Geology Can Drive the Diversity–Ecosystem Functioning Relationship in River Benthic Diatoms by Selecting for Species Functional Traits
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Analysis of Samples
2.3. Data Analysis
2.4. Model Simulations
3. Results
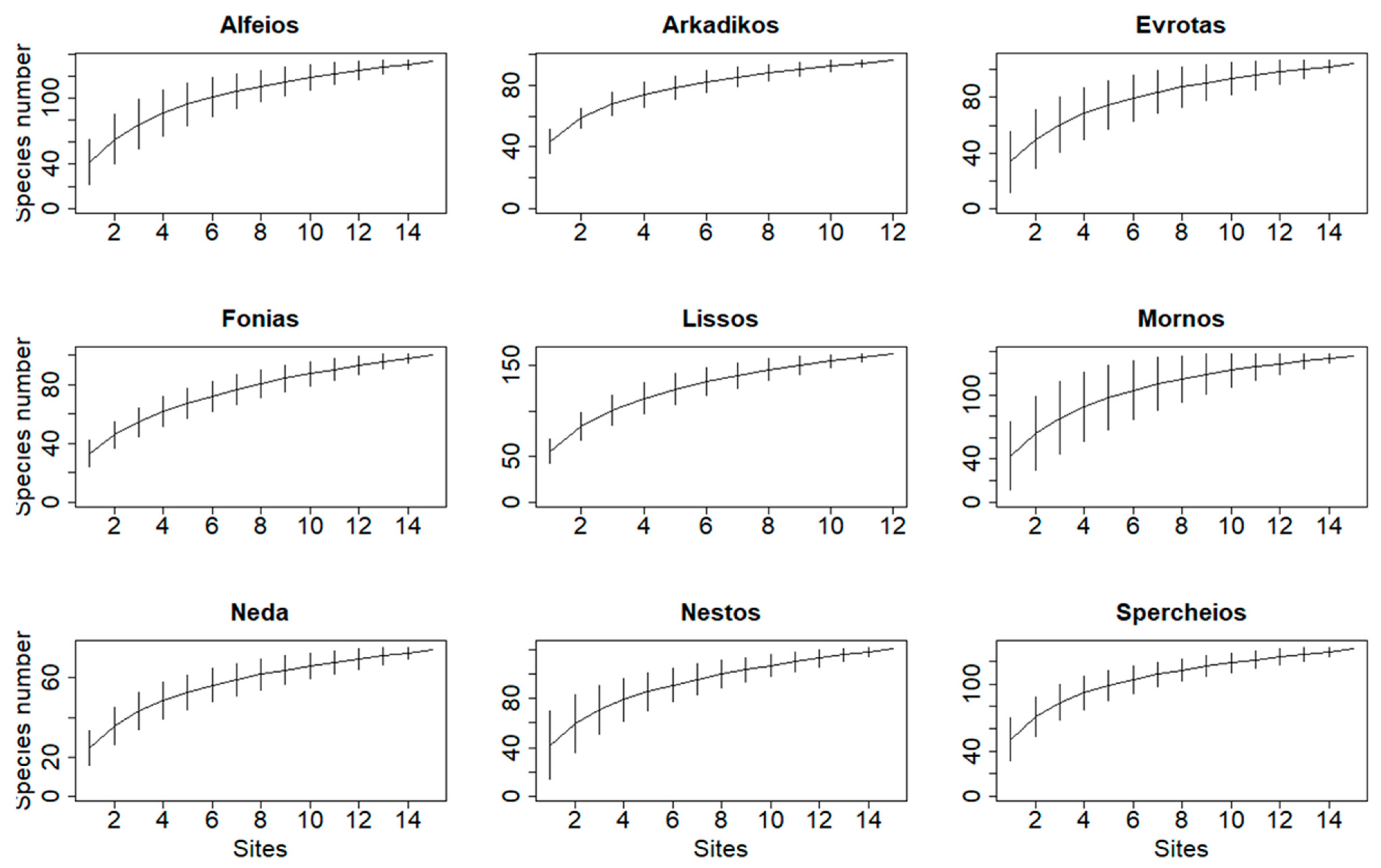
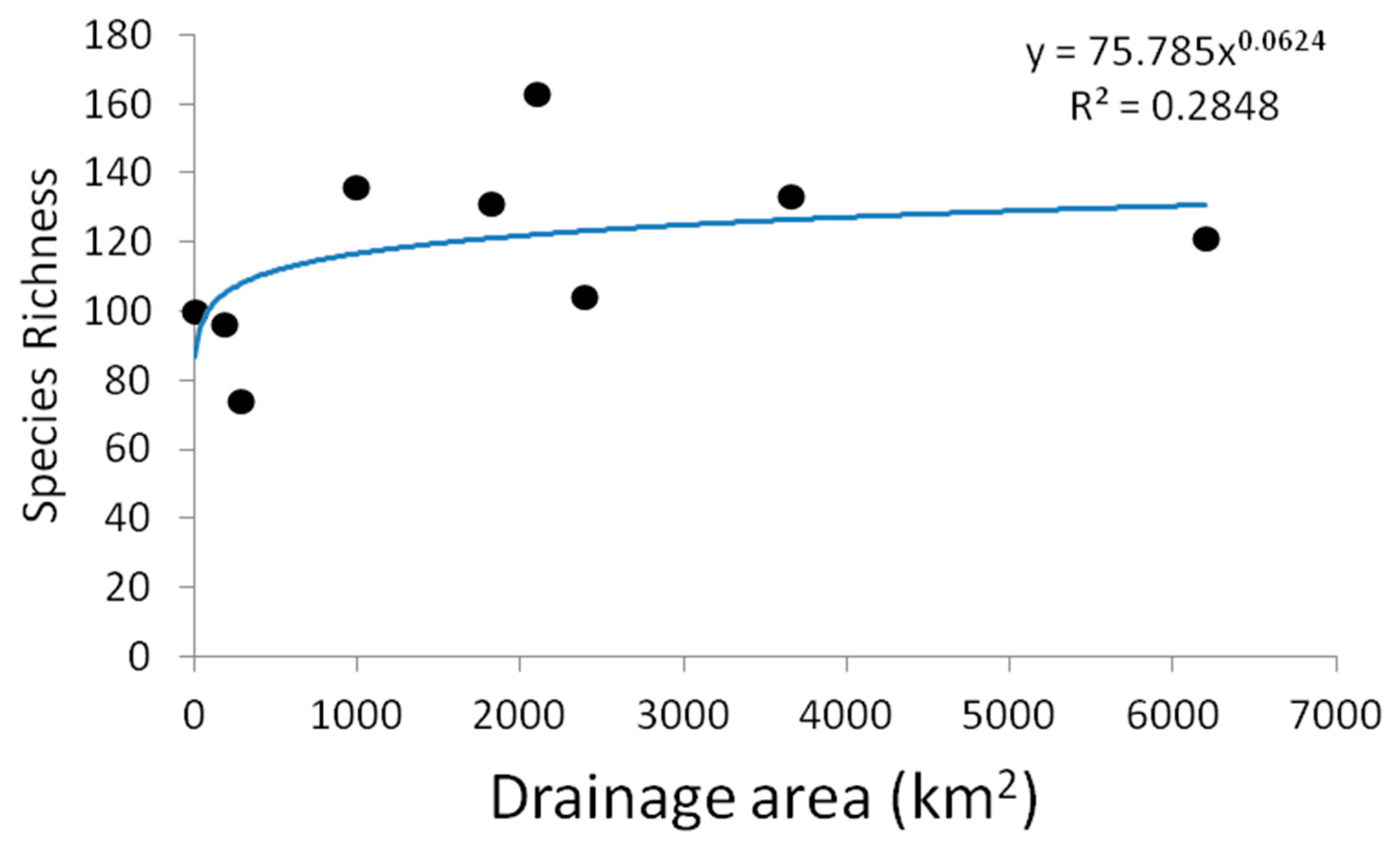
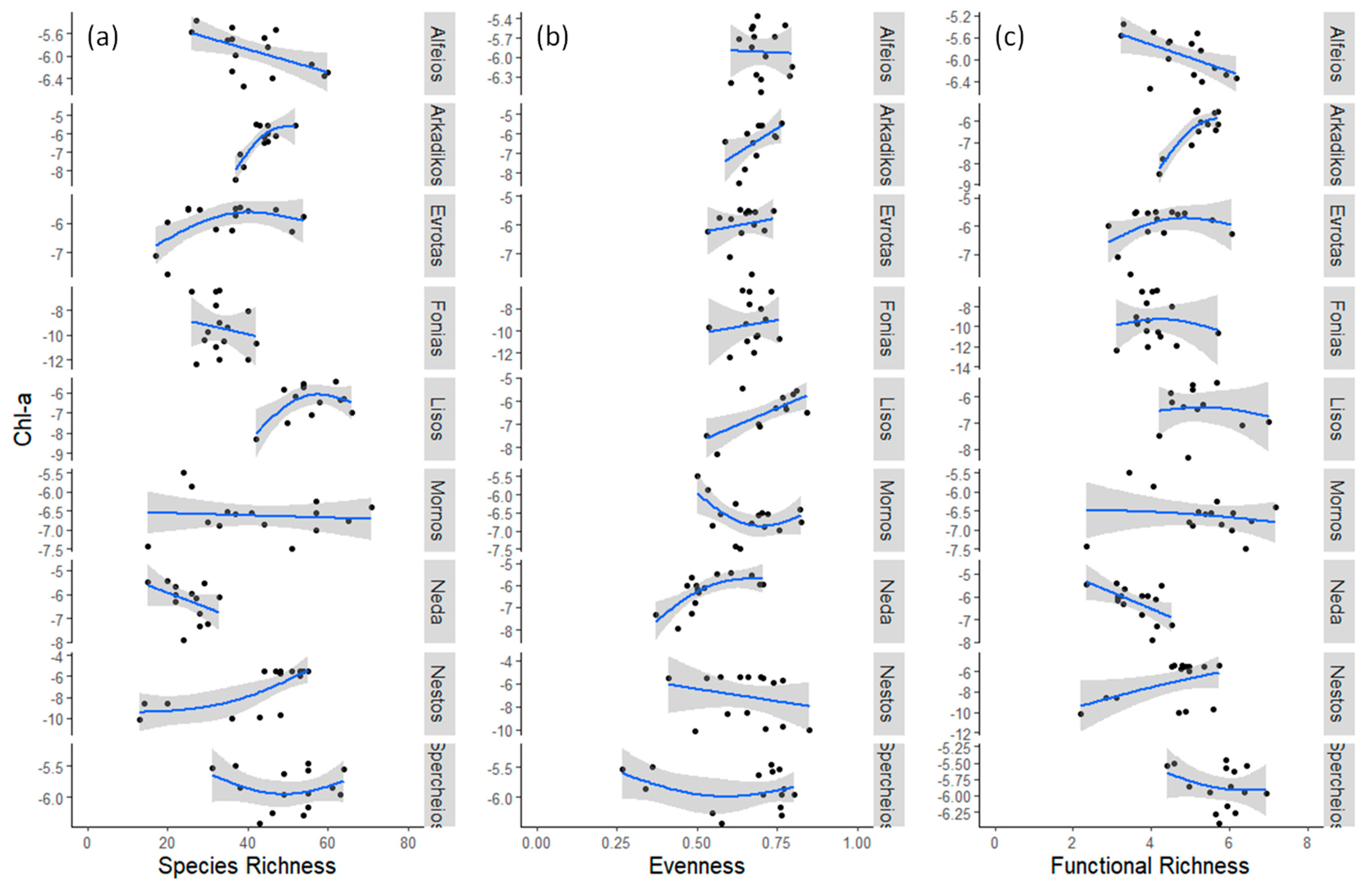
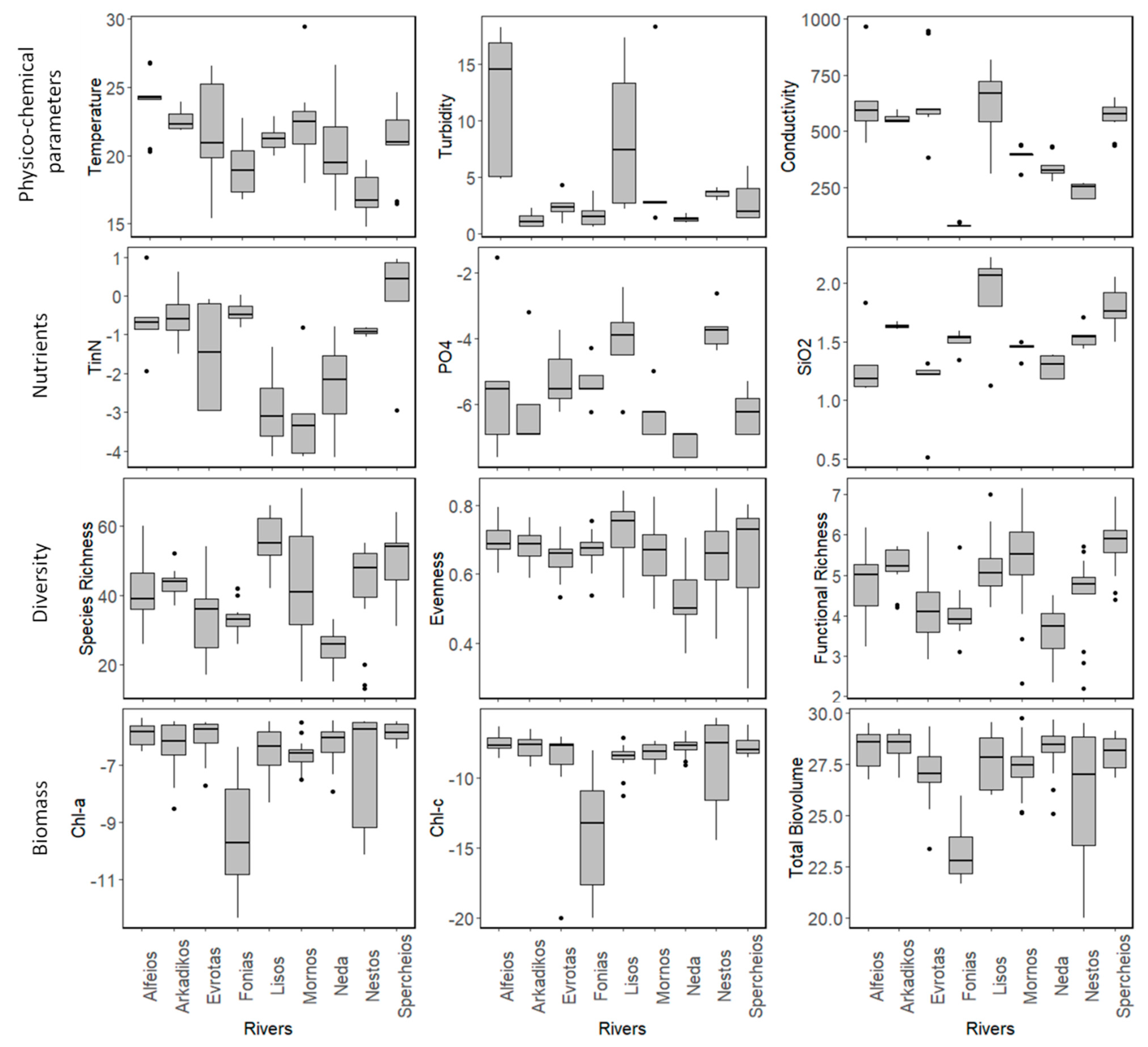
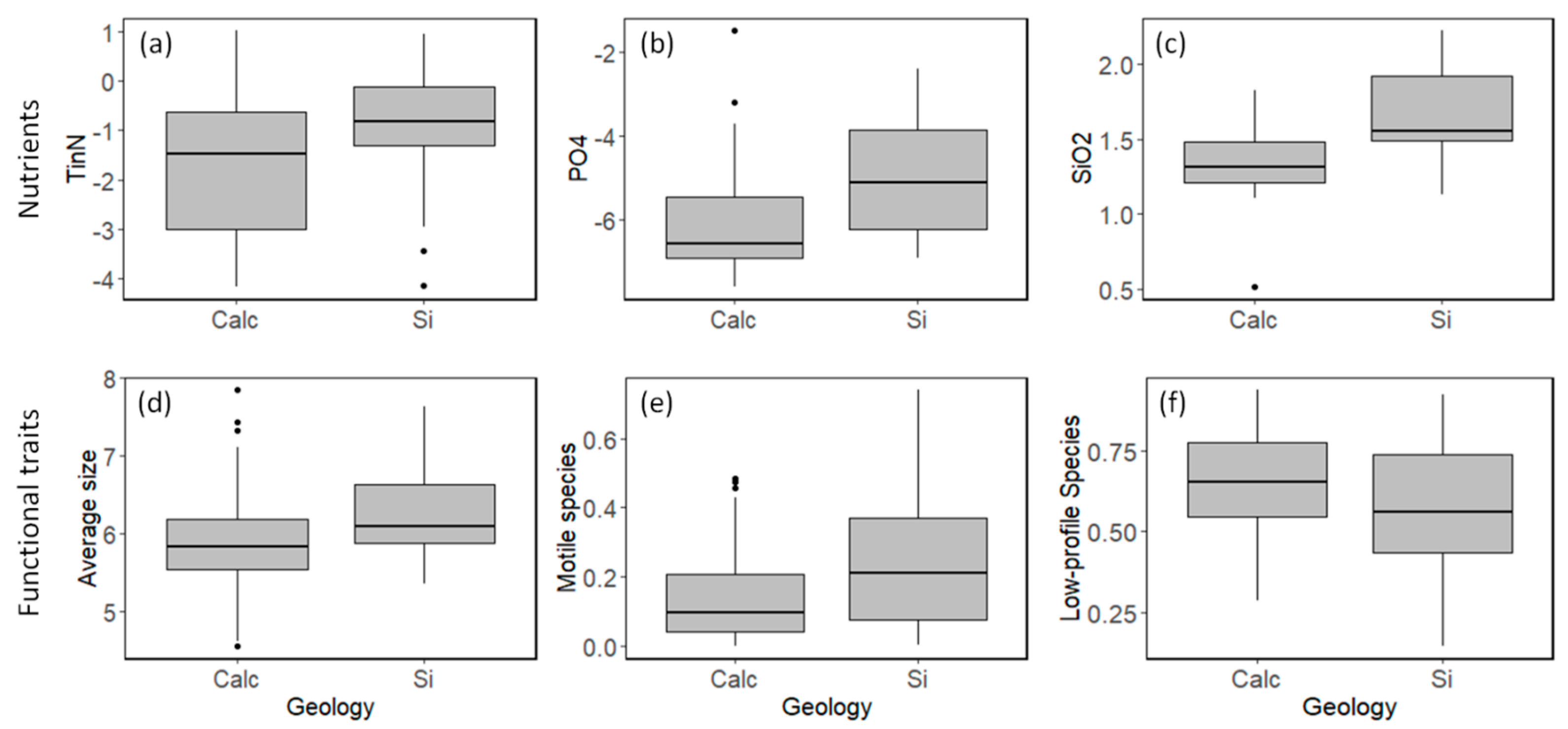
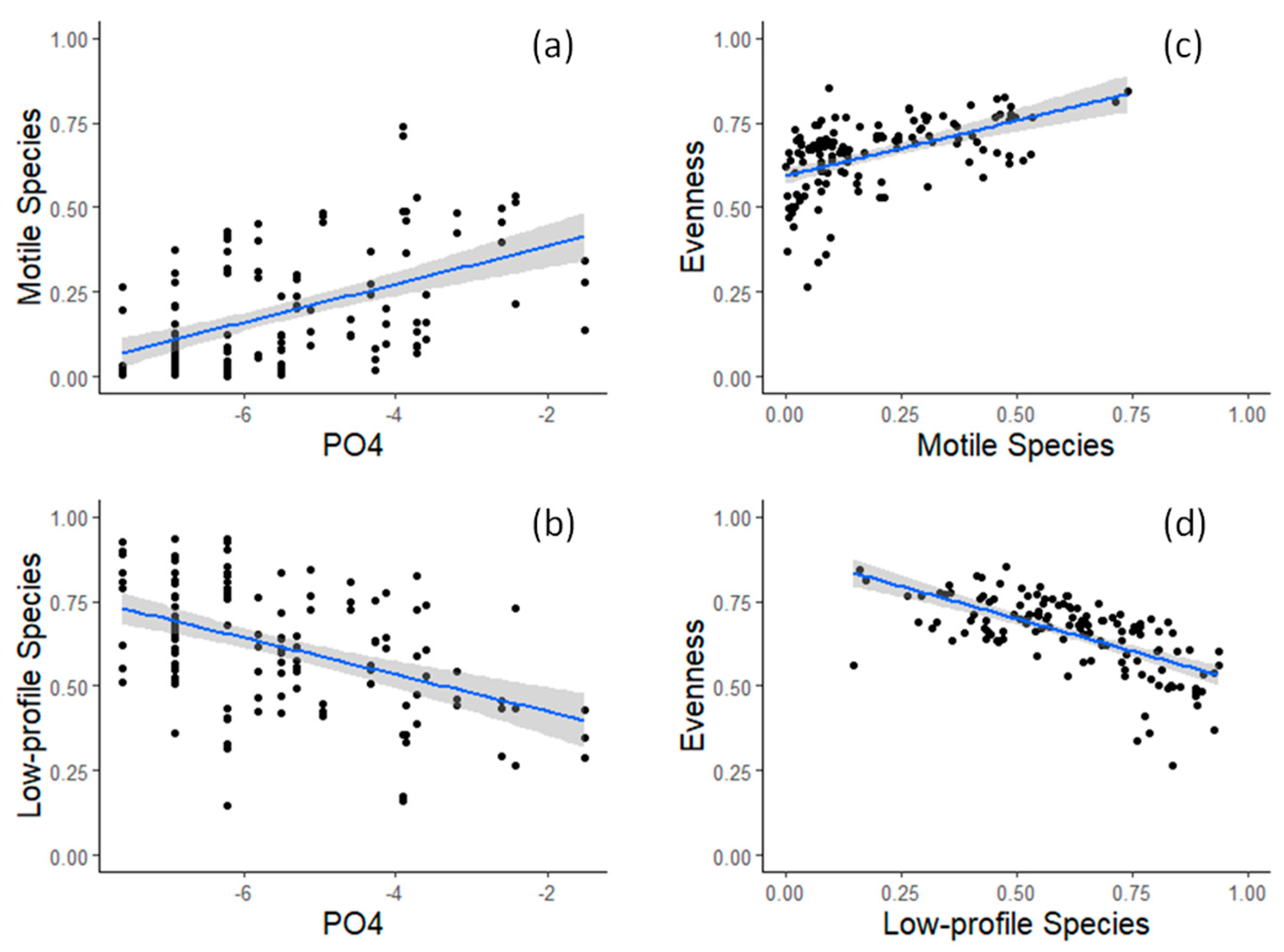
3.1. Field Observations
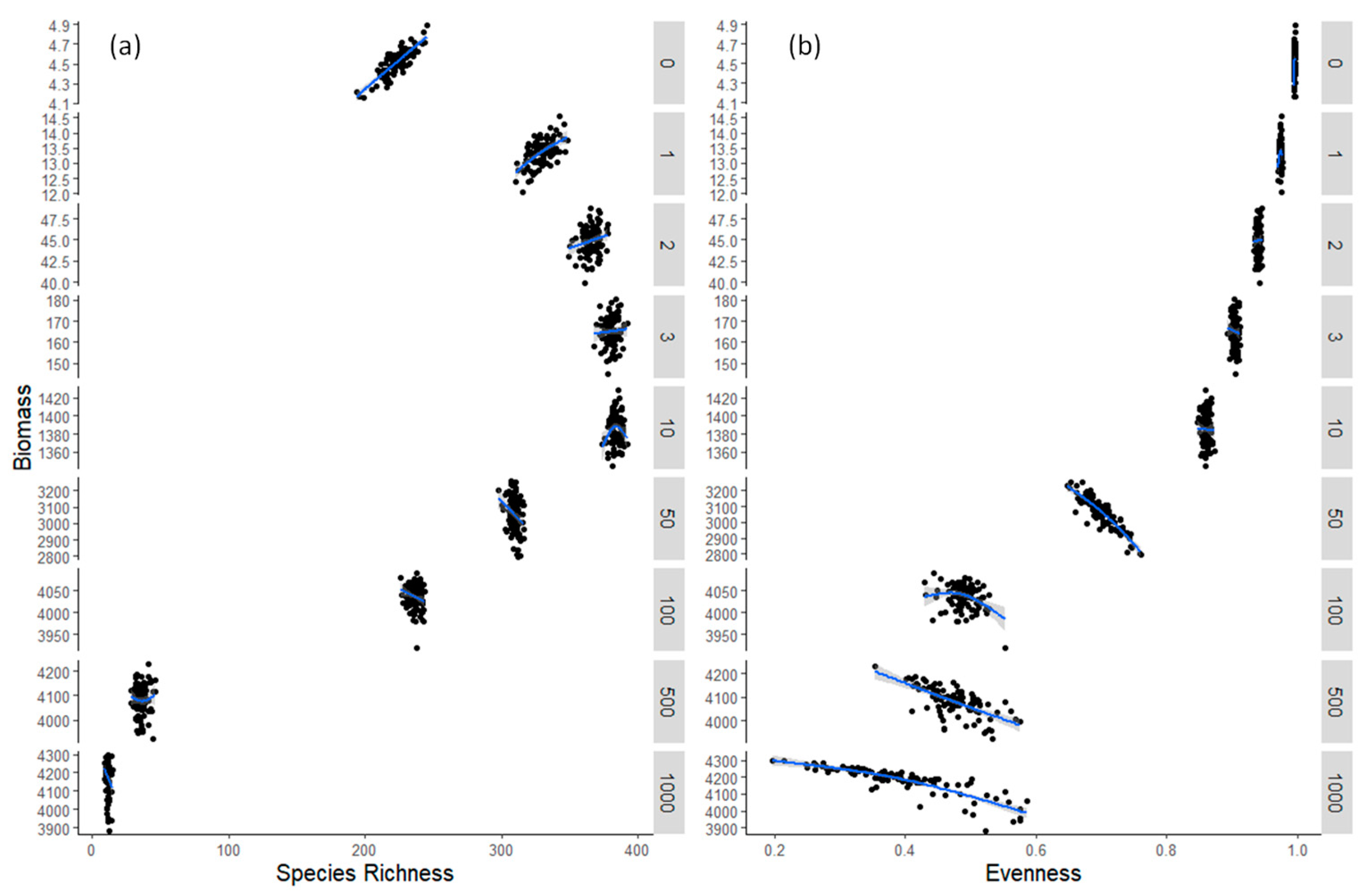
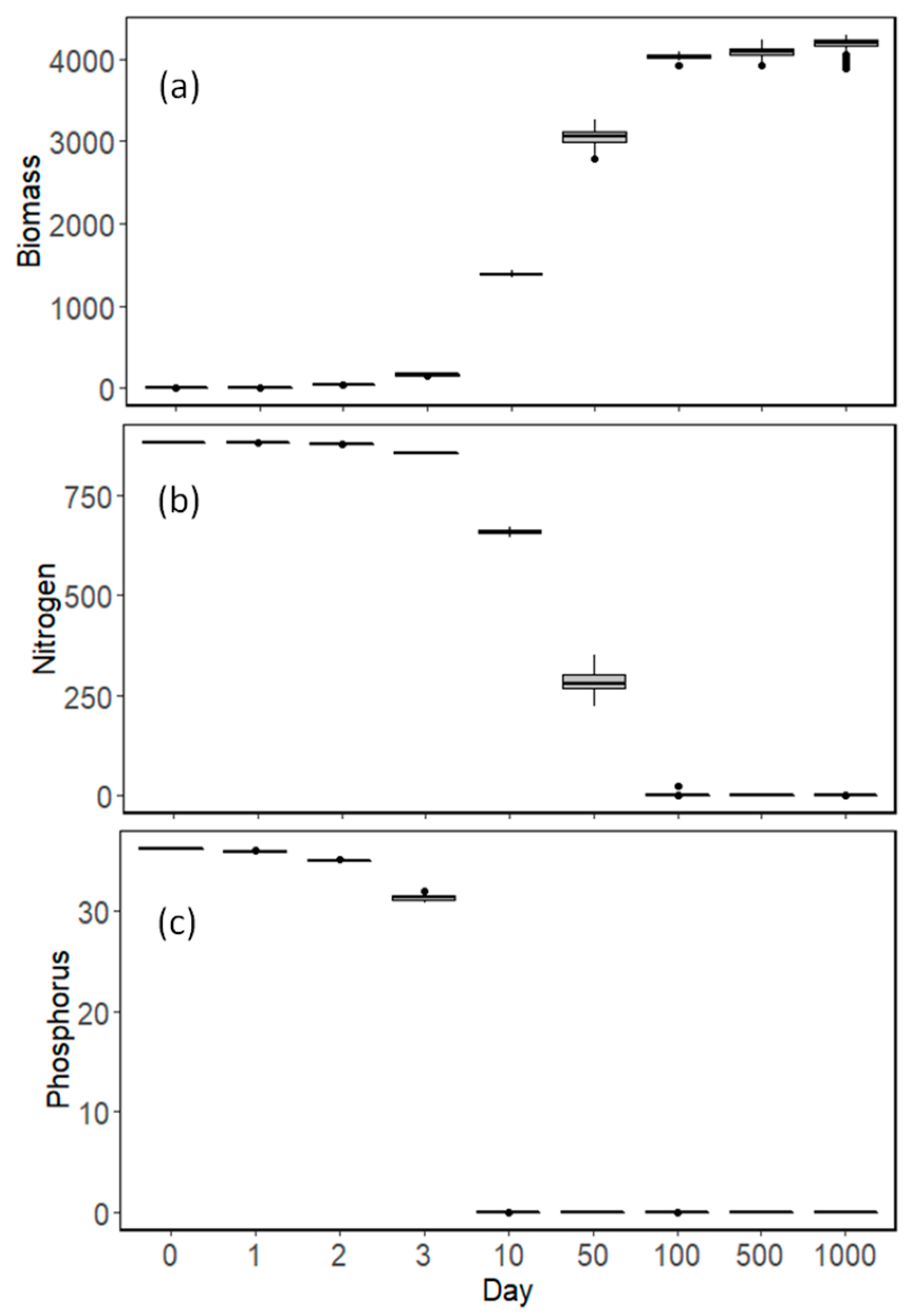
3.2. Model Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| River | Site | Latitude | Longitude | Sampling Date |
|---|---|---|---|---|
| Alfeios | 1 | 37.36144 | 22.0947 | 2 July 2020 |
| Alfeios | 2 | 37.39018 | 22.08635 | 2 July 2020 |
| Alfeios | 3 | 37.47971 | 22.04998 | 2 July 2020 |
| Alfeios | 4 | 37.63421 | 21.64196 | 3 July 2020 |
| Alfeios | 5 | 37.64135 | 21.47642 | 3 July 2020 |
| Arkadikos | 1 | 37.26862 | 21.78473 | 21 July 2020 |
| Arkadikos | 2 | 37.2796 | 21.7412 | 21 July 2020 |
| Arkadikos | 3 | 37.28775 | 21.72578 | 21 July 2020 |
| Arkadikos | 4 | 37.29342 | 21.697 | 21 July 2020 |
| Evrotas | 1 | 37.17217 | 22.30336 | 1 July 2020 |
| Evrotas | 2 | 37.09295 | 22.42634 | 1 July 2020 |
| Evrotas | 3 | 37.06522 | 22.45116 | 1 July 2020 |
| Evrotas | 4 | 36.99387 | 22.51856 | 1 July 2020 |
| Evrotas | 5 | 36.97334 | 22.58183 | 1 July 2020 |
| Fonias | 1 | 40.45111 | 25.6258 | 22 August 2020 |
| Fonias | 2 | 40.4561 | 25.62369 | 22 August 2020 |
| Fonias | 3 | 40.45862 | 25.62405 | 22 August 2020 |
| Fonias | 4 | 40.48059 | 25.64669 | 22 August 2020 |
| Fonias | 5 | 40.49182 | 25.65536 | 22 August 2020 |
