The B Cell Response and Formation of Allergenic and Anti-Allergenic Antibodies in Food Allergy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Low and High-Affinity IgE Play Opposing Roles in Food Allergy
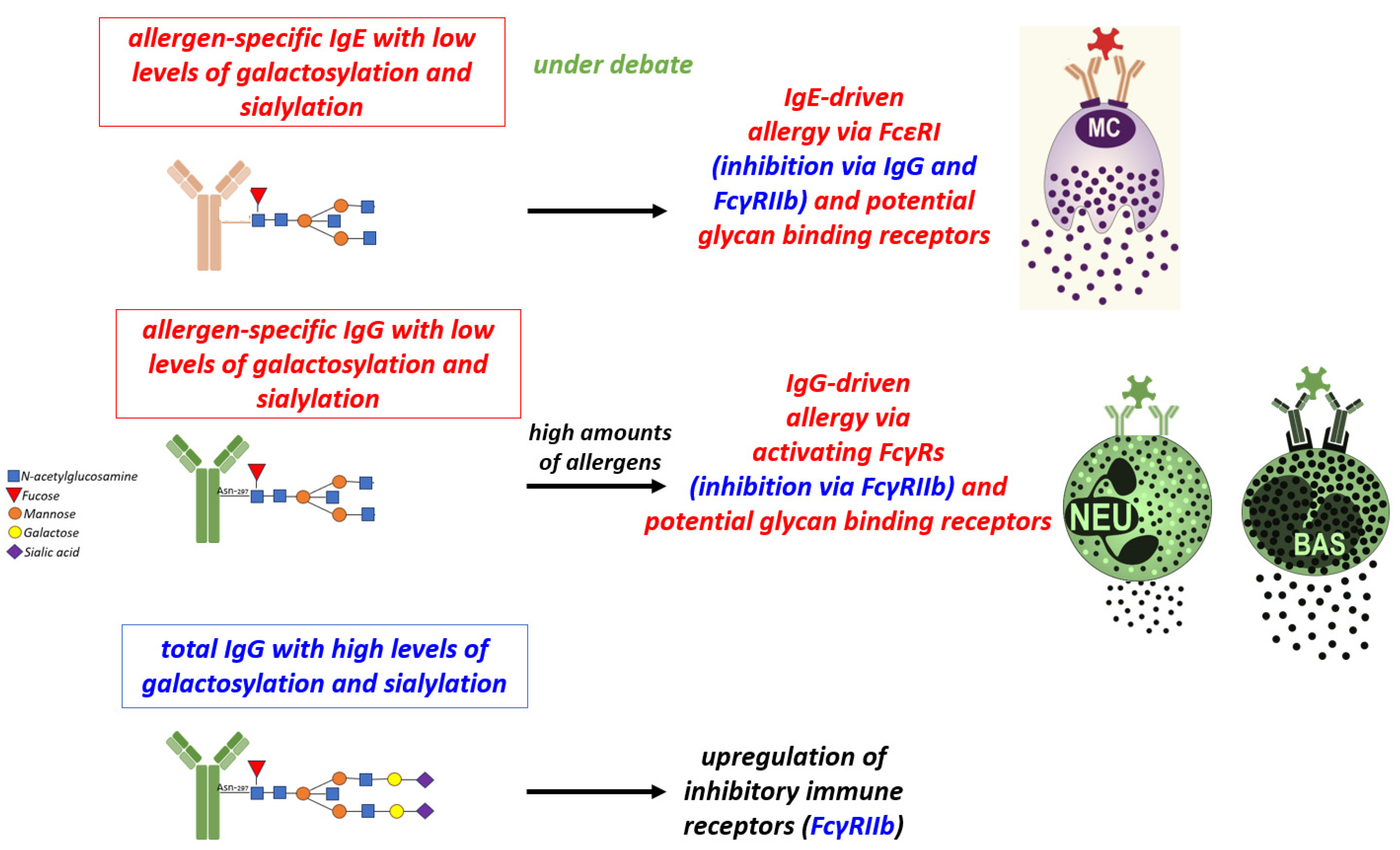
3. The Role of Antibody Isotypes, their Subclasses and Antibody Fc Glycosylation in Food Allergy
3.1. Mechanisms of IgG-Mediated Suppression of Allergy
3.2. Mechanisms of IgA-Mediated Suppression of Allergy
4. The Impact of Antibody Ig-Fc Glycosylation on Allergy Development
5. Development of Antibodies in Food Allergy
5.1. T Cell Activation
5.2. Production of Unmutated, Low-Affinity IgE
5.3. Production of Mutated, High-Affinity IgE
5.4. Regulation of IgG to IgE Ratios
6. Development of Differentially Glycosylated Antibodies
7. The Impact of Distinct Antibody Types in Type 1 Allergic Reactions to Aero-Allergens
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jackson, K.D.; Howie, L.D.; Akinbami, O.J. Trends in Allergic Conditions among Children: United States, 1997–2011; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Washington, DC, USA, 2013. [Google Scholar]
- Simons, F.E.R.; Ardusso, L.R.F.; Bilo, M.B.; Dimov, V.; Ebisawa, M.; El-Gamal, Y.M.; Ledford, D.K.; Lockey, R.F.; Ring, J.; Sanchez-Borges, M.; et al. 2012 Update: World Allergy Organization Guidelines for the Assessment and Management of Anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 389–399. [Google Scholar] [CrossRef]
- Seth, D.; Poowutikul, P.; Pansare, M.; Kamat, D. Food Allergy: A Review. Pediatr. Ann. 2020, 49, e50–e58. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, J.D.; Thomas, K.O.; Iweala, O.I. The Meat of the Matter: Understanding and Managing Alpha-Gal Syndrome. ImmunoTargets Ther. 2022, 11, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Commins, S.P.; Satinover, S.M.; Hosen, J.; Mozena, J.; Borish, L.; Lewis, B.D.; Woodfolk, J.A.; Platts-Mills, T.A. Delayed Anaphylaxis, Angioedema, or Urticaria after Consumption of Red Meat in Patients with IgE Antibodies Specific for Galactose-α-1, 3-Galactose. J. Allergy Clin. Immunol. 2009, 123, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.T.; Meize-Grochowski, R. Epinephrine Auto-Injectors for Anaphylaxis Treatment in the School Setting: A Discussion Paper. SAGE Open Nurs. 2019, 5, 2377960819845246. [Google Scholar]
- Gupta, R.S.; Springston, E.E.; Warrier, M.R.; Smith, B.; Kumar, R.; Pongracic, J.; Holl, J.L. The Prevalence, Severity, and Distribution of Childhood Food Allergy in the United States. Pediatrics 2011, 128, e9–e17. [Google Scholar] [CrossRef]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Blumenstock, J.A.; Jiang, J.; Davis, M.M.; Nadeau, K.C. The Public Health Impact of Parent-Reported Childhood Food Allergies in the United States. Pediatrics 2018, 142, e20181235. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and Severity of Food Allergies among US Adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef]
- Kurukulaaratchy, R.J.; Karmaus, W.; Arshad, S.H. Gender and Atopy Influences on the Natural History of Rhinitis. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 7. [Google Scholar] [CrossRef]
- Nielsen, S.C.A.; Roskin, K.M.; Jackson, K.J.L.; Joshi, S.A.; Nejad, P.; Lee, J.-Y.; Wagar, L.E.; Pham, T.D.; Hoh, R.A.; Nguyen, K.D.; et al. Shaping of Infant B Cell Receptor Repertoires by Environmental Factors and Infectious Disease. Sci. Transl. Med. 2019, 11, eaat2004. [Google Scholar] [CrossRef]
- Younoszai, M.K.; Lynch, A. In Vivo D-Glucose Absorption in the Developing Rat Small Intestine. Pediatr. Res. 1975, 9, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Drozdowski, L.A.; Clandinin, T.; Thomson, A.B.R. Ontogeny, Growth and Development of the Small Intestine: Understanding Pediatric Gastroenterology. World J. Gastroenterol. WJG 2010, 16, 787. [Google Scholar] [PubMed]
