Quantitative Proteomics Analysis Reveals the Effect of a MarR Family Transcriptional Regulator AHA_2124 on Aeromonas hydrophila
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Strains, Plasmids and Cultivation
2.2. Construction of the Aeromonas hydrophila AHA_2124 Deletion Mutant
2.3. Measurement of Hemolytic and Extracellular Proteolytic Activity
2.4. Bacterial Swimming and Swarming Motility Assays
2.5. Protein Sample Preparation
2.6. DIA-Based LC-MS/MS
2.7. Western Blot
2.8. Bioinformatics Analysis
3. Results
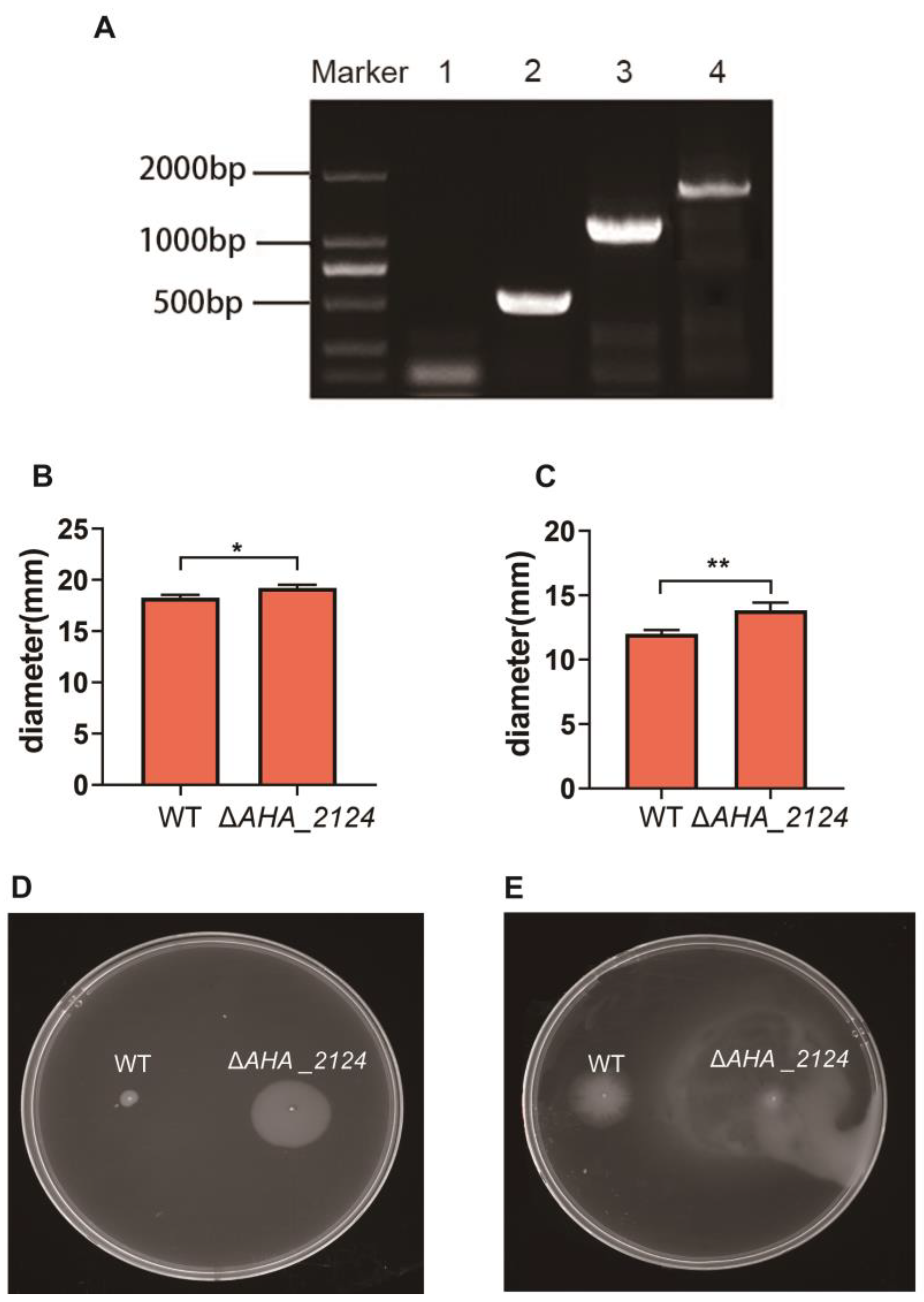
3.1. Construction and Phenotype Characterization of the AHA_2124 Deletion Strain in Aeromonas hydrophila
3.2. Proteomics Analysis of Protein Abundance between ΔAHA_2124 and Aeromonas hydrophila WT Strain
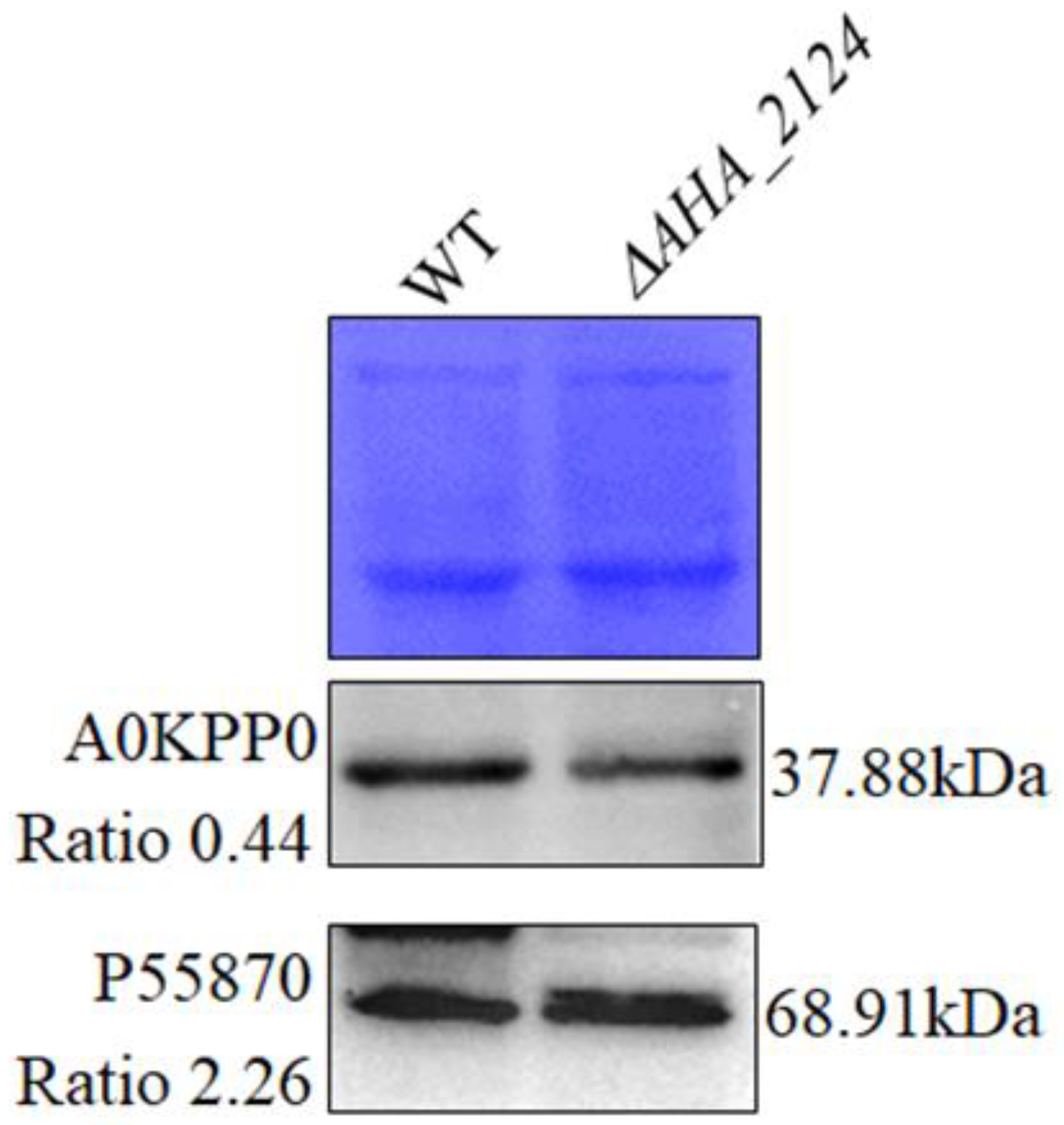
3.3. Validation of Proteomics Results
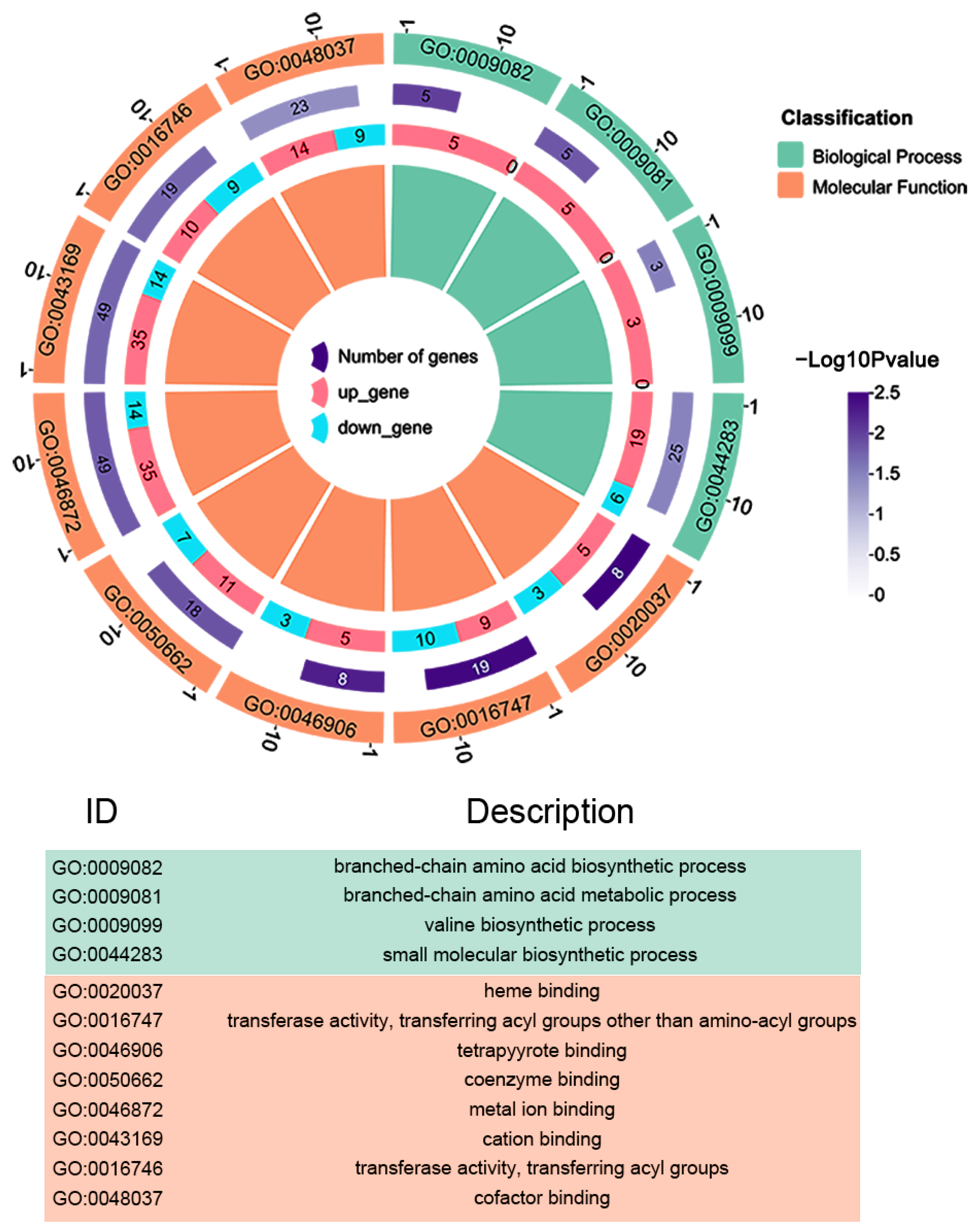
3.4. Functional Analysis of Differentially Abundant Proteins between Δ AHA_2124 and WT Strain
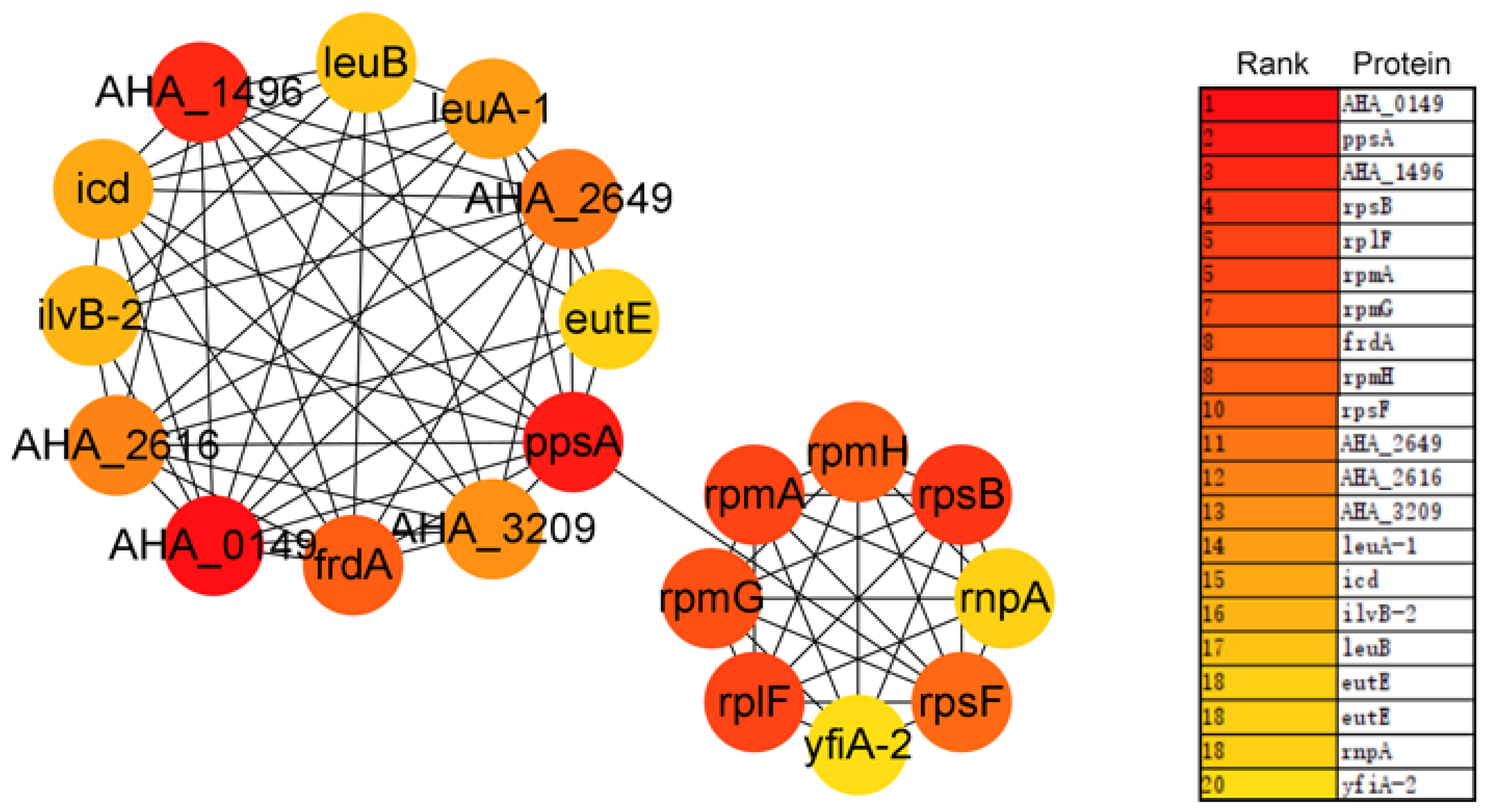
3.5. Protein–Protein Interaction Network of DAPs between WT and ΔAHA_2124 Strain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, Pathogenicity, and Infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef]
- Lin, X.; Lu, J.; Qian, C.; Lin, H.; Li, Q.; Zhang, X.; Liu, H.; Sun, Z.; Zhou, D.; Lu, W.; et al. Molecular and Functional Characterization of a Novel Plasmid-Borne blaNDM-Like Gene, blaAFM-1, in a Clinical Strain of Aeromonas hydrophila. Infect. Drug Resist. 2021, 14, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, S.; Dong, Y.; Lu, C.; Liu, Y. Isolation and Characterization of Bacteriophages against Virulent Aeromonas hydrophila. BMC Microbiol. 2020, 20, 141. [Google Scholar] [CrossRef]
- Grove, A. Regulation of Metabolic Pathways by MarR Family Transcription Factors. Comput. Struct. Biotechnol. J. 2017, 15, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Igbinosa, I.H.; Igumbor, E.U.; Aghdasi, F.; Tom, M.; Okoh, A.I. Emerging Aeromonas Species Infections and Their Significance in Public Health. Sci. World J. 2012, 2012, 625023. [Google Scholar] [CrossRef]
