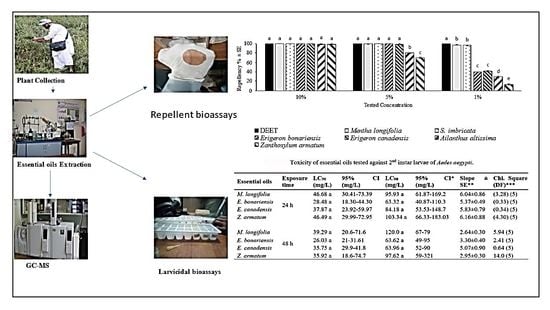
Chemical Composition, Larvicidal and Repellent Activities of Wild Plant Essential Oils against Aedes aegypti
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material
2.2. Extraction of Essential Oils
2.3. Rearing of Ae. aegypti
2.4. Mosquito Repellency Bioassay
2.5. Larvicidal Activity Bioassay
2.6. Chemical Analysis of the Essential Oils
2.7. Statistical Analysis
3. Results
3.1. Yield of Essential Oils
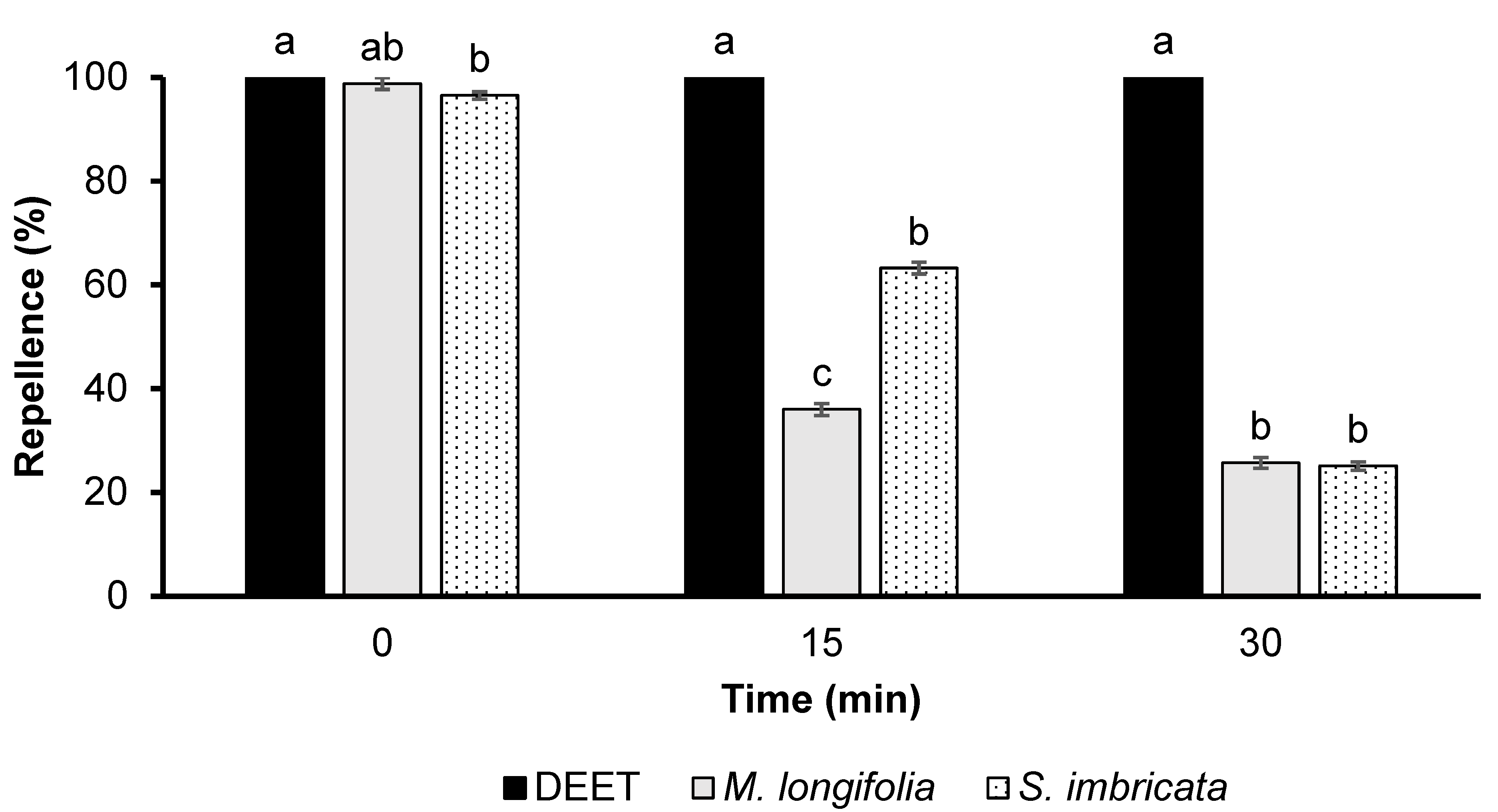
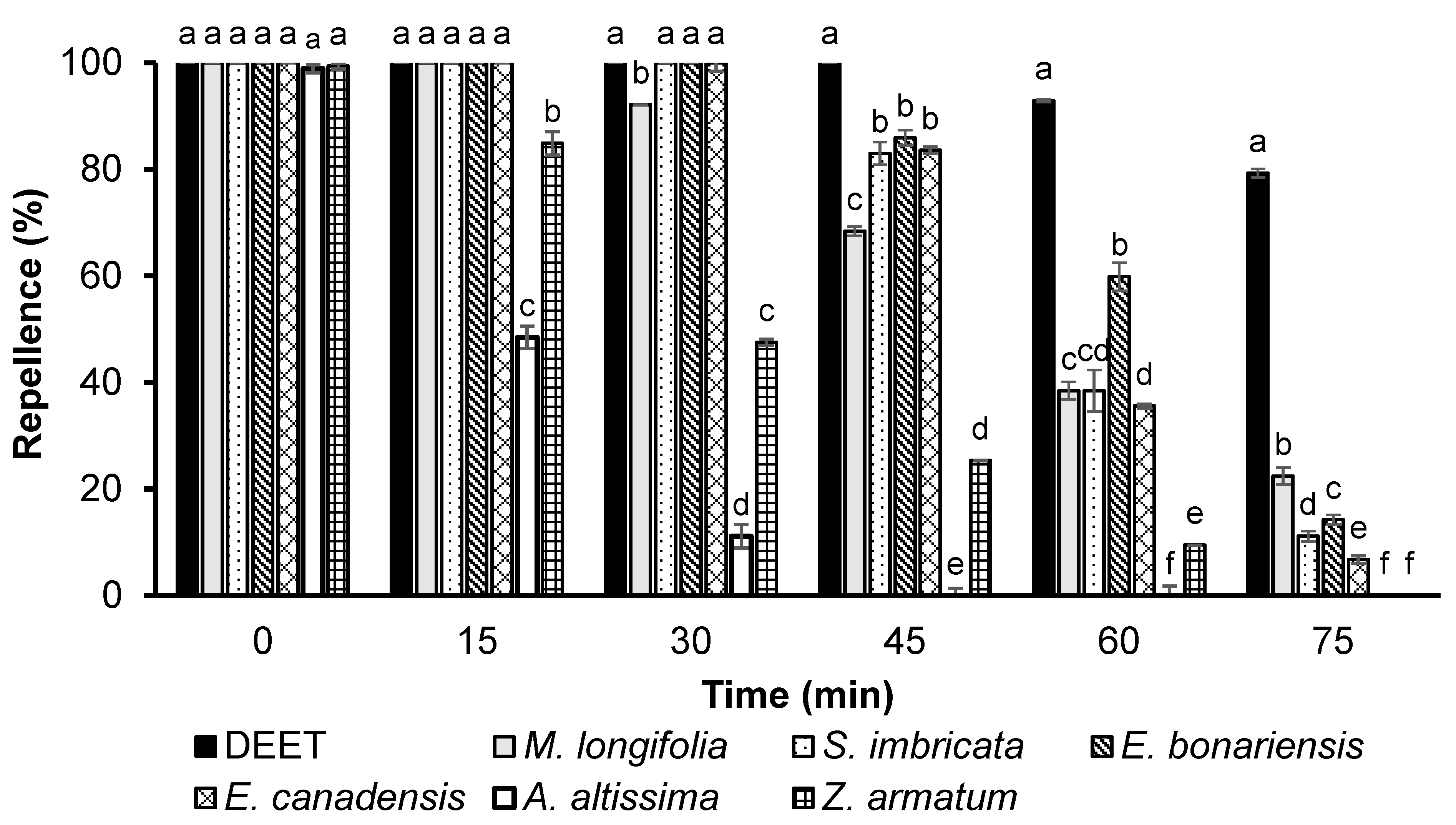
3.2. Repellency of Essential Oils
3.2.1. Time Span Repellency at a Dose of 33.3 µg/cm2
3.2.2. Time Span Repellency at a Dose of 166.5 µg/cm2
3.2.3. Time Span Repellency at a Dose of 330 µg/cm2
3.3. Larvicidal Activity of Essential Oils
3.4. Composition of Essential Oils
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dehghani, R.; Mousavi, G.A.; Ghasemi, B.; Ghasemi, M.; Saheb, M.; Mohamadi, R. A survey on residential areas infestation to house pests (Arthropods) in Kashan. Sci. Inf. Database 2013, 15, 36–39. [Google Scholar]
- WHO. Dengue and severe dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. (accessed on 13 November 2020).
