Defining the Role of Metastasis-Initiating Cells in Promoting Carcinogenesis in Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Heterogeneity of Metastasis
3. Metastasis-Initiating Cells
3.1. Origins of MICs
3.2. Characteristics of MICs
3.2.1. Markers
CD24
CD44
CD133
CD117
ROR1
ALDH
SOX2
3.2.2. Multi-Omic Profiles
Genomic Profiles
Epigenomic Landscape
Transcriptomic Landscape
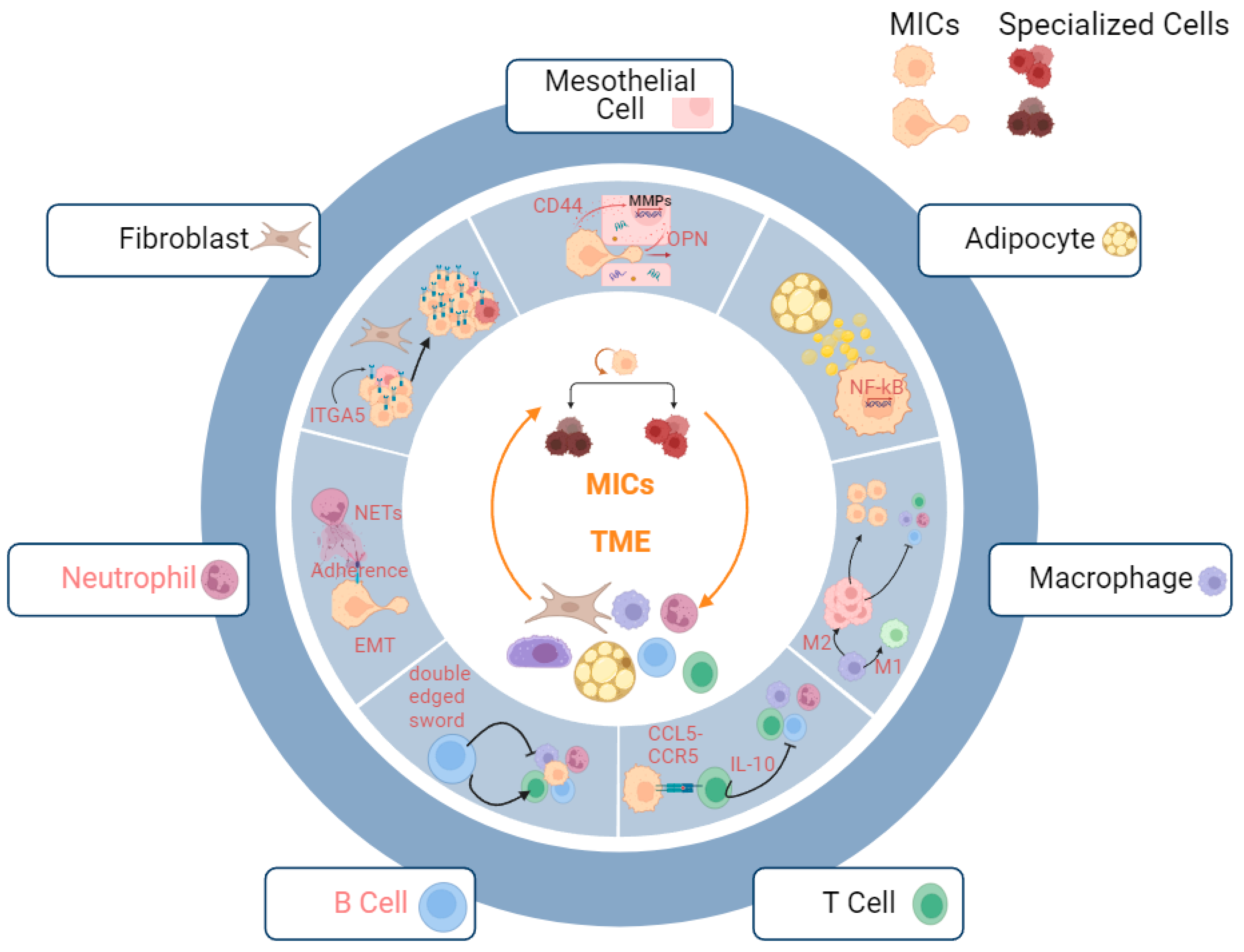
3.2.3. Metastatic Niche
Fibroblasts
Mesothelial Cells
Adipocytes
Macrophages
T Cells
B Cells
Neutrophils
3.3. Therapeutic Strategies
3.3.1. Immunotherapy
3.3.2. Epigenetic Therapy
3.3.3. Targeted Therapy
3.3.4. Anti-EMT Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO–ESGO Consensus Conference Recommendations on Ovarian Cancer: Pathology and Molecular Biology, Early and Advanced Stages, Borderline Tumours and Recurrent Disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Kuroki, L.; Guntupalli, S.R. Treatment of Epithelial Ovarian Cancer. BMJ 2020, 371, m3773. [Google Scholar] [CrossRef]
- Tan, D.S.; Agarwal, R.; Kaye, S.B. Mechanisms of Transcoelomic Metastasis in Ovarian Cancer. Lancet Oncol. 2006, 7, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Dehghani, S.; Nosrati, R.; Ghanei, M.; Salmaninejad, A.; Rajaie, S.; Hasanzadeh, M.; Pasdar, A. Current Insights into the Metastasis of Epithelial Ovarian Cancer—Hopes and Hurdles. Cell Oncol. 2020, 43, 515–538. [Google Scholar] [CrossRef] [PubMed]
- Al-Ayoubi, A.; Tarcsafalvi, A.; Zheng, H.; Sakati, W.; Eblen, S.T. ERK Activation and Nuclear Signaling Induced by the Loss of Cell/Matrix Adhesion Stimulates Anchorage-independent Growth of Ovarian Cancer Cells. J. Cell Biochem. 2008, 105, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Moss, N.M.; Barbolina, M.V.; Liu, Y.; Sun, L.; Munshi, H.G.; Stack, M.S. Ovarian Cancer Cell Detachment and Multicellular Aggregate Formation Are Regulated by Membrane Type 1 Matrix Metalloproteinase: A Potential Role in I.p. Metastatic Dissemination. Cancer Res. 2009, 69, 7121–7129. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, L.; Hu, A.; Wang, Y.; Liu, Y.; Yang, J.; Du, N.; An, X.; Wu, C.; Liu, C. Loss of 4.1N in Epithelial Ovarian Cancer Results in EMT and Matrix-Detached Cell Death Resistance. Protein Cell 2021, 12, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, B.T.; Qamar, L.; Yamamoto, T.M.; McMellen, A.; Watson, Z.L.; Richer, J.K.; Behbakht, K.; Schlaepfer, I.R.; Bitler, B.G. Targeting Fatty Acid Oxidation to Promote Anoikis and Inhibit Ovarian Cancer Progression. Mol. Cancer Res. 2020, 18, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Caneba, C.A.; Bellance, N.; Yang, L.; Pabst, L.; Nagrath, D. Pyruvate Uptake Is Increased in Highly Invasive Ovarian Cancer Cells under Anoikis Conditions for Anaplerosis, Mitochondrial Function, and Migration. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1036–E1052. [Google Scholar] [CrossRef]
- Frankel, A.; Rosen, K.; Filmus, J.; Kerbel, R.S. Induction of Anoikis and Suppression of Human Ovarian Tumor Growth in vivo by Down-Regulation of Bcl-X(L). Cancer Res. 2001, 61, 4837–4841. [Google Scholar] [PubMed]
- Kipps, E.; Tan, D.S.P.; Kaye, S.B. Meeting the Challenge of Ascites in Ovarian Cancer: New Avenues for Therapy and Research. Nat. Rev. Cancer 2013, 13, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Uno, K.; Iyoshi, S.; Yoshihara, M.; Kitami, K.; Mogi, K.; Fujimoto, H.; Sugiyama, M.; Koya, Y.; Yamakita, Y.; Nawa, A.; et al. Metastatic Voyage of Ovarian Cancer Cells in Ascites with the Assistance of Various Cellular Components. Int. J. Mol. Sci. 2022, 23, 4383. [Google Scholar] [CrossRef]
- Motohara, T.; Masuda, K.; Morotti, M.; Zheng, Y.; El-Sahhar, S.; Chong, K.Y.; Wietek, N.; Alsaadi, A.; Karaminejadranjbar, M.; Hu, Z.; et al. An Evolving Story of the Metastatic Voyage of Ovarian Cancer Cells: Cellular and Molecular Orchestration of the Adipose-Rich Metastatic Microenvironment. Oncogene 2019, 38, 2885–2898. [Google Scholar] [CrossRef]
- Hilliard, T.S.; Kowalski, B.; Iwamoto, K.; Agadi, E.A.; Liu, Y.; Yang, J.; Asem, M.; Klymenko, Y.; Johnson, J.; Shi, Z.; et al. Host Mesothelin Expression Increases Ovarian Cancer Metastasis in the Peritoneal Microenvironment. Int. J. Mol. Sci. 2021, 22, 12443. [Google Scholar] [CrossRef]
- Sun, L.; Wang, D.; Li, X.; Zhang, L.; Zhang, H.; Zhang, Y. Extracellular Matrix Protein ITGBL1 Promotes Ovarian Cancer Cell Migration and Adhesion through Wnt/PCP Signaling and FAK/SRC Pathway. Biomed. Pharmacother. 2016, 81, 145–151. [Google Scholar] [CrossRef]
- Chen, X.; Brewer, M.A.; Zou, C.; Campagnola, P.J. Adhesion and Migration of Ovarian Cancer Cells on Crosslinked Laminin Fibers Nanofabricated by Multiphoton Excited Photochemistry. Integr. Biol. 2009, 1, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Uruski, P.; Mikuła-Pietrasik, J.; Pakuła, M.; Budkiewicz, S.; Drzewiecki, M.; Gaiday, A.N.; Wierzowiecka, M.; Naumowicz, E.; Moszyński, R.; Tykarski, A.; et al. Malignant Ascites Promote Adhesion of Ovarian Cancer Cells to Peritoneal Mesothelium and Fibroblasts. Int. J. Mol. Sci. 2021, 22, 4222. [Google Scholar] [CrossRef]
- Furukawa, S.; Soeda, S.; Kiko, Y.; Suzuki, O.; Hashimoto, Y.; Watanabe, T.; Nishiyama, H.; Tasaki, K.; Hojo, H.; Abe, M.; et al. MCP-1 Promotes Invasion and Adhesion of Human Ovarian Cancer Cells. Anticancer Res. 2013, 33, 4785–4790. [Google Scholar]
- Ween, M.P.; Oehler, M.K.; Ricciardelli, C. Role of Versican, Hyaluronan and CD44 in Ovarian Cancer Metastasis. Int. J. Mol. Sci. 2011, 12, 1009–1029. [Google Scholar] [CrossRef]
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Rueger, R. Mechanisms and Targets Involved in Dissemination of Ovarian Cancer. Cancer Genom. Proteom. 2016, 13, 407–423. [Google Scholar] [CrossRef]
- Lee, J.-G.; Ahn, J.-H.; Kim, T.J.; Lee, J.H.; Choi, J.-H. Mutant P53 Promotes Ovarian Cancer Cell Adhesion to Mesothelial Cells via Integrin Β4 and Akt Signals. Sci. Rep. 2015, 5, 12642. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.A.; Kaur, S.; Coussens, L.M.; Lengyel, E. The Initial Steps of Ovarian Cancer Cell Metastasis Are Mediated by MMP-2 Cleavage of Vitronectin and Fibronectin. J. Clin. Investig. 2008, 118, 1367–1379. [Google Scholar] [CrossRef]
- Kenny, H.A.; Lengyel, E. MMP-2 Functions as an Early Response Protein in Ovarian Cancer Metastasis. Cell Cycle 2009, 8, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Sawada, K.; Tiwari, P.; Mui, K.; Gwin, K.; Lengyel, E. Ligand-Independent Activation of c-Met by Fibronectin and A5β1-Integrin Regulates Ovarian Cancer Invasion and Metastasis. Oncogene 2011, 30, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Mitra, A.K.; Radjabi, A.R.; Bhaskar, V.; Kistner, E.O.; Tretiakova, M.; Jagadeeswaran, S.; Montag, A.; Becker, A.; Kenny, H.A.; et al. Loss of E-Cadherin Promotes Ovarian Cancer Metastasis via A5-Integrin, Which Is a Therapeutic Target. Cancer Res. 2008, 68, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.A.E.; Agarwal, K.; Dasari, S.; Mitra, A.K. Productive Cross-Talk with the Microenvironment: A Critical Step in Ovarian Cancer Metastasis. Cancers 2019, 11, 1608. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Fang, Y.; Mitra, A.K. Cancer Associated Fibroblasts: Naughty Neighbors That Drive Ovarian Cancer Progression. Cancers 2018, 10, 406. [Google Scholar] [CrossRef]
- Mitra, A.K. Ovarian Cancer Metastasis: A Unique Mechanism of Dissemination. Tumor Metastasis 2016. [Google Scholar] [CrossRef]
- Yin, X.; Jing, Y.; Cai, M.-C.; Ma, P.; Zhang, Y.; Xu, C.; Zhang, M.; Di, W.; Zhuang, G. Clonality, Heterogeneity and Evolution of Synchronous Bilateral Ovarian Cancer. Cancer Res. 2017, 77, 6551–6561. [Google Scholar] [CrossRef]