- Davidson, N.O.; Hausman, A.M.; Ifkovits, C.A.; Buse, J.B.; Gould, G.W.; Burant, C.F.; Bell, G.I. Human Intestinal Glucose Transporter Expression and Localization of GLUT5. Am. J. Physiol.-Cell Physiol. 1992, 262, C795–C800. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.; Haverson, K.; Inman, C.; Harris, C.; Jones, P.; Corfield, G.; Miller, B.; Stokes, C. The Development of the Mucosal Immune System Pre-and Post-Weaning: Balancing Regulatory and Effector Function. Proc. Nutr. Soc. 2005, 64, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Ierodiakonou, D.; Garcia-Larsen, V.; Logan, A.; Groome, A.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Jarrold, K.; Reeves, T.; et al. Timing of Allergenic Food Introduction to the Infant Diet and Risk of Allergic or Autoimmune Disease: A Systematic Review and Meta-Analysis. JAMA 2016, 316, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, K.; Barman, M.; Brekke, H.K.; Hesselmar, B.; Johansen, S.; Sandberg, A.-S.; Wold, A. Late Introduction of Fish and Eggs Is Associated with Increased Risk of Allergy Development–Results from the FARMFLORA Birth Cohort. Food Nutr. Res. 2017, 61, 1393306. [Google Scholar] [CrossRef]
- Savage, J.; Sicherer, S.; Wood, R. The Natural History of Food Allergy. J. Allergy Clin. Immunol. Pract. 2016, 4, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Urisu, A. Natural History and Prevention of Food Allergy. In Food Allergy E-Book: Expert Consult Basic; Elsevier Health Science: Amsterdam, The Netherlands, 2011; p. 251. [Google Scholar]
- Chatchatee, P.; Järvinen, K.-M.; Bardina, L.; Beyer, K.; Sampson, H.A. Identification of IgE-and IgG-Binding Epitopes on As1-Casein: Differences in Patients with Persistent and Transient Cow’s Milk Allergy. J. Allergy Clin. Immunol. 2001, 107, 379–383. [Google Scholar] [CrossRef]
- Järvinen, K.-M.; Beyer, K.; Vila, L.; Bardina, L.; Mishoe, M.; Sampson, H.A. Specificity of IgE Antibodies to Sequential Epitopes of Hen’s Egg Ovomucoid as a Marker for Persistence of Egg Allergy. Allergy 2007, 62, 758–765. [Google Scholar] [CrossRef]
- Vila, L.; Beyer, K.; Järvinen, K.-M.; Chatchatee, P.; Bardina, L.; Sampson, H.A. Role of Conformational and Linear Epitopes in the Achievement of Tolerance in Cow’s Milk Allergy. Clin. Exp. Allergy 2001, 31, 1599–1606. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Wood, R.A.; Vickery, B.P.; Jones, S.M.; Liu, A.H.; Fleischer, D.M.; Dawson, P.; Mayer, L.; Burks, A.W.; Grishin, A.; et al. The Natural History of Egg Allergy in an Observational Cohort. J. Allergy Clin. Immunol. 2014, 133, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Kostara, M.; Chondrou, V.; Sgourou, A.; Douros, K.; Tsabouri, S. HLA Polymorphisms and Food Allergy Predisposition. J. Pediatr. Genet. 2020, 9, 77–86. [Google Scholar] [CrossRef]
- Grotenboer, N.S.; Ketelaar, M.E.; Koppelman, G.H.; Nawijn, M.C. Decoding Asthma: Translating Genetic Variation in IL33 and IL1RL1 into Disease Pathophysiology. J. Allergy Clin. Immunol. 2013, 131, 856–865. [Google Scholar] [CrossRef]
- Berin, M.C. Mechanisms That Define Transient versus Persistent Food Allergy. J. Allergy Clin. Immunol. 2019, 143, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, A.B.; Qamar, N.; Erickson, K.A.; Kwasny, M.J.; Cai, M.; Szychlinski, C.; Singh, A.M.; Fuleihan, R.L. Cytokine Responses to Egg Protein in Previously Allergic Children Who Developed Tolerance Naturally. Ann. Allergy Asthma Immunol. 2014, 113, 667–670. [Google Scholar] [CrossRef]
- Frischmeyer-Guerrerio, P.A.; Guerrerio, A.L.; Chichester, K.L.; Bieneman, A.P.; Hamilton, R.A.; Wood, R.A.; Schroeder, J.T. Dendritic Cell and T Cell Responses in Children with Food Allergy. Clin. Exp. Allergy 2011, 41, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.P.; Haynes, L.; Sayles, P.C.; Duso, D.K.; Eaton, S.M.; Lepak, N.M.; Johnson, L.L.; Swain, S.L.; Lund, F.E. Reciprocal Regulation of Polarized Cytokine Production by Effector B and T Cells. Nat. Immunol. 2000, 1, 475–482. [Google Scholar] [CrossRef]
- Song, Z.; Yuan, W.; Zheng, L.; Wang, X.; Kuchroo, V.K.; Mohib, K.; Rothstein, D.M. B Cell IL-4 Drives Th2 Responses in Vivo, Ameliorates Allograft Rejection, and Promotes Allergic Airway Disease. Front. Immunol. 2022, 13, 762390. [Google Scholar] [CrossRef]