- Silva, N.M.; Santos, N.C.; Martins, I.C. Dengue and Zika viruses: Epidemiological history, potential therapies, and promising vaccines. Trop Med. Infect. Dis. 2020, 5, 150. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Coffeng, L.E.; Brady, O.J.; Hay, S.I.; Bedi, N.; Bensenor, I.M.; Castañeda-Orjuela, C.A. The global burden of dengue: An analysis from the global burden of disease study 2013. Lancet Infect. Dis. 2016, 16, 712–723. [Google Scholar] [CrossRef]
- Khan, J.; Anwar, F.; Shah, S.S.; Qamar, Z.; Ullah, W.; Ali, A.; Hussain, M.; Ali, I.; Ali, F.; Ullah, F. Dengue virus epidemics: A recent report of 2018 from district Swat, Khyber-Pakhtunkhwa Pakistan. Int. J. Mosquito Res. 2021, 8, 105–108. [Google Scholar]
- Imran, M.; Hamid, Y.; Mazher, A.; Ahmad, S.R. Geo-spatially modelling dengue epidemics in urban cities: A case study of Lahore, Pakistan. Geocarto Int. 2021, 36, 197–211. [Google Scholar] [CrossRef]
- Wharton-Smith, A.; Green, J.; Loh, E.C.; Gorrie, A.; Omar, S.F.S.; Bacchus, L.; Lum, L.C.S. Using clinical practice guidelines to manage dengue: A qualitative study in a Malaysian hospital. BMC Infect. Dis. 2019, 19, 45. [Google Scholar] [CrossRef]
- Al Zahrani, M.R.; Gharsan, F.N.; Al-Ghamd, K.M.; Mahyoub, J.A.; Alghamdi, T.S. Toxicity of two groups of pesticides against the mosquito Aedes aegypti. GSC GSC Biol. Pharm Sci. 2020, 13, 148–155. [Google Scholar] [CrossRef]
- Junkum, A.; Intirach, J.; Chansang, A.; Champakaew, D.; Chaithong, U.; Jitpakdi, A.; Riyong, D.; Somboon, P.; Pitasawat, B. Enhancement of temephos and deltamethrin toxicity by Petroselinum crispum oil and its main constituents against Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2021, 58, 1298–1315. [Google Scholar] [CrossRef]
- Devine, G.J.; Vazquez-Prokopec, G.M.; Bibiano-Marín, W.; Pavia-Ruz, N.; Che-Mendoza, A.; Medina-Barreiro, A.; Villegas, J.; Gonzalez-Olvera, G.; Dunbar, M.W.; Ong, O. The entomological impact of passive metofluthrin emanators against indoor Aedes aegypti: A randomized field trial. PLoS Negl. Trop Dis. 2021, 15, e0009036. [Google Scholar] [CrossRef]
- Samal, R.R.; Kumar, S. Cuticular thickening associated with insecticide resistance in dengue vector, Aedes aegypti L. Int. J. Trop. Insect Sci. 2021, 41, 809–820. [Google Scholar] [CrossRef]
- Dos Santos, C.R.; de Melo Rodovalho, C.; Jablonka, W.; Martins, A.J.; Lima, J.B.P.; dos Santos Dias, L.; da Silva Neto, M.A.C.; Atella, G.C. Insecticide resistance, fitness and susceptibility to Zika infection of an interbred Aedes aegypti population from Rio de Janeiro, Brazil. Parasit Vectors 2020, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.; Cohren, S.; Costa, R. Epidemiological profile of diseases caused by Aedes aegypti in sanitary districts of São Luis, Brazil. Eur. J. Public Health 2020, 30, ckaa166-141. [Google Scholar] [CrossRef]
- Fernando, H.S.D.; Saavedra-Rodriguez, K.; Perera, R.; Black, W.C.; De Silva, B.N.K. Resistance to commonly used insecticides and underlying mechanisms of resistance in Aedes aegypti (L.) from Sri Lanka. Parasit Vectors 2020, 13, 407. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.U.; Cosme, L.V.; Costa, M.M.; Carrara, L.; Lima, J.B.P.; Martins, A.J.J.P.n.t.d. Insecticide resistance and genetic structure of Aedes aegypti populations from Rio de Janeiro State, Brazil. PLoS Neglec. Tropic. Dis. 2021, 15, e0008492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; De Laender, F.; Van den Brink, P.J. Community composition modifies direct and indirect effects of pesticides in freshwater food webs. Sci. Total Environ. 2020, 739, 139531. [Google Scholar] [CrossRef]
- Ali, S.; Ullah, M.I.; Sajjad, A.; Shakeel, Q.; Hussain, A. Environmental and health effects of pesticide residues. In Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2021; pp. 311–336. [Google Scholar]
