Effects of Zearalenone on Apoptosis and Copper Accumulation of Goat Granulosa Cells In Vitro
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Counting Kit—8 Assays
2.3. Cell Proliferation Analysis
2.4. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.5. Protein Extraction and Western Blot Analysis
2.6. Flow Cytometry Analysis of the Cell Cycle and Apoptosis
2.7. Atomic Absorption Spectrum (AAS) Measurement of Copper
2.8. Statistical Analysis
3. Results
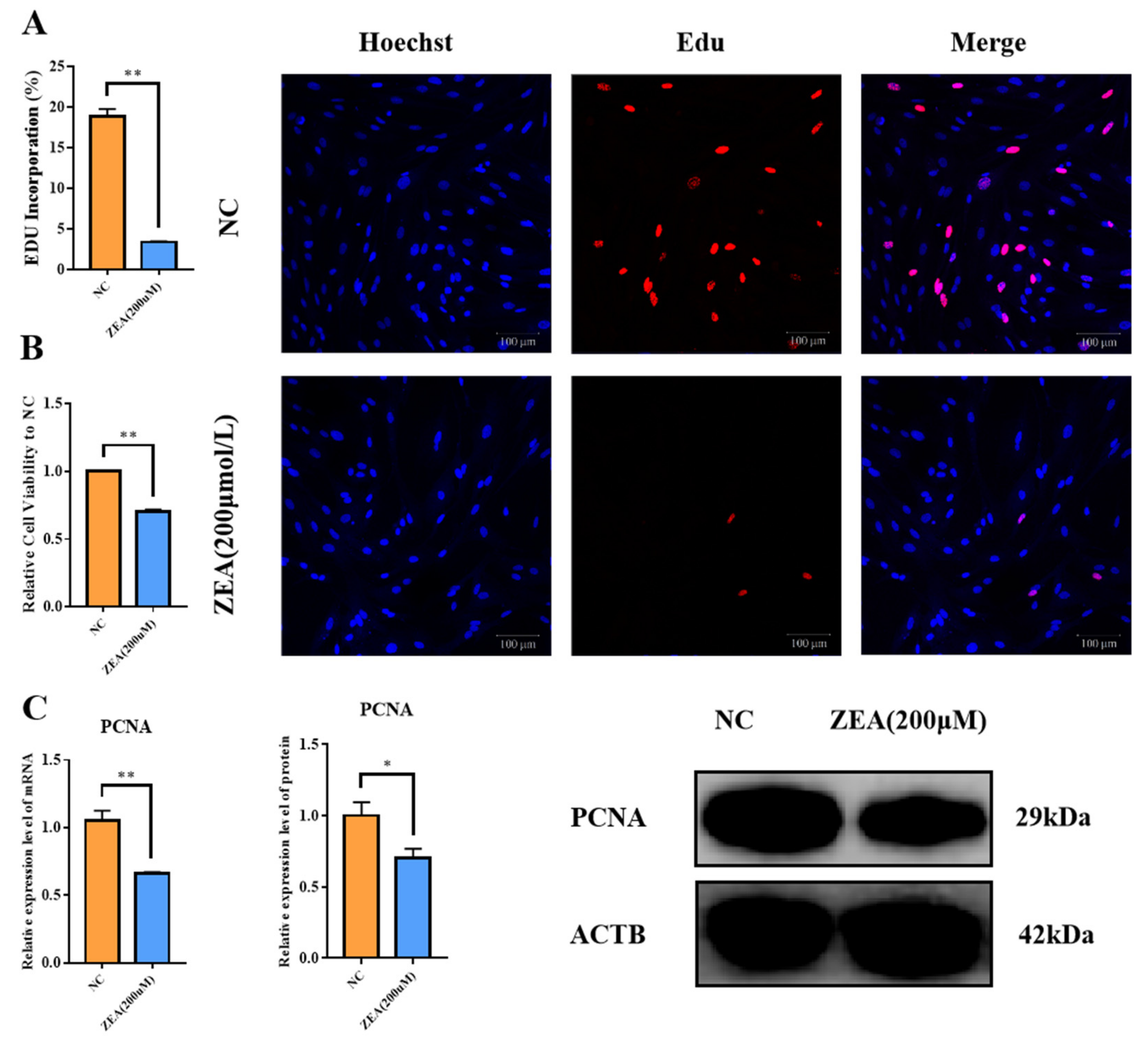
3.1. ZEA Treatment Decreased Proliferation and Cell Viability in GCs
3.2. Cell Cycle Arrest with ZEA Treatment
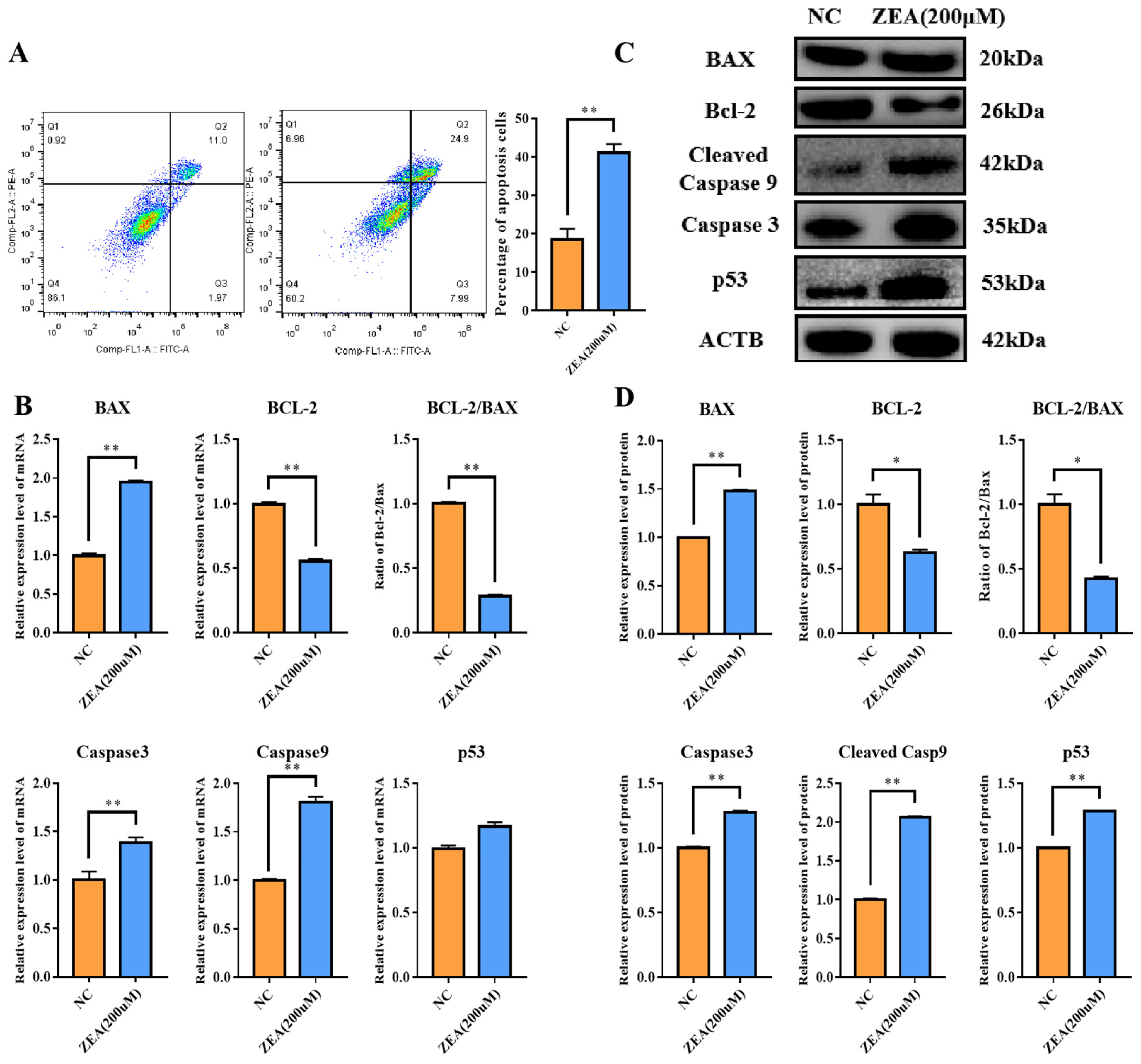
3.3. ZEA Treatment Promoted Cell Apoptosis
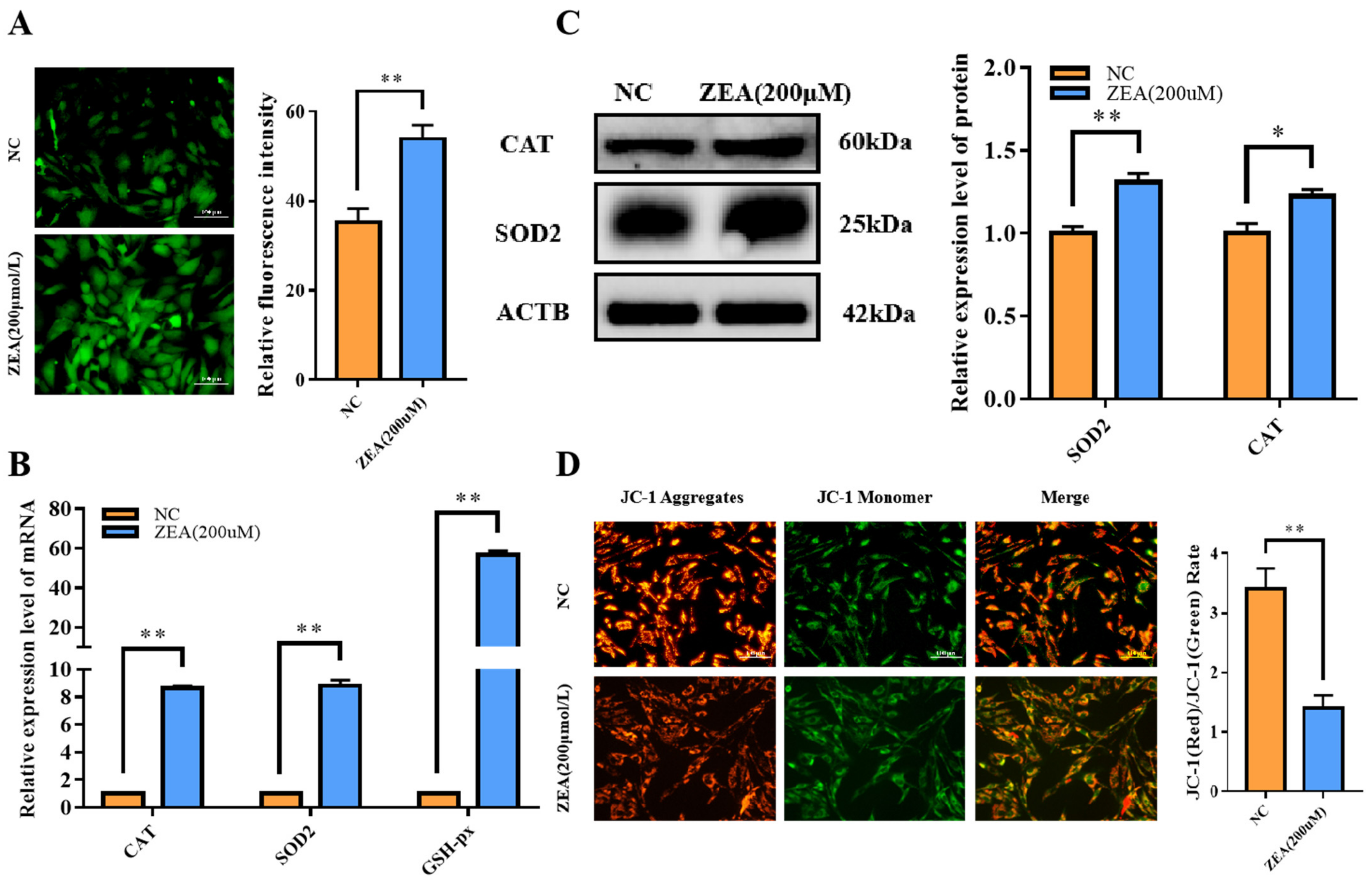
3.4. ZEA Treatment Triggered Oxidative Stress and Mitochondrial Dysfunction in GCs
3.5. ZEA Treatment Disturbed the Estrogen Synthesis in GCs
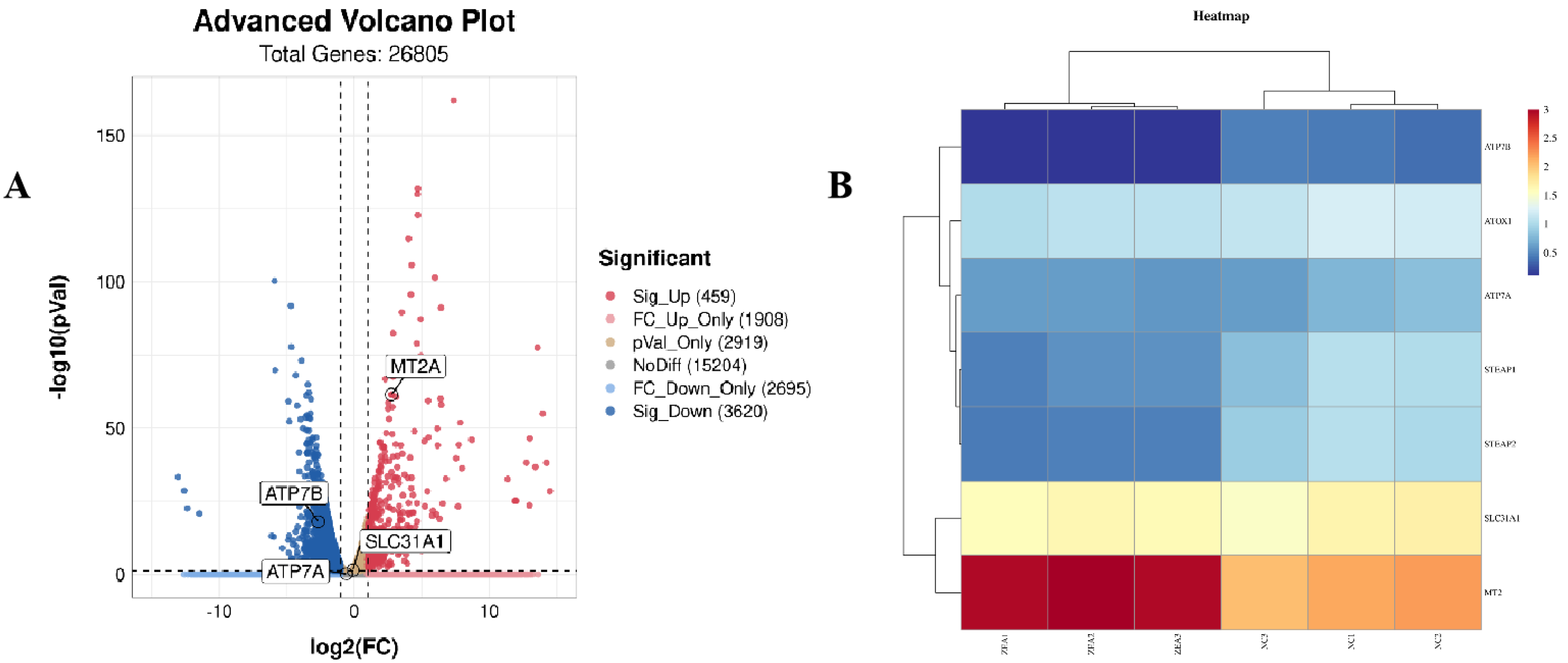
3.6. ZEA Treatment Increased Copper Accumulation in GCs
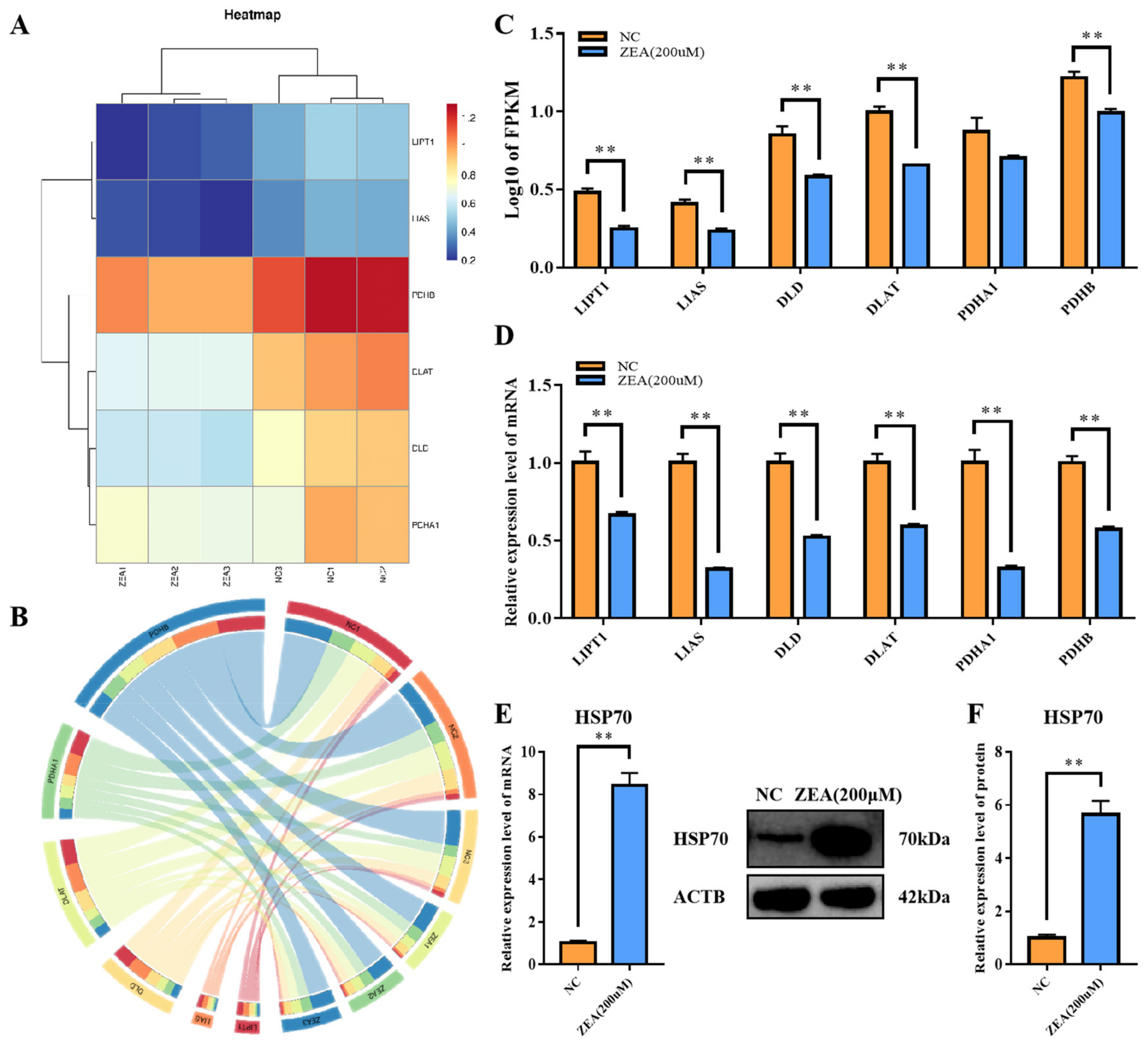
3.7. ZEA Treatment Interfered with the TCA Cycle Related Enzyme Gene Expression and Rose Protein Toxic Stress in GCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humpf, H.U.; Voss, K.A. Effects of thermal food processing on the chemical structure and toxicity of fumonisin mycotoxins. Mol. Nutr. Food Res. 2004, 48, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Jia, S.; Xue, D.; Rajput, S.A.; Liu, M.; Qi, D.; Wang, S. Dual