Purification and Properties of Polyphenol Oxidase of Dried Volvariella bombycina
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. Chemicals
2.3. Preparation of Crude PPO Extract
2.4. Purification of PPO from Dried V. bombycina
2.4.1. Precipitation with Ammonium Sulfate
2.4.2. Dialysis
2.4.3. Affinity-Column Chromatography of PPO
2.4.4. Determination of Protein Concentration
2.4.5. Determination of Dried V. bombycina PPO Activity
2.5. Physicochemical Properties of PPO
2.5.1. Determination of Dried V. bombycina PPO Substrate Specificity
2.5.2. Effects of pH on Dried V. bombycina PPO Activity
2.5.3. Effects of Temperature on Dried V. bombycina PPO Activity
2.5.4. Electrophoresis
2.5.5. Kinetic Properties of Dried V. bombycina PPO
3. Results and Discussion
3.1. Purification Profile of PPO of Dried V. bombycina
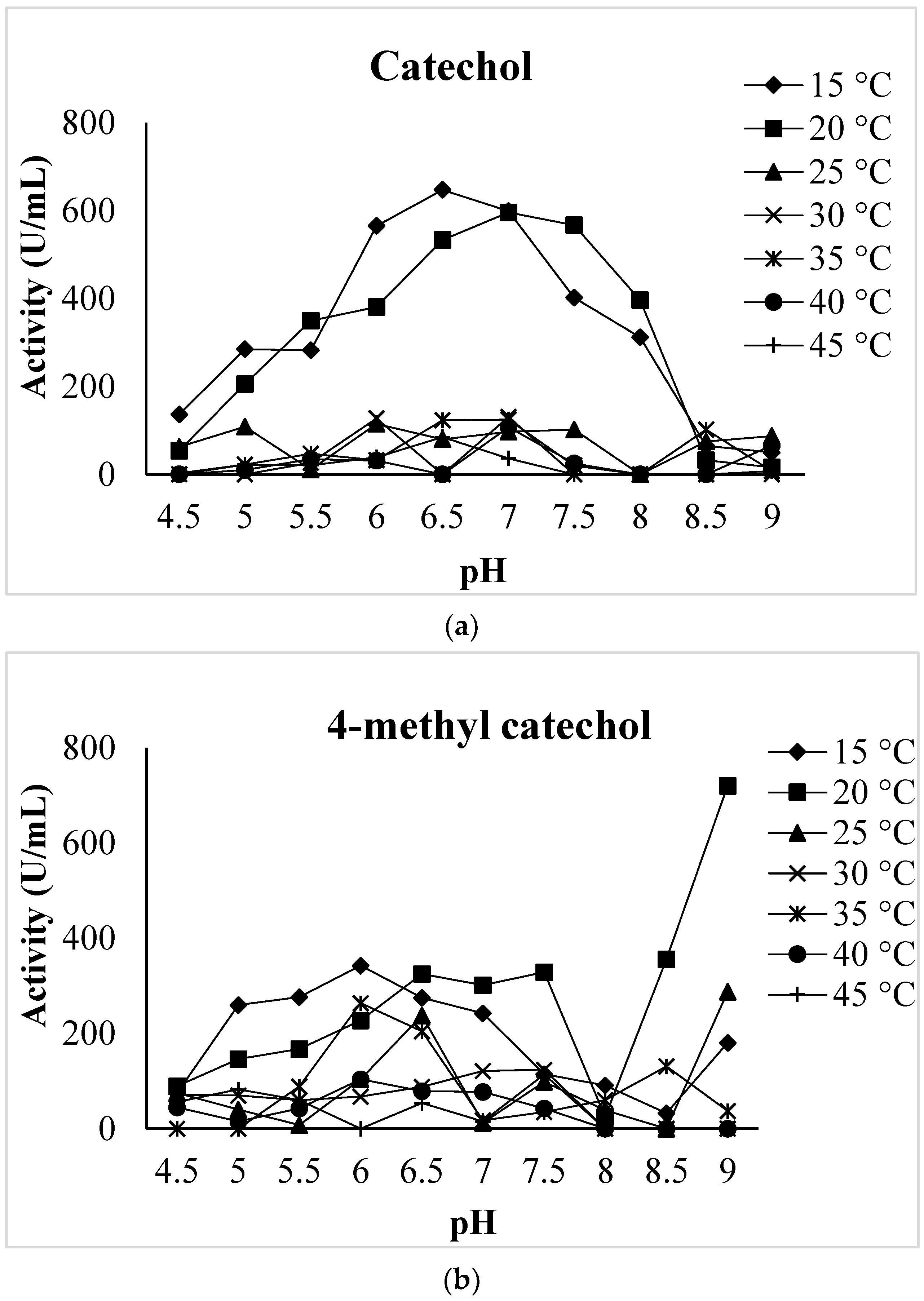
3.2. Effects of pH and Temperature on Dried V. bombycina PPO Activity
3.3. Kinetic Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wasser, S.P.; Weis, A.L. Medicinal Properties of Substances Occurring in Higher Basidiomycetes Mushrooms: Current Perspectives (Review). Int. J. Med. Mushrooms 1999, 1, 31–62. [Google Scholar] [CrossRef]
- Chang, S.-T. World Production of Cultivated Edible and Medicinal Mushrooms in 1997 with Emphasis on Lentinus edodes (Berk.) Sing, in China. Int. J. Med. Mushrooms 1999, 1, 291–301. [Google Scholar] [CrossRef]
- Yun, W.; Hall, I.R. Edible Ectomycorrhizal Mushrooms: Challenges and Achievements. Can. J. Bot. 2004, 82, 1063–1073. [Google Scholar] [CrossRef]
- Kalač, P. A Review of Chemical Composition and Nutritional Value of Wild-Growing and Cultivated Mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, J.; Chen, H.; Liu, X.; Cheng, W.; Ma, X.; Tong, P. Purification and Characterization of Polyphenol Oxidase from Agaricus bisporus. Int. J. Food Prop. 2013, 16, 1483–1493. [Google Scholar] [CrossRef]
- Chiu, S.W.; Moore, D. Development of the Basidiome of Volvariella bombycina. Mycol. Res. 1990, 94, 327–337. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Shrestha, M.; Poudel, B.H.; Adhikari, M.K. In Vitro Cultivation of Newly Reported Wild Edible Mushroom Volvariella bomybycina from Nepal. Nepal J. Biotechnol. 2017, 5, 27–31. [Google Scholar] [CrossRef][Green Version]
- Kim, J.H.; Kim, H.W.; Kim, J.W.; Choi, E.C.; Kim, B.K. Studies on Constituents of the Higher Fungi of Korea (LI). Antitumor Components Extracted from the Cultured Mycelia of Volvariella bombycina. J. Korean Cancer Res. Assoc. 1985, 17, 205–216. [Google Scholar]
- Badalyan, S.M. Edible and Medicinal Higher Basidiomycetes Mushrooms as a Source of Natural Antioxidants. Int. J. Med. Mushrooms 2003, 5, 153–162. [Google Scholar] [CrossRef]
- Xu, G.-H.; Choo, S.-J.; Kim, Y.-H.; Ryoo, I.-J.; Seok, S.-J.; Ahn, J.-S.; Yoo, I.-D. Secondary Metabolites of Volvariella bombycina and Their Inhibitory Effects on Melanogenesis. J. Microbiol. Biotechnol. 2010, 20, 78–81. [Google Scholar] [CrossRef]
