Simple Summary
Aggressive invasive species can outcompete native species in contests over resources, which can lead to the exclusion of a native species by the invader. Invasive freshwater crayfish are often more aggressive than their native counterparts, however contests between invasive and native crayfish are typically investigated under laboratory conditions and are rarely examined in a natural setting. We used a baited underwater camera in a stream located in New South Wales, Australia to examine behavioural interactions between an invasive crayfish and a critically endangered native crayfish to determine which species was dominant. We found the native species dominant over the invader when larger, however when the species were size-matched the dominance of the native was lost and neither species exhibited a significant pattern of dominance. This outcome indicates the invasive crayfish represents a threat to the native since it may be able to outcompete the native over resources. Further, this outcome contrasts to previous laboratory findings, highlighting the importance of field observations in establishing the extent of impact an invader may be having on a native species.
Abstract
Competitive behavioural interactions between invasive and native freshwater crayfish are recognised as a key underlying mechanism behind the displacement of natives by invaders. However, in situ investigations into behavioural interactions between invasive and native crayfish are scarce. In Australian freshwater systems, the invasive Cherax destructor has spread into the ranges of many native Euastacus species, including the critically endangered Euastacus dharawalus. Staged contests between the two species in a laboratory setting found E. dharawalus to be the dominant competitor, however, this has yet to be corroborated in situ. Here, we used baited remote underwater video (BRUV) to examine in situ intra- and inter-specific behavioural interactions between E. dharawalus and C. destructor. We sought to evaluate patterns of dominance and differential contest dynamics between the species to provide indications of competition between the two species. We found E. dharawalus to be dominant over C. destructor based on pooled interspecific interaction data and size-grouped interactions where C. destructor was the smaller opponent. Alarmingly, however, when C. destructor was within a 10% size difference the dominance of E. dharawalus was lost, contrasting with the outcomes of the laboratory-staged study. In addition, we report that small C. destructor initiated significantly more contests than larger conspecifics and larger E. dharawalus, a pattern that was not observed in smaller E. dharawalus. Further, intraspecific interactions between C. destructor were significantly longer in duration than intraspecific interactions between E. dharawalus, indicating a willingness to continue fighting. Concerningly, these outcomes point towards inherent and greater aggressiveness in C. destructor relative to E. dharawalus and that only larger E. dharawalus hold a competitive advantage over C. destructor. Therefore, we conclude that C. destructor represents a substantial threat to E. dharawalus through competitive behavioural interactions. Further, due to the disparity between our findings and those produced from laboratory-staged contests, we recommend the use of in situ studies when determining the behavioural impacts of invasive crayfish on natives.
1. Introduction
Aggressive interactions between animals occur to gain access to resources or to establish dominance over others. Intraspecific aggression is caused by competition over mates or limited resources [1], whereas interspecific aggression is caused by resource competition or interference competition [2]. An individual’s ability to acquire or retain a resource (its resource holding potential [RHP]) can be influenced by a range of factors [3]. One of the key factors is body size, with larger animals typically having a greater RHP than smaller individuals (e.g., [4,5,6]). Other factors known to mediate RHP include sex, reproductive state, age, and prior residency [7,8,9,10,11].
In aggressive interactions between heterospecifics, RHP may be influenced by species-specific traits that cause competitive asymmetries between species. For instance, if one species is naturally larger in size, they will likely possess a competitive advantage over the other [12,13,14]. Further, if a species exhibits greater inherent aggressiveness than a heterospecific this can also translate to a competitive advantage [15,16,17]. Aggressive encounters between invasive and native species are often asymmetric since invaders are often more inherently aggressive than natives [18,19,20,21] or possess competitively advantageous traits such as larger weaponry [22,23,24]. In some instances, greater relative aggressiveness of an invasive species can even overcome invasive–native size differences [25].
Collectively, invasive freshwater crayfish are often reported to be more aggressive than their native counterparts [26,27,28,29,30]. This, coupled with other traits such as larger relative body or chelae size [23], greater fecundity [31], growth rate [32] and tolerance of unfavourable environmental conditions [33] can lead to the competitive exclusion of natives by invasive crayfish [34]. Competitive exclusion is considered a key mechanism behind population declines and displacement of native crayfish by invasives [35,36]. However, there are instances of invasive–native crayfish co-occurrence where no evidence of competitive exclusion has been found [37,38]. Further, in contests staged over food and shelter resources, invasive crayfish have demonstrated no or even reduced competitive advantage over the native species [39,40].
Competitive interactions between invasive and native freshwater crayfish have been extensively studied under laboratory conditions, primarily by pairing contestants and observing interactions over limited resources such as food or shelters (e.g., [27,40,41,42,43]) However, such contests staged under laboratory conditions may not necessarily represent how these interactions occur in a natural setting [44]. For example, Bergman & Moore [44] reported that agonistic interactions between crayfish were much shorter and less intense than those staged in laboratory settings. There are a limited number of investigations into these competitive interactions in situ. Further, most in situ studies into the occurrence of competition between invasive and native crayfish have used indirect methods (e.g., dietary overlap, comparison of habitat use) to assess the likelihood of competition between species (e.g., [38,45,46]). Whilst these studies provide valuable insight into potential competitive interactions, they are unable to provide confirmation of competition through direct observation and hence any negative impacts invasive crayfish may have on natives is either assumed or based on indirect evidence. For this reason, direct in situ observations of aggressive interactions are important for establishing the extent invasive crayfish impact natives. To our knowledge, there have been just two studies to directly observe invasive–native crayfish competition in situ. These employed caged field experiments where contests between size and sex-matched invasive and native crayfish were staged [37,47]. However, there has yet to be any direct observational (non-manipulative) in situ study into aggressive interactions between invasive and native crayfish.
In Australian freshwater systems, the invasive Cherax destructor has proliferated beyond its natural range of the Murray–Darling Basin and is now present in all Australian states and territories [48]. It now encroaches on the natural ranges of many native Euastacus species [49,50] and owing to its aggressive tendencies [51] and life history traits such as rapid maturation, protracted spawning period and high fecundity [32], C. destructor is considered a significant threat to many members of the genus Euastacus. Staged laboratory contests between C. destructor and certain Euastacus species found C. destructor to be aggressively dominant (E. spinifer: [30]); (E. armatus: [41]). Conversely, in other studies the Euastacus species was reported as the dominant competitor (E. dharawalus: [40]); (E. spinifer: [43]).