effects of zearalenone on aflatoxin B1-induced liver and mammary gland toxicity in pregnant and lactating rats. Ecotoxicol. Environ. Saf. 2022, 245, 114115. [Google Scholar] [CrossRef]
- Gao, D.; Cao, X.; Ren, H.; Wu, L.; Yan, Y.; Hua, R.; Xing, W.; Lei, M.; Liu, J. Immunotoxicity and uterine transcriptome analysis of the effect of zearalenone (ZEA) in sows during the embryo attachment period. Toxicol. Lett. 2022, 357, 33–42. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Sun, X.F.; Wu, R.Y.; Cheng, S.F.; Zhang, G.L.; Zhai, Q.Y.; Liu, X.L.; Zhao, Y.; Shen, W.; Li, L. Zearalenone exposure elevated the expression of tumorigenesis genes in mouse ovarian granulosa cells. Toxicol. Appl. Pharmacol. 2018, 356, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Wu, R.Y.; Sun, X.F.; Cheng, S.F.; Zhang, R.Q.; Zhang, T.Y.; Zhang, X.F.; Zhao, Y.; Shen, W.; Li, L. Mycotoxin zearalenone exposure impairs genomic stability of swine follicular granulosa cells in vitro. Int. J. Biol. Sci. 2018, 14, 294–305. [Google Scholar] [CrossRef]
- Zhu, X.; Zeng, Z.; Chen, Y.; Li, R.; Tang, X.; Zhu, X.; Huo, J.; Liu, Y.; Zhang, L.; Chen, J. Genotoxicity of three mycotoxin contaminants of rice: 28-day multi-endpoint assessment in rats. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2021, 867, 503369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Wang, C.C.; Leung, L.K. Effect of zeranol on expression of apoptotic and cell cycle proteins in murine placentae. Toxicology 2013, 314, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wen, J.; Cheng, J.; Shen, W.; Zhou, S.; Yan, W.; Shen, L.; Luo, A.; Wang, S. Age-associated up-regulation of EGR1 promotes granulosa cell apoptosis during follicle atresia in mice through the NF-kappaB pathway. Cell Cycle 2016, 15, 2895–2905. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001, 45 (Suppl. 3), S116–S124. [Google Scholar] [CrossRef]
- Fitzpatrick, S.L.; Funkhouser, J.M.; Sindoni, D.M.; Stevis, P.E.; Deecher, D.C.; Bapat, A.R.; Merchenthaler, I.; Frail, D.E. Expression of estrogen receptor-beta protein in rodent ovary. Endocrinology 1999, 140, 2581–2591. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Zhu, C.C.; Xu, Y.X.; Cui, X.S.; Kim, N.H.; Sun, S.C. Zearalenone exposure affects mouse oocyte meiotic maturation and granulosa cell proliferation. Environ. Toxicol. 2015, 30, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Lei, C.; Liao, J.; Li, Q.; Shi, J.; Zhang, H.; Guo, J.; Han, Q.; Hu, L.; Li, Y.; Pan, J.; et al. Copper induces mitochondria-mediated apoptosis via AMPK-mTOR pathway in hypothalamus of Pigs. Ecotoxicol. Environ. Saf. 2021, 220, 112395. [Google Scholar] [CrossRef] [PubMed]