- Karnan, M.; Tamilkani, P.; Senthilkumar, G.; Vijayalakshmi, S.; Panneerselvam, A. Volvariella bombycina of Tamil Nadu. Int. J. Inf. Res. Rev. 2016, 3, 2175–2178. [Google Scholar]
- Badalyan, C.M.; Gasparyan, A.V.; Garibyan, N.G. Investigation of the Antioxidant Activity of Some Basidial Macromycetes. Mikologiya i Fitopatologiya 2003, 37, 63–68. [Google Scholar]
- Xu, G.-H.; Kim, Y.-H.; Choo, S.-J.; Ryoo, I.-J.; Zheng, C.-J.; Seok, S.-J.; Kim, W.-G.; Yoo, I.-D. Isodeoxyhelicobasidin, a Novel Human Neutrophil Elastase Inhibitor from the Culture Broth of Volvariella bombycina. J. Antibiot. 2009, 62, 333–334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karnan, M.; Tamilkani, P.; Senthilkumar, G.; Vijayalakshmi, S.; Panneerselvam, A. Cultivation, Nutrition, Biochemicals and Enzyme Analysis of Paddy Straw. Int. J. Curr. Res. 2016, 8, 27303–27308. [Google Scholar]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.; Pavan, S.; Montemurro, C. Polyphenol Oxidases in Crops: Biochemical, Physiological and Genetic Aspects. Int. J. Med. Mushrooms 2017, 18, 377. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M. Polyphenol Oxidases in Plants and Fungi: Going Places? A Review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef]
- Yuan, Q.; Guo, H.; Ding, J.; Jiao, C.; Qi, Y.; Zafar, H.; Ma, X.; Raza, F.; Han, J. Polyphenol Oxidase as a Promising Alternative Therapeutic Agent for Cancer Therapy. Molecules 2022, 27, 1515. [Google Scholar] [CrossRef]
- Zaidi, K.U.; Ali, A.S.; Ali, S.A. Purification and Characterization of Melanogenic Enzyme Tyrosinase from Button Mushroom. Enzym. Res. 2014, 2014, 120739. [Google Scholar] [CrossRef]
- del Marmol, V.; Beermann, F. Tyrosinase and Related Proteins in Mammalian Pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Wichers, H.J.; Gerritsen, Y.A.M.; Chapelon, C.G.J. Tyrosinase Isoforms from the Fruitbodies of Agaricus bisporus. Phytochemistry 1996, 43, 333–337. [Google Scholar] [CrossRef]
- Espín, J.C.; Morales, M.; García-Ruiz, P.A.; Tudela, J.; García-Cánovas, F. Improvement of a Continuous Spectrophotometric Method for Determining the Monophenolase and Diphenolase Activities of Mushroom Polyphenol Oxidase. J. Agric. Food Chem. 1997, 45, 1084–1090. [Google Scholar] [CrossRef]
- Seo, S.-Y.; Sharma, V.K.; Sharma, N. Mushroom Tyrosinase: Recent Prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Flurkey, W.H. Purification and Characterization of Tyrosinase from Gill Tissue of Portabella Mushrooms. Phytochemistry 2004, 65, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, Ş.; Yemenicioğlu, A. Partial Purification and Kinetic Characterization of Mushroom Stem Polyphenoloxidase and Determination of Its Storage Stability in Different Lyophilized Forms. Process Biochem. 2007, 42, 943–950. [Google Scholar] [CrossRef][Green Version]