| Lissos | 1 | 41.13642 | 25.53514 | 7 September 2020 |
| Lissos | 2 | 41.02474 | 25.3223 | 7 September 2020 |
| Lissos | 3 | 41.0249 | 25.48959 | 7 September 2020 |
| Lissos | 4 | 41.0148 | 25.26305 | 7 September 2020 |
| Mornos | 1 | 38.59818 | 22.18833 | 10 July 2020 |
| Mornos | 2 | 38.51151 | 22.07488 | 10 July 2020 |
| Mornos | 3 | 38.50764 | 21.99866 | 10 July 2020 |
| Mornos | 4 | 38.50438 | 22.02188 | 10 July 2020 |
| Mornos | 5 | 38.38779 | 21.86056 | 10 July 2020 |
| Neda | 1 | 37.40079 | 21.9485 | 21 July 2020 |
| Neda | 2 | 37.4053 | 21.92258 | 21 July 2020 |
| Neda | 3 | 37.39259 | 21.84667 | 21 July 220 |
| Neda | 4 | 37.39526 | 21.72911 | 21 July 2020 |
| Neda | 5 | 37.38446 | 21.68998 | 21 July 2020 |
| Nestos | 1 | 41.41019 | 24.10549 | 4 September 2020 |
| Nestos | 2 | 41.26262 | 24.50997 | 4 September 2020 |
| Nestos | 3 | 41.17856 | 24.70111 | 4 September 2020 |
| Nestos | 4 | 41.08417 | 24.77134 | 5 September 2020 |
| Nestos | 5 | 40.99428 | 24.7438 | 5 September 2020 |
| Spercheios | 1 | 38.94828 | 21.94711 | 27 August 2020 |
| Spercheios | 2 | 38.94361 | 22.21083 | 27 August 2020 |
| Spercheios | 3 | 38.90667 | 22.28583 | 27 August 2020 |
| Spercheios | 4 | 38.89611 | 22.3225 | 27 August 2020 |
| Spercheios | 5 | 38.86722 | 22.36333 | 27 August 2020 |
| Parameter | Explanation | Value/Range | Unit |
|---|---|---|---|
| i | species number | 400 | |
| j | number of resources | 2 | |
| μmax | maximum growth rate | 0.3–1.7 | d−1 |
| KP | half-saturation constant for phosphorus | 0.02–0.2 | μM |
| KN | half-saturation constant for nitrogen | 0.2–2 | μM |
| cP | intracellular content for P | 0.00397–0.055 | μM |
| cN | intracellular content for N | 0.055–0.244 | μM |
| SN | input nitrogen concentration | 882 | μM |