- Baert, T.; Ferrero, A.; Sehouli, J.; O´Donnell, D.M.; González-Martín, A.; Joly, F.; van der Velden, J.; Blecharz, P.; Tan, D.S.P.; Querleu, D.; et al. The Systemic Treatment of Recurrent Ovarian Cancer Revisited. Ann. Oncol. 2021, 32, 710–725. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Berchuck, A.; Birrer, M.; Chien, J.; Cramer, D.W.; Dao, F.; Dhir, R.; DiSaia, P.; Gabra, H.; Glenn, P.; et al. Integrated Genomic Analyses of Ovarian Carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Masoodi, T.; Siraj, S.; Siraj, A.K.; Azam, S.; Qadri, Z.; Parvathareddy, S.K.; Tulbah, A.; Al-Dayel, F.; AlHusaini, H.; AlOmar, O.; et al. Genetic Heterogeneity and Evolutionary History of High-Grade Ovarian Carcinoma and Matched Distant Metastases. Br. J. Cancer 2020, 122, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.P. Next-Generation Sequencing and Sequence Data Analysis; Bentham Science Publishers: Sharjah, United Arab Emirates, 2015; pp. 97–104. [Google Scholar] [CrossRef]
- Single-Cell Sequencing. Nature 2013, 493, 136. Available online: https://www.nature.com/articles/493136c#article-info. (accessed on 22 November 2023). [CrossRef]
- Nawy, T. Single-Cell Sequencing. Nat. Methods 2014, 11, 18. [Google Scholar] [CrossRef]
- Olbrecht, S.; Busschaert, P.; Qian, J.; Vanderstichele, A.; Loverix, L.; Gorp, T.V.; Nieuwenhuysen, E.V.; Han, S.; den Broeck, A.V.; Coosemans, A.; et al. High-Grade Serous Tubo-Ovarian Cancer Refined with Single-Cell RNA Sequencing: Specific Cell Subtypes Influence Survival and Determine Molecular Subtype Classification. Genome Med. 2021, 13, 111. [Google Scholar] [CrossRef]
- Izar, B.; Tirosh, I.; Stover, E.H.; Wakiro, I.; Cuoco, M.S.; Alter, I.; Rodman, C.; Leeson, R.; Su, M.-J.; Shah, P.; et al. A Single-Cell Landscape of High-Grade Serous Ovarian Cancer. Nat. Med. 2020, 26, 1271–1279. [Google Scholar] [CrossRef]
- Zhang, K.; Erkan, E.P.; Jamalzadeh, S.; Dai, J.; Andersson, N.; Kaipio, K.; Lamminen, T.; Mansuri, N.; Huhtinen, K.; Carpén, O.; et al. Longitudinal Single-Cell RNA-Seq Analysis Reveals Stress-Promoted Chemoresistance in Metastatic Ovarian Cancer. Sci. Adv. 2022, 8, eabm1831. [Google Scholar] [CrossRef]
- Qian, J.; Olbrecht, S.; Boeckx, B.; Vos, H.; Laoui, D.; Etlioglu, E.; Wauters, E.; Pomella, V.; Verbandt, S.; Busschaert, P.; et al. A Pan-Cancer Blueprint of the Heterogeneous Tumor Microenvironment Revealed by Single-Cell Profiling. Cell Res. 2020, 30, 745–762. [Google Scholar] [CrossRef]
- Olalekan, S.; Xie, B.; Back, R.; Eckart, H.; Basu, A. Characterizing the Tumor Microenvironment of Metastatic Ovarian Cancer by Single-Cell Transcriptomics. Cell Rep. 2021, 35, 109165. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Alexander, M. Untersuchungen Über Blut Und Bindegewebe. Arch. Für Mikrosk. Anat. 1922, 73, 444–561. [Google Scholar]
- Becker, A.J.; Mcculloch, E.A.; Till, J.E. Cytological Demonstration of the Clonal Nature of Spleen Colonies Derived from Transplanted Mouse Marrow Cells. Nature 1963, 197, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, D.; Dick, J.E. Human Acute Myeloid Leukemia Is Organized as a Hierarchy That Originates from a Primitive Hematopoietic Cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Bapat, S.A.; Mali, A.M.; Koppikar, C.B.; Kurrey, N.K. Stem and Progenitor-Like Cells Contribute to the Aggressive Behavior of Human Epithelial Ovarian Cancer. Cancer Res. 2005, 65, 3025–3029. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Obradović, M.M.S.; Hoffmann, M.; Harper, K.L.; Sosa, M.S.; Werner-Klein, M.; Nanduri, L.K.; Werno, C.; Ehrl, C.; Maneck, M.; et al. Early Dissemination Seeds Metastasis in Breast Cancer. Nature 2016, 540, 552–558. [Google Scholar] [CrossRef]
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.-C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.-M.; Nephew, K.P. Identification and Characterization of Ovarian Cancer-Initiating Cells from Primary Human Tumors. Cancer Res. 2008, 68, 4311–4320. [Google Scholar] [CrossRef]
- Massagué, J.; Ganesh, K. Metastasis-Initiating Cells and Ecosystems. Cancer Discov. 2021, 11, 971–994. [Google Scholar] [CrossRef] [PubMed]
- Riso, P.L.; Villa, C.E.; Gasparoni, G.; Vingiani, A.; Luongo, R.; Manfredi, A.; Jungmann, A.; Bertolotti, A.; Borgo, F.; Garbi, A.; et al. A Cell-of-Origin Epigenetic Tracer Reveals Clinically Distinct Subtypes of High-Grade Serous Ovarian Cancer. Genome Med. 2020, 12, 94. [Google Scholar] [CrossRef]
- Zhang, S.; Dolgalev, I.; Zhang, T.; Ran, H.; Levine, D.A.; Neel, B.G. Both Fallopian Tube and Ovarian Surface Epithelium Are Cells-of-Origin for High-Grade Serous Ovarian Carcinoma. Nat. Commun. 2019, 10, 5367. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.F.; Majdic, O.; Gadd, S.; Knapp, W. Signal Transduction in Lymphocytic and Myeloid Cells via CD24, a New Member of Phosphoinositol-Anchored Membrane Molecules. J. Immunol. 1990, 144, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Terai, Y.; Tanabe, A.; Ono, Y.J.; Hayashi, M.; Maeda, K.; Fujiwara, S.; Ashihara, K.; Nakamura, M.; Tanaka, Y.; et al. CD24 Expression Is a Marker for Predicting Clinical Outcome and Regulates the Epithelial-Mesenchymal Transition in Ovarian Cancer via Both the Akt and ERK Pathways. Oncol. Rep. 2017, 37, 3189–3200. [Google Scholar] [CrossRef]
- Gao, M.-Q.; Choi, Y.-P.; Kang, S.; Youn, J.H.; Cho, N.-H. CD24+ Cells from Hierarchically Organized Ovarian Cancer Are Enriched in Cancer Stem Cells. Oncogene 2010, 29, 2672–2680. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Choi, Y.P.; Gao, M.-Q.; Kang, S.; Kim, B.G.; Lee, J.H.; Kwon, M.J.; Shin, Y.K.; Cho, N.H. CD24+ Ovary Cancer Cells Exhibit an Invasive Mesenchymal Phenotype. Biochem. Biophys. Res. Commun. 2013, 432, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Lee, S.; Park, H.; Lee, H.J.; Cho, N.H.; Kim, J. Susceptibility of CD24+ Ovarian Cancer Cells to Anti-Cancer Drugs and Natural Killer Cells. Biochem. Biophys. Res. Commun. 2012, 427, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Ojeda, D.; Wu, R.; McLean, K.; Chen, Y.-C.; Talpaz, M.; Yoon, E.; Cho, K.R.; Buckanovich, R.J. CD24+ Ovarian Cancer Cells Are Enriched for Cancer-Initiating Cells and Dependent on JAK2 Signaling for Growth and Metastasis. Mol. Cancer Ther. 2015, 14, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.J.; Fogg, K.C.; Patel, H.A.; Krause, H.B.; Mancha, A.-S.; Patankar, M.S.; Weisman, P.S.; Barroilhet, L.; Kreeger, P.K. Alternatively Activated Macrophages Upregulate Mesothelial Expression of P-Selectin to Enhance Adhesion of Ovarian Cancer Cells. Cancer Res. 2018, 78, 3560–3573. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Wang, H. Role of CD24 in Anoikis Resistance of Ovarian Cancer Cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2015, 35, 390–396. [Google Scholar] [CrossRef]
- Herrera-Gayol, A.; Jothy, S. The impact of hyaluronan on the in vitro invasive properties of human breast cancer cell lines with high CD44 expression. Hyaluronan 2002, 1, 443–446. [Google Scholar] [CrossRef]
- Sano, N.; Kitazawa, K.; Sugisaki, T. Localization and Roles of CD44, Hyaluronic Acid and Osteopontin in IgA Nephropathy. Nephron 2001, 89, 416–421. [Google Scholar] [CrossRef]
- Weber, G.F.; Ashkar, S.; Glimcher, M.J.; Cantor, H. Receptor-Ligand Interaction Between CD44 and Osteopontin (Eta-1). Science 1996, 271, 509–512. [Google Scholar] [CrossRef]
- Ishii, S.; Ford, R.; Thomas, P.; Nachman, A.; Steele, G.; Jessup, J.M. CD44 Participates in the Adhesion of Human Colorectal Carcinoma Cells to Laminin and Type IV Collagen. Surg. Oncol. 1993, 2, 255–264. [Google Scholar] [CrossRef]