- Fazelian, N.; Movafeghi, A.; Yousefzadi, M.; Rahimzadeh, M. Cytotoxic impacts of CuO nanoparticles on the marine microalga Nannochloropsis oculata. Environ. Sci. Pollut. Res. Int. 2019, 26, 17499–17511. [Google Scholar] [CrossRef]
- Khan, A.; Khan, M.Z.; Dou, J.; Xu, H.; Liu, L.; Zhu, H.; Wang, Y. SOD1 Gene Silencing Promotes Apoptosis and Suppresses Proliferation of Heat-Stressed Bovine Granulosa Cells via Induction of Oxidative Stress. Vet. Sci. 2021, 8, 326. [Google Scholar] [CrossRef]
- Filannino, A.; Stout, T.A.; Gadella, B.M.; Sostaric, E.; Pizzi, F.; Colenbrander, B.; Dell’Aquila, M.E.; Minervini, F. Dose-response effects of estrogenic mycotoxins (zearalenone, alpha- and beta-zearalenol) on motility, hyperactivation and the acrosome reaction of stallion sperm. Reprod. Biol. Endocrinol. 2011, 9, 134. [Google Scholar] [CrossRef]
- Scott, P.M. Mycotoxins in Feeds and Ingredients and their Origin. J. Food Prot. 1978, 41, 385–398. [Google Scholar] [CrossRef]
- Fushimi, Y.; Takagi, M.; Monniaux, D.; Uno, S.; Kokushi, E.; Shinya, U.; Kawashima, C.; Otoi, T.; Deguchi, E.; Fink-Gremmels, J. Effects of Dietary Contamination by Zearalenone and Its Metabolites on Serum Anti-Mullerian Hormone: Impact on the Reproductive Performance of Breeding Cows. Reprod. Domest. Anim. 2015, 50, 834–839. [Google Scholar] [CrossRef]