One species under considerable threat from C. destructor is the critically endangered E. dharawalus [52,53,54]. This species is restricted to an eight-kilometre stretch of Wildes Meadow Creek located in the Southern highlands region of New South Wales (NSW), Australia. Cherax destructor has proliferated extensively throughout Wildes Meadow Creek and in some sections is found in much greater abundance than E. dharawalus [55]. Lopez et al., [40] staged size-matched laboratory contests over food between C. destructor and E. dharawalus and reported that E. dharawalus won more contests and was more intensely aggressive than C. destructor. However, an in situ investigation into the activity patterns of E. dharawalus in the presence versus absence of C. destructor found overlap in habitat use of both species and reported a significant reduction in activity of E. dharawalus following the removal of C. destructor [56], indicating some degree of in situ competitive impact by C. destructor on E. dharawalus. Therefore, to better determine the broader impact of C. destructor on E. dharawalus through direct competition we used baited remote underwater video (BRUV) to examine in situ aggressive intra- and interspecific interactions between the species over a bait source. Specifically, we aimed to determine (1) whether E. dharawalus is dominant over C. destructor in a natural setting, (2) if intraspecific contest dynamics differ from interspecific contest dynamics between the species and, (3) the effect of crayfish size on interspecific and intraspecific interactions.
2. Materials and Methods
2.1. Study Site
The study was conducted along Wildes Meadow Creek (WMC), a small stream located in the Southern Highlands region of New South Wales (NSW), Australia. It has an approximate total length of 12 km, however, the construction of Fitzroy Falls reservoir in 1974 divided the creek into two sections of ~7.5 km above and 750 m below the reservoir. The section of creek above the reservoir is situated on agricultural land with adjacent fields used as cattle pastures or for crop farming. The majority of the riparian margins in the upper section have been cleared or are now dominated by invasive willow (Salix spp.) and blackberry (Rubus spp.), however some remnants of native riparian margins are still present. Stream width varies from 0.5–7 m and maximum depth in pools is 2–3 m with shallower riffle sections between. Substratum varies between mud and silt, cobbles, clay, and bedrock. Water temperature ranges from a minimum of 7 °C in winter to a maximum of 24.9 °C in summer. A total of nine separate survey locations in the upper section of WMC were selected for this study (Supplementary Figure S1).
2.2. Behavioural Observations
Behavioural interactions and abundance of E. dharawalus and C. destructor were quantified using baited remote underwater video (BRUV) (NSW DPI permit No. F95/269-9.0). The BRUV apparatus consisted of a GoPro Hero 6 camera attached to a brick and PVC pipe. A bait bag containing two pilchards (Sardinops spp.) was attached to the end of the PVC pipe within the field of view of the video (Supplementary Video S1). Pilchards were selected as the bait type since oily bait such as this are reported to be the most effective at attracting aquatic species to BRUVs [57]. Further, there are numerous native (Galaxias maculatus, Prototroctes maraena) and non-native (Oncorhynchus mykiss, Cyprinus carpio, Gambusia holbrooki) fish species present in the study system that would elicit a similar competitive response to that produced by a resource such as pilchard. BRUVs were deployed at six locations in July 2020, nine locations in December 2020, and eight locations in December 2021. Each BRUV was deployed once at each location for 30 min with depths ranging between 0.5 and 1.3 m. Deployments conducted in July 2020 did not capture any crayfish on video, likely due to the cold temperatures in WMC at this time of the year, therefore these were omitted from the analysis. Further, for two deployments conducted in December 2021 the water was too turbid to determine any abundance measures or behavioural interactions between crayfish, therefore these videos were also omitted from the analysis and a total of 15 videos were used in the analysis. The abundance of E. dharawalus and C. destructor for each survey was determined by counting the maximum number of each species seen together at any one time over the 30-min time frame (MaxN).
All intraspecific and interspecific interactions between C. destructor and E. dharawalus were analysed to determine five metrics; (1) duration of interaction, (2) maximum intensity level reached, (3) interaction conclusion, (4) interaction outcome and (5) the initiator of the interaction. An interaction was defined as an encounter between a pair of crayfish within equal to or less than one body length of each other. To determine maximum intensity level reached, each aggressive and submissive behaviour exhibited by crayfish was assigned an intensity score (Table 1) [44]. More aggressive behaviours were assigned higher positive values, whereas submissive retreat behaviours such as a tailflip response were assigned negative values (Table 1). The conclusion of each interaction was recorded as the retreat response exhibited by the “losing” crayfish (tailflip or slowly back away; Table 1) or if no retreat occurred and the crayfish shared the bait the conclusion was recorded as “ignore” (intensity 0; Table 1). An interaction ended when individuals were greater than one body length away from each other and neither individual continued pursuit of the other. Alternatively, if opponents remained within one body length of each other but exhibited an “ignore” response, such an interaction was deemed to end if the “ignore” response lasted for over 30 s, further, these 30 s were included in the interaction duration time. To determine interaction outcome, the “losing” individual was deemed the crayfish that exhibited either a tail-flip or retreat response. If an interaction concluded in an “ignore” response it was excluded from the analysis of contest outcome. Further, if a retreat was caused by an external factor such as an approach by another crayfish, the interaction was excluded in the analysis of metrics (1), (3) and (4). The crayfish deemed as the initiator of the interaction was the individual to make the first threat display or contact with the opponent. Interactions where both crayfish ignored the other despite being within or equal to one body length away (intensity 0; Table 1) were omitted from this part of the analysis. Interactions between crayfish that moved beyond the field of view were not included in the analysis of metrics (1), (2), (3) or (4) since it was not possible to determine the interaction duration, maximum intensity reached nor the contest outcome/conclusion while off-screen.

Table 1.
Ethogram of aggressive behaviours in crayfish adapted from Bergman & Moore [44].
To determine effect of size on interspecific interaction outcome and initiation, interactions were grouped based on relative size differences. Where possible, size differences were quantified by estimating the percent difference between interacting crayfish (measured from rostrum to rear of carapace) as measured on the video screen [58]. If the size difference between crayfish was less than 10%, these interactions were considered as “size-matched” since contests outcomes between crayfish within a 10% size difference have been found to be random [59]. If the size difference between crayfish was greater than 10% these were grouped into ‘small Cherax versus large Euastacus’ or ‘small Euastacus versus large Cherax’ depending on the smaller opponent. Further, for interspecific contests where a size difference was apparent, in addition to recording which species lost the contest, whether the contest initiator and losing individual was larger or smaller than their opponent was also recorded. For intraspecific interactions, relative size differences were also recorded, and interaction initiation and outcome were recorded as whether the initiating individual and the losing individual was larger or smaller than their opponent. If an estimate of size difference between crayfish could not be obtained due to the positioning of one or both individuals in the video, or, if an interaction occurred at the edge of the field of view where crayfish size may have been altered by lens distortion, the interaction was removed from this part of the analysis.