- Knutson, J.R.; Iida, J.; Fields, G.B.; McCarthy, J.B. CD44/Chondroitin Sulfate Proteoglycan and Alpha 2 Beta 1 Integrin Mediate Human Melanoma Cell Migration on Type IV Collagen and Invasion of Basement Membranes. Mol. Biol. Cell 1996, 7, 383–396. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Localization of Matrix Metalloproteinase 9 to the Cell Surface Provides a Mechanism for CD44-Mediated Tumor Invasion. Gene Dev. 1999, 13, 35–48. [Google Scholar] [CrossRef]
- Chellaiah, M.A.; Ma, T. Membrane Localization of Membrane Type 1 Matrix Metalloproteinase by CD44 Regulates the Activation of Pro-Matrix Metalloproteinase 9 in Osteoclasts. Biomed. Res. Int. 2013, 2013, 302392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Brown, R.L.; Wei, Y.; Zhao, P.; Liu, S.; Liu, X.; Deng, Y.; Hu, X.; Zhang, J.; Gao, X.D.; et al. CD44 Splice Isoform Switching Determines Breast Cancer Stem Cell State. Gene Dev. 2019, 33, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.K.; Nishi, T.; Saya, H. Novel Variants of CD44 Arising from Alternative Splicing: Changes in the CD44 Alternative Splicing Pattern of MCF-7 Breast Carcinoma Cells Treated with Hyaluronidase. Mol. Carcinog. 1993, 7, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Wreesmann, V.B.; Bourguignon, L.Y.W. Association of CD44 V3-containing Isoforms with Tumor Cell Growth, Migration, Matrix Metalloproteinase Expression, and Lymph Node Metastasis in Head and Neck Cancer. Head Neck 2007, 29, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Tjhay, F.; Motohara, T.; Tayama, S.; Narantuya, D.; Fujimoto, K.; Guo, J.; Sakaguchi, I.; Honda, R.; Tashiro, H.; Katabuchi, H. CD44 Variant 6 Is Correlated with Peritoneal Dissemination and Poor Prognosis in Patients with Advanced Epithelial Ovarian Cancer. Cancer Sci. 2015, 106, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gu, C.; Huang, K.; Zhang, Z.; Ye, M.; Fan, W.; Han, W.; Meng, Y. The Prognostic Value and Clinicopathological Significance of CD44 Expression in Ovarian Cancer: A Meta-Analysis. Arch. Gynecol. Obstet. 2016, 294, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-F.; Hu, P.; Fang, S.-Q. Understanding the Role of CD44V6 in Ovarian Cancer. Oncol. Lett. 2017, 14, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Mitra, T.; Chaudhuri, S.R.; Roy, S.S. Mesenchymal Splice Isoform of CD44 (CD44s) Promotes EMT/Invasion and Imparts Stem-like Properties to Ovarian Cancer Cells. J. Cell Biochem. 2018, 119, 3373–3383. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, C.; Zhu, H.; Wang, Y.; Wang, X.; Cheng, X.; Ge, W.; Lu, W. Exosome-Mediated Transfer of CD44 from High-Metastatic Ovarian Cancer Cells Promotes Migration and Invasion of Low-Metastatic Ovarian Cancer Cells. J. Ovarian Res. 2021, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Coskun, V.; Wu, H.; Blanchi, B.; Tsao, S.; Kim, K.; Zhao, J.; Biancotti, J.C.; Hutnick, L.; Krueger, R.C.; Fan, G.; et al. CD133+ Neural Stem Cells in the Ependyma of Mammalian Postnatal Forebrain. Proc. Natl. Acad. Sci. USA 2008, 105, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, R.M. Erythropoiesis from Adult but Not Fetal Blood-Derived CD133+ Stem Cells Depends Strongly on Interleukin-3. Growth Factors 2009, 22, 45–50. [Google Scholar] [CrossRef]
- Handgretinger, R.; Gordon, P.R.; Leimig, T.; Chen, X.; Bühring, H.; Niethammer, D.; Kuçi, S. Biology and Plasticity of CD133+ Hematopoietic Stem Cells. Ann. N. Y. Acad. Sci. 2003, 996, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fei, H.; Lin, Q.; Liang, F.; You, Y.; Li, M.; Wu, M.; Qu, Y.; Li, P.; Yuan, Y.; et al. ZEB2 Facilitates Peritoneal Metastasis by Regulating the Invasiveness and Tumorigenesis of Cancer Stem-like Cells in High-Grade Serous Ovarian Cancers. Oncogene 2021, 40, 5131–5141. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, A.; Song, H.; Tao, J.; Yang, H.; Zuo, M. Prognostic Value of Cancer Stem Cell Marker CD133 in Ovarian Cancer: A Meta-Analysis. Int. J. Clin. Exp. Med. 2014, 8, 3080–3088. [Google Scholar]
- Roy, L.; Bobbs, A.; Sattler, R.; Kurkewich, J.L.; Dausinas, P.B.; Nallathamby, P.; Dahl, K.D.C. CD133 Promotes Adhesion to the Ovarian Cancer Metastatic Niche. Cancer Growth Metastasis 2018, 11, 1179064418767882. [Google Scholar] [CrossRef]
- Su, Y.-J.; Lin, W.-H.; Chang, Y.-W.; Wei, K.-C.; Liang, C.-L.; Chen, S.-C.; Lee, J.-L. Polarized Cell Migration Induces Cancer Type-Specific CD133/Integrin/Src/Akt/GSK3β/β-Catenin Signaling Required for Maintenance of Cancer Stem Cell Properties. Oncotarget 2015, 6, 38029–38045. [Google Scholar] [CrossRef]
- Massa, S.; Balciunaite, G.; Ceredig, R.; Rolink, A.G. Critical Role for C-kit (CD117) in T Cell Lineage Commitment and Early Thymocyte Development in vitro. Eur. J. Immunol. 2006, 36, 526–532. [Google Scholar] [CrossRef]
- Matsuda, R.; Takahashi, T.; Nakamura, S.; Sekido, Y.; Nishida, K.; Seto, M.; Seito, T.; Sugiura, T.; Ariyoshi, Y.; Takahashi, T. Expression of the C-Kit Protein in Human Solid Tumors and in Corresponding Fetal and Adult Normal Tissues. Am. J. Pathol. 1993, 142, 339–346. [Google Scholar]
- Natali, P.G.; Nicotra, M.R.; Sures, I.; Santoro, E.; Bigotti, A.; Ullrich, A. Expression of C-Kit Receptor in Normal and Transformed Human Nonlymphoid Tissues. Cancer Res. 1992, 52, 6139–6143. [Google Scholar] [PubMed]
- Luo, L.; Zeng, J.; Liang, B.; Zhao, Z.; Sun, L.; Cao, D.; Yang, J.; Shen, K. Ovarian Cancer Cells with the CD117 Phenotype Are Highly Tumorigenic and Are Related to Chemotherapy Outcome. Exp. Mol. Pathol. 2011, 91, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Al-Shami, S.A.; Al-Kaabi, M.M.; Mahdi, A.K.; Al-Attar, Z. Immunohistochemical Expression of CD117 in Borderline, Low- and High-Grade Ovarian Surface Epithelial Tumours: A Clinicopathological Study. Malays. J. Pathol. 2023, 45, 229–236. [Google Scholar]
- Conic, I.; Stanojevic, Z.; Velickovic, L.J.; Stojnev, S.; Petrovic, A.R.; Krstic, M.; Stanojevic, M.; Bogdanović, D.; Stefanovic, V. Epithelial Ovarian Cancer with CD117 Phenotype Is Highly Aggressive and Resistant to Chemotherapy. J. Obstet. Gynaecol. Res. 2015, 41, 1630–1637. [Google Scholar] [CrossRef]
- Robinson, M.; Gilbert, S.F.; Waters, J.A.; Lujano-Olazaba, O.; Lara, J.; Alexander, L.J.; Green, S.E.; Burkeen, G.A.; Patrus, O.; Sarwar, Z.; et al. Characterization of SOX2, OCT4 and NANOG in Ovarian Cancer Tumor-Initiating Cells. Cancers 2021, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Shnaider, P.V.; Petrushanko, I.Y.; Aleshikova, O.I.; Babaeva, N.A.; Ashrafyan, L.A.; Borovkova, E.I.; Dobrokhotova, J.E.; Borovkov, I.M.; Shender, V.O.; Khomyakova, E. Expression Level of CD117 (KIT) on Ovarian Cancer Extracellular Vesicles Correlates with Tumor Aggressiveness. Front. Cell Dev. Biol. 2023, 11, 1057484. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ye, X.; Bordeaux, A.; Hettich, S.; Lin, S.; Han, F.; Jia, Y. MiR-26b Regulates Cell Proliferation and Apoptosis of CD117+CD44+ Ovarian Cancer Stem Cells by Targeting PTEN. Eur. J. Histochem. 2021, 65, 3186. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Cheng, H.; Liu, Y.; Liu, S.; Lowe, S.; Li, Y.; Bentley, R.; King, B.; Tuason, J.P.W.; Zhou, Q.; et al. Metformin Anticancer: Reverses Tumor Hypoxia Induced by Bevacizumab and Reduces the Expression of Cancer Stem Cell Markers CD44/CD117 in Human Ovarian Cancer SKOV3 Cells. Front. Pharmacol. 2022, 13, 955984. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Su, J.; Feng, C.; Liu, Y.; Zhao, L.; Tian, Y. Chemokine CCL20 Promotes the Paclitaxel Resistance of CD44+CD117+ Cells via the Notch1 Signaling Pathway in Ovarian Cancer. Mol. Med. Rep. 2021, 24, 635. [Google Scholar] [CrossRef]
- Paganoni, S.; Ferreira, A. Neurite Extension in Central Neurons: A Novel Role for the Receptor Tyrosine Kinases Ror1 and Ror2. J. Cell Sci. 2005, 118, 433–446. [Google Scholar] [CrossRef]