- Hurdayal, R.; Ndlovu, H.H.; Revaz-Breton, M.; Parihar, S.P.; Nono, J.K.; Govender, M.; Brombacher, F. IL-4–Producing B Cells Regulate T Helper Cell Dichotomy in Type 1-and Type 2-Controlled Diseases. Proc. Natl. Acad. Sci. USA 2017, 114, E8430–E8439. [Google Scholar] [CrossRef]
- Hammad, H.; Plantinga, M.; Deswarte, K.; Pouliot, P.; Willart, M.A.M.; Kool, M.; Muskens, F.; Lambrecht, B.N. Inflammatory Dendritic Cells—Not Basophils—Are Necessary and Sufficient for Induction of Th2 Immunity to Inhaled House Dust Mite Allergen. J. Exp. Med. 2010, 207, 2097–2111. [Google Scholar] [CrossRef]
- Looney, T.J.; Lee, J.-Y.; Roskin, K.M.; Hoh, R.A.; King, J.; Glanville, J.; Liu, Y.; Pham, T.D.; Dekker, C.L.; Davis, M.M.; et al. Human B-Cell Isotype Switching Origins of IgE. J. Allergy Clin. Immunol. 2016, 137, 579–586. [Google Scholar] [CrossRef] [PubMed]
- He, J.-S.; Narayanan, S.; Subramaniam, S.; Ho, W.Q.; Lafaille, J.J.; de Lafaille, M.A.C. Biology of IgE Production: IgE Cell Differentiation and the Memory of IgE Responses. In IgE Antibodies: Generation and Function; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–19. [Google Scholar]
- Udoye, C.C.; Rau, C.N.; Freye, S.M.; Almeida, L.N.; Vera-Cruz, S.; Othmer, K.; Korkmaz, R.Ü.; Clauder, A.-K.; Lindemann, T.; Niebuhr, M.; et al. B-Cell Receptor Physical Properties Affect Relative IgG1 and IgE Responses in Mouse Egg Allergy. Mucosal Immunol. 2022, 15, 1375–1388. [Google Scholar] [CrossRef]
- He, J.-S.; Subramaniam, S.; Narang, V.; Srinivasan, K.; Saunders, S.P.; Carbajo, D.; Wen-Shan, T.; Hidayah Hamadee, N.; Lum, J.; Lee, A.; et al. IgG1 Memory B Cells Keep the Memory of IgE Responses. Nat. Commun. 2017, 8, 641. [Google Scholar] [CrossRef]
- Takhar, P.; Smurthwaite, L.; Coker, H.A.; Fear, D.J.; Banfield, G.K.; Carr, V.A.; Durham, S.R.; Gould, H.J. Allergen Drives Class Switching to IgE in the Nasal Mucosa in Allergic Rhinitis. J. Immunol. 2005, 174, 5024–5032. [Google Scholar] [CrossRef]
- Hoh, R.A.; Joshi, S.A.; Lee, J.-Y.; Martin, B.A.; Varma, S.; Kwok, S.; Nielsen, S.C.A.; Nejad, P.; Haraguchi, E.; Dixit, P.S.; et al. Origins and Clonal Convergence of Gastrointestinal IgE+ B Cells in Human Peanut Allergy. Sci. Immunol. 2020, 5, eaay4209. [Google Scholar] [CrossRef]
- Manz, R.A.; Hauser, A.E.; Hiepe, F.; Radbruch, A. Maintenance of Serum Antibody Levels. Annu Rev. Immunol. 2005, 23, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Geha, R.S.; Jabara, H.H.; Brodeur, S.R. The Regulation of Immunoglobulin E Class-Switch Recombination. Nat. Rev. Immunol. 2003, 3, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Robinson, M.J.; Chen, X.; Smith, G.A.; Taunton, J.; Liu, W.; Allen, C.D.C. Regulation of B Cell Fate by Chronic Activity of the IgE B Cell Receptor. Elife 2016, 5, e21238. [Google Scholar] [CrossRef] [PubMed]
- Croote, D.; Darmanis, S.; Nadeau, K.C.; Quake, S.R. High-Affinity Allergen-Specific Human Antibodies Cloned from Single IgE B Cell Transcriptomes. Science 2018, 362, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Laffleur, B.; Duchez, S.; Tarte, K.; Denis-Lagache, N.; Péron, S.; Carrion, C.; Denizot, Y.; Cogné, M. Self-Restrained B Cells Arise Following Membrane IgE Expression. Cell Rep. 2015, 10, 900–909. [Google Scholar] [CrossRef] [PubMed]
- MacGlashan, D., Jr. IgE Receptor and Signal Transduction in Mast Cells and Basophils. Curr. Opin. Immunol. 2008, 20, 717–723. [Google Scholar] [CrossRef]
- Metzger, H. The Receptor with High Affinity for IgE. Immunol. Rev. 1992, 125, 37–48. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and Mast Cells in Allergic Disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef]
- Mita, H.; Yasueda, H.; Akiyama, K. Affinity of IgE Antibody to Antigen Influences Allergen-induced Histamine Release. Clin. Exp. Allergy 2000, 30, 1583–1589. [Google Scholar] [CrossRef]
- Chang, X. Low-Affinity but High-Avidity Interactions May Offer an Explanation for IgE-Mediated Allergen Cross-Reactivity. Allergy 2021, 76, 2565–2574. [Google Scholar] [CrossRef]