2.3. Statistical Analysis
Where applicable, normality of the data was checked via visual inspection of Q-Q plots and histograms using R studio (version 2022.07.1). Due to right-skewed data, a log +1 transformation was performed on crayfish abundance (MaxN) and a log transformation was performed on (1) interaction duration (s). To determine if the abundance (MaxN) of E. dharawalus and C. destructor varied significantly between the two species and between the two survey periods (December 2020 and December 2021), a linear mixed model (LMM) (LME4 package; [60]) was used with site ID included as a random effect.
To determine the effect of interaction type (interspecific, Cherax intraspecific, and Euastacus intraspecific) on (1) interaction duration (response variable) a linear model (LM) was used followed by a Tukey’s post hoc test. To determine if there was significant variation in the (2) maximum intensity levels reached and (3) interaction conclusion between and within interaction types, generalised linear models (GLM) were used with a negative binomial distribution and log link function specified for both metrics. To determine where significant differences occurred between interaction types, we used the Marascuilo procedure [61] to perform post hoc comparisons between the proportion of interactions to reach each intensity and conclusion level. Post hoc comparisons were also performed within each interaction type to determine significant variation between the intensity levels and interaction conclusions.
For interspecific interactions, a GLM with binomial distribution and logit link function specified was used to investigate the effect of species (categorical fixed effect) on the (4) interaction outcome (win/loss) (response variable). This was repeated to determine the effect of species on (5) interaction initiation (yes/no) (response variable).
To determine the effect of size difference grouping (categorical fixed effect) on interspecific interaction outcome and initiation (losing or initiating species: Euastacus/Cherax) a GLM with binomial distribution and logit link function specified was used for both metrics. Since only one interaction in which E. dharawalus was smaller than C. destructor was observed (Table 2), the ‘small Euastacus versus large Cherax’ grouping was excluded from the analyses. Finally, to determine the effect of size on contest initiation and outcome in intraspecific contests, Pearson Chi-squared tests were performed on the frequency of interactions initiated and lost by smaller and larger opponents.

Table 2.
The number of intraspecific interactions between Cherax destructor (C) and Euastacus dharawalus (E) and the total number of interspecific interactions observed. The number of interspecific interactions that were size-matched, small C. destructor versus large E. dharawalus and small E. dharawalus versus large C. destructor are also listed.
3. Results
There were no differences in the abundance (MaxN) of E. dharawalus (mean ± SE: 1.24 ± 0.32) and C. destructor (mean ± SE: 1.82 ± 0.49) (LMM: F1,32 = 1.20, p = 0.281) nor did abundance of both species vary between the survey periods (F1,32 = 1.26, p = 0.269). In total, 131 interactions were observed between crayfish and, there was significant variation in the type of interaction observed (χ221,131 = 11.80, p = 0.002). Intraspecific interactions between E. dharawalus were observed significantly less frequently than intraspecific interactions between C. destructor (p = 0.004) and interspecific interactions (p = 0.002) (Table 2).
3.1. Contest Dynamics: Duration, Intensity, and Conclusion
The mean interaction duration across all interaction types was 35.2 ± 4.7 s (mean ± SE, n = 131). Interaction duration (s) differed significantly between interaction types (LM: F2128 = 3.80, p = 0.025). Intraspecific E. dharawalus interactions were significantly shorter (mean ± SE: 18.57 ± 4.52) than intraspecific C. destructor interactions (mean ± SE: 35.63 ± 4.63) (p = 0.02) (Figure 1). However, there was no significant difference in interactions durations for intraspecific E. dharawalus interactions and interspecific interactions (p = 0.08) (Figure 1).
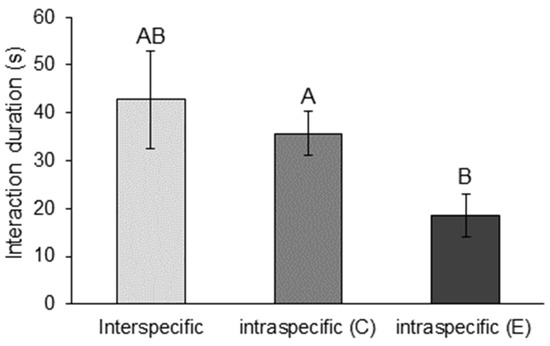
Figure 1.
Mean time (s) (±SE) crayfish spent interacting in interspecific interactions and intraspecific interactions between Cherax destructor (C) and Euastacus dharawalus (E). Different letters denote significant differences based on Tukey’s post hoc tests.
There were no interactions between crayfish that reached the highest intensity level (i.e., level five; Table 1). Maximum intensity levels for interspecific, intraspecific C. destructor and intraspecific E. dharawalus interactions ranged from level zero intensity to level four intensity. Further, for all interaction types the most common maximum intensity level was four (Figure 2). Maximum intensity levels reached during interactions varied significantly between interaction types (GLM: χ212,131 = 11.81, p = 0.003). Post hoc tests confirmed that the proportion of interactions to reach level one intensity in intraspecific C. destructor interactions was significantly lower than for E. dharawalus intraspecific interactions (Figure 2). Further, the proportion of interactions to reach level three intensity in intraspecific E. dharawalus interactions was significantly less than the proportion of C. destructor intraspecific interactions and interspecific interactions to reach intensity level three (Figure 2). However, there was no significant variation between the interaction types for any other maximum intensity level (Figure 2). Maximum intensity levels also varied significantly within each interaction type (χ28131 = 32.97, p < 0.001). In interspecific interactions, the proportion of interactions to reach intensity four was significantly greater than the proportion to reach intensity zero (Figure 2). In intraspecific C. destructor interactions, the proportion of interactions to reach intensity four was significantly greater than the proportion to reach intensities zero and one (Figure 2). In intraspecific E. dharawalus interactions, the proportion of interactions to reach intensity four was significantly greater than the proportion to reach intensity three (Figure 2).
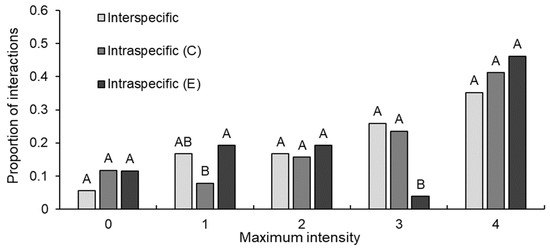
Figure 2.
Proportion of interactions that reached each maximum intensity level for interspecific interactions between Euastacus dharawalus and Cherax destructor, intraspecific interactions between C. destructor (C) and intraspecific interaction between E. dharawalus (E). Different letters denote significant differences between the interaction types for each intensity level based on the Marascuilo procedure for multiple comparisons of proportions.