| SP | input phosphorus concentration | 36.2 | μM |
| D | nutrient flushing rate | 0.1 | d−1 |
| mi | species-specific flushing-induced mortality (D/μmax) | 0.3–0.06 | d−1 |
| total initial biomass | 4 × 106–5 × 106 | cells/cm2 | |
| threshold abundance for a species’ survival | 0.01 × 106 | cells/cm2 | |
| range of initial abundance of each species | 0.000009 × 106–0.0225 × 106 | cells/cm2 |
References
- van der Plas, F. Biodiversity and Ecosystem Functioning in Naturally Assembled Communities. Biol. Rev. 2019, 94, 1220–1245. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D. Prospects for Sustaining Freshwater Biodiversity in the 21st Century: Linking Ecosystem Structure and Function. Curr. Opin. Environ. Sustain. 2010, 2, 422–430. [Google Scholar] [CrossRef]
- Srivastava, D.S.; Vellend, M. Biodiversity-Ecosystem Function Research: Is It Relevant to Conservation? Annu. Rev. Ecol. Evol. Syst. 2005, 36, 267–294. [Google Scholar] [CrossRef]
- Truchy, A.; Angeler, D.G.; Sponseller, R.A.; Johnson, R.K.; McKie, B.G. Linking Biodiversity, Ecosystem Functioning and Services, and Ecological Resilience: Towards an Integrative Framework for Improved Management. Adv. Ecol. Res. 2015, 53, 55–96. [Google Scholar] [CrossRef]
- Daam, M.A.; Teixeira, H.; Lillebø, A.I.; Nogueira, A.J.A. Establishing Causal Links between Aquatic Biodiversity and Ecosystem Functioning: Status and Research Needs. Sci. Total Environ. 2019, 656, 1145–1156. [Google Scholar] [CrossRef]
- Ptacnik, R.; Solimini, A.G.; Andersen, T.; Tamminen, T.; Brettum, P.; Lepistö, L.; Willén, E.; Rekolainen, S. Diversity Predicts Stability and Resource Use Efficiency in Natural Phytoplankton Communities. Proc. Natl. Acad. Sci. USA 2008, 105, 5134–5138. [Google Scholar] [CrossRef]