- Stratev, D.; Odeyemi, O.A. Antimicrobial Resistance of Aeromonas hydrophila Isolated from Different Food Sources: A mini-review. J. Infect. Public Health 2016, 9, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen-Ivey, C.R.; Figueras, M.J.; McGarey, D.; Liles, M.R. Virulence Factors of Aeromonas hydrophila: In the Wake of Reclassification. Front. Microbiol. 2016, 7, 1337. [Google Scholar] [CrossRef] [PubMed]
- Ran, C.; Qin, C.; Xie, M.; Zhang, J.; Li, J.; Xie, Y.; Wang, Y.; Li, S.; Liu, L.; Fu, X.; et al. Aeromonas veronii and aerolysin are Important for the Pathogenesis of Motile Aeromonad Septicemia in Cyprinid Fish. Environ. Microbiol. 2018, 20, 3442–3456. [Google Scholar] [CrossRef]
- Mandin, P.; Guillier, M. Expanding Control in Bacteria Interplay between Small RNAs and Transcriptional Regulators to Control Gene Expression. Curr. Opin. Microbiol. 2013, 16, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Vogel, V.; Hauber, S.; Bartel, J.; Alkhnbashi, O.S.; Maaß, S.; Schwarz, T.S.; Backofen, R.; Becher, D.; Duggin, I.G. CdrS Is a Global Transcriptional Regulator Influencing Cell Division in Haloferax volcanii. mBio 2021, 12, e0141621. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Q.; Xu, Z.; Chen, B.; Zhang, A.; Sun, X.; Jin, M. Screening of Virulence-Related Transcriptional Regulators in Streptococcus suis. Genes 2020, 11, 972. [Google Scholar] [CrossRef]
- Chaparian, R.R.; Ball, A.S.; van Kessel, J.C. Hierarchical Transcriptional Control of the LuxR Quorum-Sensing Regulon of Vibrio harveyi. J. Bacteriol. 2020, 202, e00047-20. [Google Scholar] [CrossRef]