Between interaction types, there were significant differences in the interaction conclusion (GLM: χ24117 = 9.51, p = 0.009). A significantly greater proportion of intraspecific C. destructor interactions concluded with intensity level zero than did interspecific interactions and intraspecific E. dharawalus interactions (Figure 3). Further, the proportion of intraspecific E. dharawalus interactions to conclude with intensity zero was significantly greater than those in interspecific interactions (Figure 3). A slow back away response was observed significantly more often in interspecific interactions than in intraspecific C. destructor interactions and intraspecific E. dharawalus interactions (Figure 3). Further, a significantly greater proportion of intraspecific C. destructor interactions concluded with a slow back away response than did intraspecific E. dharawalus interactions (Figure 3). Finally, a significantly greater proportion of intraspecific E. dharawalus interactions concluded with a tailflip response than intraspecific C. destructor interactions and interspecific interactions (Figure 3). There were significant differences in interaction conclusion within interaction types (χ26117 = 21.50, p < 0.001). Interspecific interactions concluded with a significantly greater proportion of back away and tail flip responses than ignore responses (Figure 3). Intraspecific E. dharawalus interactions concluded with a significantly greater proportion of tailflip responses than back away and ignore responses (Figure 3). However, there was no significant differences in the interaction conclusions in intraspecific C. destructor interactions (Figure 3).
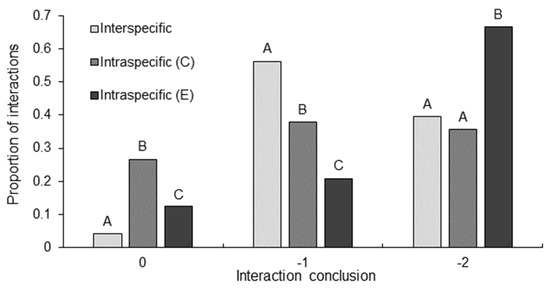
Figure 3.
Proportion of interactions that concluded with ignore (0), back away (−1) or tailflip away (−2) in interspecific interactions Euastacus dharawalus and Cherax destructor, intraspecific interactions between C. destructor (C) and intraspecific interaction between E. dharawalus (E). Different letters denote significant differences between the interaction types for each conclusion level based on the Marascuilo procedure for multiple comparisons of proportions.
3.2. Interspecific Interaction Outcome and Initiation
Regardless of relative size, we found C. destructor was significantly more likely to initiate interspecific interactions than E. dharawalus (GLM: χ21108 = 5.38, p = 0.02), with C. destructor initiating 61% of interactions and E. dharawalus only initiating 39% (Figure 4). Euastacus dharawalus was significantly more likely to win interactions than C. destructor (GLM: χ21,88 = 16.97, p < 0.001), with E. dharawalus winning 73% of interspecific interactions (Figure 4).
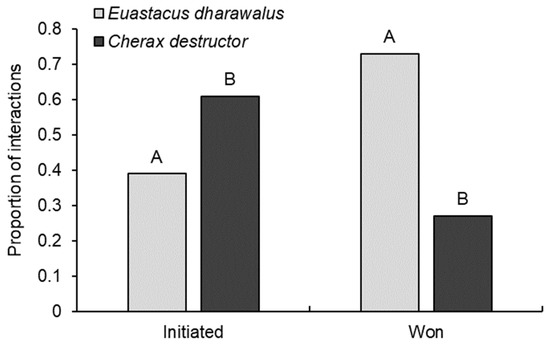
Figure 4.
The proportion of interspecific interactions initiated and won by Euastacus dharawalus and Cherax destructor. Different letters denote significant differences based on Tukey’s post hoc tests.
3.3. Effect of Size on Interaction Outcome and Initiation
Relative size difference between opponents in interspecific interactions had a significant effect on both interaction initiation (GLM: χ21,35 = 7.44, p = 0.006) and outcome (GLM: χ21,35 = 8.73, p = 0.003). Euastacus dharawalus was significantly more likely to win against smaller C. destructor than size-matched C. destructor, winning 87% of interactions where C. destructor was the smaller opponent and only 38% of size-matched interactions (Figure 5). Further, C. destructor was significantly more likely to win when size-matched against E. dharawalus than when the smaller opponent. The only interaction where C. destructor was larger than E. dharawalus was won by E. dharawalus.
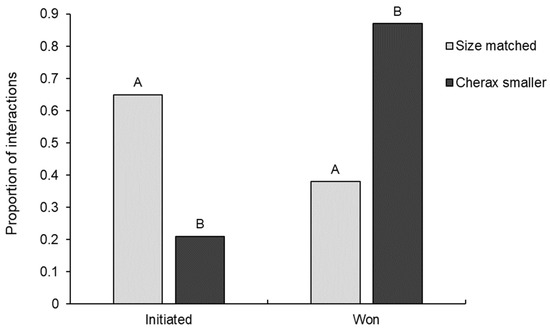
Figure 5.
The proportion of interspecific interactions initiated and won by Euastacus dharawalus in interactions where Cherax destructor was the smaller opponent and interactions where opponents were size matched (size difference of <10%). Different letters denote significant differences based on Tukey’s post hoc tests. “Cherax larger” was only observed on one occasion so has not been displayed here.
Euastacus dharawalus was significantly more likely to initiate an interaction when size-matched with C. destructor than when C. destructor was smaller with E. dharawalus initiating 65% of size-matched interactions and only 21% of interactions where C. destructor was the smaller opponent (Figure 5). Conversely, C. destructor was significantly more likely to initiate an interaction when smaller than E. dharawalus than when size matched, initiating 79% of interactions as the smaller opponent and only 35% when size-matched with E. dharawalus.
In intraspecific interactions, the larger opponent was significantly more likely to win than the smaller opponent in intraspecific C. destructor interactions (χ21,10 = 10, p = 0.002) and intraspecific E. dharawalus interactions (χ21,17 = 17, p < 0.001) with the larger opponent winning 100% of the interactions for both interaction types. In intraspecific C. destructor interactions, smaller opponents were also significantly more likely to initiate an interaction (χ21,10 = 6.4, p = 0.011), initiating 90% of interactions. However, in intraspecific E. dharawalus interactions, smaller opponents were not significantly more likely to initiate an interaction than larger opponents (χ21,17 = 0.53, p = 0.467), with smaller opponents initiating 59% of interactions.