- Tseng, H.-C.; Lyu, P.-C.; Lin, W. Nuclear Localization of Orphan Receptor Protein Kinase (Ror1) Is Mediated through the Juxtamembrane Domain. BMC Cell Biol. 2010, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Widhopf, G.F.; Cui, B.; Ghia, E.M.; Chen, L.; Messer, K.; Shen, Z.; Briggs, S.P.; Croce, C.M.; Kipps, T.J. ROR1 Can Interact with TCL1 and Enhance Leukemogenesis in Eµ-TCL1 Transgenic Mice. Proc. Natl. Acad. Sci. USA 2014, 111, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Borcherding, N.; Kusner, D.; Liu, G.-H.; Zhang, W. ROR1, an Embryonic Protein with an Emerging Role in Cancer Biology. Protein Cell 2014, 5, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Paganoni, S.; Bernstein, J.; Ferreira, A. Ror1-Ror2 Complexes Modulate Synapse Formation in Hippocampal Neurons. Neuroscience 2010, 165, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qiu, J.; Ye, C.; Yang, D.; Gao, L.; Su, Y.; Tang, X.; Xu, N.; Zhang, D.; Xiong, L.; et al. ROR1 Expression Correlated with Poor Clinical Outcome in Human Ovarian Cancer. Sci. Rep. 2014, 4, 5811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cui, B.; Lai, H.; Liu, G.; Ghia, E.M.; Widhopf, G.F.; Zhang, Z.; Wu, C.C.N.; Chen, L.; Wu, R.; et al. Ovarian Cancer Stem Cells Express ROR1, Which Can Be Targeted for Anti–Cancer-Stem-Cell Therapy. Proc. Natl. Acad. Sci. USA 2014, 111, 17266–17271. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Hacker, N.; Ford, C. Silencing ROR1 and ROR2 Inhibits Invasion and Adhesion in an Organotypic Model of Ovarian Cancer Metastasis. Oncotarget 2017, 8, 112727–112738. [Google Scholar] [CrossRef]
- Rodriguez-Torres, M.; Allan, A.L. Aldehyde Dehydrogenase as a Marker and Functional Mediator of Metastasis in Solid Tumors. Clin. Exp. Metastas 2016, 33, 97–113. [Google Scholar] [CrossRef]
- Marcato, P.; Dean, C.A.; Giacomantonio, C.A.; Lee, P.W.K. Aldehyde Dehydrogenase: Its Role as a Cancer Stem Cell Marker Comes down to the Specific Isoform. Cell Cycle 2011, 10, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishnan, V.; Fang, F.; Given, T.C.; Podicheti, R.; Chchterbinine, M.; Sriramkumar, S.; O’Hagan, H.M.; Hurley, T.D.; Nephew, K.P. A Novel ALDH1A1 Inhibitor Blocks Platinum-Induced Senescence and Stemness in Ovarian Cancer. Biorxiv 2022, 14, 3437. [Google Scholar] [CrossRef]
- Sharbatoghli, M.; Shamshiripour, P.; Fattahi, F.; Kalantari, E.; Shams, Z.H.; Panahi, M.; Totonchi, M.; Asadi-Lari, Z.; Madjd, Z.; Zanjani, L.S. Co-Expression of Cancer Stem Cell Markers, SALL4/ALDH1A1, Is Associated with Tumor Aggressiveness and Poor Survival in Patients with Serous Ovarian Carcinoma. J. Ovarian Res. 2022, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Sterzyńska, K.; Klejewski, A.; Wojtowicz, K.; Świerczewska, M.; Nowacka, M.; Kaźmierczak, D.; Andrzejewska, M.; Rusek, D.; Brązert, M.; Brązert, J.; et al. Mutual Expression of ALDH1A1, LOX, and Collagens in Ovarian Cancer Cell Lines as Combined CSCs- and ECM-Related Models of Drug Resistance Development. Int. J. Mol. Sci. 2018, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Landen, C.N.; Goodman, B.; Katre, A.A.; Steg, A.D.; Nick, A.M.; Stone, R.L.; Miller, L.D.; Mejia, P.V.; Jennings, N.B.; Gershenson, D.M.; et al. Targeting Aldehyde Dehydrogenase Cancer Stem Cells in Ovarian Cancer. Mol. Cancer Ther. 2010, 9, 3186–3199. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.; Mitra, A.; Tripathi, K.; Finan, M.A.; Scalici, J.; McClellan, S.; da Silva, L.M.; Reed, E.; Shevde, L.A.; Palle, K.; et al. ALDH1A1 Maintains Ovarian Cancer Stem Cell-Like Properties by Altered Regulation of Cell Cycle Checkpoint and DNA Repair Network Signaling. PLoS ONE 2014, 9, e107142. [Google Scholar] [CrossRef]
- Jiang, Y.-X.; Siu, M.K.-Y.; Wang, J.-J.; Mo, X.-T.; Leung, T.H.-Y.; Chan, D.W.; Cheung, A.N.-Y.; Ngan, H.Y.-S.; Chan, K.K.-L. Ascites-Derived ALDH+CD44+ Tumour Cell Subsets Endow Stemness, Metastasis and Metabolic Switch via PDK4-Mediated STAT3/AKT/NF-ΚB/IL-8 Signalling in Ovarian Cancer. Br. J. Cancer 2020, 123, 275–287. [Google Scholar] [CrossRef]
- Cui, T.; Srivastava, A.K.; Han, C.; Wu, D.; Wani, N.; Liu, L.; Gao, Z.; Qu, M.; Zou, N.; Zhang, X.; et al. DDB2 Represses Ovarian Cancer Cell Dedifferentiation by Suppressing ALDH1A1. Cell Death Dis. 2018, 9, 561. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Huang, J.; Chen, T.; Liu, X.; Jiang, J.; Li, J.; Li, D.; Liu, X.S.; Li, W.; Kang, J.; Pei, G. More Synergetic Cooperation of Yamanaka Factors in Induced Pluripotent Stem Cells than in Embryonic Stem Cells. Cell Res. 2009, 19, 1127–1138. [Google Scholar] [CrossRef]
- Wang, H.F.; He, H.X. Regulation of Yamanaka Factors during H5N1 Virus Infection in A549 Cells and HEK293T Cells. Biotechnol. Biotechnol. Equip. 2018, 32, 1548–1557. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.; Chen, T.; Wang, Y.; Xin, S.; Li, J.; Pei, G.; Kang, J. Yamanaka Factors Critically Regulate the Developmental Signaling Network in Mouse Embryonic Stem Cells. Cell Res. 2008, 18, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Plath, K.; Lowry, W.E. Progress in Understanding Reprogramming to the Induced Pluripotent State. Nat. Rev. Genet. 2011, 12, 253–265. [Google Scholar] [CrossRef]
- Wen, Y.; Hou, Y.; Huang, Z.; Cai, J.; Wang, Z. SOX2 Is Required to Maintain Cancer Stem Cells in Ovarian Cancer. Cancer Sci. 2017, 108, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ji, X.; Chen, J.; Yan, D.; Zhang, Z.; Wang, Q.; Xi, X.; Feng, Y. SOX2 Enhances the Migration and Invasion of Ovarian Cancer Cells via Src Kinase. PLoS ONE 2014, 9, e99594. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, X.; Jones, H.M.; Chan, L.L.-Y.; Song, F.; Zhang, W.; Bae-Jump, V.L.; Zhou, C. Evaluation of the Antitumor Effects of C-Myc-Max Heterodimerization Inhibitor 100258-F4 in Ovarian Cancer Cells. J. Transl. Med. 2014, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Dutta, P.; Sahay, O.; Gopalakrishnan, K.; Muhury, S.R.; Parameshwar, P.; Shetty, P.; Santra, M.K. Feedback-regulated Transcriptional Repression of FBXO31 by C-Myc Triggers Ovarian Cancer Tumorigenesis. Int. J. Cancer 2022, 150, 1512–1524. [Google Scholar] [CrossRef]
- Dimova, I.; Raitcheva, S.; Dimitrov, R.; Doganov, N.; Toncheva, D. Correlations between C-Myc Gene Copy-Number and Clinicopathological Parameters of Ovarian Tumours. Eur. J. Cancer 2006, 42, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, J.; Fang, C.; Zhang, Z.; Feng, Y.; Xi, X. OCT4 Mediates FSH-Induced Epithelial–Mesenchymal Transition and Invasion through the ERK1/2 Signaling Pathway in Epithelial Ovarian Cancer. Biochem. Biophys. Res. Commun. 2015, 461, 525–532. [Google Scholar] [CrossRef]
- Wu, D.; Xie, W.; Wang, H.; Chen, W.; Chen, X.; Sun, H. OCT4 Promotes Ovarian Cancer Cell Metastasis and Angiogenesis via Modulating VEGFR2/LRPPRC Pathway. Preprint 2021. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Liu, W.; Zhao, G.; Lee, S.; Balogh, A.; Zou, Y.; Guo, Y.; Zhang, Z.; Gu, W.; et al. Doxycycline Inducible Kruppel-Like Factor 4 Lentiviral Vector Mediates Mesenchymal to Epithelial Transition in Ovarian Cancer Cells. PLoS ONE 2014, 9, e105331. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shen, A.; Ouyang, X.; Zhao, G.; Du, Z.; Huo, W.; Zhang, T.; Wang, Y.; Yang, C.; Dong, P.; et al. KLF4 Expression Enhances the Efficacy of Chemotherapy Drugs in Ovarian Cancer Cells. Biochem. Biophys. Res. Commun. 2017, 484, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Mazzoldi, E.L.; Pastò, A.; Pilotto, G.; Minuzzo, S.; Piga, I.; Palumbo, P.; Carella, M.; Frezzini, S.; Nicoletto, M.O.; Amadori, A.; et al. Comparison of the Genomic Profile of Cancer Stem Cells and Their Non-Stem Counterpart: The Case of Ovarian Cancer. J. Clin. Med. 2020, 9, 368. [Google Scholar] [CrossRef]
