Klotho Null Mutation Indirectly Leads to Age-Related Lacrimal Gland Degeneration in Mutant Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Klotho Mutant Mice and Genotyping
2.2. Tear Volume Assessment
2.3. Histological Preparation and Hematoxylin-Eosin Stain
2.4. Masson’s Trichrome Stain
2.5. Immunohistochemistry
2.6. Statistics
3. Results
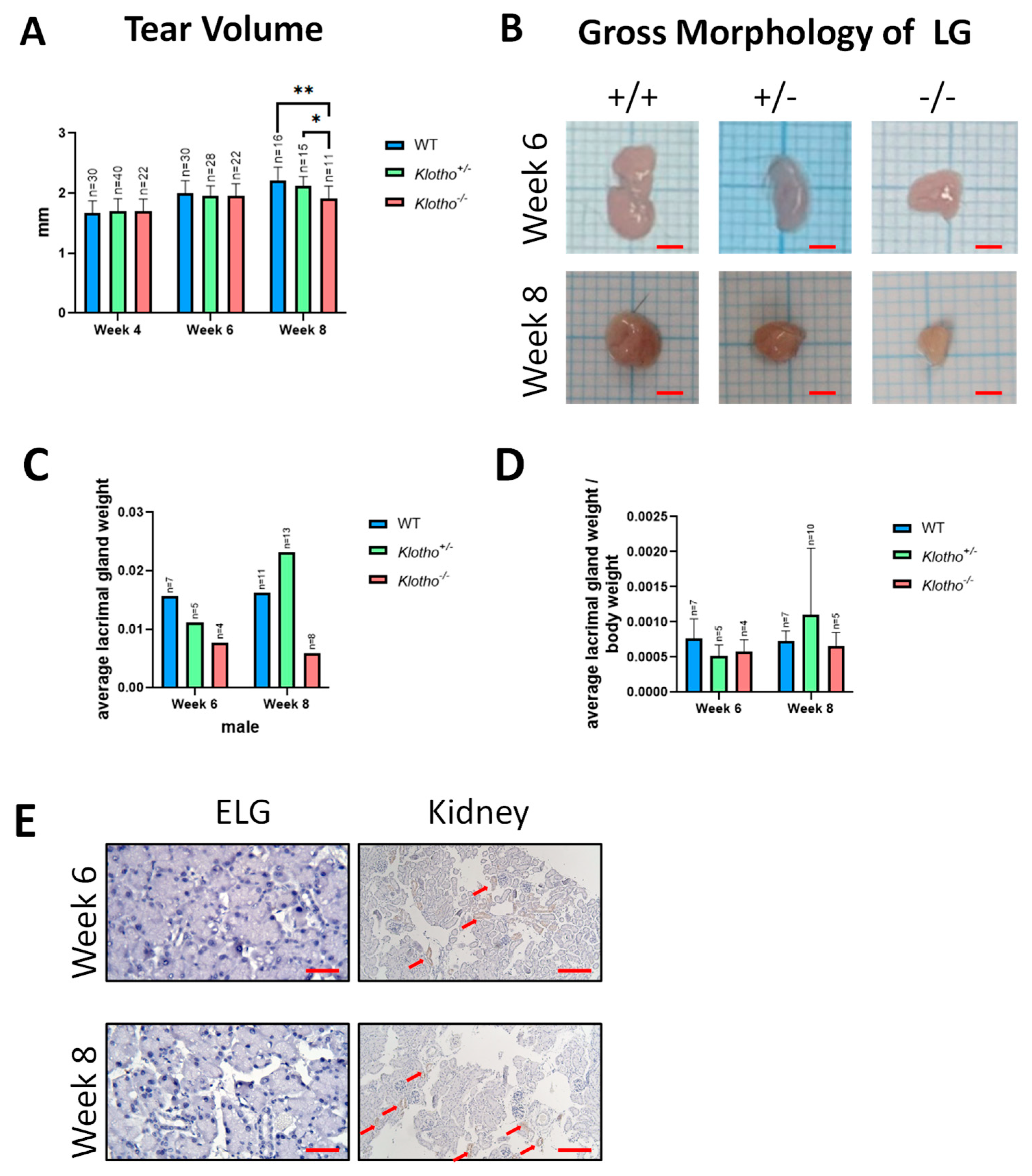
3.1. Decreased Tear Secretion and LG Size and Lack of Klotho Protein within LG
3.2. Lacrimal Gland Atrophy and Capsular Thickening
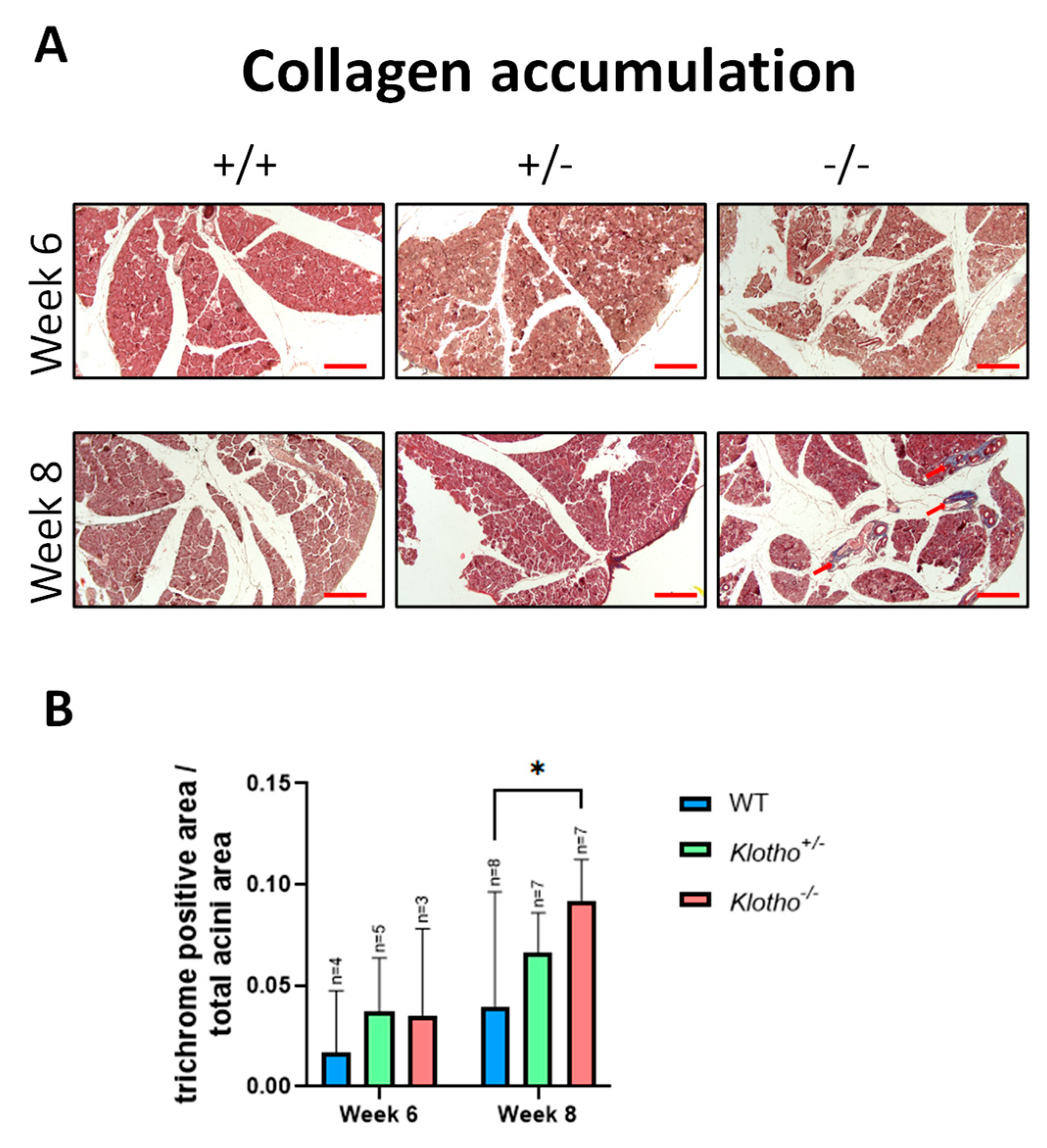
3.3. Deposition of Collagen Fibers
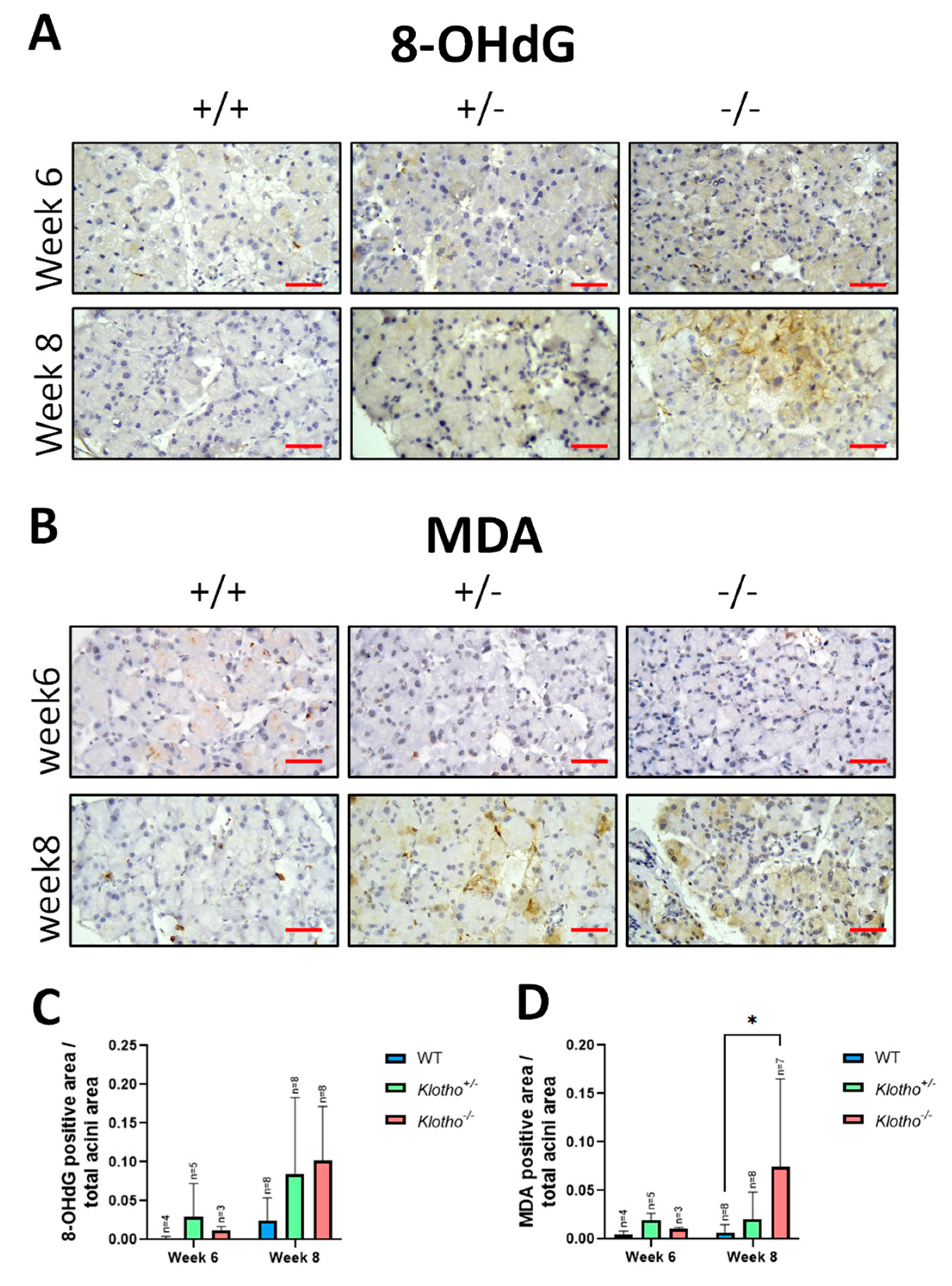
3.4. Increased Oxidative Stress
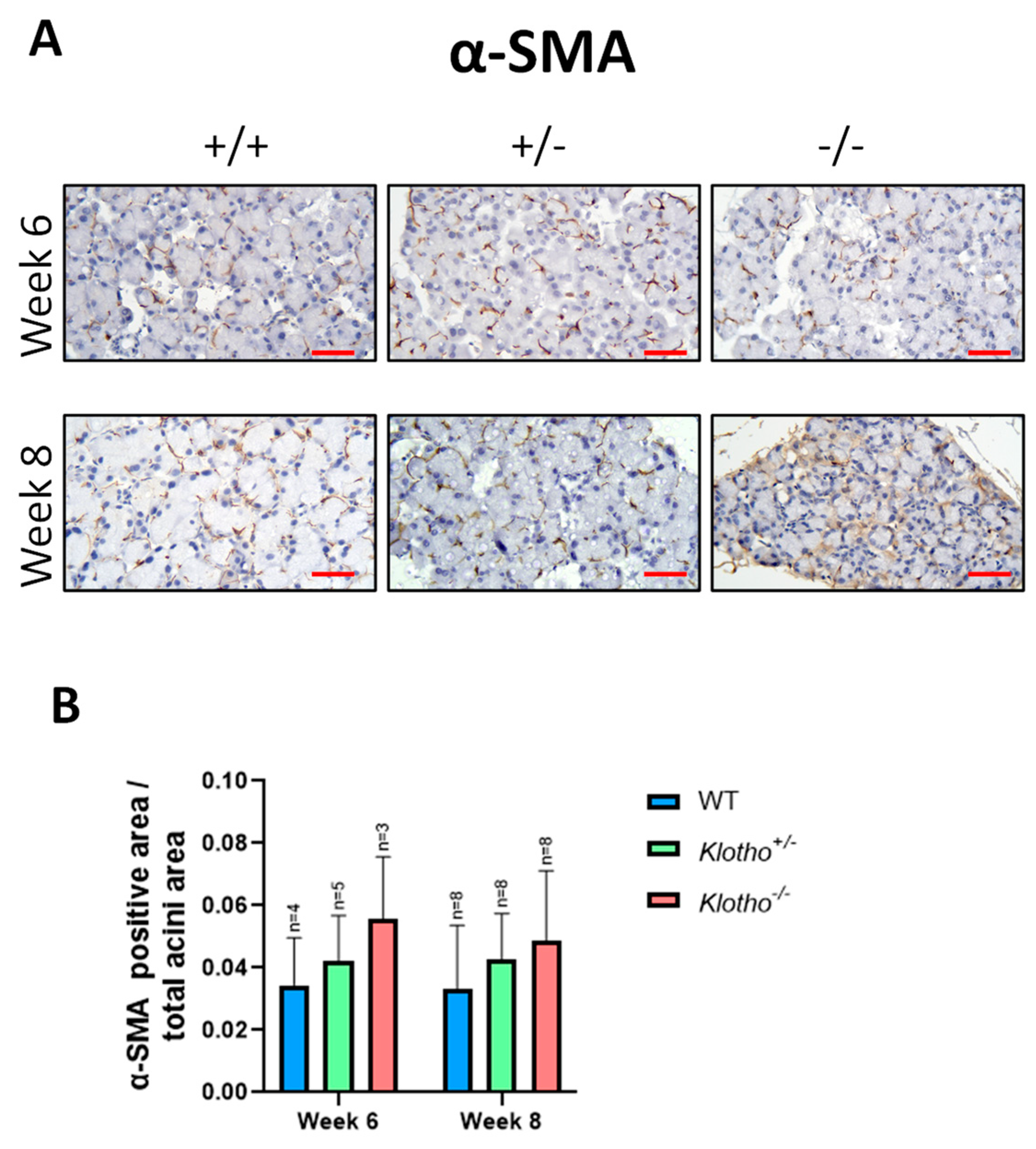
3.5. Alteration of α-SMA Expression
3.6. Elevated MMP-2 and MMP-9 Expression
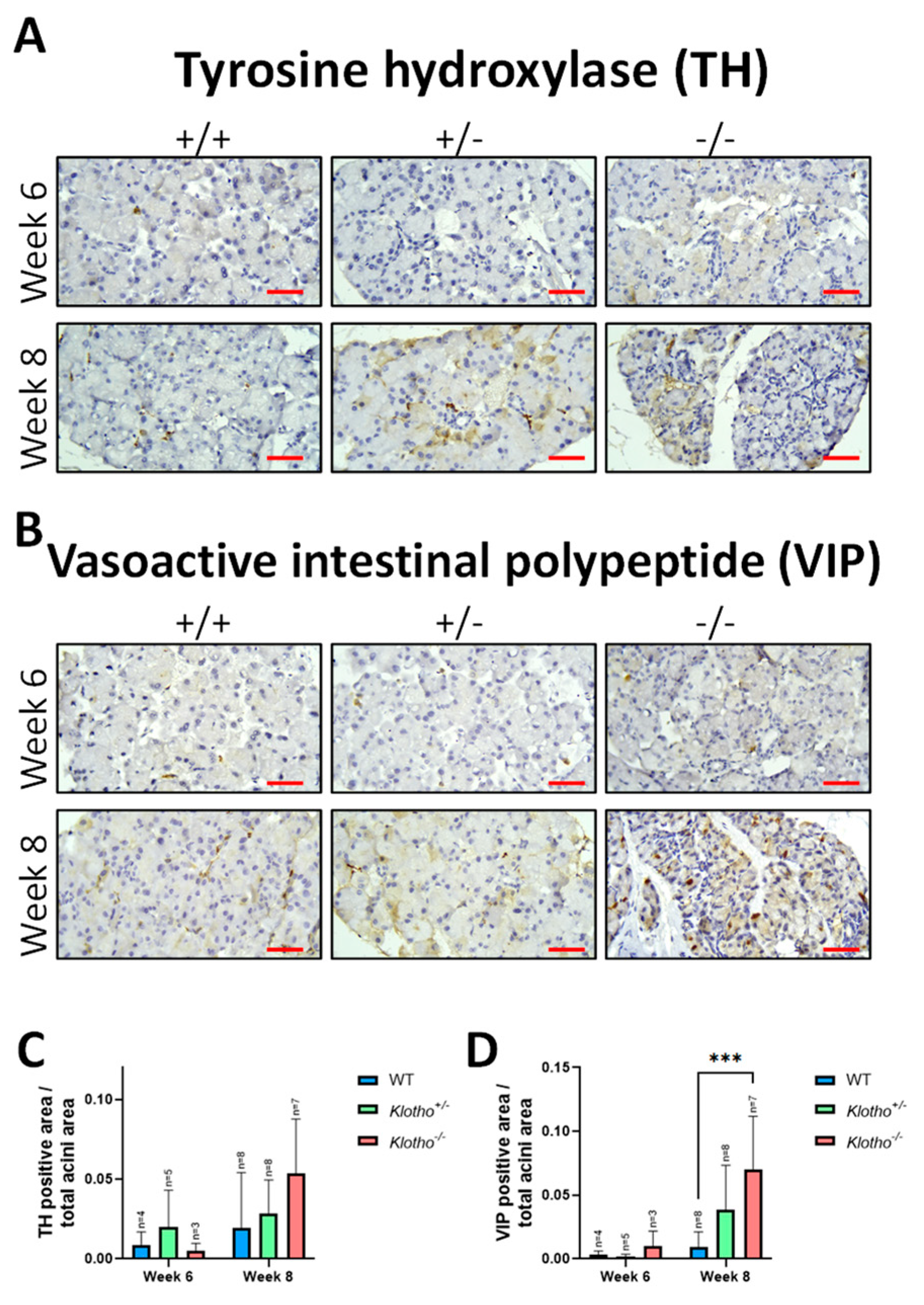
3.7. Increased Expression of Tyrosine Hydroxylase and Vasoactive Intestinal Polypeptide
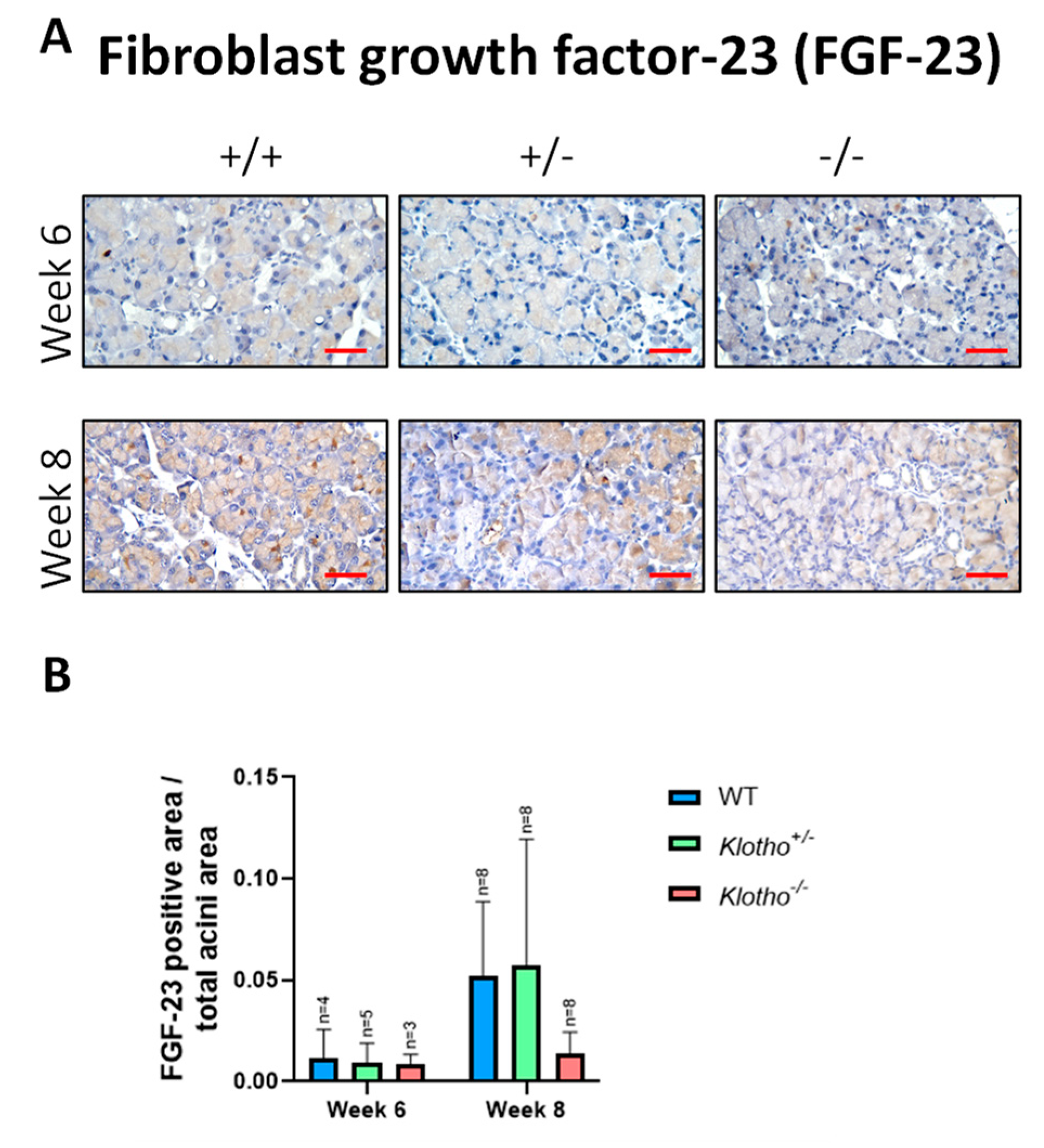
3.8. Decreased FGF-23 Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sacca, S.C.; Cutolo, C.A.; Ferrari, D.; Corazza, P.; Traverso, C.E. The Eye, Oxidative Damage and Polyunsaturated Fatty Acids. Nutrients 2018, 10, 668. [Google Scholar] [CrossRef]
- Ayaki, M.; Negishi, K.; Kawashima, M.; Uchino, M.; Kaido, M.; Tsubota, K. Age Is a Determining Factor of Dry Eye-Related Signs and Symptoms. Diagnostics 2020, 10, 193. [Google Scholar] [CrossRef]