4. Discussion
Examining in situ competitive behavioural interactions between invasive and native species is key in understanding the mechanisms underpinning the competitive exclusion of natives by invaders. Overall, our findings do not indicate consistent dominance of the native E. dharawalus over the invasive C. destructor. Based on pooled interspecific interactions, E. dharawalus possessed an advantage over the invader. However, when interspecific interactions were separated by relative size difference between opponents, E. dharawalus lost its advantage in size-matched interactions against C. destructor. Our findings also reveal patterns of inherent and greater aggressiveness in C. destructor. Cherax destructor exhibited a greater propensity to initiate interactions than E. dharawalus, further, even smaller C. destructor demonstrated a willingness to initiate interactions with larger E. dharawalus or conspecifics. Conversely, we found E. dharawalus was more likely to initiate an interaction with a similar-sized C. destructor than with a smaller C. destructor. Further, intraspecific interactions between C. destructor were significantly longer than intraspecific interactions between E. dharawalus, indicating a willingness to continue fighting in C. destructor. In contrast, however, intraspecific interactions between C. destructor concluded less intensely than those between E. dharawalus and interspecific interactions.
Our findings surrounding the competitive dominance of E. dharawalus over smaller C. destructor are not surprising given the role size asymmetries play in contest outcome in freshwater crayfish [27,39]. However, the loss of this advantage for the native in size-matched interspecific contests is both concerning and contrary to our expectations. In staged size-matched laboratory contest between E. dharawalus and C. destructor, Lopez et al. [40] reported that E. dharawalus was competitively dominant over C. destructor. The inconsistency between our findings and those of Lopez et al. [40] indicates the dominance of E. dharawalus under laboratory conditions is not reflected in a natural setting. It is necessary to note that sex can play a role in contest outcome in freshwater crayfish [27], however, since it was not possible to control for the effect of sex in the present study, we are unable to discern its effect on the patterns of dominance here. However, Lopez et al. [40] did not find any effect of sex on the aggressive behaviours of E. dharawalus or C. destructor. Based on the frequency of interspecific interactions in which C. destructor was the smaller opponent and E. dharawalus the larger, it is evident E. dharawalus possesses a size advantage over C. destructor in situ as the naturally larger-bodied species. However, since only one interaction between a larger C. destructor and smaller E. dharawalus was observed by the present study, it is not possible to assess the behavioural impacts larger C. destructor may have on juvenile E. dharawalus here. It would therefore be important for future studies to investigate interactions between larger C. destructor and smaller E. dharawalus.
The initiation of more interactions by smaller C. destructor relative to larger E. dharawalus and conspecifics is an unexpected outcome given contests between crayfish are more often initiated by larger more dominant individuals [62]. However, initiation of contests by smaller C. destructor may be explained by the ‘Napoleon complex’, where likely losers are expected to initiate a contest, even without a payoff asymmetry. Just & Morris [63] suggest that if a resource exceeds the cost of losing a contest, the cost of displaying is adequately small, and assessment of RHP is reasonably accurate but not perfect, a likely loser is prompted to initiate a contest, while a likely winner will wait for the adversary to attack or retreat. However, a pattern of contest initiation by smaller individuals was only apparent in C. destructor and not E. dharawalus, therefore we consider the willingness of smaller C. destructor to initiate an interaction with a larger opponent, despite the risk of injury, is a likely consequence of inherent aggressiveness in C. destructor.
The prolonged interaction durations in intraspecific C. destructor interactions compared to those between E. dharawalus may be an indication of a motivation to continue fighting in C. destructor, thus further suggesting inherent aggressiveness in the invasive species. Further, the longer interaction duration of intraspecific interactions between invasive species compared to those between the native are consistent with previous research to examine invasive–native crayfish interactions. In staged laboratory contests between native Euastacus spinifer and invasive C. destructor, O’Hea Miller et al. [30] reported that C. destructor spent significantly more time interacting than did E. spinifer in intraspecific contests. Further, Hudina et al. [28], reported the same pattern with the invasive Pacifastacus leniusculus interacting significantly longer in intraspecific contests than those between native Astacus leptodactylus. These patterns of contest initiation and duration also suggest asymmetry between the aggressiveness of the C. destructor and E. dharawalus. In invasive species, aggressiveness is regarded as key behavioural trait in their successful establishment and proliferation [64]. Further, greater relative aggressiveness of invasive than native crayfish is common (e.g., [26,28,29,30]). Hence, the patterns of contest initiation and duration exhibited by C. destructor offer evidence of significant aggression in this invasive crayfish.
In contrast to C. destructor, E. dharawalus was more likely to initiate interspecific interactions with size-matched opponents than with smaller opponents. Alternative to greater inherent aggression in the invasive species, the differential patterns of contest initiation and duration exhibited by C. destructor and E. dharawalus may be an indication of greater self or opponent assessment of RHP in E. dharawalus relative to C. destructor. Self and opponent assessment is commonly reported in decapod crustaceans (e.g., [65,66,67]), however the degree of assessment used may not be consistent across all species. As a smaller opponent typically has a lesser RHP [62] they may not be perceived as a risk to the resource acquisition for a larger E. dharawalus, therefore initiating an interaction may be an unnecessary cost. Further, a larger individual can benefit from leaving contest initiation to the smaller opponent as the smaller opponent may retreat and no contest need occur [63]. However, a similar sized opponent is likely to have a comparable RHP, and hence more likely to be considered a threat to resource acquisition. If follows then that, initiating an interaction is in the best interest of E. dharawalus. This pattern of assessment behaviour varies to that displayed by C. destructor, thereby suggesting some difference in opponent assessment between the species.
Intraspecific interactions between C. destructor concluded less intensely than did interspecific interactions and intraspecific E. dharawalus interactions. The retreat behaviour exhibited by an opponent at the conclusion of a contest is a consequence of the level of threat perceived by the retreating individual [68]. In crayfish, slow back away and ignore responses are indicative of lower threat perception, whereas a quicker tailflip retreat indicates high level threat perception. Hence, C. destructor that lost intraspecific interactions perceived opponents as less of a threat than did losing individuals in interspecific interactions and intraspecific E. dharawalus interactions. It is evident, however, that retreat intensity did not correspond to the intensity reached during interactions since we observed little variation in the maximum intensity levels reached between the interaction types. This contrasts to the findings of Bergman & Moore [44] who reported in situ interactions that reached higher intensities were also more likely to end in a tailflip response. Therefore, while the intensity at which E. dharawalus and C. destructor interact does not depend on their opponent, the intensity at which an interaction concludes does.
The amount of intra- and inter-specific interactions to reach a maximum intensity level of four as well as the average interaction duration (35.2 s) contrasts to previous in situ observations of crayfish interactions. Observations of intraspecific crayfish interactions over shelters in situ were reported to never reach a maximum intensity of four but were most likely to reach intensity level one and lasted an average of 17 s [58]. Further, interactions between crayfish over shelters, detritus and macrophyte resources in situ lasted an average of 5.3 s and reached maximum intensity level three more frequently than they did intensity four [44]. It is possible that these conflicting results are a consequence of the high value nature of the resource being contested over in the present study. It is necessary to note that due to the use of pilchards as bait, the contests in the present study do not represent an entirely natural in situ competitive scenario, since this resource would be uncommon in the study system. However, such scenarios are likely to occur over carcasses of the native or non-native fish species present in the study system. Resources that are perceived as high value should elicit an increased investment in a contest by opponents [69] and a resource such as pilchard or other fish carcasses are likely perceived as high value in the study system. We contend that crayfish are likely to interact for longer and more intensely over such a resource.