- Blankenship, L.T.; Dickey, J.F.; Bodine, A.B. In vitro mycotoxin binding to bovine uterine steroid hormone receptors. Theriogenology 1982, 17, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Dell’Aquila, M.E.; Maritato, F.; Minoia, P.; Visconti, A. Toxic effects of the mycotoxin zearalenone and its derivatives on in vitro maturation of bovine oocytes and 17 beta-estradiol levels in mural granulosa cell cultures. Toxicol. In Vitro 2001, 15, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Wan, S.; Wang, S.; Khan, A.; Guo, J.; Zheng, X.; Li, H.; Sun, N. Scutellarin protects mouse ovarian granulosa cells from injury induced by the toxin zearalenone. Food Funct. 2021, 12, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, X.L.; Zhang, F.L.; Tian, Y.; Zhu, M.; Meng, L.Y.; Dyce, P.W.; Shen, W.; Li, L. Whole-transcriptome analysis of the toxic effects of zearalenone exposure on ceRNA networks in porcine granulosa cells. Environ. Pollut. 2020, 261, 114007. [Google Scholar] [CrossRef]
- Chen, F.; Wen, X.; Lin, P.; Chen, H.; Wang, A.; Jin, Y. HERP depletion inhibits zearalenone-induced apoptosis through autophagy activation in mouse ovarian granulosa cells. Toxicol. Lett. 2019, 301, 1–10. [Google Scholar] [CrossRef]
- Yao, X.; Wang, Z.; El-Samahy, M.A.; Ren, C.; Liu, Z.; Wang, F.; You, P. Roles of vitamin D and its receptor in the proliferation and apoptosis of luteinised granulosa cells in the goat. Reprod. Fertil. Dev. 2020, 32, 335–348. [Google Scholar] [CrossRef]