- Xiong, H.; Dolpady, J.; Wabl, M.; Curotto de Lafaille, M.A.; Lafaille, J.J. Sequential Class Switching Is Required for the Generation of High Affinity IgE Antibodies. J. Exp. Med. 2012, 209, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Leach, S.; Liu, W.; Ralston, E.; Scheffel, J.; Zhang, W.; Lowell, C.A.; Rivera, J. Molecular Editing of Cellular Responses by the High-Affinity Receptor for IgE. Science 2014, 343, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, M.M.; Phillips, R.; Brown, S.J.; Cro, S.; Cornelius, V.; Carlsen, K.C.L.; Skjerven, H.O.; Rehbinder, E.M.; Lowe, A.J.; Dissanayake, E.; et al. Skin Care Interventions in Infants for Preventing Eczema and Food Allergy. Cochrane Database Syst. Rev. 2022, 11, CD013534. [Google Scholar]
- Gowthaman, U.; Chen, J.S.; Zhang, B.; Flynn, W.F.; Lu, Y.; Song, W.; Joseph, J.; Gertie, J.A.; Xu, L.; Collet, M.A.; et al. Identification of a T Follicular Helper Cell Subset That Drives Anaphylactic IgE. Science 2019, 365, eaaw6433. [Google Scholar] [CrossRef] [PubMed]
- Burrows, B.; Martinez, F.D.; Halonen, M.; Barbee, R.A.; Cline, M.G. Association of Asthma with Serum IgE Levels and Skin-Test Reactivity to Allergens. N. Engl. J. Med. 1989, 320, 271–277. [Google Scholar] [CrossRef]
- Fitzsimmons, C.M.; Falcone, F.H.; Dunne, D.W. Helminth Allergens, Parasite-Specific IgE, and Its Protective Role in Human Immunity. Front. Immunol. 2014, 5, 61. [Google Scholar] [CrossRef]
- Plum, T.; Binzberger, R.; Thiele, R.; Shang, F.; Postrach, D.; Fung, C.; Fortea, M.; Stakenborg, N.; Wang, Z.; Tappe-Theodor, A.; et al. Mast Cells Link Immune Sensing to Antigen-Avoidance Behaviour. Nature 2023, 620, 634–642. [Google Scholar] [CrossRef]
- Florsheim, E.B.; Bachtel, N.D.; Cullen, J.; Lima, B.G.C.; Godazgar, M.; Carvalho, F.; Chatain, C.P.; Zimmer, M.R.; Zhang, C.; Gautier, G.; et al. Immune Sensing of Food Allergens Promotes Avoidance Behaviour. Nature 2023, 620, 643–650. [Google Scholar] [CrossRef]
- Udoye, C.C.; Manz, R.A. IgE-Mast Cell Mediated Allergy a Sensor of Food Quality. Signal Transduct. Target. Ther. 2023. [Google Scholar]
- Ramsey, N.; Berin, M.C. Pathogenesis of IgE-mediated Food Allergy and Implications for Future Immunotherapeutics. Pediatr. Allergy Immunol. 2021, 32, 1416–1425. [Google Scholar] [CrossRef]
- Karsten, C.M.; Pandey, M.K.; Figge, J.; Kilchenstein, R.; Taylor, P.R.; Rosas, M.; McDonald, J.U.; Orr, S.J.; Berger, M.; Petzold, D.; et al. Anti-Inflammatory Activity of IgG1 Mediated by Fc Galactosylation and Association of FcγRIIB and Dectin-1. Nat. Med. 2012, 18, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, Z.Y. Induction and Suppression of Allergic Diarrhea and Systemic Anaphylaxis in a Murine Model of Food Allergy. J. Allergy Clin. Immunol. 2012, 129, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Petry, J.; Rahmöller, J.; Dühring, L.; Lilienthal, G.-M.; Lehrian, S.; Buhre, J.S.; Bartsch, Y.C.; Epp, A.; Lunding, H.B.; Moremen, K.W.; et al. Enriched Blood IgG Sialylation Attenuates IgG-Mediated and IgG-Controlled-IgE-Mediated Allergic Reactions. J. Allergy Clin. Immunol. 2021, 147, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Strait, R.T.; Posgai, M.T.; Mahler, A.; Barasa, N.; Jacob, C.O.; Köhl, J.; Ehlers, M.; Stringer, K.; Shanmukhappa, S.K.; Witte, D.; et al. IgG1 Protects against Renal Disease in a Mouse Model of Cryoglobulinaemia. Nature 2015, 517, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Bieber, K.; Hundt, J.E.; Yu, X.; Ehlers, M.; Petersen, F.; Karsten, C.M.; Köhl, J.; Kridin, K.; Kalies, K.; Kasprick, A.; et al. Autoimmune Pre-Disease. Autoimmun. Rev. 2022, 22, 103236. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.; Winkler, A.; Lorenz, A.K.; Holecska, V.; Blanchard, V.; Eiglmeier, S.; Schoen, A.-L.; Bitterling, J.; Stoehr, A.D.; Petzold, D.; et al. T Cell–Independent B Cell Activation Induces Immunosuppressive Sialylated IgG Antibodies. J. Clin. Investig. 2013, 123, 3788–3796. [Google Scholar] [CrossRef]