5. Conclusions
This study is the first to examine in situ behavioural interactions between invasive and native freshwater crayfish of varying relative sizes. Although our findings point to overall competitive dominance of the critically endangered E. dharawalus over the invasive C. destructor in situ, this is overshadowed by several other concerning findings. First, the loss of competitive dominance of E. dharawalus in size-matched contests with C. destructor indicates E. dharawalus only possesses an advantage over C. destructor when it is the larger opponent. Further, the willingness of smaller C. destructor to initiate contests as well as continue fighting for prolonged periods suggests either reduced discernment of self and opponent assessment of RHP in C. destructor relative to E. dharawalus, or greater inherent aggressiveness of C. destructor compared E. dharawalus (although we need to further examine how smaller E. dharawalus behave towards larger invasive opponents). Based on our findings, we therefore consider that C. destructor represents a substantial threat to E. dharawalus through competitive behavioural interactions and expect their impact on smaller juvenile E. dharawalus to be highly deleterious—although more data are needed to support this notion. Further, we consider that C. destructor presents a serious threat to other Euastacus species and therefore recommend that preventative action is taken to halt the further spread of C. destructor in Australia. Moreover, we recommend all possible measures to eradicate the C. destructor population from the range of E. dharawalus are taken. Finally, the contrast between our findings and those of the laboratory study by Lopez et al. (2019), highlights that outcomes of laboratory investigations into aggressive interactions between invasive and native crayfish may not be reflected in a natural setting. Therefore, we recommend the use of both ex- and in situ examinations of invasive–native crayfish behavioural interactions when determining the impact of an invader on a native species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology12010018/s1, Figure S1: Map indicating Wildes Meadow Creek and the positions of the nine selected survey sites. Video S1: Interspecific interaction between Euastacus dharawalus and Cherax destructor.
Author Contributions
Conceptualization, S.B.O.M., A.R.D. and M.Y.L.W.; data curation, S.B.O.M.; formal analysis, S.B.O.M.; funding acquisition, A.R.D. and M.Y.L.W.; investigation, S.B.O.M.; methodology, S.B.O.M., A.R.D. and M.Y.L.W.; project administration, S.B.O.M.; resources, S.B.O.M.; software, S.B.O.M.; supervision, A.R.D. and M.Y.L.W.; validation, A.R.D. and M.Y.L.W.; visualization, S.B.O.M.; writing—original draft, S.B.O.M.; writing—review and editing, S.B.O.M., A.R.D. and M.Y.L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Local Land Services (South East) through funding from the Australian Government’s national landcare program (SE02862).
Institutional Review Board Statement
This study was conducted with approval from the New South Wales Department of Primary Industries (Permit number F95/269-9.0, 24 March 2020) and with approval from the animal ethics committee of University of Wollongong (protocol code AE18/18, 17 January 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available from authors upon request.
Acknowledgments
We thank Keegan Bowen for his assistance with field surveys. We also thank Local Land Services for providing the funding for this research and the local land holders and Water NSW who allowed access through their properties to conduct this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hardy, I.C.; Briffa, M. Animal Contests, 1st ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Peiman, K.S.; Robinson, B.W. Ecology and evolution of resource-related heterospecific aggression. Q. Rev. Biol. 2010, 85, 133–158. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.A. Assessment strategy and evolution of fighting behaviour. J. Theor. Biol. 1974, 47, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.B.; Halliday, T.R. Deep croaks and fighting assessment in toads Bufo bufo. Nature 1978, 274, 683–685. [Google Scholar] [CrossRef]
- Tarpy, D.R.; Mayer, M.K. The effects of size and reproductive quality on the outcomes of duels between honey bee queens (Apis mellifera L.). Ethol. Ecol. Evol. 2009, 21, 146–153. [Google Scholar] [CrossRef]
- Colella, D.J.; Paijmans, K.C.; Wong, Y.L.W. Size, sex and social experience: Experimental tests of multiple factors mediating contest behaviour in a rockpool fish. Ethology 2018, 125, 369–379. [Google Scholar] [CrossRef]
- Figler, M.H.; Blank, G.S.; Peeke, H.V.S. Maternal aggression and post-hatch care in red swamp crayfish, Procambarus clarkii (Girard): The influences of presence of offspring, fostering and maternal moulting. Mar. Freshw. Behav. Physiol. 1997, 30, 173–194. [Google Scholar] [CrossRef]
- Chamorro-Florescano, I.A.; Favila, M.E.; Macías-Ordóñezm, R. Ownership, size and reproductive status affect the outcome of food ball contests in a dung roller beetle: When do enemies share? Evol. Ecol. 2011, 25, 277–289. [Google Scholar] [CrossRef]
- Snell-Rood, E.C.; Cristol, D.A. Prior residence influences contest outcome in flocks of non-breeding birds. Ethology 2005, 111, 441–454. [Google Scholar] [CrossRef]
- Tsai, Y.J.J.; Barrows, E.M.; Weiss, M.R. Why do larger and older males win contests in the parasitoid wasp Nasonia vitripennis? Anim. Behav. 2014, 91, 151–159. [Google Scholar] [CrossRef]
- Yang, S.; Premel, V.; Richards-Zawacki, C.L. Prior residence effect determines success of male-male territorial competition in a color polymorphic poison frog. Ethology 2020, 126, 1131–1140. [Google Scholar] [CrossRef]
- Petren, K.; Case, T.J. An experimental demonstration of exploitation competition in an ongoing invasion. Ecology 1996, 77, 118–132. [Google Scholar] [CrossRef]
- Chock, R.Y.; Shier, D.M.; Grether, G.F. Body size, not phylogenetic relationship or residency, drives interspecific dominance in a little pocket mouse community. Anim. Behav. 2018, 137, 197–204. [Google Scholar] [CrossRef]