- Otani, H.; Stogios, P.J.; Xu, X.; Nocek, B.; Li, S.N.; Savchenko, A.; Eltis, L.D. The Activity of CouR, a MarR Family Transcriptional Regulator, is Modulated through a Novel Molecular Mechanism. Nucleic Acids Res. 2016, 44, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Weatherspoon-Griffin, N.; Wing, H.J. Characterization of SlyA in Shigella flexneri Identifies a Novel Role in Virulence. Infect. Immun. 2016, 84, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, W.; Sun, L.; Wang, Y.; Lin, Y.; Lin, X. Quantitative Proteomics Reveals the Molecular Mechanism of Aeromonas hydrophila in Enoxacin Stress. J. Proteom. 2020, 211, 103561. [Google Scholar] [CrossRef] [PubMed]
- Arimi, S.M.; Park, R.W.; Fricker, C.R. Study of Haemolytic Activity of Some Campylobacter spp. on Blood Agar Plates. J. Appl. Bacteriol. 1990, 69, 384–389. [Google Scholar] [CrossRef]
- Cole, S.J.; Hall, C.L.; Schniederberend, M.; Farrow Iii, J.M.; Goodson, J.R.; Pesci, E.C.; Kazmierczak, B.I.; Lee, V.T. Host Suppression of Quorum Sensing during Catheter-associated Urinary Tract Infections. Nat. Commun. 2018, 9, 4436. [Google Scholar] [CrossRef]
- Wiśniewski, J.R. Filter Aided Sample Preparation-A tutorial. Anal. Chim. Acta 2019, 1090, 23–30. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Zhang, L.; Cai, Q.; Lin, Y.; Lin, L.; Lin, X. Proteomics Analysis Reveals the Effect of Aeromonas hydrophila Sirtuin CobB on Biological Functions. J. Proteom. 2020, 225, 103848. [Google Scholar] [CrossRef]
- Zhao, X.L.; Chen, Z.G.; Yang, T.C.; Jiang, M.; Wang, J.; Cheng, Z.X.; Yang, M.J.; Zhu, J.X.; Zhang, T.T.; Li, H.; et al. Glutamine Promotes Antibiotic Uptake to Kill Multidrug-resistant Uropathogenic Bacteria. Sci. Transl. Med. 2021, 13, eabj0716. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Cai, Y.; Dang, L.T.; Burke, H.M.S.; Revote, J.; Charitakis, N.; Bienroth, D.; Nim, H.T.; Li, Y.F.; Ramialison, M. MonaGO: A Novel Gene Ontology Enrichment Analysis Visualisation System. BMC Bioinform. 2022, 23, 69. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Bader, G.D.; Hogue, C.W. An Automated Method for Finding Molecular Complexes in Large Protein Interaction Networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying Hub Objects and Sub-networks from Complex Interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef] [PubMed]