- Filstrup, C.T.; King, K.B.S.; McCullough, I.M. Evenness Effects Mask Richness Effects on Ecosystem Functioning at Macro-Scales in Lakes. Ecol. Lett. 2019, 22, 2120–2129. [Google Scholar] [CrossRef]
- Olli, K.; Ptacnik, R.; Klais, R.; Tamminen, T. Phytoplankton Species Richness along Coastal and Estuarine Salinity Continua. Am. Nat. 2019, 194, E41–E51. [Google Scholar] [CrossRef]
- Ribeiro, L.; Benyoucef, I.; Poulin, M.; Jesus, B.; Rosa, P.; Méléder, V.; Du, G.Y.; Barillé, L. Spatio-Temporal Variation of Microphytobenthos Biomass, Diversity and Assemblage Structure in the Loire Estuary, France. Aquat. Microb. Ecol. 2021, 87, 61–77. [Google Scholar] [CrossRef]
- Virta, L.; Gammal, J.; Järnström, M.; Bernard, G.; Soininen, J.; Norkko, J.; Norkko, A. The Diversity of Benthic Diatoms Affects Ecosystem Productivity in Heterogeneous Coastal Environments. Ecology 2019, 100, e02765. [Google Scholar] [CrossRef]
- Smeti, E.; von Schiller, D.; Karaouzas, I.; Laschou, S.; Vardakas, L.; Sabater, S.; Tornés, E.; Monllor-Alcaraz, L.S.; Guillem-Argiles, N.; Martinez, E.; et al. Multiple Stressor Effects on Biodiversity and Ecosystem Functioning in a Mediterranean Temporary River. Sci. Total Environ. 2019, 647, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, S.A.; Potapova, M.G.; Bishop, I.W.; Lee, S.S.; Gasperak, T.S.; Jovanoska, E.; Furey, P.C.; Edlund, M.B. Diatoms.Org: Supporting Taxonomists, Connecting Communities. Diatom Res. 2021, 36, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Passy, S.I. Diatom Ecological Guilds Display Distinct and Predictable Behavior along Nutrient and Disturbance Gradients in Running Waters. Aquat. Bot. 2007, 86, 171–178. [Google Scholar] [CrossRef]