- Uwaifo, F.; John-Ohimai, F. Dangers of organophosphate pesticide exposure to human health. Matrix Sci. Med. 2020, 4, 27. [Google Scholar] [CrossRef]
- Pratiwi, M.A.M. The repellent activity test of rosemary leaf (Rosmarinus officinalis L) essential oil gel preparations influence on Aedes aegypti mosquito. In Proceedings of Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2021; p. 012016. [Google Scholar]
- Afify, A.; Potter, C. Insect repellents mediate species-specific olfactory behaviours in mosquitoes. Malar. J. 2020, 19, 127. [Google Scholar] [CrossRef]
- Qiu, H.; Jun, H.W.; Dzimianski, M.; McCall, J. Reduced transdermal absorption of N, N-diethyl-m-toluamide from a new topical insect repellent formulation. Pharm. Dev. Technol. 1997, 2, 33–42. [Google Scholar] [CrossRef]
- Calafat, A.M.; Baker, S.E.; Wong, L.-Y.; Bishop, A.M.; Morales-A, P.; Valentin-Blasini, L. Novel exposure biomarkers of N, N-diethyl-m-toluamide (DEET): Data from the 2007–2010 National Health and Nutrition Examination Survey. Environ. Int. 2016, 92, 398–404. [Google Scholar] [CrossRef]
- Oftadeh, M.; Sendi, J.J.; Ebadollahi, A.; Setzer, W.N.; Krutmuang, P. Mulberry protection through flowering-stage essential oil of Artemisia annua against the lesser mulberry pyralid, Glyphodes pyloalis Walker. Food Addit. Contam. 2021, 10, 210. [Google Scholar] [CrossRef]
- Wong, C.; Crystal, K.; Coats, J. Three molecules found in rosemary or nutmeg essential oils repel ticks (Dermacentor variabilis) more effectively than DEET in a no-human assay. Pest. Manage. Sci. 2020, 77, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.H.; Ling, S.; Zeng, X. Seriphidium brevifolium essential oil: A novel alternative to synthetic insecticides against the dengue vector Aedes albopictus. Environ. Sci Pollut Res. Int. 2020, 27, 31863–31871. [Google Scholar] [CrossRef] [PubMed]
- Stappen, I.; Wanner, J.; Tabanca, N.; Bernier, U.R.; Kendra, P.E. Blue Tansy Essential Oil: Chemical Composition, Repellent Activity Against Aedes aegypti and Attractant Activity for Ceratitis capitata. Nat. Prod.Commun. 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Upadhyay, S.; Bhuiyan, M.; Bhattacharya. A review on prospects of essential oils as biopesticide in insect-pest management. J. Pharm. Phytother. 2009, 1, 52–63. [Google Scholar]
- Azeem, M.; Zaman, T.; Abbasi, A.M.; Abid, M.; Mozūratis, R.; Alwahibi, M.S.; Elshikh, M.S. Pesticidal potential of some wild plant essential oils against grain pests Tribolium castaneum (Herbst, 1797) and Aspergillus flavus (Link, 1809). Arab J. Chem. 2022, 15, 103482. [Google Scholar] [CrossRef]
- Azeem, M.; Zaman, T.; Tahir, M.; Haris, A.; Iqbal, Z.; Binyameen, M.; Nazir, A.; Shad, S.A.; Majeed, S.; Mozūraitis, R. Chemical composition and repellent activity of native plants essential oils against dengue mosquito, Aedes aegypti. Ind Crops Prod. 2019, 140, 111609. [Google Scholar] [CrossRef]
- Johnson, H. Notes on the continuous rearing of Aedes aegypti in the laboratory. Public Health Rep. 1937, 52, 1177–1179. [Google Scholar] [CrossRef]
- Zheng, M.-L.; Zhang, D.-J.; Damiens, D.D.; Lees, R.S.; Gilles, J.R. Standard operating procedures for standardized mass rearing of the dengue and chikungunya vectors Aedes aegypti and Aedes albopictus (Diptera: Culicidae)-II-Egg storage and hatching. Parasit Vectors. 2015, 8, 1–7. [Google Scholar]
- Morlan, H.B.; Hayes, R.O.; Schoof, H.F. Methods for mass rearing of Aedes aegypti (L.). Public Health Rep. 1963, 78, 711. [Google Scholar] [CrossRef]