- De Paiva, C.S. Effects of Aging in Dry Eye. Int. Ophthalmol. Clin. 2017, 57, 47–64. [Google Scholar] [CrossRef]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Nishiwaki, Y.; Michikawa, T.; Shirakawa, K.; Kuwahara, E.; Yamada, M.; Dogru, M.; Schaumberg, D.A.; Kawakita, T.; Takebayashi, T.; et al. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology 2011, 118, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, M.; Kawakita, T.; Okada, N.; Ogawa, Y.; Murat, D.; Nakamura, S.; Nakashima, H.; Shimmura, S.; Shinmura, K.; Tsubota, K. Calorie restriction: A new therapeutic intervention for age-related dry eye disease in rats. Biochem. Biophys. Res. Commun. 2010, 397, 724–728. [Google Scholar] [CrossRef]
- Yoon, C.H.; Ryu, J.S.; Hwang, H.S.; Kim, M.K. Comparative Analysis of Age-Related Changes in Lacrimal Glands and Meibomian Glands of a C57BL/6 Male Mouse Model. Int. J. Mol. Sci. 2020, 21, 4169. [Google Scholar] [CrossRef]
- Shikama, Y.; Kurosawa, M.; Furukawa, M.; Ishimaru, N.; Matsushita, K. Involvement of adiponectin in age-related increases in tear production in mice. Aging 2019, 11, 8329–8346. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Zhang, X. Lacrimal gland development: From signaling interactions to regenerative medicine. Dev. Dyn. 2017, 246, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Uchino, Y.; Kawakita, T.; Ishii, T.; Ishii, N.; Tsubota, K. A new mouse model of dry eye disease: Oxidative stress affects functional decline in the lacrimal gland. Cornea 2012, 31 (Suppl. S1), S63–S67. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Landini, G.; Martinelli, G.; Piccinini, F. Colour deconvolution: Stain unmixing in histological imaging. Bioinformatics 2021, 37, 1485–1487. [Google Scholar] [CrossRef]
- Hartig, S.M. Basic image analysis and manipulation in ImageJ. Curr. Protoc. Mol. Biol. 2013. [Google Scholar] [CrossRef]
- Barbosa, A.P.; Oliveira, F.R.; Rocha, F.J.D.; Muglia, V.F.; Rocha, E.M. Lacrimal gland atrophy and dry eye related to isotretinoin, androgen, and prolactin: Differential diagnosis for Sjogren’s syndrome. Arq. Bras. Oftalmol. 2021, 84, 78–82. [Google Scholar] [PubMed]
- Ortiz, G.; Chao, C.; Jamali, A.; Seyed-Razavi, Y.; Kenyon, B.; Harris, D.L.; Zoukhri, D.; Hamrah, P. Effect of Dry Eye Disease on the Kinetics of Lacrimal Gland Dendritic Cells as Visualized by Intravital Multi-Photon Microscopy. Front. Immunol. 2020, 11, 1713. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Ogawa, Y.; Shimmura, S.; Ohta, S.; Suzuki, T.; Kawamura, N.; Kuwana, M.; Kawakami, Y.; Tsubota, K. Expression and localization of aging markers in lacrimal gland of chronic graft-versus-host disease. Sci. Rep. 2013, 3, 2455. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Batista, T.M.; Tomiyoshi, L.M.; Dias, A.C.; Roma, L.P.; Módulo, C.M.; Malki, L.T.; Filho, E.B.; Deminice, R.; Jordão, A.A., Jr.; Cunha, D.A.; et al. Age-dependent changes in rat lacrimal gland anti-oxidant and vesicular related protein expression profiles. Mol. Vis. 2012, 18, 194–202. [Google Scholar]
- Nooh, H.Z.; El-Saify, G.H.; Eldien, N.M.N. Neuroprotective effects of food restriction on autonomic innervation of the lacrimal gland in the rat. Ann. Anat. 2017, 213, 8–18. [Google Scholar] [CrossRef]
- Andrade, A.S.; Salomon, T.B.; Behling, C.S.; Mahl, C.D.; Hackenhaar, F.S.; Putti, J.; Benfato, M.S. Alpha-lipoic acid restores tear production in an animal model of dry eye. Exp. Eye Res. 2014, 120, 1–9. [Google Scholar] [CrossRef]
- De Souza, R.G.; Yu, Z.; Hernandez, H.; Trujillo-Vargas, C.M.; Lee, A.; Mauk, K.E.; Cai, J.; Alves, M.R.; de Paiva, C.S. Modulation of Oxidative Stress and Inflammation in the Aged Lacrimal Gland. Am. J. Pathol. 2021, 191, 294–308. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef] [PubMed]
- Aluri, H.S.; Kublin, C.L.; Thotakura, S.; Armaos, H.; Samizadeh, M.; Hawley, D.; Thomas, W.M.; Leavis, P.; Makarenkova, H.P.; Zoukhri, D. Role of Matrix Metalloproteinases 2 and 9 in Lacrimal Gland Disease in Animal Models of Sjögren’s Syndrome. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5218–5228. [Google Scholar] [CrossRef] [PubMed]