- Zhu, L.; Yuan, H.; Guo, C.; Lu, Y.; Deng, S.; Yang, Y.; Wei, Q.; Wen, L.; He, Z. Zearalenone induces apoptosis and necrosis in porcine granulosa cells via a caspase-3- and caspase-9-dependent mitochondrial signaling pathway. J. Cell. Physiol. 2012, 227, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Roudbari, N.H.; Amidi, F.; Parivar, K. Investigating the effect of Sulforaphane on AMPK/AKT/NRF2 pathway in human granulosa-lutein cells under H(2)O(2)-induced oxidative stress. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 276, 125–133. [Google Scholar] [CrossRef]
- Malekinejad, H.; Van Tol, H.T.; Colenbrander, B.; Fink-Gremmels, J. Expression of 3alpha- and 3beta-hydroxy steroid dehydrogenase mRNA in COCs and granulosa cells determines Zearalenone biotransformation. Toxicol. In Vitro 2006, 20, 458–463. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Z.; Cui, H.; Ding, K.; Zhao, Y.; Ma, X.; Adetunji, A.O.; Min, L. Effect of Zearalenone-Induced Ferroptosis on Mice Spermatogenesis. Animals 2022, 12, 3026. [Google Scholar] [CrossRef]
- Wang, G.; Qin, S.; Zheng, Y.; Xia, C.; Zhang, P.; Zhang, L.; Yao, J.; Yi, Y.; Deng, L. T-2 Toxin Induces Ferroptosis by Increasing Lipid Reactive Oxygen Species (ROS) and Downregulating Solute Carrier Family 7 Member 11 (SLC7A11). J. Agric. Food Chem. 2021, 69, 15716–15727. [Google Scholar] [CrossRef] [PubMed]