- Gouzi, H.; Depagne, C.; Coradin, T. Kinetics and Thermodynamics of the Thermal Inactivation of Polyphenol Oxidase in an Aqueous Extract from Agaricus bisporus. J. Agric. Food Chem. 2012, 60, 500–506. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, S.; Kaur, S.; Sodhi, H. Biochemical Characterization with Kinetic Studies of Melanogenic Enzyme Tyrosinase from White Button Mushroom, Agaricus bisporus. Indian J. Biochem. Biophys. 2022, 59, 718–725. [Google Scholar] [CrossRef]
- Mueller, L.A.; Hinz, U.; Zrÿd, J.-P. Characterization of a Tyrosinase from Amanita Muscaria Involved in Betalain Biosynthesis. Phytochemistry 1996, 42, 1511–1515. [Google Scholar] [CrossRef]
- Colak, A.; Sahin, E.; Yildirim, M.; Sesli, E. Polyphenol Oxidase Potentials of Three Wild Mushroom Species Harvested from Lişer High Plateau, Trabzon. Food Chem. 2007, 103, 1426–1433. [Google Scholar] [CrossRef]
- Li, T.; Zhang, N.; Yan, S.; Jiang, S.; Yin, H. A Novel Tyrosinase from Armillaria Ostoyae with Comparable Monophenolase and Diphenolase Activities Suffers Substrate Inhibition. Appl. Environ. Microbiol. 2021, 87, e00275-21. [Google Scholar] [CrossRef]
- Özel, A.; Colak, A.; Arslan, O.; Yildirim, M. Purification and Characterisation of a Polyphenol Oxidase from Boletus erythropus and Investigation of Its Catalytic Efficiency in Selected Organic Solvents. Food Chem. 2010, 119, 1044–1049. [Google Scholar] [CrossRef]
- Tsivinska, M.V.; Antonyuk, V.O.; Stoika, R.S. Isolation and Properties of Polyphenol Oxidase from Basidiocarps of Lactarius pergamenus Fr. (Fr.) Fungi. Ukr. Biochem. J. 2015, 87, 56–65. [Google Scholar] [CrossRef][Green Version]
- Öz, F.; Colak, A.; Özel, A.; Sağlam Ertunga, N.; Sesli, E. Purification and Characterization of a Mushroom Polyphenol Oxidase and Its Activity in Organic Solvents. J. Food Biochem. 2013, 37, 36–44. [Google Scholar] [CrossRef]
- Dedeoglu, N.; Guler, O.O. Differential in Vitro Inhibition of Polyphenoloxidase from a Wild Edible Mushroom Lactarius salmonicolor. J. Enzym. Inhib. Med. Chem. 2009, 24, 464–470. [Google Scholar] [CrossRef] [PubMed]
- de Faria, R.O.; Moure, V.R.; Balmant, W. The Tyrosinase Produced by Lentinula boryana (Berk. & Mont.) Pegler Suffers Substrate Inhibition by L-DOPA. Food Technol. Biotechnol. 2007, 45, 334–340. [Google Scholar]
- Kanda, K.; Sato, T.; Ishii, S.; Enei, H.; Ejiri, S. Purification and Properties of Tyrosinase Isozymes from the Gill of Lentinus edodes Fruiting Body. Biosci. Biotechnol. Biochem. 1996, 60, 1273–1278. [Google Scholar] [CrossRef]
- Li, Y.; Ding, S.; Yang, J. Extraction of Polyphenol Oxidase in Shiitake Mushrooms (Lentinus edodes) and Its Enzymatic Characteristics. J. Res. Agric. Anim. Sci. 2019, 6, 30–34. [Google Scholar]