- Ali, A.; Wang, Y.-H.; Khan, I.A. Larvicidal and biting deterrent activity of essential oils of Curcuma longa, ar-turmerone, and curcuminoids against Aedes aegypti and Anopheles quadrimaculatus (Culicidae: Diptera). J. Med. Entomol. 2015, 52, 979–986. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Med. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Litchfield, J.j.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar]
- Hassan, E.M.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M.; Elshamy, A.I.; Farag, M.A.; Alamery, S.F.; Omer, E.A. Comparative chemical profiles of the essential oils from different varieties of Psidium guajava L. Molecules 2021, 26, 119. [Google Scholar] [CrossRef]
- Nezhadasad Aghbash, B.; Dehghan, G.; Movafeghi, A.; Talebpour, A.H.; Pouresmaeil, M.; Maggi, F.; Sabzi Nojadeh, M. Chemical compositions and biological activity of essential oils from four populations of Satureja macrantha CA Mey. J. Essent. Oil. Res. 2021, 33, 1–10. [Google Scholar] [CrossRef]
- Al-Sarar, A. Chemical Composition, Adulticidal and Repellent Activity of Essential Oils From Mentha longifolia L. and Lavandula dentata L. against Culex pipiens L. Plant. Prot. Pathol. 2014, 5, 817–826. [Google Scholar] [CrossRef]
- Saljoqi, A.U.R.; Afridi, M.K.; Khan, S.A. Effects of six plant extracts on rice weevil Sitophilus oryzae L. in the stored wheat grains. J. Agric. Biol Sci. 2006, 1, 1–5. [Google Scholar]
- Odeyemi, O.; Masika, P.; Afolayan, A. Insecticidal activities of essential oil from the leaves of Mentha longifolia L. subsp. capensis against Sitophilus zeamais (Motschulsky)(Coleoptera: Curculionidae). Afr. Entomol. 2008, 16, 220–225. [Google Scholar] [CrossRef]
- Motazedian, N.; Ravan, S.; Bandani, A. Toxicity and repellency effects of three essential oils against T etranychus urticae Koch (Acari: Tetranychidae). J. Agric. Sci Technol. Health Care. 2012, 14, 275–284. [Google Scholar]
- Koc, S.; Oz, E.; Cetin, H. Repellent activities of some Labiatae plant essential oils against the saltmarsh mosquito Ochlerotatus caspius (Pallas, 1771)(Diptera: Culicidae). Parasitol Res. 2012, 110, 2205–2209. [Google Scholar] [CrossRef]
- Saeidi, M.; Moharramipour, S. Insecticidal and repellent activities of Artemisia khorassanica, Rosmarinus officinalis and Mentha longifolia essential oils on Tribolium confusum. J. Crop. Prot. 2013, 2, 23–31. [Google Scholar]
- Koliopoulos, G.; Pitarokili, D.; Kioulos, E.; Michaelakis, A.; Tzakou, O. Chemical composition and larvicidal evaluation of Mentha, Salvia, and Melissa essential oils against the West Nile virus mosquito Culex pipiens. Parasitol. Res. 2010, 107, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Prajapati, V.; Ahmad, A.; Aggarwal, K.K.; Khanuja, S.P. Piperitenone oxide as toxic, repellent, and reproduction retardant toward malarial vector Anopheles stephensi (Diptera: Anophelinae). J. Med. Entomol. 2004, 41, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Giatropoulos, A.; Kimbaris, A.; Michaelakis, A.; Papachristos, D.P.; Polissiou, M.G.; Emmanouel, N. Chemical composition and assessment of larvicidal and repellent capacity of 14 Lamiaceae essential oils against Aedes albopictus. Parasitol. Res. 2018, 117, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Kumar, A.; Tewari, K.S. Chemical composition and mosquito repellent activity of the essential oil of Plectranthus incanus link. Facta Univ. Ser. Phys. Chem. Technol. 2011, 9, 57–64. [Google Scholar] [CrossRef]
- Binder, R.G.; Chan, B.G.; Elliger, C.A. Antibiotic Effects of C10–C12 Fatty Acid Esters on Pink Bollworm, Bollworm and Tobacco Budworm. Agric. Biol. Chem. 1979, 43, 2467–2471. [Google Scholar] [CrossRef][Green Version]
- Hoi, T.M.; Huong, L.T.; Chinh, H.V.; Hau, D.V.; Satyal, P.; Tai, T.A.; Dai, D.N.; Hung, N.H.; Hien, V.T.; Setzer, W.N. Essential oil compositions of three invasive Conyza species collected in Vietnam and their larvicidal activities against Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus. Molecules 2020, 25, 4576. [Google Scholar] [CrossRef]