- Alekshun, M.N.; Levy, S.B.; Mealy, T.R.; Seaton, B.A.; Head, J.F. The Crystal Structure of MarR, a Regulator of Multiple Antibiotic Resistance, at 2.3 A Resolution. Nat. Struct. Biol. 2001, 8, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Li, M.; Liu, L.; Zhang, J.; Zhang, H. Functional Analysis of Bucella Reveals Transcriptional Regulation of MarR. Microb. Pathog. 2020, 144, 104201. [Google Scholar] [CrossRef]
- Kotecka, K.; Kawalek, A.; Kobylecki, K.; Bartosik, A.A. The MarR-Type Regulator PA3458 Is Involved in Osmoadaptation Control in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2021, 22, 3982. [Google Scholar] [CrossRef]
- Xu, S.; Wang, X.; Zhang, F.; Jiang, Y.; Zhang, Y.; Cheng, M.; Yan, X.; Hong, Q.; He, J.; Qiu, J. PicR as a MarR Family Transcriptional Repressor Multiply Controls the Transcription of Picolinic Acid Degradation Gene Cluster pic in Alcaligenes faecalis JQ135. Appl. Environ. Microbiol. 2022, 88, e0017222. [Google Scholar] [CrossRef]
- Sahu, I.; Das, B.K.; Marhual, N.; Samanta, M.; Mishra, B.K.; Eknath, A.E. Toxicity of Crude Extracellular Products of Aeromonas hydrophila on Rohu, Labeo rohita (Ham.). Indian J. Microbiol. 2011, 51, 515–520. [Google Scholar] [CrossRef]
- Yu, H.B.; Kaur, R.; Lim, S.; Wang, X.H.; Leung, K.Y. Characterization of Extracellular Proteins Produced by Aeromonas hydrophila AH-1. Proteomics 2007, 7, 436–449. [Google Scholar] [CrossRef]
- Esteve, C.; Birbeck, T.H. Secretion of Haemolysins and Proteases by Aeromonas hydrophila EO63: Separation and Characterization of the Serine Protease (caseinase) and the Metalloprotease (elastase). J. Appl. Microbiol. 2004, 96, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Ingavale, S.; van Wamel, W.; Luong, T.T.; Lee, C.Y.; Cheung, A.L. Rat/MgrA, a Regulator of Autolysis, is a Regulator of Virulence Genes in Staphylococcus aureus. Infect. Immun. 