- Hoogstraat, M.; de Pagter, M.S.; Cirkel, G.A.; van Roosmalen, M.J.; Harkins, T.T.; Duran, K.; Kreeftmeijer, J.; Renkens, I.; Witteveen, P.O.; Lee, C.C.; et al. Genomic and Transcriptomic Plasticity in Treatment-Naïve Ovarian Cancer. Genome Res. 2014, 24, 200–211. [Google Scholar] [CrossRef] [PubMed]
- van de Haar, J.; Hoes, L.R.; Roepman, P.; Lolkema, M.P.; Verheul, H.M.W.; Gelderblom, H.; de Langen, A.J.; Smit, E.F.; Cuppen, E.; Wessels, L.F.A.; et al. Limited Evolution of the Actionable Metastatic Cancer Genome under Therapeutic Pressure. Nat. Med. 2021, 27, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Zucconi, F. Epigenetic regulation in plants. Isr. J. Plant Sci. 1988, 37, 131–144. [Google Scholar] [CrossRef]
- Göndör, A. Epigenetic Regulation. Br. J. Surg. 2008, 95, 939–940. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.J.; Benarroch, E.E. Epigenetic Regulation. Neurology 2014, 82, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Sun, Y.E. Epigenetic Regulation of Stem Cell Differentiation. Pediatr. Res. 2006, 59, 21R–25R. [Google Scholar] [CrossRef]
- Seto, E. Histone Modifications. Methods 2003, 31, 1–2. [Google Scholar] [CrossRef]
- Peterson, C.L.; Laniel, M.-A. Histones and Histone Modifications. Curr. Biol. 2004, 14, R546–R551. [Google Scholar] [CrossRef]
- Sang, F. DNA Modifications. Methods Mol. Biol. 2020, 2198, 441–450. [Google Scholar] [CrossRef]
- Tang, F.; Yuan, B.-F. DNA Modification Detection Methods. In DNA Modification Detection Methods; Springer Protocols Handbooks; Humana: New York, NY, USA, 2021; pp. 181–194. [Google Scholar] [CrossRef]
- Lucia, F.D.; Dean, C. Long Non-Coding RNAs and Chromatin Regulation. Curr. Opin. Plant Biol. 2011, 14, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Carninci, P. Long Non-coding RNA Modifies Chromatin. Bioessays 2011, 33, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Saitoh, N. Non-Coding RNAs and Chromatin Domains. Curr. Opin. Cell Biol. 2019, 58, 26–33. [Google Scholar] [CrossRef]
- Efroni, S.; Duttagupta, R.; Cheng, J.; Dehghani, H.; Hoeppner, D.J.; Dash, C.; Bazett-Jones, D.P.; Grice, S.L.; McKay, R.D.G.; Buetow, K.H.; et al. Global Transcription in Pluripotent Embryonic Stem Cells. Cell Stem Cell 2008, 2, 437–447. [Google Scholar] [CrossRef]
- Berry, N.B.; Bapat, S.A. Ovarian Cancer Plasticity and Epigenomics in the Acquisition of a Stem-like Phenotype. J. Ovarian Res. 2008, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Teschendorff, A.E.; Enver, T. Single-Cell Entropy for Accurate Estimation of Differentiation Potency from a Cell’s Transcriptome. Nat. Commun. 2017, 8, 15599. [Google Scholar] [CrossRef]
- Kannan, S.; Farid, M.; Lin, B.L.; Miyamoto, M.; Kwon, C. Transcriptomic Entropy Benchmarks Stem Cell-Derived Cardiomyocyte Maturation against Endogenous Tissue at Single Cell Level. Biorxiv 2020, 17, e1009305. [Google Scholar] [CrossRef]
- Banerji, C.R.S.; Severini, S.; Caldas, C.; Teschendorff, A.E. Intra-Tumour Signalling Entropy Determines Clinical Outcome in Breast and Lung Cancer. PLoS Comput. Biol. 2015, 11, e1004115. [Google Scholar] [CrossRef]
- Feng, L.; Sun, Y.-D.; Li, C.; Li, Y.-X.; Chen, L.-N.; Zeng, R. Pan-Cancer Network Disorders Revealed by Overall and Local Signaling Entropy. J. Mol. Cell Biol. 2021, 13, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, H.; Jeong, H.S.; Keith, K.; Maegawa, S.; Calendo, G.; Madzo, J.; Jelinek, J.; Issa, J.-P.J. DNA Methylation Entropy as a Measure of Stem Cell Replication and Aging. Genome Biol. 2023, 24, 27. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Shen, S.S.; Margueron, R.; Reinberg, D. Nucleosome-Binding Activities within JARID2 and EZH1 Regulate the Function of PRC2 on Chromatin. Gene Dev. 2013, 27, 2663–2677. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhu, B. Chapter 10—Regulation of PRC2 Activity. In Polycomb Group Proteins; Pirrotta, V., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 225–258. ISBN 978-0-12-809737-3. [Google Scholar]
- Wang, W.; Cho, H.; Kim, D.; Park, Y.; Moon, J.H.; Lim, S.J.; Yoon, S.M.; McCane, M.; Aicher, S.A.; Kim, S.; et al. PRC2 Acts as a Critical Timer That Drives Oligodendrocyte Fate over Astrocyte Identity by Repressing the Notch Pathway. Cell Rep. 2020, 32, 108147. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Wang, W.; Ozes, A.; Fang, F.; Sandusky, G.E.; Nephew, K.P. EZH2-Mediated Downregulation of the Tumor Suppressor DAB2IP Maintains Ovarian Cancer Stem Cells. Cancer Res. 2020, 80, 4371–4385. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Convery, P.A.; Matsumura, N.; Whitaker, R.S.; Kondoh, E.; Perry, T.; Huang, Z.; Bentley, R.C.; Mori, S.; Fujii, S.; et al. Epigenetic Regulation of CD133 and Tumorigenicity of CD133+ Ovarian Cancer Cells. Oncogene 2009, 28, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ramadoss, S.; Sen, S.; Ramachandran, I.; Roy, S.; Chaudhuri, G.; Farias-Eisner, R. Lysine-Specific Demethylase KDM3A Regulates Ovarian Cancer Stemness and Chemoresistance. Oncogene 2017, 36, 1537–1545. [Google Scholar] [CrossRef]
- Lamouille, S.; Subramanyam, D.; Blelloch, R.; Derynck, R. Regulation of Epithelial–Mesenchymal and Mesenchymal–Epithelial Transitions by MicroRNAs. Curr. Opin. Cell Biol. 2013, 25, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Banyard, J.; Bielenberg, D.R. The Role of EMT and MET in Cancer Dissemination. Connect. Tissue Res. 2015, 56, 403–413. [Google Scholar] [CrossRef]
- Hei, B.; Wang, J.; Wu, G.; Ouyang, J.; Liu, R. Verbascoside Suppresses the Migration and Invasion of Human Glioblastoma Cells via Targeting C-Met-Mediated Epithelial-Mesenchymal Transition. Biochem. Biophys. Res. Commun. 2019, 514, 1270–1277. [Google Scholar] [CrossRef]
- Tang, H.; Massi, D.; Hemmings, B.A.; Mandalà, M.; Hu, Z.; Wicki, A.; Xue, G. AKT-Ions with a TWIST between EMT and MET. Oncotarget 2016, 7, 62767–62777. [Google Scholar] [CrossRef]
- Loret, N.; Denys, H.; Tummers, P.; Berx, G. The Role of Epithelial-to-Mesenchymal Plasticity in Ovarian Cancer Progression and Therapy Resistance. Cancers 2019, 11, 838. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Shao, M.; Schilder, J.; Guise, T.; Mohammad, K.S.; Matei, D. Tissue Transglutaminase Links TGF-β, Epithelial to Mesenchymal Transition and a Stem Cell Phenotype in Ovarian Cancer. Oncogene 2012, 31, 2521–2534. [Google Scholar] [CrossRef] [PubMed]
- Teeuwssen, M.; Fodde, R. Wnt Signaling in Ovarian Cancer Stemness, EMT, and Therapy Resistance. J. Clin. Med. 2019, 8, 1658. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Bai, X.; Feng, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Inhibition of PI3K/Akt/MTOR Signaling Pathway Alleviates Ovarian Cancer Chemoresistance through Reversing Epithelial-Mesenchymal Transition and Decreasing Cancer Stem Cell Marker Expression. BMC Cancer 2019, 19, 618. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Zhao, S.; Sun, Z.; Jiang, N.; Shang, Y.; Wang, Y.; Gu, J.; Li, S. BRMS1L Suppresses Ovarian Cancer Metastasis via Inhibition of the β-Catenin-Wnt Pathway. Exp. Cell Res. 2018, 371, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, W.; Luo, J.; Wu, Y.; Xu, Y.; Chen, T.; Zhang, W.; Fu, F. Cysteine-Rich Intestinal Protein 1 Served as an Epithelial Ovarian Cancer Marker via Promoting Wnt/β-Catenin-Mediated EMT and Tumour Metastasis. Dis. Markers 2021, 2021, 3566749. [Google Scholar] [CrossRef] [PubMed]
- Bocci, F.; Zhou, P.; Nie, Q. Single-Cell RNA-Seq Analysis Reveals the Acquisition of Cancer Stem Cell Traits and Increase of Cell–Cell Signaling during EMT Progression. Cancers 2021, 13, 5726. [Google Scholar] [CrossRef]