- Lamprecht, P.; Kerstein, A.; Klapa, S.; Schinke, S.; Karsten, C.M.; Yu, X.; Ehlers, M.; Epplen, J.T.; Holl-Ulrich, K.; Wiech, T.; et al. Pathogenetic and Clinical Aspects of Anti-Neutrophil Cytoplasmic Autoantibody-Associated Vasculitides. Front. Immunol. 2018, 9, 680. [Google Scholar] [CrossRef]
- Cabral-Marques, O.; Riemekasten, G. Functional Autoantibodies Directed against Cell Surface Receptors in Systemic Sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 160–168. [Google Scholar] [CrossRef]
- Khodoun, M.V.; Kucuk, Z.Y.; Strait, R.T.; Krishnamurthy, D.; Janek, K.; Clay, C.D.; Morris, S.C.; Finkelman, F.D. Rapid Desensitization of Mice with Anti-FcγRIIb/FcγRIII mAb Safely Prevents IgG-Mediated Anaphylaxis. J. Allergy Clin. Immunol. 2013, 132, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Beutier, H.; Gillis, C.M.; Iannascoli, B.; Godon, O.; England, P.; Sibilano, R.; Reber, L.L.; Galli, S.J.; Cragg, M.S.; van Rooijen, N.; et al. IgG Subclasses Determine Pathways of Anaphylaxis in Mice. J. Allergy Clin. Immunol. 2017, 139, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Strait, R.T.; Morris, S.C.; Yang, M.; Qu, X.-W.; Finkelman, F.D. Pathways of Anaphylaxis in the Mouse. J. Allergy Clin. Immunol. 2002, 109, 658–668. [Google Scholar] [CrossRef]
- Kanagaratham, C.; Ansari, Y.S.E.; Lewis, O.L.; Oettgen, H.C. IgE and IgG Antibodies as Regulators of Mast Cell and Basophil Functions in Food Allergy. Front. Immunol. 2020, 11, 3000. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; James, L.K.; Bahnson, H.T.; Shamji, M.H.; Couto-Francisco, N.C.; Islam, S.; Houghton, S.; Clark, A.T.; Stephens, A.; Turcanu, V.; et al. IgG4 Inhibits Peanut-Induced Basophil and Mast Cell Activation in Peanut-Tolerant Children Sensitized to Peanut Major Allergens. J. Allergy Clin. Immunol. 2015, 135, 1249–1256. [Google Scholar] [CrossRef]
- Akdis, C.A.; Akdis, M. Mechanisms of Allergen-Specific Immunotherapy and Immune Tolerance to Allergens. World Allergy Organ. J. 2015, 8, 17. [Google Scholar] [CrossRef]
- Shamji, M.H.; Valenta, R.; Jardetzky, T.; Verhasselt, V.; Durham, S.R.; Würtzen, P.A.; van Neerven, R.J.J. The Role of Allergen-specific IgE, IgG and IgA in Allergic Disease. Allergy 2021, 76, 3627–3641. [Google Scholar] [CrossRef]
- Ansari, Y.S.E.; Kanagaratham, C.; Burton, O.T.; Santos, J.V.; Hollister, B.-M.A.; Lewis, O.L.; Renz, H.; Oettgen, H.C. Allergen-Specific IgA Antibodies Block IgE-Mediated Activation of Mast Cells and Basophils. Front. Immunol. 2022, 13, 881655. [Google Scholar] [CrossRef]
- Strait, R.T.; Morris, S.C.; Finkelman, F.D. IgG-Blocking Antibodies Inhibit IgE-Mediated Anaphylaxis in Vivo through Both Antigen Interception and FcγRIIb Cross-Linking. J. Clin. Investig. 2006, 116, 833–841. [Google Scholar] [CrossRef]
- Vickery, B.P.; Lin, J.; Kulis, M.; Fu, Z.; Steele, P.H.; Jones, S.M.; Scurlock, A.M.; Gimenez, G.; Bardina, L.; Sampson, H.A.; et al. Peanut Oral Immunotherapy Modifies IgE and IgG4 Responses to Major Peanut Allergens. J. Allergy Clin. Immunol. 2013, 131, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, R.; Karagiannis, S.N.; Jordakieva, G.; Jensen-Jarolim, E. The Role of IgG4 in the Fine Tuning of Tolerance in IgE-Mediated Allergy and Cancer. Int. J. Mol. Sci. 2020, 21, 5017. [Google Scholar] [CrossRef] [PubMed]
- Lilienthal, G.-M.; Rahmöller, J.; Petry, J.; Bartsch, Y.C.; Leliavski, A.; Ehlers, M. Potential of Murine IgG1 and Human IgG4 to Inhibit the Classical Complement and Fcγ Receptor Activation Pathways. Front. Immunol. 2018, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Coker, H.A.; Durham, S.R.; Gould, H.J. Local Somatic Hypermutation and Class Switch Recombination in the Nasal Mucosa of Allergic Rhinitis Patients. J. Immunol. 2003, 171, 5602–5610. [Google Scholar] [CrossRef] [PubMed]
- Strait, R.T.; Mahler, A.; Hogan, S.; Khodoun, M.; Shibuya, A.; Finkelman, F.D. Ingested Allergens Must Be Absorbed Systemically to Induce Systemic Anaphylaxis. J. Allergy Clin. Immunol. 2011, 127, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; DiLillo, D.J.; Bournazos, S.; Giddens, J.P.; Ravetch, J.V.; Wang, L.-X. Modulating IgG Effector Function by Fc Glycan Engineering. Proc. Natl. Acad. Sci. USA 2017, 114, 3485–3490. [Google Scholar] [CrossRef]
