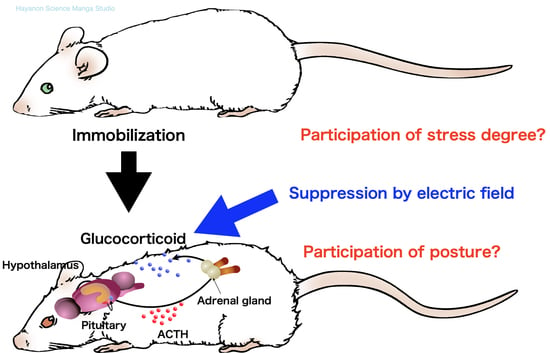
Suppression of Glucocorticoid Response in Stressed Mice Using 50 Hz Electric Field According to Immobilization Degree and Posture
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
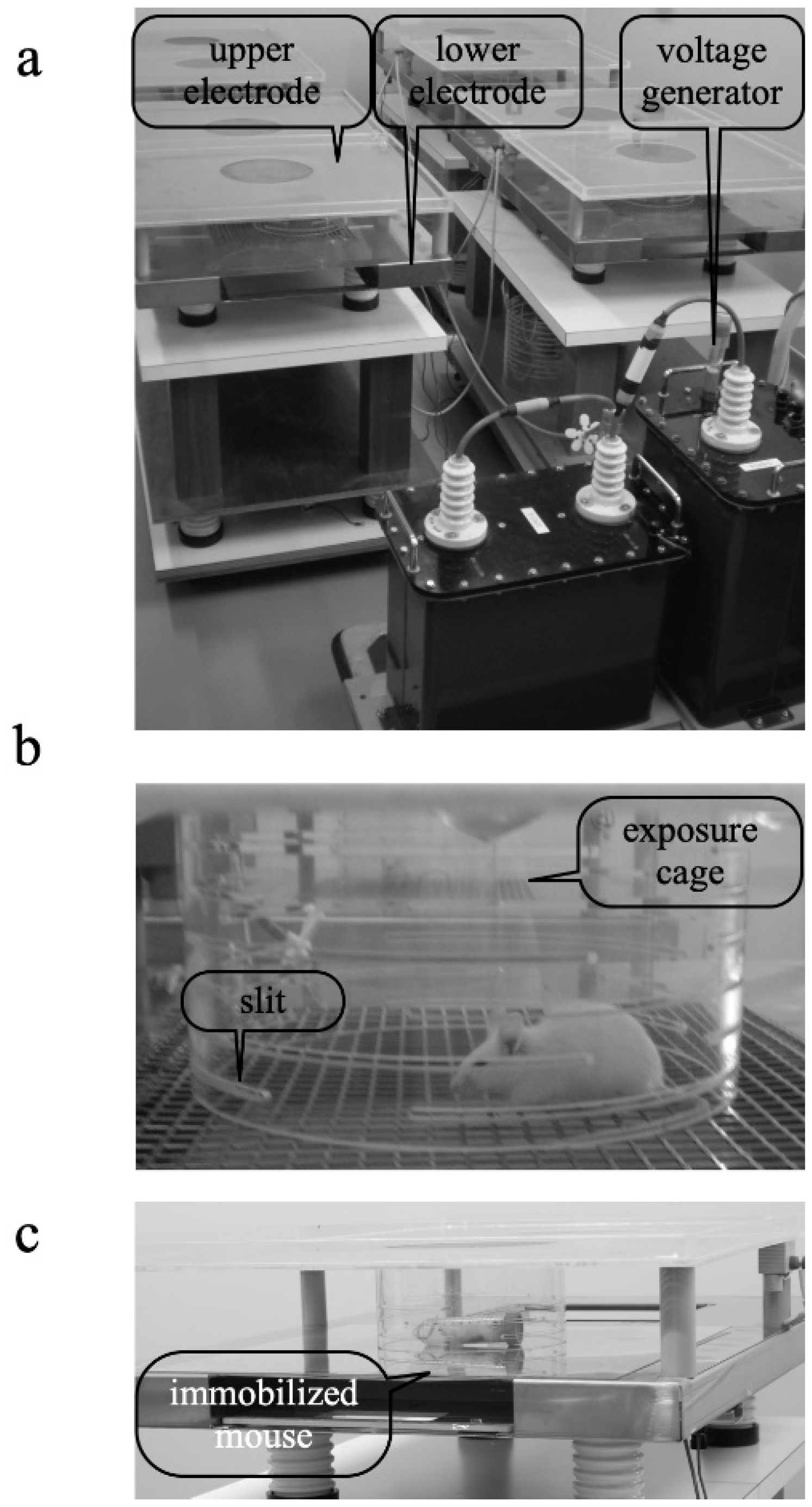
2.2. EF-Treatment System
2.3. Immobilization Stress