- Saki, N.; Akin, M.; Alici, E.H.; Arabaci, G. Partial Purification and Characterization of Polyphenol Oxidase from the Wild Edible Mushroom Lepiota procera Using Three-Phase Partitioning. Int. J. Food Eng. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Kolcuoğlu, Y. Purification and Comparative Characterization of Monophenolase and Diphenolase Activities from a Wild Edible Mushroom (Macrolepiota gracilenta). Process Biochem. 2012, 47, 2449–2454. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, D.; Sharma, A.; Bansal, S. Thermo Stable Tyrosinase Purified from Pleurotus djamor Grown in Biomimetic Calcium Carbonate: A Biological Strategy to Industrial Waste Remediation. Environ. Technol. Innov. 2021, 21, 101294. [Google Scholar] [CrossRef]
- Murniati, A.; Buchari, B.; Gandasasmita, S.; Nurachman, Z.; Nurhanifah, N. Characterization of Polyphenol Oxidase Application as Phenol Removal in Extracts of Rejected White Oyster Mushrooms (Pleurotus ostreatus). Orient. J. Chem. 2018, 34, 1457–1468. [Google Scholar] [CrossRef]
- Keskin, S.; Ertunga, N.S.; Colak, A.; Akatin, M.Y.; Özel, A.; Kolcuoglu, Y. Characterization of a Polyphenol Oxidase Having Monophenolase and Diphenolase Activities from a Wild Edible Mushroom, Russula delica. Asian J. Chem. 2012, 24, 1203–1208. [Google Scholar]
- Ahlawat, O.P.; Gupta, P.; Kamal, S.; Dhar, B.L. Development of Molecular and Biochemical Markers for Selecting a Potential High Yielding Strain of Paddy Straw Mushroom (Volvariella volvacea). J. Plant Biochem. Biotechnol. 2008, 17, 57–63. [Google Scholar] [CrossRef]
- Shaffer, R.L. Volvariella in North America. Mycologia 1957, 49, 545–549. [Google Scholar] [CrossRef]
- Ajana, M.; Ouabbou, A.; El kholfy, S.; Nmichi, A.; Ouazzani Touhami, A.; Benkirane, R.; Douira, A. Some New Observations on the Volvariella Genus Speg. 1898. Int. J. Environ. Agric. Biotechnol. 2017, 2, 940–945. [Google Scholar]
- Arslan, O.; Erzengin, M.; Sinan, S.; Ozensoy, O. Purification of Mulberry (Morus alba L.) Polyphenol Oxidase by Affinity Chromatography and Investigation of Its Kinetic and Electrophoretic Properties. Food Chem. 2004, 88, 479–484. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Lineweaver, H.; Burk, D. The Determination of Enzyme Dissociation Constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Panadare, D.; Rathod, V.K. Extraction and Purification of Polyphenol Oxidase: A Review. Biocatal. Agric. Biotechnol. 2018, 14, 431–437. [Google Scholar] [CrossRef]
- Kaya, E.D.; Bağci, O. Purification and Biochemical Characterization of Polyphenol Oxidase Extracted from Kirmizi Kismis Grape (Vitis vinifera L.). J. Food Biochem. 2021, 45, e13627. [Google Scholar] [CrossRef] [PubMed]
- Kawamura-Konishi, Y.; Tsuji, M.; Hatana, S.; Asanuma, M.; Kakuta, D.; Kawano, T.; Mukouyama, E.B.; Goto, H.; Suzuki, H. Purification, Characterization, and Molecular Cloning of Tyrosinase from Pholiota nameko. Biosci. Biotechnol. Biochem. 2007, 71, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, M.; Mattera, M. Polyphenoloxidase Activity and Polyphenol Levels in Organically and Conventionally Grown Peach (Prunus persica L., cv. Regina Bianca) and Pear (Pyrus communis L., cv. Williams). Food Chem. 2001, 72, 419–424. [Google Scholar] [CrossRef]