- Brari, J.; Kumar, V. Insecticidal efficacy of essential oils from Artemisia maritima L. and Zanthoxylum armatum DC. and their two major constituents against Plodia interpunctella (Hubner). Int. J. Life Sci. Res. 2021, 9, 71–79. [Google Scholar]
- Hieu, T.T.; Kim, S.I.; Kwon, H.W.; Ahn, Y.J.J.P.m.s. Enhanced repellency of binary mixtures of Zanthoxylum piperitum pericarp steam distillate or Zanthoxylum armatum seed oil constituents and Calophyllum inophyllum nut oil and their aerosols to Stomoxys calcitrans. Pest. Manage. Sci. 2010, 66, 1191–1198. [Google Scholar] [CrossRef]
- Singh, A.; Dhami, A.; Palariya, D.; Prakash, O.; Kumar, R.; Kumar, R.; Pant, A. Methyl nonyl ketone and linalool rich essential oils from three accessions of Zanthoxylum armatum (DC.) and their biological activities. Int. J. Herb. Med. 2019, 7, 20–28. [Google Scholar]
- Schlyter, F.; Smitt, O.; Sjödin, K.; Högberg, H.E.; Löfqvist, J. Carvone and less volatile analogues as repellent and deterrent antifeedants against the pine weevil, Hylobius abietis. J. Appl. Entomol. 2004, 128, 610–619. [Google Scholar] [CrossRef]
- Frank, T.; Biert, K.; Speiser, B. Feeding deterrent effect of carvone, a compound from caraway seeds, on the slug Arion lusitanicus. Ann. Appl. Biol. 2002, 141, 93–100. [Google Scholar] [CrossRef]
- Obeng-Ofori, D.; Reichmuth, C.; Bekele, A.; Hassanali, A. Toxicity and protectant potential of camphor, a major component of essential oil of Ocimum kilimandscharicum, against four stored product beetles. Int. J. Pest. Manag. 1998, 44, 203–209. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Guo, S.-S.; Cao, J.-Q.; Pang, X.; Geng, Z.-F.; Wang, Y.; Zhang, Z.; Du, S.-S. Insecticidal and repellent activity of essential oil from Amomum villosum Lour. and its main compounds against two stored-product insects. Int. J. Food Prop. 2018, 21, 2265–2275. [Google Scholar] [CrossRef]
- Lu, J.; Wu, S. Bioactivity of essential oil from Ailanthus altissima bark against 4 major stored-grain insects. Afr. J. Microbiol. Res. 2010, 4, 154–157. [Google Scholar]
- Haroon, M.; Maduka, U.; Sujarajini, V. Insecticidal effects of plant extracts of some medicinal plants against Sitophilus zeamaise mostchulsky on stored maize. In Abstracts of the 7th Annual Science Research Sessions (ASRS); Faculty of Applied Science, South Eastern University of Sri Lanka: Oluvil, Sri Lanka, 2018. [Google Scholar]
- El Ayeb-Zakhama, A.; Ben Salem, S.; Sakka-Rouis, L.; Flamini, G.; Ben Jannet, H.; Harzallah-Skhiri, F. Chemical composition and phytotoxic effects of essential oils obtained from Ailanthus altissima (Mill.) swingle cultivated in Tunisia. Chem. Biodivers 2014, 11, 1216–1227. [Google Scholar] [CrossRef]
- Kozuharova, E.; Benbassat, N.; Berkov, S.; Ionkova, I. Ailanthus altissima and Amorpha fruticosa–invasive arboreal alien plants as cheap sources of valuable essential oils. Pharmacia 2020, 67, 71. [Google Scholar] [CrossRef]
- Khani, A.; Ordouni, F.; Sahebzadeh, N.J.E.a.B. Qualitative phytochemical screening and mortality effect of ethanolic extract of Salsola imbricata on Aphis gossypii. Exp. Anim. Biol. 2018, 7, 89–96. [Google Scholar]
- Wallace, J.R.; Wylie, C.D.; Wagner, R.L. Plant extract efficacy on mosquito mortality: Preliminary studies on the effect of Ailanthus altissima extract on adult Aedes aegypti and Culex quinquefasciatus. Great Lakes Entomol. 2021, 54, 8. [Google Scholar]
- Khani, A.; Asghari, J. Insecticide activity of essential oils of Mentha longifolia, Pulicaria gnaphalodes and Achillea wilhelmsii against two stored product pests, the flour beetle, Tribolium castaneum, and the cowpea weevil, Callosobruchus maculatus. J. Insect Sci. 2012, 12, 73. [Google Scholar] [CrossRef]