- Li, Y.; Gong, W.; Ma, X.; Sun, X.; Jiang, H.; Chen, T. Smad7 Maintains Epithelial Phenotype of Ovarian Cancer Stem-like Cells and Supports Tumor Colonization by Mesenchymal-Epithelial Transition. Mol. Med. Rep. 2015, 11, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; George, J.T.; Tripathi, S.C.; Kundnani, D.L.; Lu, M.; Hanash, S.M.; Onuchic, J.N.; Jolly, M.K.; Levine, H. Testing the Gene Expression Classification of the EMT Spectrum. Biorxiv 2018, 16, 452508. [Google Scholar] [CrossRef]
- Loh, K.M.; Lim, B. Actors in the Cell Reprogramming Drama. Nature 2012, 488, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.L. Mechanism of MicroRNA-Mediated Global DNA Demethylation in Human iPS Cells. In Advances in Stem Cell Research. Stem Cell Biology and Regenerative Medicine; Baharvand, H., Aghdami, N., Eds.; Humana Press: Totowa, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Niimi, P.; Levine, M.; Meer, M. Epigenetic Trajectories of Aging and Reprogramming. Innov. Aging 2021, 5, 664–665. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Ye, P.; Ling, R.-S.; Zeng, F.; Zhu, X.-S.; Chen, L.; Huang, Y.; Xu, L.; Xie, X.-Y. Histone Demethylase KDM4C Is Required for Ovarian Cancer Stem Cell Maintenance. Stem Cells Int. 2020, 2020, 8860185. [Google Scholar] [CrossRef] [PubMed]
- Fatma, H.; Siddique, H.R. Pluripotency Inducing Yamanaka Factors: Role in Stemness and Chemoresistance of Liver Cancer. Expert. Rev. Anticancer 2021, 21, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.-L.; Leung, C.S.; Yip, K.-P.; Yeung, C.L.A.; Wong, S.T.C.; Mok, S.C. Cellular and Molecular Processes in Ovarian Cancer Metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am. J. Physiol.-Cell Physiol. 2015, 309, C444–C456. [Google Scholar] [CrossRef] [PubMed]
- Psaila, B.; Lyden, D. The Metastatic Niche: Adapting the Foreign Soil. Nat. Rev. Cancer 2009, 9, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Geng, X.; Li, Y. Milky Spots: Omental Functional Units and Hotbeds for Peritoneal Cancer Metastasis. Tumor Biol. 2016, 37, 5715–5726. [Google Scholar] [CrossRef]
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C.; et al. Towards the Introduction of the ‘Immunoscore’ in the Classification of Malignant Tumours. J. Pathol. 2014, 232, 199–209. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, M.; Yu, Y.; Sang, J.; Wang, H.; Shi, J.; Duan, P.; Ge, R. The Immune Subtypes and Landscape of Advanced-Stage Ovarian Cancer. Vaccines 2022, 10, 1451. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ Tumor-Infiltrating Lymphocytes and a High CD8+/Regulatory T Cell Ratio Are Associated with Favorable Prognosis in Ovarian Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Huang, B.; Huang, Z.; Cai, J.; Gong, L.; Zhang, Y.; Jiang, J.; Dong, W.; Wang, Z. Tumor Cell-Fibroblast Heterotypic Aggregates in Malignant Ascites of Patients with Ovarian Cancer. Int. J. Mol. Med. 2019, 44, 2245–2255. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, S.; Li, S.; Chen, Y.; Yang, L. Stanniocalcin 1 in Tumor Microenvironment Promotes Metastasis of Ovarian Cancer. Oncotargets Ther. 2019, 12, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.; Kenny, H.A.; Ashcroft, B.; Mukherjee, A.; Johnson, A.; Zhang, Y.; Helou, Y.; Batlle, R.; Liu, X.; Gutierrez, N.; et al. Fibroblasts Mobilize Tumor Cell Glycogen to Promote Proliferation and Metastasis. Cell Metab. 2019, 29, 141–155.e9. [Google Scholar] [CrossRef] [PubMed]
- Kotsiliti, E. Origin of CAFs in Colorectal Cancer. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 79. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, Z.; Xu, S.; Li, X.; Yang, X.; Jin, P.; Liu, Y.; Zhou, X.; Zhang, T.; Gong, C.; et al. Heterotypic CAF-Tumor Spheroids Promote Early Peritoneal Metastatis of Ovarian Cancer. J. Exp. Med. 2019, 216, 688–703. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Li, W.; Chen, R.; Wang, J.; Lu, X.; Li, J. Stromal POSTN Induced by TGF-Β1 Facilitates the Migration and Invasion of Ovarian Cancer. Gynecol. Oncol. 2021, 160, 530–538. [Google Scholar] [CrossRef]
- Yasuda, K.; Torigoe, T.; Mariya, T.; Asano, T.; Kuroda, T.; Matsuzaki, J.; Ikeda, K.; Yamauchi, M.; Emori, M.; Asanuma, H.; et al. Fibroblasts Induce Expression of FGF4 in Ovarian Cancer Stem-like Cells/Cancer-Initiating Cells and Upregulate Their Tumor Initiation Capacity. Lab. Investig. 2014, 94, 1355–1369. [Google Scholar] [CrossRef]
- Davidowitz, R.A.; Iwanicki, M.P.; Brugge, J.S. In Vitro Mesothelial Clearance Assay That Models the Early Steps of Ovarian Cancer Metastasis. J. Vis. Exp. Jove 2012, 60, e3888. [Google Scholar] [CrossRef]
- Davidowitz, R.A.; Selfors, L.M.; Iwanicki, M.P.; Elias, K.M.; Karst, A.; Piao, H.; Ince, T.A.; Drage, M.G.; Dering, J.; Konecny, G.E.; et al. Mesenchymal Gene Program–Expressing Ovarian Cancer Spheroids Exhibit Enhanced Mesothelial Clearance. J. Clin. Investig. 2014, 124, 2611–2625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, N.; Kreeger, P.K.; Notbohm, J. Topological Defects in the Mesothelium Suppress Ovarian Cancer Cell Clearance. Appl. Bioeng. 2021, 5, 036103. [Google Scholar] [CrossRef] [PubMed]
- Mogi, K.; Yoshihara, M.; Iyoshi, S.; Kitami, K.; Uno, K.; Tano, S.; Koya, Y.; Sugiyama, M.; Yamakita, Y.; Nawa, A.; et al. Ovarian Cancer-Associated Mesothelial Cells: Transdifferentiation to Minions of Cancer and Orchestrate Developing Peritoneal Dissemination. Cancers 2021, 13, 1352. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Chang, X.; Qi, Y.; Zhu, Z.; Yang, X. Fibrosis of Mesothelial Cell-Induced Peritoneal Implantation of Ovarian Cancer Cells. Cancer Manag. Res. 2018, 10, 6641–6647. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Yang, T.; Chen, X.; Wu, Y.; Deng, X.; He, W.; Yang, J.; Wang, Z. Malignant Ascites-Derived Exosomes Promote Proliferation and Induce Carcinoma-Associated Fibroblasts Transition in Peritoneal Mesothelial Cells. Oncotarget 2017, 8, 42262–42271. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Sawada, K.; Kinose, Y.; Yoshimura, A.; Toda, A.; Nakatsuka, E.; Hashimoto, K.; Mabuchi, S.; Morishige, K.; Kurachi, H.; et al. Exosomes Promote Ovarian Cancer Cell Invasion through Transfer of CD44 to Peritoneal Mesothelial Cells. Mol. Cancer Res. 2017, 15, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Shishido, A.; Mori, S.; Yokoyama, Y.; Hamada, Y.; Minami, K.; Qian, Y.; Wang, J.; Hirose, H.; Wu, X.; Kawaguchi, N.; et al. Mesothelial Cells Facilitate Cancer Stem-like Properties in Spheroids of Ovarian Cancer Cells. Oncol. Rep. 2018, 40, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; LeSavage, B.L.; Hubka, K.M.; Ma, C.; Natarajan, S.; Eggold, J.T.; Xiao, Y.; Fuh, K.C.; Krishnan, V.; Enejder, A.; et al. Cancer-Associated Mesothelial Cells Promote Ovarian Cancer Chemoresistance through Paracrine Osteopontin Signaling. J. Clin. Investig. 2021, 131, e146186. [Google Scholar] [CrossRef]
- Zhao, G.; Cardenas, H.; Matei, D. Ovarian Cancer—Why Lipids Matter. Cancers 2019, 11, 1870. [Google Scholar] [CrossRef]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes Promote Ovarian Cancer Metastasis and Provide Energy for Rapid Tumor Growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Condello, S.; Thomes-Pepin, J.; Ma, X.; Xia, Y.; Hurley, T.D.; Matei, D.; Cheng, J.-X. Lipid Desaturation Is a Metabolic Marker and Therapeutic Target of Ovarian Cancer Stem Cells. Cell Stem Cell 2017, 20, 303–314.e5. [Google Scholar] [CrossRef]