- Rimet, F.; Bouchez, A. Life-Forms, Cell-Sizes and Ecological Guilds of Diatoms in European Rivers. Knowl. Manag. Aquat. Ecosyst. 2012, 406, 01. [Google Scholar] [CrossRef]
- Abonyi, A.; Horváth, Z.; Ptacnik, R. Functional Richness Outperforms Taxonomic Richness in Predicting Ecosystem Functioning in Natural Phytoplankton Communities. Freshw. Biol. 2018, 63, 178–186. [Google Scholar] [CrossRef]
- Crawford, M.S.; Barry, K.E.; Clark, A.T.; Farrior, C.E.; Hines, J.; Ladouceur, E.; Lichstein, J.W.; Maréchaux, I.; May, F.; Mori, A.S.; et al. The Function-Dominance Correlation Drives the Direction and Strength of Biodiversity–Ecosystem Functioning Relationships. Ecol. Lett. 2021, 24, 1762–1775. [Google Scholar] [CrossRef]
- Smeti, E.; Roelke, D.L.; Tsirtsis, G.; Spatharis, S. Species Extinctions Strengthen the Relationship between Biodiversity and Resource Use Efficiency. Ecol. Modell. 2018, 384, 75–86. [Google Scholar] [CrossRef]
- Vallina, S.M.; Cermeno, P.; Dutkiewicz, S.; Loreau, M.; Montoya, J.M. Phytoplankton Functional Diversity Increases Ecosystem Productivity and Stability. Ecol. Modell. 2017, 361, 184–196. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 15th ed.; American Public Health Association: Washington, DC, USA, 1980. [Google Scholar]
- Jeffrey, S.W.; Humphrey, G.F. New Spectrophotometric Equations for Determining Chlorophylls a, b, C1 and C2 in Higher Plants, Algae and Natural Phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Battarbee, R.W. Diatom Analysis. In Handbook of Holocene Palaeoecology and Palaeohydrology; Berglund, B.E., Ed.; Wiley-Interscience; John Wiley & Sons Ltd: Chichester, UK, 1986; pp. 527–570. [Google Scholar]
- Cantonati, M.; Kelly, M.G.; Lange-Bertalot, H. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; Koeltz Botanical Books: Hessen, Germany, 2017. [Google Scholar]