- Tiwary, M.; Naik, S.; Tewary, D.K.; Mittal, P.; Yadav, S.J.J.o.v.b.d. Chemical composition and larvicidal activities of the essential oil of Zanthoxylum armatum DC (Rutaceae) against three mosquito vectors. J. Vector. Borne Dis. 2007, 44, 198. [Google Scholar]
- Zhang, W.; Wang, Y.; Geng, Z.; Guo, S.; Cao, J.; Zhang, Z.; Pang, X.; Chen, Z.; Du, S.; Deng, Z. Antifeedant activities of lignans from stem bark of Zanthoxylum armatum DC. against Tribolium castaneum. Molecules 2018, 23, 617. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-F.; Zhang, W.-J.; You, C.-X.; Guo, S.-S.; Geng, Z.-F.; Fan, L.; Du, S.-S.; Deng, Z.-W.; Wang, Y.-Y. Insecticidal constituents of essential oil derived from Zanthoxylum armatum against two stored-product insects. J. Oleo Sci. 2015, 64, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Abutaha, N.; Al-Mekhlafi, F.A.; Al-Keridis, L.A.; Farooq, M.; Nasr, F.A.; Al-Wadaan, M. Larvicidal potency of selected xerophytic plant extracts on Culex pipiens (Diptera: Culicidae). Entomol. Res. 2018, 48, 362–371. [Google Scholar]


| Scientific Name | Family | Part Used | Collection Coordinates | Elevation (m) | Yield (%) |
|---|---|---|---|---|---|
| Mentha longifolia | Lamiaceae | Leaves, stems | 34°11′48.9″ N 73°14′51.7″ E | 1236 | 0.36 |
| Salsola imbricata | Amaranthaceae | Leaves, stems | 30°15′59.6″ N 71°30′40.2″ E | 124 | 0.01 |
| Erigeron bonariensis | Asteraceae | Leaves, stems | 30°16′13.5″ N 71°30′44.6″ E | 124 | 0.21 |
| Erigeron canadensis | Asteraceae | Leaves, stems | 30°15′52.9″ N 71°30′04.0″ E | 1183 | 0.21 |
| Ailanthus altissima | Simaroubaceae | Leaves, stems | 30°15′52.9″ N 71°30′04.0″ E | 124 | 0.04 |
| Zanthoxylum armatum | Rutaceae | Leaves | 34°11′48.4″ N 73°15′43.0″ E | 1370 | 0.76 |
| Essential Oils | Exposure Time | LC50 (mg/L) | 95% CI (mg/L) | LC90 (mg/L) | 95% CI (mg/L) | Slope ± SE | Chi-Square (df) |
|---|---|---|---|---|---|---|---|
| M. longifolia | 24 h | 46.7 a | 30.41–73.39 | 95.9a | 61.87–169.2 | 6.04 ± 0.86 | 3.28 (5) |
| S. imbricata | 132.3 b | 83.94–208.4 | 294.0 b | 187.8–516.75 | 7.83 ± 1.10 | 0.31 (5) | |
| E. bonariensis | 28.5 a | 18.30–44.30 | 63.3a | 40.87–110.3 | 5.37 ± 0.49 | 0.33 (5) | |
| E. canadensis | 37.9 a | 23.92–59.97 | 84.2 a | 53.53–148.7 | 5.83 ± 0.79 | 0.34 (5) | |
| A. altissima | 356.1 c | 231.3–551.2 | 791.4 b | 513.62–1376 | 9.42 ± 1.29 | 0.99 (5) | |
| Z. armatum | 46.5 a | 29.99–72.95 | 103.3 a | 66.33–183.03 | 6.16 ± 0.88 | 4.30 (5) | |
| M. longifolia | 48 h | 39.3 a | 20.6–71.6 | 120.0 a | 67–79 | 2.64 ± 0.30 | 5.94 (5) |
| S. imbricata | 124.2 b | 106–145 | 223.9 b | 185–304 | 5.00 ± 0.78 | 2.41 (5) | |
| E. bonariensis | 26.1 a | 21–31.61 | 63.6 a | 49–95 | 3.30 ± 0.40 | 2.41 (5) | |
| E. canadensis | 35.7 a | 29.9–41.8 | 63.9 a | 52–90 | 5.07 ± 0.90 | 0.64 (5) | |
| A. altissima | 333.6 c | 161–631 | 909.5 c | 517–7792 | 2.94 ± 0.40 | 7.20 (5) | |
| Z. armatum | 35.9 a | 18.6–74.7 | 97.6 a | 59–321 | 2.95 ± 0.30 | 14.0 (5) |
| RI | Compound | CAS Number | M. longifolia | S. imbricata | E. bonariensis | E. canadensis | A. altissima |
|---|---|---|---|---|---|---|---|
| 929 | α-Pinene * | 80-56-8 | 0.4 | 1.1 | |||
| 970 | Sabinene | 3387-41-5 | 0.3 | 0.6 | 1.1 | ||
| 972 | β-Pinene * | 127-91-3 | 0.5 | ||||
| 990 | β-Myrcene * | 123-35-3 | 2.0 | 0.7 | 0.1 | 0.7 | tr |
| 1003 | α-Phellandrene | 99-83-2 | 0.2 | 0.1 | |||
| 1008 | 3-Carene | 13466-78-9 | 0.3 | ||||
| 1026 | Limonene * | 138-86-3 | 4.6 | 2.2 | 2.1 | 28.4 | 0.5 |
| 1028 | Eucalyptol * | 470-82-6 | 1.1 | 2.3 | 0.5 | 1.7 | |
| 1038 | cis-β-Ocimene * | 3338-55-4 | 0.5 | ||||
| 1047 | trans-β-Ocimene * | 3779-61-1 | 0.1 | 0.8 | 5.0 | ||