- Volesky, B.; Holan, Z.R. Biosorption of heavy metals. Biotechnol. Prog. 1995, 11, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Okamoto, A.; Hashimoto, K.; Nakamura, R. Sulfur-Mediated Electron Shuttling Sustains Microbial Long-Distance Extracellular Electron Transfer with the Aid of Metallic Iron Sulfides. Langmuir 2015, 31, 7427–7434. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, F.; Cheng, L.; Bo, X.; Liu, T.; Li, M. Fabrication of manganese borate/iron carbide encapsulated in nitrogen and boron co-doped carbon nanowires as the accelerated alkaline full water splitting bi-functional electrocatalysts. J. Colloid. Interface Sci. 2022, 629 Pt B, 179–192. [Google Scholar] [CrossRef]
- Szekacs, I.; Tokarz, P.; Horvath, R.; Kovacs, K.; Kubas, A.; Shimura, M.; Brasun, J.; Murzin, V.; Caliebe, W.; Szewczuk, Z.; et al. In vitro SOD-like activity of mono- and di-copper complexes with a phosphonate substituted SALAN-type ligand. Chem. Biol. Interact. 2019, 306, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Kwok, M.L.; Chan, K.M. Functional characterization of copper transporters zCtr1, zAtox1, zAtp7a and zAtp7b in zebrafish liver cell line ZFL. Metallomics 2019, 11, 1532–1546. [Google Scholar] [CrossRef]
- Petris, M.J. The SLC31 (Ctr) copper transporter family. Pflug. Arch. 2004, 447, 752–755. [Google Scholar] [CrossRef]
- Tadini-Buoninsegni, F.; Bartolommei, G.; Moncelli, M.R.; Inesi, G.; Galliani, A.; Sinisi, M.; Losacco, M.; Natile, G.; Arnesano, F. Translocation of platinum anticancer drugs by human copper ATPases ATP7A and ATP7B. Angew. Chem. Int. Ed. Engl. 2014, 53, 1297–1301. [Google Scholar] [CrossRef]