- Petchey, O.L.; Gaston, K.J. Functional Diversity (FD), Species Richness and Community Composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume Calculation for Pelagic and Benthic Microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Sommer, U.; Charalampous, E.; Genitsaris, S.; Moustaka-Gouni, M. Benefits, Costs and Taxonomic Distribution of Marine Phytoplankton Body Size. J. Plankton Res. 2017, 39, 494–508. [Google Scholar] [CrossRef]
- Skoulikidis, N.T.; Amaxidis, Y.; Bertahas, I.; Laschou, S.; Gritzalis, K. Analysis of Factors Driving Stream Water Composition and Synthesis of Management Tools—A Case Study on Small/Medium Greek Catchments. Sci. Total Environ. 2006, 362, 205–241. [Google Scholar] [CrossRef] [PubMed]
- Skoulikidis, N.; Amaxidis, Y. Origin and Dynamics of Dissolved and Particulate Nutrients in a Minimally Disturbed Mediterranean River with Intermittent Flow. J. Hydrol. 2009, 373, 218–229. [Google Scholar] [CrossRef]
- Karaouzas, I.; Theodoropoulos, C.; Vardakas, L.; Zogaris, S.; Skoulikidis, N. The Evrotas River Basin: 10 Years of Ecological Monitoring. In The Rivers of Greece; Springer: Berlin/Heidelberg, Germany, 2018; pp. 279–326. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; et al. Vegan: Community Ecology Package; Wiley Online Library: Hoboken, NJ, USA, 2020. [Google Scholar]
- Cardoso, P.; Mammola, S.; Rigal, F.; Carvalho, J. BAT: Biodiversity Assessment Tools; University of Helsinki: Helsinki, Finland, 2021. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Sievert, C. Interactive Web-Based Data Visualization with R, Plotly, and Shiny; Chapman and Hall/CRC: London, UK, 2020. [Google Scholar]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Sarthou, G.; Timmermans, K.R.; Blain, S.; Tréguer, P. Growth Physiology and Fate of Diatoms in the Ocean: A Review. J. Sea Res. 2005, 53, 25–42. [Google Scholar] [CrossRef]