- Umazume, T.; Thomas, W.M.; Campbell, S.; Aluri, H.; Thotakura, S.; Zoukhri, D.; Makarenkova, H.P. Lacrimal Gland Inflammation Deregulates Extracellular Matrix Remodeling and Alters Molecular Signature of Epithelial Stem/Progenitor Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8392–8402. [Google Scholar] [CrossRef] [PubMed]
- Schenke-Layland, K.; Xie, J.; Angelis, E.; Starcher, B.; Wu, K.; Riemann, I.; MacLellan, W.R.; Hamm-Alvarez, S.F. Increased degradation of extracellular matrix structures of lacrimal glands implicated in the pathogenesis of Sjogren’s syndrome. Matrix Biol. 2008, 27, 53–66. [Google Scholar] [CrossRef]
- Draper, C.E.; Adeghate, E.; Lawrence, P.A.; Pallot, D.J.; Garner, A.; Singh, J. Age-related changes in morphology and secretory responses of male rat lacrimal gland. J. Auton. Nerv. Syst. 1998, 69, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Makarenkova, H.P.; Dartt, D.A. Myoepithelial Cells: Their Origin and Function in Lacrimal Gland Morphogenesis, Homeostasis, and Repair. Curr. Mol. Biol. Rep. 2015, 1, 115–123. [Google Scholar] [CrossRef]