2005, 73, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Kovacikova, G.; Lin, W.; Skorupski, K. Vibrio cholerae AphA uses a Novel Mechanism for Virulence Gene Activation that Involves Interaction with the LysR-type Regulator AphB at the tcpPH Promoter. Mol. Microbiol. 2004, 53, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Linehan, S.A.; Rytkönen, A.; Yu, X.J.; Liu, M.; Holden, D.W. SlyA Regulates Function of Salmonella pathogenicity island 2 (SPI-2) and Expression of SPI-2-associated genes. Infect. Immun. 2005, 73, 4354–4362. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.; Rosa, L.; Cutone, A.; Lepanto, M.S.; Franchitto, A.; Onori, P.; Gaudio, E.; Valenti, P. Viral Hepatitis and Iron Dysregulation: Molecular Pathways and the Role of Lactoferrin. Molecules 2020, 25, 1997. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Yeh, W.H.; Tang, H.J.; Chen, J.W.; Shu, H.Y.; Su, Y.C.; Wang, S.T.; Kuo, C.J.; Chuang, Y.C.; Chen, C.C.; et al. UvrY is Required for the Full Virulence of Aeromonas dhakensis. Virulence 2020, 11, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Heroven, A.K.; Dersch, P. RovM, a Novel LysR-type Regulator of the Virulence Activator Gene rovA, Controls Cell Invasion, Virulence and Motility of Yersinia pseudotuberculosis. Mol. Microbiol. 2006, 62, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.C.; Khan, S. The Bacterial Flagellar Motor. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 509–539. [Google Scholar] [CrossRef]
- Luterbach, C.L.; Mobley, H.L.T. Cross Talk between MarR-Like Transcription Factors Coordinates the Regulation of Motility in Uropathogenic Escherichia coli. Infect. Immun. 2018, 86, e00338-18. [Google Scholar] [CrossRef]
- Zhou, J.N.; Zhang, H.B.; Lv, M.F.; Chen, Y.F.; Liao, L.S.; Cheng, Y.Y.; Liu, S.Y.; Chen, S.H.; He, F.; Cui, Z.N.; et al. SlyA Regulates Phytotoxin Production and Virulence in Dickeya zeae EC1. Mol. Plant Pathol. 2016, 17, 1398–1408. [Google Scholar] [CrossRef]