- Roelke, D.L.; Spatharis, S. Phytoplankton Succession in Recurrently Fluctuating Environments. PLoS ONE 2015, 10, e0121392. [Google Scholar] [CrossRef]
- Dürr, H.H.; Meybeck, M.; Hartmann, J.; Laruelle, G.G.; Roubeix, V. Global Spatial Distribution of Natural Riverine Silica Inputs to the Coastal Zone. Biogeosciences 2011, 8, 597–620. [Google Scholar] [CrossRef]
- von Wandruszka, R. Phosphorus Retention in Calcareous Soils and the Effect of Organic Matter on Its Mobility. Geochem. Trans. 2006, 7, 1–8. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial Phosphorus Limitation: Mechanisms, Implications, and Nitrogen-Phosphorus Interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Loreau, M. From Populations to Ecosystems: Theoretical Foundations for a New Ecological Synthesis (MPB-46); Princeton University Press: Princeton, NJ, USA, 2010; ISBN 9781400834167. [Google Scholar]
- Li, T.; Zhou, P.; Ding, Y.; Tang, Q.; Zhou, S.; Liu, Y. Distribution Characteristics and Source Analysis of Nitrogen and Phosphorus in Different Rivers in Two Water Period: A Case Study of Pi River and Shiting River in the Upper Reaches of Tuo River in China. Int. J. Environ. Res. Public Health 2022, 19, 12433. [Google Scholar] [CrossRef] [PubMed]
- von Schiller, D.; Acuña, V.; Aristi, I.; Arroita, M.; Basaguren, A.; Bellin, A.; Boyero, L.; Butturini, A.; Ginebreda, A.; Kalogianni, E.; et al. River Ecosystem Processes: A Synthesis of Approaches, Criteria of Use and Sensitivity to Environmental Stressors. Sci. Total Environ. 2017, 596–597, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Nimick, D.A.; Gammons, C.H.; Parker, S.R. Diel Biogeochemical Processes and Their Effect on the Aqueous Chemistry of Streams: A Review. Chem. Geol. 2011, 283, 3–17. [Google Scholar] [CrossRef]