- Yeo, S.C.; Cheung, C.K.; Barratt, J. New Insights into the Pathogenesis of IgA Nephropathy. Pediatr. Nephrol. 2018, 33, 763–777. [Google Scholar] [CrossRef]
- Epp, A.; Hobusch, J.; Bartsch, Y.C.; Petry, J.; Lilienthal, G.-M.; Koeleman, C.A.M.; Eschweiler, S.; Möbs, C.; Hall, A.; Morris, S.C.; et al. Sialylation of IgG Antibodies Inhibits IgG-Mediated Allergic Reactions. J. Allergy Clin. Immunol. 2018, 141, 399–402. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-Inflammatory Activity of Immunoglobulin G Resulting from Fc Sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Buhre, J.S.; Becker, M.; Ehlers, M. IgG Subclass and Fc Glycosylation Shifts Are Linked to the Transition from Pre-to Inflammatory Autoimmune Conditions. Front. Immunol. 2022, 13, 1006939. [Google Scholar] [CrossRef] [PubMed]
- Seeling, M.; Pöhnl, M.; Kara, S.; Horstmann, N.; Riemer, C.; Wöhner, M.; Liang, C.; Brückner, C.; Eiring, P.; Werner, A.; et al. Immunoglobulin G-Dependent Inhibition of Inflammatory Bone Remodeling Requires Pattern Recognition Receptor Dectin-1. Immunity 2023, 56, 1046–1063. [Google Scholar] [CrossRef] [PubMed]
- Plomp, R.; Hensbergen, P.J.; Rombouts, Y.; Zauner, G.; Dragan, I.; Koeleman, C.A.M.; Deelder, A.M.; Wuhrer, M. Site-Specific N-Glycosylation Analysis of Human Immunoglobulin e. J. Proteome Res. 2014, 13, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Shade, K.-T.C.; Platzer, B.; Washburn, N.; Mani, V.; Bartsch, Y.C.; Conroy, M.; Pagan, J.D.; Bosques, C.; Mempel, T.R.; Fiebiger, E.; et al. A Single Glycan on IgE Is Indispensable for Initiation of Anaphylaxis. J. Exp. Med. 2015, 212, 457–467. [Google Scholar] [CrossRef]
- Seeling, M.; Brückner, C.; Nimmerjahn, F. Differential Antibody Glycosylation in Autoimmunity: Sweet Biomarker or Modulator of Disease Activity? Nat. Rev. Rheumatol. 2017, 13, 621–630. [Google Scholar] [CrossRef]
- Shade, K.-T.C.; Conroy, M.E.; Washburn, N.; Kitaoka, M.; Huynh, D.J.; Laprise, E.; Patil, S.U.; Shreffler, W.G.; Anthony, R.M. Sialylation of Immunoglobulin E Is a Determinant of Allergic Pathogenicity. Nature 2020, 582, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Dühring, L.; Petry, J.; Lilienthal, G.-M.; Bartsch, Y.C.; Kubiak, M.; Pfeufer, C.; Lehrian, S.; Buhre, J.S.; Lunding, H.B.; Kern, C.; et al. Sialylation of IgE Reduces FcεRIα Interaction and Mast Cell and Basophil Activation in Vitro and Increases IgE Half-life In Vivo. Allergy 2023, 78, 2301–2305. [Google Scholar] [CrossRef]
- Gao, P.; Simpson, J.L.; Zhang, J.; Gibson, P.G. Galectin-3: Its Role in Asthma and Potential as an Anti-Inflammatory Target. Respir. Res. 2013, 14, 136. [Google Scholar] [CrossRef]
- Duan, S.; Koziol-White, C.J.; Jester, W.F.; Smith, S.A.; Nycholat, C.M.; Macauley, M.S.; Panettieri, R.A.; Paulson, J.C. CD33 Recruitment Inhibits IgE-Mediated Anaphylaxis and Desensitizes Mast Cells to Allergen. J. Clin. Investig. 2021, 129, 125456. [Google Scholar] [CrossRef]
- Adler, L.N.; Jiang, W.; Bhamidipati, K.; Millican, M.; Macaubas, C.; Hung, S.; Mellins, E.D. The Other Function: Class II-Restricted Antigen Presentation by B Cells. Front. Immunol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Izadi, N.; Luu, M.; Ong, P.Y.; Tam, J.S. The Role of Skin Barrier in the Pathogenesis of Food Allergy. Children 2015, 2, 382–402. [Google Scholar] [CrossRef] [PubMed]
- Jakwerth, C.A.; Ordovas-Montanes, J.; Blank, S.; Schmidt-Weber, C.B.; Zissler, U.M. Role of Respiratory Epithelial Cells in Allergic Diseases. Cells 2022, 11, 1387. [Google Scholar] [CrossRef] [PubMed]