- Maddocks, S.E.; Oyston, P.C.F. Structure and Function of the LysR-type Transcriptional Regulator (LTTR) Family Proteins. Microbiol. Read. Engl. 2008, 154 Pt 12, 3609–3623. [Google Scholar] [CrossRef]
- Calvo, J.M.; Matthews, R.G. The Leucine-responsive Regulatory Protein, a Global Regulator of Metabolism in Escherichia coli. Microbiol. Rev. 1994, 58, 466–490. [Google Scholar] [CrossRef]
- Deng, W.; Wang, H.; Xie, J. Regulatory and Pathogenesis Roles of Mycobacterium Lrp/AsnC Family Transcriptional Factors. J. Cell. Biochem. 2011, 112, 2655–2662. [Google Scholar] [CrossRef]
- Fu, Y.; Cai, Q.; Wang, Y.; Li, W.; Yu, J.; Yang, G.; Lin, W.; Lin, X. Four LysR-type Transcriptional Regulator Family Proteins (LTTRs) Involved in Antibiotic Resistance in Aeromonas hydrophila. World J. Microbiol. Biotechnol. 2019, 35, 127. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, L.; Wang, G.; Lin, Y.; Ramanathan, S.; Yang, G.; Lin, W.; Lin, X. The LysR-Type Transcriptional Regulator YeeY Plays Important Roles in the Regulatory of Furazolidone Resistance in Aeromonas hydrophila. Front. Microbiol. 2020, 11, 577376. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, L.; Srinivasan, R.; Lin, M.; Gong, L.; Lin, X. Unveiling a Virulence-Regulating Mechanism in Aeromonas hydrophila: A Quantitative Exoproteomic Analysis of an AraC-Like Protein. Front. Immunol. 2023, 14, 1191209. [Google Scholar] [CrossRef]
- Guo, S.; Vance, T.D.R.; Stevens, C.A.; Voets, I.; Davies, P.L. RTX Adhesins are Key Bacterial Surface Megaproteins in the Formation of Biofilms. Trends Microbiol. 2019, 27, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Suigo, L.; Chojnacki, M.; Zanotto, C.; Sebastián-Pérez, V.; Morghen, C.G.; Casiraghi, A.; Dunman, P.M.; Valoti, E.; Straniero, V. Staphylococcus aureus RnpA Inhibitors: Computational-Guided Design, Synthesis and Initial Biological Evaluation. Antibiotics 2021, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- Olson, P.D.; Kuechenmeister, L.J.; Anderson, K.L.; Daily, S.; Beenken, K.E.; Roux, C.M.; Reniere, M.L.; Lewis, T.L.; Weiss, W.J.; Pulse, M.; et al. Small Molecule Inhibitors of Staphylococcus aureus RnpA Alter Cellular mRNA Turnover, Exhibit Antimicrobial Activity, and Attenuate Pathogenesis. PLoS Pathog. 2011, 7, e1001287. [Google Scholar] [CrossRef]