- You, S.; Avidan, O.; Tariq, A.; Ahluwalia, I.; Stark, P.C.; Kublin, C.L.; Zoukhri, D. Role of epithelial-mesenchymal transition in repair of the lacrimal gland after experimentally induced injury. Investig. Ophthalmol. Vis. Sci. 2012, 53, 126–135. [Google Scholar] [CrossRef]
- Okada, N.; Kawakita, T.; Ito, M.; Tsubota, K. Aquaporins 8 and 9 as Possible Markers for Adult Murine Lacrimal Gland Cells. BioMed Res. Int. 2021, 2021, 6888494. [Google Scholar] [CrossRef]
- Abughanam, G.; Elkashty, O.A.; Liu, Y.; Bakkar, M.O.; Tran, S.D. Mesenchymal Stem Cells Extract (MSCsE)-Based Therapy Alleviates Xerostomia and Keratoconjunctivitis Sicca in Sjogren’s Syndrome-Like Disease. Int. J. Mol. Sci. 2019, 20, 4750. [Google Scholar] [CrossRef]
- Yoshida, K.; Wang, X.; Bhawal, U.K. Dec1 deficiency restores the age-related dysfunctions of submandibular glands. J. Physiol. Pharmacol. 2021, 72, 571–581. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Hu, L.; Wu, X.; Zhao, B.; Fan, Z.; Zhang, C.; Wang, J.; Wang, S. Age associated decrease of sialin in salivary glands. Biotech. Histochem. Off. Publ. Biol. Stain Comm. 2018, 93, 505–511. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Richter, B.; Faul, C. FGF23 Actions on Target Tissues-With and Without Klotho. Front. Endocrinol. 2018, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Lin, D.P.; Chang, L.S.; Huang, T.P.; Liu, H.J.; Luk, C.P.; Lo, Y.L.; Chang, H.H. Dietary alpha-lipoic acid prevents UVB-induced corneal and conjunctival degeneration through multiple effects. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6757–6766. [Google Scholar] [CrossRef][Green Version]