| 1056 | γ-Terpinene | 99-85-4 | tr | 0.2 | 0.1 | ||
| 1086 | Terpinolene | 586-62-9 | 0.1 | 0.1 | 0.1 | ||
| 1099 | Linalool * | 78-70-6 | 0.3 | 0.2 | 0.3 | ||
| 1103 | Nonanal | 124-19-6 | 0.5 | 0.5 | |||
| 1141 | Camphor * | 76-22-2 | 0.1 | 20.4 | tr | 0.1 | |
| 1163 | Borneol * | 507-70-0 | 0.2 | 1.4 | 0.1 | ||
| 1175 | 4-Terpineol | 562-74-3 | 0.8 | tr | 0.1 | ||
| 1189 | α-Terpineol | 98-55-5 | 0.1 | 0.3 | tr | 0.1 | |
| 1194 | cis-Dihydrocarvone | 3792-53-8 | 1.1 | ||||
| 1237 | Pulegone * | 89-82-7 | 4.1 | ||||
| 1242 | Carvone * | 99-49-0 | tr | 39.9 | 0.1 | 0.5 | |
| 1252 | Piperitone | 89-81-6 | 6.9 | 0.1 | |||
| 1260 | Piperitone oxide | 57130-28-6 | 45.5 | ||||
| 1272 | Isopiperitenone | 529-01-1 | 0.2 | ||||
| 1285 | Bornyl acetate | 76-49-3 | 0.2 | ||||
| 1290 | Piperitenone oxide # | 2.6 | |||||
| 1293 | Thymol * | 89-83-8 | 0.2 | 0.2 | tr | 0.9 | |
| 1298 | 2-Hydroxypiperitone | 490-03-9 | 0.4 | 0.2 | 0.3 | ||
| 1312 | 2-Methoxy-4-vinylphenol | 7786-61-0 | 2.1 | 0.2 | |||
| 1337 | δ-Elemene | 20307-84-0 | 0.2 | ||||
| 1342 | α-Guaiene | 3691-12-1 | 0.2 | ||||
| 1349 | α-Cubebene | 17699-14-8 | 0.1 | 0.2 | 0.2 | ||
| 1358 | Eugenol * | 97-53-0 | 0.2 | 29.9 | |||
| 1378 | Piperitenone oxide * | 35178-55-3 | 30.1 | 0.3 | |||
| 1389 | β-Cubebene | 13744-15-5 | 0.2 | 0.2 | |||
| 1391 | β-Elemen | 515-13-9 | 0.1 | 0.2 | 0.2 | 0.4 | |
| 1404 | Methyleugenol | 93-15-2 | 0.6 | 20.3 | |||
| 1414 | p-Menthane-1,2,3-triol | 22555-61-9 | 3.6 | ||||
| 1418 | trans-β-Caryophyllene * | 87-44-5 | 1.8 | 0.3 | 1.9 | 0.7 | 2.8 |
| 1436 | trans-α-Bergamotene | 13474-59-4 | 0.2 | 3.6 | |||
| 1452 | α-Humulene | 6753-98-6 | 1.1 | 0.3 | 0.6 | ||
| 1458 | trans-β-Farnesene | 18794-84-8 | 10.2 | 2.5 | |||
| 1476 | γ-Muurolene | 30021-74-0 | 0.2 | 0.2 | |||
| 1480 | Germacrene D | 23986-74-5 | 0.3 | 4.6 | 6.4 | 6.5 | |
| 1485 | β-Selinene | 17066-67-0 | 3.9 | 0.2 | |||
| 1496 | Capilline | 520-74-1 | 3.7 | 0.6 | 23.7 | ||
| 1508 | trans-α-Farnesene | 502-61-4 | 0.9 | 0.3 | 1.3 | ||
| 1516 | cis-Lachnophyllum ester | 505-01-1 | 0.1 | 24.9 | 16.3 | ||
| 1523 | δ-Cadinene | 483-76-1 | 0.4 | ||||
| 1526 | Matricaria ester | 505-02-2 | 43.1 | 31.7 | |||
| 1549 | Hedycaryol | 21657-90-9 | 2.6 | ||||
| 1563 | Nerolidol | 142-50-7 | 0.3 | 0.3 | |||
| 1576 | Spathulenol | 77171-55-2 | 0.3 | 2.0 | tr | 0.4 | |
| 1582 | Caryophyllene oxide | 1139-30-6 | 0.4 | 0.3 | |||
| 1646 | α-Eudesmol | 473-16-5 | 0.3 | 1.2 | |||
| 1654 | Juniper camphor | 473-04-1 | 3.5 | ||||
| 1715 | Pentadecanal | 2765-11-9 | 0.4 | 0.2 | |||
| 1817 | Hexadecanal | 629-80-1 | 1.9 | ||||
| Total Identified | 98.7 | 94.4 | 98.2 | 98.8 | 95.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, M.G.; Haris, A.; Binyameen, M.; Nazir, A.; Mozūratis, R.; Azeem, M. Chemical Composition, Larvicidal and Repellent Activities of Wild Plant Essential Oils against Aedes aegypti. Biology 2023, 12, 8. https://doi.org/10.3390/biology12010008
Abbas MG, Haris A, Binyameen M, Nazir A, Mozūratis R, Azeem M. Chemical Composition, Larvicidal and Repellent Activities of Wild Plant Essential Oils against Aedes aegypti. Biology. 2023; 12(1):8. https://doi.org/10.3390/biology12010008
Chicago/Turabian StyleAbbas, Muhammad Ghazanfar, Abdullah Haris, Muhammad Binyameen, Abdul Nazir, Raimondas Mozūratis, and Muhammad Azeem. 2023. "Chemical Composition, Larvicidal and Repellent Activities of Wild Plant Essential Oils against Aedes aegypti" Biology 12, no. 1: 8. https://doi.org/10.3390/biology12010008
APA StyleAbbas, M. G., Haris, A., Binyameen, M., Nazir, A., Mozūratis, R., & Azeem, M. (2023). Chemical Composition, Larvicidal and Repellent Activities of Wild Plant Essential Oils against Aedes aegypti. Biology, 12(1), 8. https://doi.org/10.3390/biology12010008