- Newstead, S. Recent Advances in Understanding Proton Coupled Peptide Transport via the POT family. Curr. Opin. Struct. Biol. 2017, 45, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Rentsch, D. Uptake and Partitioning of Amino Acids and Peptides. Mol. Plant 2010, 3, 997–1011. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Primer Sequence (5′→3′) |
|---|---|
| P1 | CGATCCCAAGCTTCTTCTAGACCGACTGCCTGCTGCGTC |
| P2 | TCAGGTCAGAGAAATGTGCGGTTGCG |
| P3 | CGCACATTTCTCTGACCTGAACAACTTTGCCACCA |
| P4 | CATGAATTCCCGGGAGAGCTCCTGCCGACCATCAGGCAG |
| P5 | GGATCTTCCAGAGATGTGCATGTTCAGACAAACGGC |
| P6 | CTGCCGTTCGACGATTCATGAGGGTCGCTCGCTAT |
| P7 | GGATCTTCCAGAGATGAGCCGCTGTTCGACGCC |
| P8 | CTGCCGTTCGACGATTGCCCAGGCCAAGGGCAT |
| Accession | Gene | Descriptions | p-Value | log2 (ΔAHA_2124/WT) |
|---|---|---|---|---|
| A0KI69 | AHA_1431 | Pts system, fructose-specific iiabc component | 1.11 × 10−23 | 10.11 |
| A0KFI7 | AHA_0480 | Uncharacterized protein | 6.83 × 10−10 | 7.95 |
| A0KK36 | polB | DNA polymerase | 6.49 × 10−9 | 7.64 |
| A0KLW8 | AHA_2761 | 4Fe-4S binding domain protein | 1.96 × 10−14 | 7.18 |
| A0KEP9 | ubiA | 4-hydroxybenzoate octaprenyltransferase | 9.89 × 10−15 | 6.94 |
| A0KQJ3 | AHA_4120 | Probable nitrite transporter | 2.23 × 10−11 | 5.90 |
| A0KFS4 | AHA_0567 | Heat shock protein 15 | 7.26 × 10−8 | 3.78 |
| A0KK31 | lpxM | Lipid A biosynthesis myristoyltransferase | 2.49 × 10−6 | 3.77 |
| A0KM07 | AHA_2803 | Uncharacterized protein | 7.30 × 10−4 | 3.67 |
| A0KLP4 | AHA_2687 | Microbial serine proteinase | 2.54 × 10−3 | 3.27 |
| A0KQ31 | AHA_3948 | Uncharacterized protein | 1.55 × 10−9 | 2.72 |
| A0KL71 | selB | Selenocysteine-specific translation elongation factor | 1.49 × 10−6 | 2.58 |
| A0KL58 | fdhA | Formate dehydrogenase, alpha subunit, selenocysteine-containing | 4.54 × 10−4 | 2.55 |