- Schweer, D.; McAtee, A.; Neupane, K.; Richards, C.; Ueland, F.; Kolesar, J. Tumor-Associated Macrophages and Ovarian Cancer: Implications for Therapy. Cancers 2022, 14, 2220. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, Z.; Fu, L.; Xu, T. Macrophage Polarization in the Development and Progression of Ovarian Cancers: An Overview. Front. Oncol. 2019, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Lin, Y.; Yicong, W.; Chengyan, L.; Shulin, Z.; Wenjun, C. Effect of Macrophages on Biological Function of Ovarian Cancer Cells in Tumor Microenvironment in Vitro. Arch. Gynecol. Obstet. 2020, 302, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Hensler, M.; Kasikova, L.; Fiser, K.; Rakova, J.; Skapa, P.; Laco, J.; Lanickova, T.; Pecen, L.; Truxova, I.; Vosahlikova, S.; et al. M2-like Macrophages Dictate Clinically Relevant Immunosuppression in Metastatic Ovarian Cancer. J. Immunother. Cancer 2020, 8, e000979. [Google Scholar] [CrossRef]
- Travers, M.; Brown, S.M.; Dunworth, M.; Holbert, C.E.; Wiehagen, K.R.; Bachman, K.E.; Foley, J.R.; Stone, M.L.; Baylin, S.B.; Casero, R.A.; et al. DFMO and 5-Azacytidine Increase M1 Macrophages in the Tumor Microenvironment of Murine Ovarian Cancer. Cancer Res. 2019, 79, 3445–3454. [Google Scholar] [CrossRef]
- Madeddu, C.; Gramignano, G.; Kotsonis, P.; Coghe, F.; Atzeni, V.; Scartozzi, M.; Macciò, A. Microenvironmental M1 Tumor-Associated Macrophage Polarization Influences Cancer-Related Anemia in Advanced Ovarian Cancer: Key Role of Interleukin-6. Haematologica 2018, 103, e388–e391. [Google Scholar] [CrossRef]
- Yin, M.; Li, X.; Tan, S.; Zhou, H.J.; Ji, W.; Bellone, S.; Xu, X.; Zhang, H.; Santin, A.D.; Lou, G.; et al. Tumor-Associated Macrophages Drive Spheroid Formation during Early Transcoelomic Metastasis of Ovarian Cancer. J. Clin. Investig. 2016, 126, 4157–4173. [Google Scholar] [CrossRef]
- Raghavan, S.; Mehta, P.; Xie, Y.; Lei, Y.L.; Mehta, G. Ovarian Cancer Stem Cells and Macrophages Reciprocally Interact through the WNT Pathway to Promote Pro-Tumoral and Malignant Phenotypes in 3D Engineered Microenvironments. J. Immunother. Cancer 2019, 7, 190. [Google Scholar] [CrossRef]
- Song, M.; Yeku, O.O.; Rafiq, S.; Purdon, T.; Dong, X.; Zhu, L.; Zhang, T.; Wang, H.; Yu, Z.; Mai, J.; et al. Tumor Derived UBR5 Promotes Ovarian Cancer Growth and Metastasis through Inducing Immunosuppressive Macrophages. Nat. Commun. 2020, 11, 6298. [Google Scholar] [CrossRef]
- Chu, Y.; Dai, E.; Li, Y.; Han, G.; Pei, G.; Ingram, D.R.; Thakkar, K.; Qin, J.-J.; Dang, M.; Le, X.; et al. Pan-Cancer T Cell Atlas Links a Cellular Stress Response State to Immunotherapy Resistance. Nat. Med. 2023, 29, 1550–1562. [Google Scholar] [CrossRef]
- Westergaard, M.C.W.; Andersen, R.; Chong, C.; Kjeldsen, J.W.; Pedersen, M.; Friese, C.; Hasselager, T.; Lajer, H.; Coukos, G.; Bassani-Sternberg, M.; et al. Tumour-Reactive T Cell Subsets in the Microenvironment of Ovarian Cancer. Br. J. Cancer 2019, 120, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Labarriere, N. PD-1 Expression on Tumor-Specific T Cells: Friend or Foe for Immunotherapy? Oncoimmunology 2017, 7, e1364828. [Google Scholar] [CrossRef] [PubMed]
- Weng, D.; Song, B.; Durfee, J.; Sugiyama, V.; Wu, Z.; Koido, S.; Calderwood, S.K.; Gong, J. Induction of Cytotoxic T Lymphocytes against Ovarian Cancer-initiating Cells. Int. J. Cancer 2011, 129, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Li, Y.; Li, M.; Lei, M.; Wu, M.; Qu, Y.; Yuan, Y.; Chen, T.; Jiang, H. Ovarian Cancer Stem Cells Promote Tumour Immune Privilege and Invasion via CCL5 and Regulatory T Cells. Clin. Exp. Immunol. 2018, 191, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Toker, A.; Nguyen, L.T.; Stone, S.C.; Yang, C.; Katz, S.R.; Shaw, P.A.; Clarke, B.A.; Ghazarian, D.A.; Habeeb, A.S.A.; Easson, A.M.; et al. Regulatory T Cells in Ovarian Cancer Are Characterized by a Highly Activated Phenotype Distinct from That in Melanoma. Clin. Cancer Res. 2018, 24, 5685–5696. [Google Scholar] [CrossRef]
- Reth, M. Regulation of B-Cell Development by Pre-B-Cell Receptors. Curr. Biol. 1991, 1, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Kantor, A.B. The Development and Repertoire of B-1 Cells (CD5 B Cells). Immunol. Today 1991, 12, 389–391. [Google Scholar] [CrossRef]
- Blocking Development of B Cells. Science 1999, 286, 1813j. [CrossRef]
- Kroese, F.G.M.; Ammerlaan, W.A.M.; Deenen, G.J. Location and Function of B-Cell Lineagesa. Ann. N. Y. Acad. Sci. 1992, 651, 44–58. [Google Scholar] [CrossRef]
- Montfort, A.; Pearce, O.M.T.; Maniati, E.; Vincent, B.; Bixby, L.M.; Böhm, S.; Dowe, T.; Wilkes, E.H.; Chakravarty, P.; Thompson, R.; et al. A Strong B Cell Response Is Part of the Immune Landscape in Human High-Grade Serous Ovarian Metastases. Clin. Cancer Res. 2016, 23, 250–262. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; Zhao, L.; Wang, X.; Gimple, R.C.; Xu, L.; Wang, Y.; Rich, J.N.; Zhou, S. Plasma Cells Shape the Mesenchymal Identity of Ovarian Cancers through Transfer of Exosome-Derived MicroRNAs. Sci. Adv. 2021, 7, eabb0737. [Google Scholar] [CrossRef]
- Ragonnaud, E.; Moritoh, K.; Bodogai, M.; Gusev, F.; Garaud, S.; Chen, C.; Wang, X.; Baljinnyam, T.; Becker, K.G.; Maul, R.W.; et al. Tumor-Derived Thymic Stromal Lymphopoietin Expands Bone Marrow B-Cell Precursors in Circulation to Support Metastasis. Cancer Res. 2019, 79, 5826–5838. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Malanchi, I. Neutrophils Support Lung Colonization of Metastasis-Initiating Breast Cancer Cells. Nature 2015, 528, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ko, S.Y.; Mohamed, M.S.; Kenny, H.A.; Lengyel, E.; Naora, H. Neutrophils Facilitate Ovarian Cancer Premetastatic Niche Formation in the Omentum. J. Exp. Med. 2019, 216, 176–194. [Google Scholar] [CrossRef]
- Muqaku, B.; Pils, D.; Mader, J.C.; Aust, S.; Mangold, A.; Muqaku, L.; Slany, A.; Favero, G.D.; Gerner, C. Neutrophil Extracellular Trap Formation Correlates with Favorable Overall Survival in High Grade Ovarian Cancer. Cancers 2020, 12, 505. [Google Scholar] [CrossRef]
- Mayer, C.; Darb-Esfahani, S.; Meyer, A.-S.; Hübner, K.; Rom, J.; Sohn, C.; Braicu, I.; Sehouli, J.; Hänsch, G.M.; Gaida, M.M. Neutrophil Granulocytes in Ovarian Cancer—Induction of Epithelial-To-Mesenchymal-Transition and Tumor Cell Migration. J. Cancer 2016, 7, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Pyzer, A.R.; Cole, L.; Rosenblatt, J.; Avigan, D.E. Myeloid-Derived Suppressor Cells as Effectors of Immune Suppression in Cancer: MDSC Mediate Immune Suppression in Cancer. Int. J. Cancer 2016, 139, 1915–1926. [Google Scholar] [CrossRef]
- Sarkar, S.; Bristow, C.A.; Dey, P.; Rai, K.; Perets, R.; Ramirez-Cardenas, A.; Malasi, S.; Huang-Hobbs, E.; Haemmerle, M.; Wu, S.Y.; et al. PRKCI Promotes Immune Suppression in Ovarian Cancer. Gene Dev. 2017, 31, 1109–1121. [Google Scholar] [CrossRef]
- Evans, C.; Dalgleish, A.G.; Kumar, D. Review Article: Immune Suppression and Colorectal Cancer. Aliment. Pharmacol. Ther. 2006, 24, 1163–1177. [Google Scholar] [CrossRef]
- Ullrich, S.E. Sunlight and Skin Cancer: Lessons from the Immune System. Mol. Carcinog. 2007, 46, 629–633. [Google Scholar] [CrossRef]
- Coward, J.; Frentzas, S.; Mislang, A.; Gao, B.; Lemech, C.; Jin, X.; Li, B.; Wang, M.; Kwek, K.Y.; Zhou, Y.; et al. 427 Efficacy and Safety of AK112, an Anti-PD-1/VEGF-A Bispecific Antibody, in Patients with Platinum-Resistant/Refractory Epithelial Ovarian Cancer in a Phase 1 Study. J. Immunother. Cancer 2021, 9, A457. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Shapira-Frommer, R.; Santin, A.D.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Provencher, D.M.; et al. Antitumor Activity and Safety of Pembrolizumab in Patients with Advanced Recurrent Ovarian Cancer: Results from the Phase II KEYNOTE-100 Study. Ann. Oncol. 2019, 30, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients with Platinum-Resistant Ovarian Cancer. J. Clin. Oncol. 2015, 33, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.S.; Nichols, E.; Cimino-Mathews, A.; Peer, C.; Cao, L.; Lee, M.-J.; Kohn, E.C.; Annunziata, C.M.; Lipkowitz, S.; Trepel, J.B.; et al. A Phase I Study of the PD-L1 Inhibitor, Durvalumab, in Combination with a PARP Inhibitor, Olaparib, and a VEGFR1–3 Inhibitor, Cediranib, in Recurrent Women’s Cancers with Biomarker Analyses. J. Immunother. Cancer 2019, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.; Kamrava, M.; Rogatko, A.; Li, A.J.; Cass, I.; Karlan, B.Y.; Rimel, B.J. Phase II Trial of Pembrolizumab with Cisplatin and Gemcitabine in Women with Recurrent Platinum-Resistant Ovarian Cancer. Gynecol. Oncol. 2019, 154, 16–17. [Google Scholar] [CrossRef]