- Amiot, M.J.; Tacchini, M.; Aubert, S.Y.; Oleszek, W. Influence of Cultivar, Maturity Stage, and Storage Conditions on Phenolic Composition and Enzymic Browning of Pear Fruits. J. Agric. Food Chem. 1995, 43, 1132–1137. [Google Scholar] [CrossRef]
- Zhang, X.; Flurkey, W.H. Phenoloxidases in Portabella Mushrooms. J. Food Sci. 1997, 62, 97–100. [Google Scholar] [CrossRef]
- Gouzi, H.; Benmansour, A. Partial Purification and Characterization of Polyphenol Oxidase Extracted from Agaricus bisporus (J.E.Lange) Imbach. Int. J. Chem. React. Eng. 2007, 5, 1–11. [Google Scholar] [CrossRef]
- Yoruk, R.; Marshall, M.R. Physicochemical Properties and Function of Plant Polyphenol Oxidase: A Review1. J. Food Biochem. 2003, 27, 361–422. [Google Scholar] [CrossRef]
- Mazzafera, P.; Robinson, S.P. Characterization of Polyphenol Oxidase in Coffee. Phytochemistry 2000, 55, 285–296. [Google Scholar] [CrossRef]
- García-Molina, F.; Peñalver, M.J.; Fenoll, L.G.; Rodríguez-López, J.N.; Varón, R.; García-Cánovas, F.; Tudela, J. Kinetic Study of Monophenol and O-Diphenol Binding to Oxytyrosinase. J. Mol. Catal. B Enzym. 2005, 32, 185–192. [Google Scholar] [CrossRef]




| Purification Steps | Volume (mL) | Activity (U/mL) | Total Activity | Total Protein (mg) | Specific Activity (U/mg Protein) | Purification Fold |
|---|---|---|---|---|---|---|
| Crude Extract | 36 | 616.25 | 22,185.00 | 13.0138 | 1704.73 | |
| Ammonium sulfate precipitation | 10 | 488.75 | 4887.50 | 9.7475 | 501.41 | 0.29 |
| Dialysis | 10 | 1290.00 | 12,900.00 | 8.5905 | 1501.65 | 0.88 |
| Affinity chromatography | 2 | 275.00 | 550.00 | 0.0095 | 57,698.99 | 33.85 |
| Substrates | Optimum pH | Optimum Temperature (°C) | Activity (U/mL) |
|---|---|---|---|
| Catechol | 6.5 | 15 | 647.50 |
| 4-methyl catechol | 9.0 | 20 | 720.00 |
| Pyrogallol | 8.0 | 15 | 26,692.50 |
| Substrates | Km (mM) | Vmax (U/mL) | Vmax/Km |
|---|---|---|---|
| Catechol | 1.67 | 833.33 | 500.00 |
| 4-methyl catechol | 3.17 | 158.73 | 50.00 |
| Pyrogallol | 2.67 | 3333.33 | 1250.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarsenova, A.; Demir, D.; Çağlayan, K.; Abiyev, S.; Darbayeva, T.; Eken, C. Purification and Properties of Polyphenol Oxidase of Dried Volvariella bombycina. Biology 2023, 12, 53. https://doi.org/10.3390/biology12010053
Sarsenova A, Demir D, Çağlayan K, Abiyev S, Darbayeva T, Eken C. Purification and Properties of Polyphenol Oxidase of Dried Volvariella bombycina. Biology. 2023; 12(1):53. https://doi.org/10.3390/biology12010053
Chicago/Turabian StyleSarsenova, Assemgul, Dudu Demir, Kardelen Çağlayan, Sardarbek Abiyev, Talshen Darbayeva, and Cafer Eken. 2023. "Purification and Properties of Polyphenol Oxidase of Dried Volvariella bombycina" Biology 12, no. 1: 53. https://doi.org/10.3390/biology12010053
APA StyleSarsenova, A., Demir, D., Çağlayan, K., Abiyev, S., Darbayeva, T., & Eken, C. (2023). Purification and Properties of Polyphenol Oxidase of Dried Volvariella bombycina. Biology, 12(1), 53. https://doi.org/10.3390/biology12010053