- van Splunter, M.; Liu, L.; van Neerven, R.J.J.; Wichers, H.J.; Hettinga, K.A.; Jong, N.W.D. Mechanisms Underlying the Skin-Gut Cross Talk in the Development of IgE-Mediated Food Allergy. Nutrients 2020, 12, 3830. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Antonicelli, L. Does Sensitization to Foods in Adults Occur Always in the Gut? Int. Arch. Allergy Immunol. 2010, 154, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Khodoun, M.V.; Tomar, S.; Tocker, J.E.; Wang, Y.H.; Finkelman, F.D. Prevention of Food Allergy Development and Suppression of Established Food Allergy by Neutralization of Thymic Stromal Lymphopoietin, IL-25, and IL-33. J. Allergy Clin. Immunol. 2018, 141, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Divekar, R.; Kita, H. Recent Advances in Epithelium-Derived Cytokines (IL-33, IL-25 and TSLP) and Allergic Inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 98. [Google Scholar] [CrossRef]
- Whetstone, C.E.; Ranjbar, M.; Omer, H.; Cusack, R.P.; Gauvreau, G.M. The Role of Airway Epithelial Cell Alarmins in Asthma. Cells 2022, 11, 1105. [Google Scholar] [CrossRef]
- MacLennan, I.C.M.; Toellner, K.-M.; Cunningham, A.F.; Serre, K.; Sze, D.M.-Y.; Zúñiga, E.; Cook, M.C.; Vinuesa, C.G. Extrafollicular Antibody Responses. Immunol. Rev. 2003, 194, 8–18. [Google Scholar] [CrossRef]
- Elsner, R.A.; Shlomchik, M.J. Germinal Center and Extrafollicular B Cell Responses in Vaccination, Immunity, and Autoimmunity. Immunity 2020, 53, 1136–1150. [Google Scholar] [CrossRef]
- Mackay, F.; Figgett, W.A.; Saulep, D.; Lepage, M.; Hibbs, M.L. B-cell Stage and Context-dependent Requirements for Survival Signals from BAFF and the B-cell Receptor. Immunol. Rev. 2010, 237, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Finney, J.; Yeh, C.-H.; Kelsoe, G.; Kuraoka, M. Germinal Center Responses to Complex Antigens. Immunol. Rev. 2018, 284, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Shlomchik, M.J.; Weisel, F. Germinal Center Selection and the Development of Memory B and Plasma Cells. Immunol. Rev. 2012, 247, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, R.; Benson, M.J.; Vries, V.C.D.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular Mechanism and Function of CD40/CD40L Engagement in the Immune System. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. Follicular Helper CD4 T Cells (Tfh). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Berin, M.C. Targeting Type 2 Immunity and the Future of Food Allergy Treatment. J. Exp. Med. 2023, 220, e20221104. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, L.M.; Tarlinton, D.M. Regulation of Germinal Center Responses, Memory B Cells and Plasma Cell Formation—An Update. Curr. Opin. Immunol. 2016, 39, 59–67. [Google Scholar] [CrossRef]
- Chong, A.S.; Ansari, M.J. Heterogeneity of Memory B Cells. Am. J. Transplant. 2018, 18, 779–784. [Google Scholar] [CrossRef]
- Wade-Vallance, A.K.; Allen, C.D.C. Intrinsic and Extrinsic Regulation of IgE B Cell Responses. Curr. Opin. Immunol. 2021, 72, 221–229. [Google Scholar] [CrossRef]
- Schmitt, M.E.R. The B-Cell Antigen Receptor of IgE-Switched Plasma Cells Regulates Memory IgE Responses. J. Allergy Clin. Immunol. 2020, 146, 642–651. [Google Scholar] [CrossRef]
- Newman, R.; Tolar, P. Chronic Calcium Signaling in IgE+ B Cells Limits Plasma Cell Differentiation and Survival. Immunity 2021, 54, 2756–2771. [Google Scholar] [CrossRef] [PubMed]
- Asrat, S.; Kaur, N.; Liu, X.; Ben, L.-H.; Kajimura, D.; Murphy, A.J.; Sleeman, M.A.; Limnander, A.; Orengo, J.M. Chronic Allergen Exposure Drives Accumulation of Long-Lived IgE Plasma Cells in the Bone Marrow, Giving Rise to Serological Memory. Sci. Immunol. 2020, 5, eaav8402. [Google Scholar] [CrossRef]
- Luger, E.O. Induction of Long-Lived Allergen-Specific Plasma Cells by Mucosal Allergen Challenge. J. Allergy Clin. Immunol. 2009, 124, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Scheerens, H. Targeting IgE Production in Mice and Humans. Curr. Opin. Immunol. 2014, 31, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.; Panetta, V.; Cappella, A.; Hofmaier, S.; Hatzler, L.; Rohrbach, A.; Tsilochristou, O.; Bauer, C.-P.; Hoffmann, U.; Forster, J.; et al. IgG and IgG4 to 91 Allergenic Molecules in Early Childhood by Route of Exposure and Current and Future IgE Sensitization: Results from the Multicentre Allergy Study Birth Cohort. J. Allergy Clin. Immunol. 2016, 138, 1426–1433. [Google Scholar] [CrossRef]