- Shinomiya, K.; Ueta, M.; Kinoshita, S. A new dry eye mouse model produced by exorbital and intraorbital lacrimal gland excision. Sci. Rep. 2018, 8, 1483. [Google Scholar] [CrossRef]
- Kuo, Y.K.; Lin, I.C.; Chien, L.N.; Lin, T.Y.; How, Y.T.; Chen, K.H.; Dusting, G.J.; Tseng, C.L. Dry Eye Disease: A Review of Epidemiology in Taiwan, and its Clinical Treatment and Merits. J. Clin. Med. 2019, 8, 1227. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, M.; Del Regno, P.; Gharbiya, M.; Sacchetti, M.; Plateroti, R.; Lambiase, A. Analysis of the Pathogenic Factors and Management of Dry Eye in Ocular Surface Disorders. Int. J. Mol. Sci. 2017, 18, 1764. [Google Scholar] [CrossRef]
- El-Fadaly, A.B.; El-Shaarawy, E.A.; Rizk, A.A.; Nasralla, M.M.; Shuaib, D.M. Age-related alterations in the lacrimal gland of adult albino rat: A light and electron microscopic study. Ann. Anat. 2014, 196, 336–351. [Google Scholar] [CrossRef]


| Primer Sequence | PCR Product Length | Genotype | |
|---|---|---|---|
| Forward | 5′-GATGGGGTCGACGTCA-3′ | 186 bp only | Wildtype (+/+) |
| Reverse | 5′-TAAAGGAGGAAAGCCATTGTC-3′ | ||