- O’Cearbhaill, R.E.; Homicsko, K.; Wolfer, A.; DiSilvestro, P.A.; O’Malley, D.M.; Sabbatini, P.; Orcurto, A.; Barras, D.; Shohara, L.; Ricciardi, T.; et al. A Phase I/II Study of Chemo-Immunotherapy with Durvalumab (Durva) and Pegylated Liposomal Doxorubicin (PLD) in Platinum-Resistant Recurrent Ovarian Cancer (PROC): Genomic Sequencing and Updated Efficacy Results. Gynecol. Oncol. 2020, 159, 41. [Google Scholar] [CrossRef]
- Liu, J.F.; Herold, C.; Gray, K.P.; Penson, R.T.; Horowitz, N.; Konstantinopoulos, P.A.; Castro, C.M.; Hill, S.J.; Curtis, J.; Luo, W.; et al. Assessment of Combined Nivolumab and Bevacizumab in Relapsed Ovarian Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 1731. [Google Scholar] [CrossRef]
- Lokhorst, H.M.; Liebowitz, D. Adoptive T-Cell Therapy. Semin. Hematol. 1999, 36, 26–29. [Google Scholar]
- Gottschalk, S.; Rooney, C.M. Adoptive T-Cell Immunotherapy. Curr. Top. Microbiol. 2015, 391, 427–454. [Google Scholar] [CrossRef]
- Odunsi, K.; Cristea, M.C.; Dorigo, O.; Jazaeri, A.A.; Slomovitz, B.M.; Chagin, K.; Winkle, E.V.; Kari, G.; Iyengar, M.; Norry, E.; et al. A Phase I/IIa, Open Label, Clinical Trial Evaluating the Safety and Efficacy of Autologous T Cells Expressing Enhanced TCRs Specific for NY-ESO-1 in Patients with Recurrent or Treatment Refractory Ovarian Cancer (NCT01567891). J. Clin. Oncol. 2017, 35, TPS3094. [Google Scholar] [CrossRef]
- Yeku, O.; Purdon, T.; Spriggs, D.; Brentjens, R. Abstract PR08: Armored CAR T Cells Genetically Modified to Secrete IL-12 Show Enhanced Efficacy and Overcome a Hostile Tumor Microenvironment in Mouse Ovarian Peritoneal Carcinomatosis. Cancer Immunol. Res. 2017, 5, PR08. [Google Scholar] [CrossRef]
- Lustberg, M.B.; Ramaswamy, B. Epigenetic Therapy in Breast Cancer. Curr. Breast Cancer Rep. 2011, 3, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, K.; Grønbæk, K. Epigenetic Therapy in Hematological Cancers. APMIS 2019, 127, 316–328. [Google Scholar] [CrossRef]
- Liu, S.V.; Fabbri, M.; Gitlitz, B.J.; Laird-Offringa, I.A. Epigenetic Therapy in Lung Cancer. Front. Oncol. 2013, 3, 135. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Hu, W.; Iyer, R.; Kavanagh, J.J.; Coleman, R.L.; Levenback, C.F.; Sood, A.K.; Wolf, J.K.; Gershenson, D.M.; Markman, M.; et al. Phase 1b-2a Study to Reverse Platinum Resistance through Use of a Hypomethylating Agent, Azacitidine, in Patients with Platinum-resistant or Platinum-refractory Epithelial Ovarian Cancer. Cancer 2011, 117, 1661–1669. [Google Scholar] [CrossRef]
- Falchook, G.S.; Fu, S.; Naing, A.; Hong, D.S.; Hu, W.; Moulder, S.; Wheler, J.J.; Sood, A.K.; Bustinza-Linares, E.; Parkhurst, K.L.; et al. Methylation and Histone Deacetylase Inhibition in Combination with Platinum Treatment in Patients with Advanced Malignancies. Investig. New Drug 2013, 31, 1192–1200. [Google Scholar] [CrossRef]
- Huang, X.; Huang, J.; Leng, D.; Yang, S.; Yao, Q.; Sun, J.; Hu, J. Gefitinib-Loaded DSPE-PEG2000 Nanomicelles with CD133 Aptamers Target Lung Cancer Stem Cells. World J. Surg. Oncol. 2017, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Lee, J.-W.; Choi, W.S.; Won, J.E.; Kim, G.H.; Kim, M.G.; Wi, T.I.; Lee, J.M.; Kang, T.H.; Jung, I.D.; et al. CD44-Targeted PLGA Nanoparticles Incorporating Paclitaxel and FAK SiRNA Overcome Chemoresistance in Epithelial Ovarian Cancer. Cancer Res. 2018, 78, 6247–6256. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Chan, D.K.; Shank, J.J.; Griffith, K.A.; Fan, H.; Szulawski, R.; Yang, K.; Reynolds, R.K.; Johnston, C.; McLean, K.; et al. Phase II Clinical Trial of Metformin as a Cancer Stem Cell-Targeting Agent in Ovarian Cancer. JCI Insight 2020, 5, e133247. [Google Scholar] [CrossRef]
- Ramachandran, I.; Ramadoss, S.; Sen, S.; Karuppaiyah, S.; Kumaran, R.I.; Chaudhuri, G. Inhibition of ALDH1A1 Activity in Cisplatin-Resistant Ovarian Cancer Cells Alters Their Cancer Stemness, Cell Cycle Profile and Mitochondrial Respiration Rate. J. Endocr. Soc. 2021, 5, A1023–A1024. [Google Scholar] [CrossRef]
- Huber, M.A.; Kraut, N.; Beug, H. Molecular Requirements for Epithelial-Mesenchymal Transition during Tumor Progression. Curr. Opin. Cell Biol. 2005, 17, 548–558. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, M.W.; Bensch, F.; Glaudemans, A.W.J.M.; Enting, R.H.; Bunskoek, S.; Munnink, T.H.O.; Hooge, M.N.L.; Pearlberg, J.; Gietema, J.A.; de Vries, E.G.E.; et al. 89 Zr-GC1008 PET Imaging and GC1008 Treatment of Recurrent Glioma Patients. J. Clin. Oncol. 2013, 31, 2050. [Google Scholar] [CrossRef]
- Kopetz, S.; Spira, A.I.; Wertheim, M.; Kim, E.; Tan, B.R.; Lenz, H.-J.; Nikolinakos, P.; Rich, P.; Smith, D.A.; Helwig, C.; et al. M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGF-β, in Patients with Heavily Pretreated CRC: Preliminary Results from a Phase I Trial. J. Clin. Oncol. 2018, 36, 764. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Berlin, J.D.; Cosaert, J.; Kauh, J.; Chan, E.; Piha-Paul, S.A.; Amaya, A.; Tang, S.; Driscoll, K.; Kimbung, R.; et al. A Phase 1 Study of Anti-TGFβ Receptor Type-II Monoclonal Antibody LY3022859 in Patients with Advanced Solid Tumors. Cancer Chemother. Pharmacol. 2017, 79, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Rodon, J.; Carducci, M.A.; Sepúlveda, J.M.; Azaro, A.; Calvo, E.; Seoane, J.; Brana, I.; Sicart, E.; Gueorguieva, I.; Cleverly, A.; et al. Integrated Data Review of the First-in-Human Dose (FHD) Study Evaluating Safety, Pharmacokinetics (PK), and Pharmacodynamics (PD) of the Oral Transforming Growth Factor-Beta (TGF-ß) Receptor I Kinase Inhibitor, LY2157299 Monohydrate (LY). J. Clin. Oncol. 2013, 31, 2016. [Google Scholar] [CrossRef]
- Aluri, S.; Bachiashvili, K.; Budhathoki, A.; Bhagat, T.D.; Choudhary, G.S.; Gordon, S.; Ramachandra, N.; Pradhan, K.; Maqbool, S.; Shastri, A.; et al. Clinical ALK5 Inhibitor, Vactosertib, Reverses TGFβ-1 Stimulated Smad-2 Driven Ineffective Hematopoiesis in MDS. Blood 2019, 134, 2990. [Google Scholar] [CrossRef]
- Moore, K.N.; Gunderson, C.C.; Sabbatini, P.; McMeekin, D.S.; Mantia-Smaldone, G.; Burger, R.A.; Morgan, M.A.; Kapoun, A.M.; Brachmann, R.K.; Stagg, R.; et al. A Phase 1b Dose Escalation Study of Ipafricept (OMP54F28) in Combination with Paclitaxel and Carboplatin in Patients with Recurrent Platinum-Sensitive Ovarian Cancer. Gynecol. Oncol. 2019, 154, 294–301. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Ford, J.C.; Mitra, A.K. Defining the Role of Metastasis-Initiating Cells in Promoting Carcinogenesis in Ovarian Cancer. Biology 2023, 12, 1492. https://doi.org/10.3390/biology12121492
Wang J, Ford JC, Mitra AK. Defining the Role of Metastasis-Initiating Cells in Promoting Carcinogenesis in Ovarian Cancer. Biology. 2023; 12(12):1492. https://doi.org/10.3390/biology12121492
Chicago/Turabian StyleWang, Ji, James C. Ford, and Anirban K. Mitra. 2023. "Defining the Role of Metastasis-Initiating Cells in Promoting Carcinogenesis in Ovarian Cancer" Biology 12, no. 12: 1492. https://doi.org/10.3390/biology12121492
APA StyleWang, J., Ford, J. C., & Mitra, A. K. (2023). Defining the Role of Metastasis-Initiating Cells in Promoting Carcinogenesis in Ovarian Cancer. Biology, 12(12), 1492. https://doi.org/10.3390/biology12121492