| A0KLH0 | AHA_2612 | Oligopeptide ABC transporter, permease protein OppB | 1.80 × 10−10 | −7.84 |
| A0KKJ5 | AHA_2273 | Phospholipase, patatin family | 7.18 × 10−7 | −8.19 |
| A0KEY3 | AHA_0271 | Uncharacterized protein | 6.46 × 10−13 | −8.39 |
| A0KQT0 | AHA_4209 | Phosphate acyltransferase family protein | 1.13 × 10−5 | −8.55 |
| A0KIW0 | AHA_1675 | Uncharacterized protein | 3.20 × 10−5 | −8.85 |
| A0KLV6 | AHA_2749 | Putative membrane protein | 6.70 × 10−3 | −9.41 |
| A0KFG4 | AHA_0456 | Acetyltransferase | 2.07 × 10−8 | −9.55 |
| A0KK46 | AHA_2123 | Uncharacterized protein | 2.83 × 10−12 | −9.69 |
| A0KLG9 | AHA_2611 | Oligopeptide ABC transporter, permease protein OppC | 1.44 × 10−9 | −9.72 |
| A0KLT3 | AHA_2726 | Na+/H+ antiporter family protein | 4.95 × 10−5 | −12.40 |
| A0KLQ5 | AHA_2698 | Diguanylate cyclase/phosphodiesterase domain 1 | 1.25 × 10−11 | −13.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, W.; Lu, J.; Liu, Z.; Lin, X.; Liu, Y. Quantitative Proteomics Analysis Reveals the Effect of a MarR Family Transcriptional Regulator AHA_2124 on Aeromonas hydrophila. Biology 2023, 12, 1473. https://doi.org/10.3390/biology12121473
Li Z, Li W, Lu J, Liu Z, Lin X, Liu Y. Quantitative Proteomics Analysis Reveals the Effect of a MarR Family Transcriptional Regulator AHA_2124 on Aeromonas hydrophila. Biology. 2023; 12(12):1473. https://doi.org/10.3390/biology12121473
Chicago/Turabian StyleLi, Zhen, Wanxin Li, Jinlian Lu, Ziqiu Liu, Xiangmin Lin, and Yanling Liu. 2023. "Quantitative Proteomics Analysis Reveals the Effect of a MarR Family Transcriptional Regulator AHA_2124 on Aeromonas hydrophila" Biology 12, no. 12: 1473. https://doi.org/10.3390/biology12121473
APA StyleLi, Z., Li, W., Lu, J., Liu, Z., Lin, X., & Liu, Y. (2023). Quantitative Proteomics Analysis Reveals the Effect of a MarR Family Transcriptional Regulator AHA_2124 on Aeromonas hydrophila. Biology, 12(12), 1473. https://doi.org/10.3390/biology12121473