- Graham, Z.A.; Angilletta, M.J. Claw size predicts dominance within and between invasive species of crayfish. Anim. Behav. 2020, 166, 153–161. [Google Scholar] [CrossRef]
- Robinson, S.K.; Terborgh, J. Interspecific aggression and habitat selection by Amazonian birds. J. Anim. Ecol. 1995, 64, 1–11. [Google Scholar] [CrossRef]
- Pearce, D.; Pryke, S.R.; Griffith, S.C. Interspecific aggression for nest sites: Model experiments with long-tailed finches (Poephila acuticauda) and endangered Gouldian finches (Erythrura gouldiae). Auk 2011, 128, 497–505. [Google Scholar] [CrossRef]
- Ferretti, F. Interspecific aggression between fallow and roe deer. Ethol. Ecol. Evol. 2011, 23, 179–186. [Google Scholar] [CrossRef]
- Mills, M.D.; Rader, R.B.; Belk, M.C. Complex interactions between native and invasive fish: The simultaneous effects of multiple negative interactions. Oecologia 2004, 141, 713–721. [Google Scholar] [CrossRef]
- Polo-Cavia, N.; López, P.; Martín, J. Aggressive interactions during feeding between invasive and native freshwater turtles. Biol. Invasions 2011, 13, 1387–1396. [Google Scholar] [CrossRef]
- Kakareko, T.; Kobak, J.; Grabowska, J.; Jermacz, L.; Przybylski, M.; Poznańska, M.; Pietraszewski, D.; Copp, G.H. Competitive interactions for food resources between invasive racer goby Babka gymnotrachelus and native European bullhead Cottus gobio. Biol. Invasions 2013, 15, 2519–2530. [Google Scholar] [CrossRef][Green Version]
- Culbertson, K.A.; Herrmann, N.C. Asymmetric interference competition and niche partitioning between native and invasive Anolis lizards. Oecologia 2019, 190, 811–820. [Google Scholar] [CrossRef]
- Holway, D.A. Competitive mechanisms underlying the displacement of native ants by the invasive Argentine ant. Ecology 1999, 80, 238–251. [Google Scholar] [CrossRef]
- Gherardi, F.; Cioni, A. Agonism and interference competition in freshwater decapods. Behaviour 2004, 141, 1297–1324. [Google Scholar] [CrossRef]
- Hajek, A.E.; Henry, J.C.; Standley, C.R.; Foelker, C.J. Comparing functional traits and abundance of invasive versus native woodwasps. Neobiota 2017, 36, 39–55. [Google Scholar] [CrossRef][Green Version]
- Sanches, F.H.C.; Miyai, C.A.; Costa, T.M.; Christofoletti, R.A.; Volpato, G.L.; Barreto, R.E. Aggressiveness overcomes body-size effects in fights staged between invasive and native fish species with overlapping niches. PLoS ONE 2012, 7, e29746. [Google Scholar] [CrossRef]
- Blank, G.S.; Figler, M.H. Interspecific shelter competition between the sympatric crayfish species Procambarus clarkii (Girard) and Procambarus zonangulus (Hobbs and Hobbs). J. Crustac. Biol. 1996, 16, 300–309. [Google Scholar] [CrossRef]
- Nakata, K.; Goshima, S. Competition for shelter of preferred sizes between the native crayfish species Cambaroides japonicus and the alien crayfish species Pacifastacus leniusculus in Japan in relation to prior residence, sex difference, and body size. J. Crustac. Biol. 2003, 23, 897–907. [Google Scholar] [CrossRef]
- Hudina, S.; Hock, K.; Radović, A.; Klobučar, G.; Petković, J.; Jelić, M.; Maquire, I. Species-specific differences in dynamics of agonistic interactions may contribute to the competitive advantage of the invasive signal crayfish (Pacifastacus leniusculus) over the native narrow-clawed crayfish (Astacus leptodactylus). Mar. Freshw. Behav. Physiol. 2016, 49, 147–157. [Google Scholar] [CrossRef]
- Klocker, C.A.; Strayer, D.L. Interactions among an invasive crayfish (Orconectes rusticus), a native crayfish (Orconectes limosus), and native bivalves (Sphaeriidae and Unionidae). Northeast. Nat. 2004, 11, 167–178. [Google Scholar] [CrossRef]
- O’Hea Miller, S.B.; Davis, A.R.; Wong, M.Y.L. Does habitat complexity and prior residency influence aggression between invasive and native freshwater crayfish? Ethology 2022, 128, 443–452. [Google Scholar] [CrossRef]
- Pârvulescu, L.; Pîrvu, M.; Moroşan, L.G.; Zaharia, C. Plasticity in fecundity highlights the females’ importance in the spiny-cheek crayfish invasion mechanism. Zoology 2015, 118, 424–432. [Google Scholar] [CrossRef]
- Beatty, S.; Morgan, D.; Gill, H. Role of life history strategy in the colonisation of Western Australian aquatic systems by the introduced crayfish Cherax destructor Clark, 1936. Hydrobiologia 2005, 549, 219–237. [Google Scholar] [CrossRef]
- Larson, E.R.; Magoulick, D.D.; Turner, C.; Laycock, K.H. Disturbance and species displacement: Different tolerances to stream drying and desiccation in a native and an invasive crayfish. Freshw. Biol. 2009, 54, 1899–1908. [Google Scholar] [CrossRef]
- Smith, K.R.; Roth, B.M.; Jones, M.L.; Hayes, D.B.; Hervst, S.J.; Popoff, N. Changes in the distribution of Michigan crayfishes and the influence of invasive rusty crayfish (Faxonius rusticus) on native crayfish substrate associations. Biol. Invasions 2019, 21, 637–656. [Google Scholar] [CrossRef]
- Söderback, B. Replacement of the native crayfish Astacus astacus by the introduced species Pacifastacus leniusculus in a Swedish lake: Possible causes and mechanisms. Freshw. Biol. 1995, 33, 291–304. [Google Scholar] [CrossRef]
- Gherardi, F. Crayfish invading Europe: The case study of Procambarus clarkii. Mar. Freshw. Behav. Physiol. 2006, 39, 175–191. [Google Scholar] [CrossRef]
- Rabalais, M.R.; Magoulick, D.D. Is competition with the invasive crayfish Orconectes neglectus chaenodactylus responsible for the displacement of the native crayfish Orconectes eupunctus? Biol. Invasions 2006, 8, 1039–1048. [Google Scholar] [CrossRef]
- Westhoff, J.T.; Rabeni, C.F. Resource selection and space use of a native and an invasive crayfish: Evidence for competitive exclusion? Freshw. Sci. 2013, 32, 1383–1397. [Google Scholar] [CrossRef]
- Vorburger, C.; Ribi, G. Aggression and competition for shelter between a native and an introduced crayfish in Europe. Freshw. Biol. 1999, 41, 111–119. [Google Scholar] [CrossRef]