- Jellusova, J. Metabolic Control of B Cell Immune Responses. Curr. Opin. Immunol. 2020, 63, 21–28. [Google Scholar] [CrossRef]
- Ohmi, Y.; Ise, W.; Harazono, A.; Takakura, D.; Fukuyama, H.; Baba, Y.; Narazaki, M.; Shoda, H.; Takahashi, N.; Ohkawa, Y.; et al. Sialylation Converts Arthritogenic IgG into Inhibitors of Collagen-Induced Arthritis. Nat. Commun. 2016, 7, 11205. [Google Scholar] [CrossRef]
- Bartsch, Y.C.; Eschweiler, S.; Leliavski, A.; Lunding, H.B.; Wagt, S.; Petry, J.; Lilienthal, G.-M.; Rahmöller, J.; de Haan, N.; Hölscher, A.; et al. IgG Fc Sialylation Is Regulated during the Germinal Center Reaction Following Immunization with Different Adjuvants. J. Allergy Clin. Immunol. 2020, 146, 652–666. [Google Scholar] [CrossRef]
- Buhre, J.S.; Pongracz, T.; Künsting, I.; Lixenfeld, A.S.; Wang, W.; Nouta, J.; Lehrian, S.; Schmelter, F.; Lunding, H.B.; Dühring, L.; et al. mRNA Vaccines against SARS-CoV-2 Induce Comparably Low Long-Term IgG Fc Galactosylation and Sialylation Levels but Increasing Long-Term IgG4 Responses Compared to an Adenovirus-Based Vaccine. Front. Immunol. 2023, 13, 1020844. [Google Scholar] [CrossRef]
- Tong, X.; Guan, C.; Ji, T.; Cao, C.; Jiang, J.; Liu, M.; Guo, Q.; Zhou, P.; Gong, F. Increased Circulating T Follicular Helper 13 Subset Correlates with High IgE Levels in Pediatric Allergic Asthma. Eur. J. Immunol. 2022, 52, 2010–2012. [Google Scholar] [CrossRef]
- Pfeifle, R.; Rothe, T.; Ipseiz, N.; Scherer, H.U.; Culemann, S.; Harre, U.; Ackermann, J.A.; Seefried, M.; Kleyer, A.; Uderhardt, S.; et al. Regulation of Autoantibody Activity by the IL-23–TH17 Axis Determines the Onset of Autoimmune Disease. Nat. Immunol. 2017, 18, 104–113. [Google Scholar] [CrossRef]
- Faber, M.A.; Van Gasse, A.L.; Decuyper, I.I.; Sabato, V.; Hagendorens, M.M.; Mertens, C.; Bridts, C.H.; De Clerck, L.S.; Ebo, D.G. Cross-Reactive Aeroallergens: Which Need to Cross Our Mind in Food Allergy Diagnosis? J. Allergy Clin. Immunol. Pract. 2018, 6, 1813–1823. [Google Scholar] [CrossRef]
- Liu, T.; Lai, S.; Li, W.; Jiang, Y. Prevalence of Food Allergen and Aeroallergen Sensitization among Children in Sichuan Province. Medicine 2020, 99, e21055. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.W.; Tjota, M.Y.; Sperling, A.I. The Contribution of Allergen-Specific IgG to the Development of Th2-Mediated Airway Inflammation. J. Allergy 2012, 2012, 236075. [Google Scholar] [CrossRef] [PubMed]
- Orengo, J.; Radin, A.; Kamat, V.; Badithe, A.; Ben, L.; Bennett, B.; Zhong, S.; Birchard, D.; Limnander, A.; Rafique, A.; et al. Treating Cat Allergy with Monoclonal IgG Antibodies That Bind Allergen and Prevent IgE Engagement. Nat. Commun. 2018, 9, 1421. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Kobayashi, K.; Yamamoto, M.; Nakata, K.; Takagawa, T.; Funada, Y.; Kotani, Y.; Karasuyama, H.; Yoshida, M.; Nishimura, Y. Antigen-Specific IgG Ameliorates Allergic Airway Inflammation via Fcγ Receptor IIB on Dendritic Cells. Respir. Res. 2011, 12, 42. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udoye, C.C.; Ehlers, M.; Manz, R.A. The B Cell Response and Formation of Allergenic and Anti-Allergenic Antibodies in Food Allergy. Biology 2023, 12, 1501. https://doi.org/10.3390/biology12121501
Udoye CC, Ehlers M, Manz RA. The B Cell Response and Formation of Allergenic and Anti-Allergenic Antibodies in Food Allergy. Biology. 2023; 12(12):1501. https://doi.org/10.3390/biology12121501
Chicago/Turabian StyleUdoye, Christopher C., Marc Ehlers, and Rudolf A. Manz. 2023. "The B Cell Response and Formation of Allergenic and Anti-Allergenic Antibodies in Food Allergy" Biology 12, no. 12: 1501. https://doi.org/10.3390/biology12121501
APA StyleUdoye, C. C., Ehlers, M., & Manz, R. A. (2023). The B Cell Response and Formation of Allergenic and Anti-Allergenic Antibodies in Food Allergy. Biology, 12(12), 1501. https://doi.org/10.3390/biology12121501