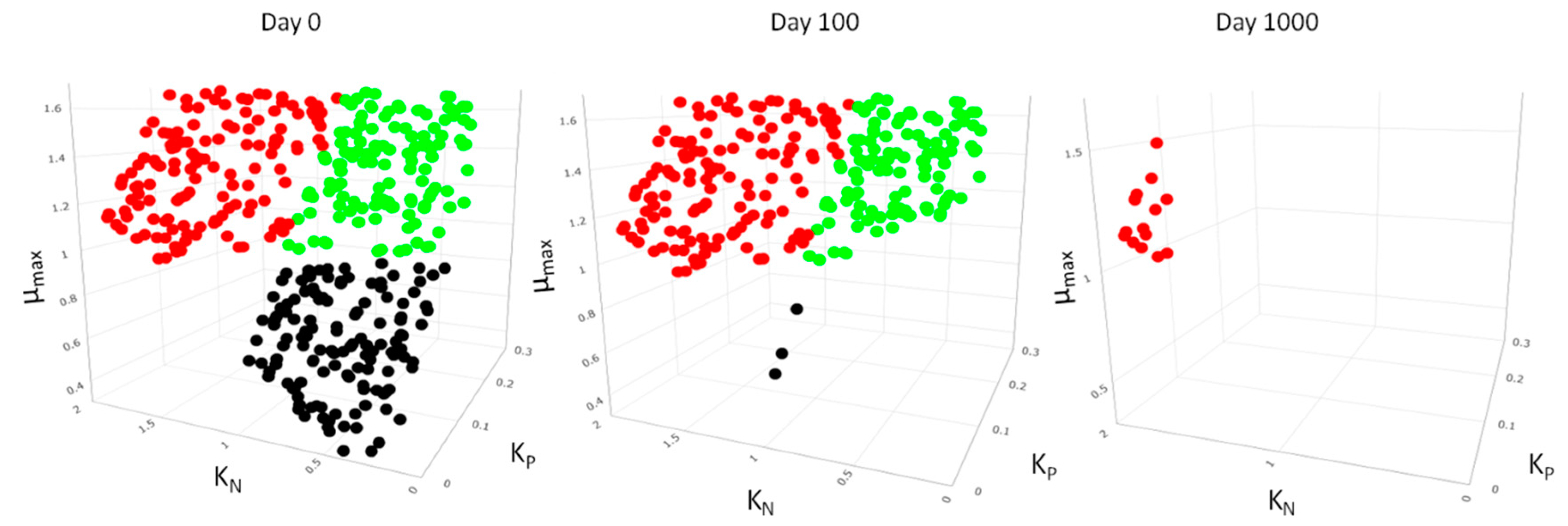
| Species Richness (S) | Evenness (J) | |||
|---|---|---|---|---|
| Day | Slope (a) | p-Value | Slope (a) | p-Value |
| 0 | 0.00286 | *** | 31.01131 | ** |
| 1 | 0.002282 | *** | 2.686801 | 0.278 |
| 2 | 0.001267 | * | 0.447689 | 0.742 |
| 3 | 0.000544 | 0.569 | −0.87027 | 0.445 |
| 10 | 0.00018 | 0.585 | −0.05598 | 0.823 |
| 50 | −0.00278 | ** | −1.21366 | *** |
| 100 | 0.000241 | 0.170 | −0.08981 | ** |
| 500 | 0.000103 | 0.787 | −0.24929 | *** |
| 1000 | −0.00434 | ** | −0.19818 | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smeti, E.; Tsirtsis, G.; Skoulikidis, N.T. Geology Can Drive the Diversity–Ecosystem Functioning Relationship in River Benthic Diatoms by Selecting for Species Functional Traits. Biology 2023, 12, 81. https://doi.org/10.3390/biology12010081
Smeti E, Tsirtsis G, Skoulikidis NT. Geology Can Drive the Diversity–Ecosystem Functioning Relationship in River Benthic Diatoms by Selecting for Species Functional Traits. Biology. 2023; 12(1):81. https://doi.org/10.3390/biology12010081
Chicago/Turabian StyleSmeti, Evangelia, George Tsirtsis, and Nikolaos Theodor Skoulikidis. 2023. "Geology Can Drive the Diversity–Ecosystem Functioning Relationship in River Benthic Diatoms by Selecting for Species Functional Traits" Biology 12, no. 1: 81. https://doi.org/10.3390/biology12010081
APA StyleSmeti, E., Tsirtsis, G., & Skoulikidis, N. T. (2023). Geology Can Drive the Diversity–Ecosystem Functioning Relationship in River Benthic Diatoms by Selecting for Species Functional Traits. Biology, 12(1), 81. https://doi.org/10.3390/biology12010081