- Lopez, L.K.; Hendry, K.; Wong, M.Y.; Davis, A.R. Insight into invasion: Interactions between a critically endangered and invasive crayfish. Austral Ecol. 2019, 44, 78–85. [Google Scholar] [CrossRef]
- Hazlett, B.A.; Lawler, S.; Geoffery, E. Agonistic behaviour of the crayfish Euastacus armatus and Cherax destructor. Mar. Freshw. Behav. Physiol. 2007, 40, 257–266. [Google Scholar] [CrossRef]
- Roessink, I.; van der Zon, K.A.E.; de Reus, S.R.M.M.; Peeters, E.R.H.M. Native European crayfish Astacus astacus competitive in staged confrontation with the invasive crayfish Faxonius limosus and Procambarus acutus. PLoS ONE 2022, 17, e0263133. [Google Scholar] [CrossRef] [PubMed]
- Cerato, S.; Davis, A.R.; Coleman, D.; Wong, M.L.Y. Reversal of competitive dominance between invasive and native freshwater crayfish species under near-future elevated water temperature. Aquat. Invasions 2019, 14, 518–530. [Google Scholar] [CrossRef]
- Bergman, D.A.; Moore, P.A. Field observations of intraspecific agonistic behaviour of two crayfish species, Orconectes rusticus and Orconectes virilis, in different habitats. Biol. Bull. 2003, 205, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Bubb, D.H.; Thom, T.J.; Lucas, M.C. Movement, dispersal and refuge use of co-occurring introduced and native crayfish. Freshw. Biol. 2006, 51, 1359–1368. [Google Scholar] [CrossRef]
- Olsson, K.; Stenroth, P.; Nyström, P.; Granéli, W. Invasions and niche width: Does niche width of an introduced crayfish differ from a native crayfish? Freshw. Biol. 2009, 54, 1731–1740. [Google Scholar] [CrossRef]
- Dresser, C.M.; Kuhlmann, M.L.; Swanson, B.J. Variation in native crayfish agonistic response to the invasion of the rusty crayfish Orconectes rusticus (Girard, 1852). J. Crustac. Biol. 2016, 36, 129–137. [Google Scholar] [CrossRef]
- Coughran, J.; McCormack, R.B.; Daly, G. Translocation of the yabby Cherax destructor into eastern drainages of New South Wales, Australia. Aust. Zool. 2009, 35, 100–103. [Google Scholar] [CrossRef]
- Coughran, J.; Daly, G. Potential threats posed by a translocation crayfish: The case of Cherax destructor in coastal drainages of New South Wales, Australia. Crust. Res. 2012, 7, 5–13. [Google Scholar] [CrossRef]
- McCormack, R.B. A Guide to Australia’s Spiny Freshwater Crayfish, 1st ed.; CSIRO Publishing: Collingwood, VIC, Australia, 2012. [Google Scholar]
- Lynas, J.; Storey, A.W.; Knott, B. Aggressive interactions between three species of freshwater crayfish of the genus Cherax (Decapoda: Parastacidae). Mar. Freshw. Behav. Physiol. 2007, 40, 105–116. [Google Scholar] [CrossRef]
- Williamson, J. Final Determination, the Fitzroy Falls Spiny Crayfish, Euastacus dharawalus, as a Critically Endangered Species; Ref. No. FD 49. File No. FSC 11/01; NSW Fisheries Scientific Committee: Wollstonecraft, Australia, 2011. [Google Scholar]
- McCormack, R.B. Conservation of imperiled crayfish, Euastacus dharawalus (Decapoda: Astacidae: Parastacidae), from the Southern highlands of New South Wales, Australia. J. Crustac. Biol. 2013, 33, 432–439. [Google Scholar] [CrossRef][Green Version]
- McCormack, R.B. Euastacus dharawalus. The IUCN Red List of Threatened Species, 2016: e.T153704A188805771. Available online: https://www.iucnredlist.org/species/153704/188805771 (accessed on 13 September 2022).
- Van Der Meulen, D.; (Department of Primary Industries, Batemans Bay, NSW, Australia). Personal communication, 2021.
- O’Hea Miller, S.B.; Davis, A.R.; Broadhurst, B.; Wong, M.Y.L. Shifts in the Movement and Habitat Use of a Critically Endangered Native Crayfish in the Presence of an Invader; University of Wollongong: Wollongong, NSW, Australia, 2022; to be submitted. [Google Scholar]
- Wraith, J.; Lynch, T.; Minchinton, T.E.; Broad, A.; Davis, A.R. Bait type affects fish assemblages and feeding guilds observed at baited remote underwater video stations. Mar. Ecol. Prog. Ser. 2013, 477, 189–199. [Google Scholar] [CrossRef]
- Martin, L.A.; Moore, P.A. Field observation of agonism in the crayfish, Orconectes rusticus: Shelter use in a natural environment. Ethology 2007, 113, 1192–1201. [Google Scholar] [CrossRef]
- Daws, A.G.; Grills, J.; Konzen, K.; Moore, P.A. Previous experiences alter the outcome of aggressive interactions between males in the crayfish, Procambarus Clarkii. Mar. Freshw. Behav. Physiol. 2002, 35, 139–148. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects model using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Marascuilo, L.A.; McSweeney, M. Nonparametric post hoc comparisons for trend. Psychol. Bull. 1967, 67, 401–412. [Google Scholar] [CrossRef]
- Pavey, C.R.; Fielder, D.R. The influence of size difference on agonistic behaviour in freshwater crayfish, Cherax cuspidatus (Decapoda: Parastacidae). J. Zool. 1996, 238, 445–457. [Google Scholar] [CrossRef]
- Just, W.; Morris, M.R. The Napoleon Complex: Why smaller males pick fights. Evol. Ecol. 2003, 17, 509–522. [Google Scholar] [CrossRef]
- Sih, A.; Cote, J.; Evans, M.; Forgarty, S.; Pruitt, J. Ecological implications of behavioural syndromes. Ecol. Lett. 2012, 15, 278–289. [Google Scholar] [CrossRef]
- Bruce, M.; Doherty, T.; Kaplan, J.; Sutherland, C.; Atema, J. American lobsters, Homarus americanus, use vision for initial opponent evaluation and subsequent memory. Bull. Mar. Sci. 2018, 94, 517–532. [Google Scholar] [CrossRef]
- Percival, D.T.; Moore, P.A. Shelter size influences self-assessment of size in crayfish, Orconectes rusticus: Consequences for agonistic fights. Behaviour 2010, 147, 103–119. [Google Scholar] [CrossRef]
- Wofford, S.J.; Earley, R.L.; Moore, P.A. Evidence for assessment disappears in mixed-sex contests of the crayfish, Orconectes virilis. Behaviour 2015, 152, 995–1018. [Google Scholar] [CrossRef]
- Lane, S.M.; Briffa, M. The price of attack: Rethinking damage costs in animal contests. Anim. Behav. 2017, 126, 23–29. [Google Scholar] [CrossRef]
- Arnott, G.; Elwood, R.W. Information gathering and decision making about resource value in animal contests. Anim. Behav. 2008, 76, 529–542. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).