- Kahlson, M.A.; Dixon, S.J. Copper-induced cell death. Science 2022, 375, 1231–1232. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Detappe, A.; Cai, K.; Keys, H.R.; Brune, Z.; Ying, W.; Thiru, P.; Reidy, M.; Kugener, G.; Rossen, J.; et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol. 2019, 15, 681–689. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Ma, J.; Wei, Z.; Yang, Y.; Li, D.; Wan, Y. Effects of Zearalenone on Apoptosis and Copper Accumulation of Goat Granulosa Cells In Vitro. Biology 2023, 12, 100. https://doi.org/10.3390/biology12010100
Liu L, Ma J, Wei Z, Yang Y, Li D, Wan Y. Effects of Zearalenone on Apoptosis and Copper Accumulation of Goat Granulosa Cells In Vitro. Biology. 2023; 12(1):100. https://doi.org/10.3390/biology12010100
Chicago/Turabian StyleLiu, Liang, Jianyu Ma, Zongyou Wei, Yingnan Yang, Dongxu Li, and Yongjie Wan. 2023. "Effects of Zearalenone on Apoptosis and Copper Accumulation of Goat Granulosa Cells In Vitro" Biology 12, no. 1: 100. https://doi.org/10.3390/biology12010100
APA StyleLiu, L., Ma, J., Wei, Z., Yang, Y., Li, D., & Wan, Y. (2023). Effects of Zearalenone on Apoptosis and Copper Accumulation of Goat Granulosa Cells In Vitro. Biology, 12(1), 100. https://doi.org/10.3390/biology12010100





