |
| D-limonene | AD model: primary rat cultures treated with the neurotoxic peptide Aβ1–42 | Increased neuronal viability Antioxidant | [57] |
| β-caryophyllene | AD model: PC-12 cells overexpressing amyloid-β protein precursor | Increased cell viability | [61] |
| β-Caryophyllene | LPS-Induced Primary Microglia M1/M2 Imbalance | Anti-inflammatory | [62] |
| carvacrol | PD: PC12 cells treated with 6-hydroxydopamine (6-OHDA) | Increased cell viability | [64] |
| carvacrol | AD: SH-SY5Y neuronal cells treated with hydrogen peroxide | Inhibition of acetylcholinesterase and butyrylcholinesterase | [65] |
| Essential Oil/EO Single Molecule | Disease Model | Effects | References |
|---|---|---|---|
| Zelkova serrata | Oxidative stress and heat shock induced in C. elegans | Increased stress resistance Decrease in ROS | [38] |
| Zelkova serrata (1S,4S-7-hydroxycalamenene) | AD model: Aβ induction in C. elegans (CL4716 strain) | Decrease in Aβ-induced toxicity Decrease in ROS | [38] |
| Rosa setate × Rosa rugosa | PD model: C. elegans (BZ555 strain for dopaminergic neurotoxicity, OW13 strain for α-synuclein expression) | Reduction in α-synuclein accumulation Decrease in dopaminergic neuron degeneration Decrease in ROS | [39] |
| Rosa setate × Rosa rugosa | C. elegans CF1553 strain (expressing antioxidant enzymes) | Increase in SOD-3 expression Decrease in ROS | [39] |
| Rosa setate × Rosa rugosa | AD model: C. elegans CL4176, CL2355 (Aβ inducible) CL2006 (Aβ constitutively expressed) | Delay of AD-like symptoms Suppression of Aβ | [40] |
| cinnamaldehyde | PD model: mice treated with 1-metil 4-fenil 1,2,3,6-tetraidro-piridina | Autophagy regulation, antioxidant effects, upregulation of Parkin, DJ-1 upregulation of glial cell-derived neurotrophic factor, modulation of the TLR/NF-κB | [41] |
| Ferulago angulata | AD model: rats treated with scopolamine | Improved memory function | [44] |
| Tetraclinis articulata | AD model: intracerebroventricular administration amyloid-β peptide 1–42 | Improved memory function | [45] |
| Pulicaria undulata | PD model: rat treated with rotenone | Decrease in iNOS activity Decrease in α-synuclein | [46] |
| Rosa damascena | PD model: mice treated with L-dopa and benserazide | Reduction in oxidative stress biomarkers (malondialdehyde, protein carbonyl content, and nitric oxide radicals | [47] |
| Lavandula angustifolia | PD model: mice treated with L-dopa and benserazide | Reduction in oxidative stress biomarkers (malondialdehyde, protein carbonyl content, and nitric oxide radicals | [47] |
| eugenol | PD model: mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) | Reduced motor disfunction (pretreatment) Worsening of motor disfunction (post-induction treatment) | [50] |
| eugenol | PD model: intracerebroventricularly injected 6-hydroxydopamine | Improvement in behavioral impairments Antioxidant activity in the striatum | [51] |
| eugenol | AD model: 5 × FAD mice | Decreased neuronal cell loss Decreased Aβ deposition | [52] |
| cinnamaldehyde | AD model: intracerebroventricular streptozotocin injection in rats | Improved recognition/spatial memory Inhibition of Aβ aggregation | [53] |
| cinnamaldehyde | AD model: 5 × FAD mice | Improvement of AD symptoms | [54] |
| cinnamaldehyde | AD model: rat treated with aluminum | Reduced loss of dendritic spines Reduced neurofibrillary degeneration Improvement in memory and intellectual performance | [55] |
| cinnamaldehyde | PD model: mice treated with MPTP | Prevention of neurodegeneration | [56] |
| D-limonene | AD model: Aβ42-expressing drosophila | Prevention of cell death Extracellular signal-regulated kinase phosphorylation Decrease in ROS Decrease in inflammation | [58] |
| D-limonene | PD model: rats treated with rotenone | Reduced dopaminergic neuronal loss Reduced inflammatory markers | [59] |
| β-caryophyllene | PD model: rats treated with rotenone | Prevention of the loss of dopaminergic neurons Reduction in lipid peroxidation Increased activity of antioxidant enzymes Decrease in inflammatory markers Decrease in activated astrocytes and microglia | [60] |
| Carvacrol | AD model: intracerebroventricular injection of amyloid Aβ1–42 in rats | Improved Aβ-associated impairments in synaptic plasticity | [66] |
| Citronellol | PD model: rats treated with rotenone | Reduced dopaminergic neuronal loss Prevented the over-expression of α-synuclein | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spisni, E.; Valerii, M.C.; Massimino, M.L. Essential Oil Molecules Can Break the Loop of Oxidative Stress in Neurodegenerative Diseases. Biology 2023, 12, 1504. https://doi.org/10.3390/biology12121504
Spisni E, Valerii MC, Massimino ML. Essential Oil Molecules Can Break the Loop of Oxidative Stress in Neurodegenerative Diseases. Biology. 2023; 12(12):1504. https://doi.org/10.3390/biology12121504
Chicago/Turabian StyleSpisni, Enzo, Maria Chiara Valerii, and Maria Lina Massimino. 2023. "Essential Oil Molecules Can Break the Loop of Oxidative Stress in Neurodegenerative Diseases" Biology 12, no. 12: 1504. https://doi.org/10.3390/biology12121504
APA StyleSpisni, E., Valerii, M. C., & Massimino, M. L. (2023). Essential Oil Molecules Can Break the Loop of Oxidative Stress in Neurodegenerative Diseases. Biology, 12(12), 1504. https://doi.org/10.3390/biology12121504