| Forward | 5′-GCAGCGCATCGCCTTCTATC-3′ | 186 bp and 455 bp | Heterozygous (+/−) |
| Reverse | 5′-ATGCTCCAGACATTCTCAGC-3′ | ||
| Forward | 5′-GCAGCGCATCGCCTTCTATC-3′ | 455 bp only | Homozygous (−/−) |
| Reverse | 5′-ATGCTCCAGACATTCTCAGC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-Y.; Song, D.-F.; Lu, T.-H.; Chen, Z.-J.; Tsai, S.-M.; Liu, Y.-J.; Chang, H.-H.; Lin, D.P.-C. Klotho Null Mutation Indirectly Leads to Age-Related Lacrimal Gland Degeneration in Mutant Mice. Biology 2023, 12, 1328. https://doi.org/10.3390/biology12101328
Wu C-Y, Song D-F, Lu T-H, Chen Z-J, Tsai S-M, Liu Y-J, Chang H-H, Lin DP-C. Klotho Null Mutation Indirectly Leads to Age-Related Lacrimal Gland Degeneration in Mutant Mice. Biology. 2023; 12(10):1328. https://doi.org/10.3390/biology12101328
Chicago/Turabian StyleWu, Chun-Yen, Da-Fong Song, Tsung-Han Lu, Zhi-Jia Chen, Su-Min Tsai, Ya-Jing Liu, Han-Hsin Chang, and David Pei-Cheng Lin. 2023. "Klotho Null Mutation Indirectly Leads to Age-Related Lacrimal Gland Degeneration in Mutant Mice" Biology 12, no. 10: 1328. https://doi.org/10.3390/biology12101328
APA StyleWu, C.-Y., Song, D.-F., Lu, T.-H., Chen, Z.-J., Tsai, S.-M., Liu, Y.-J., Chang, H.-H., & Lin, D. P.-C. (2023). Klotho Null Mutation Indirectly Leads to Age-Related Lacrimal Gland Degeneration in Mutant Mice. Biology, 12(10), 1328. https://doi.org/10.3390/biology12101328