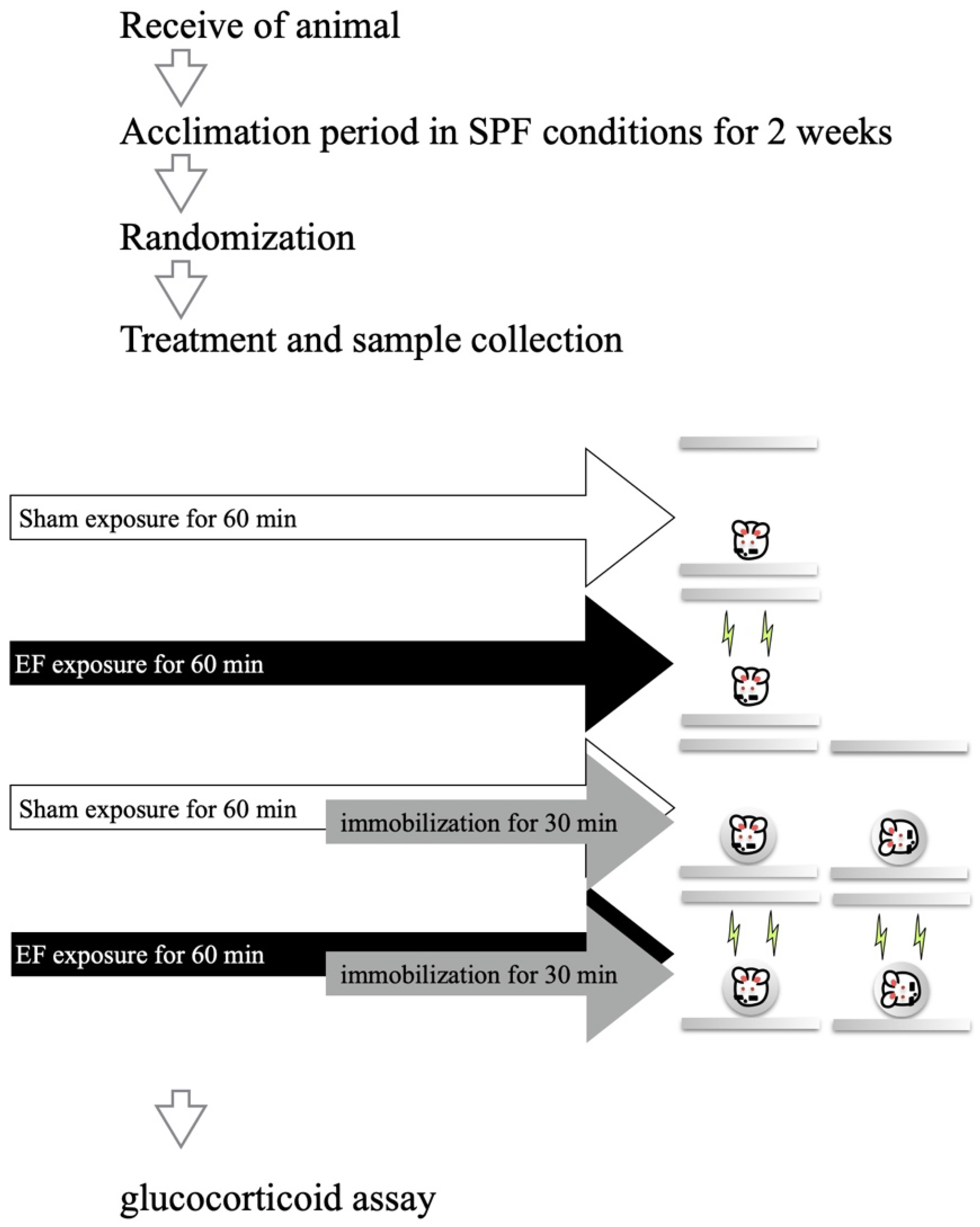
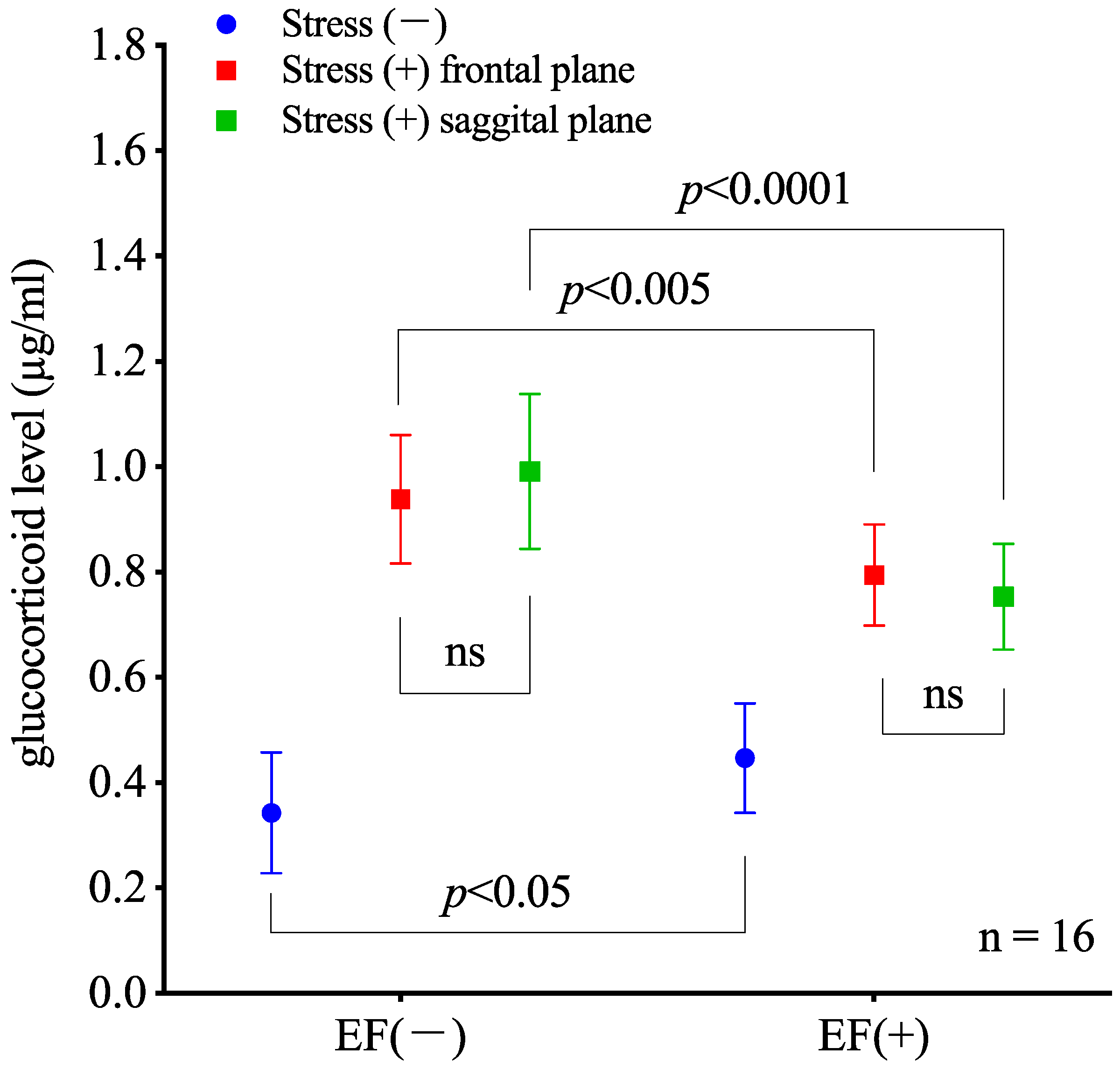
2.4. Effect of Mouse Posture on EF-Induced Suppression of Stress Response
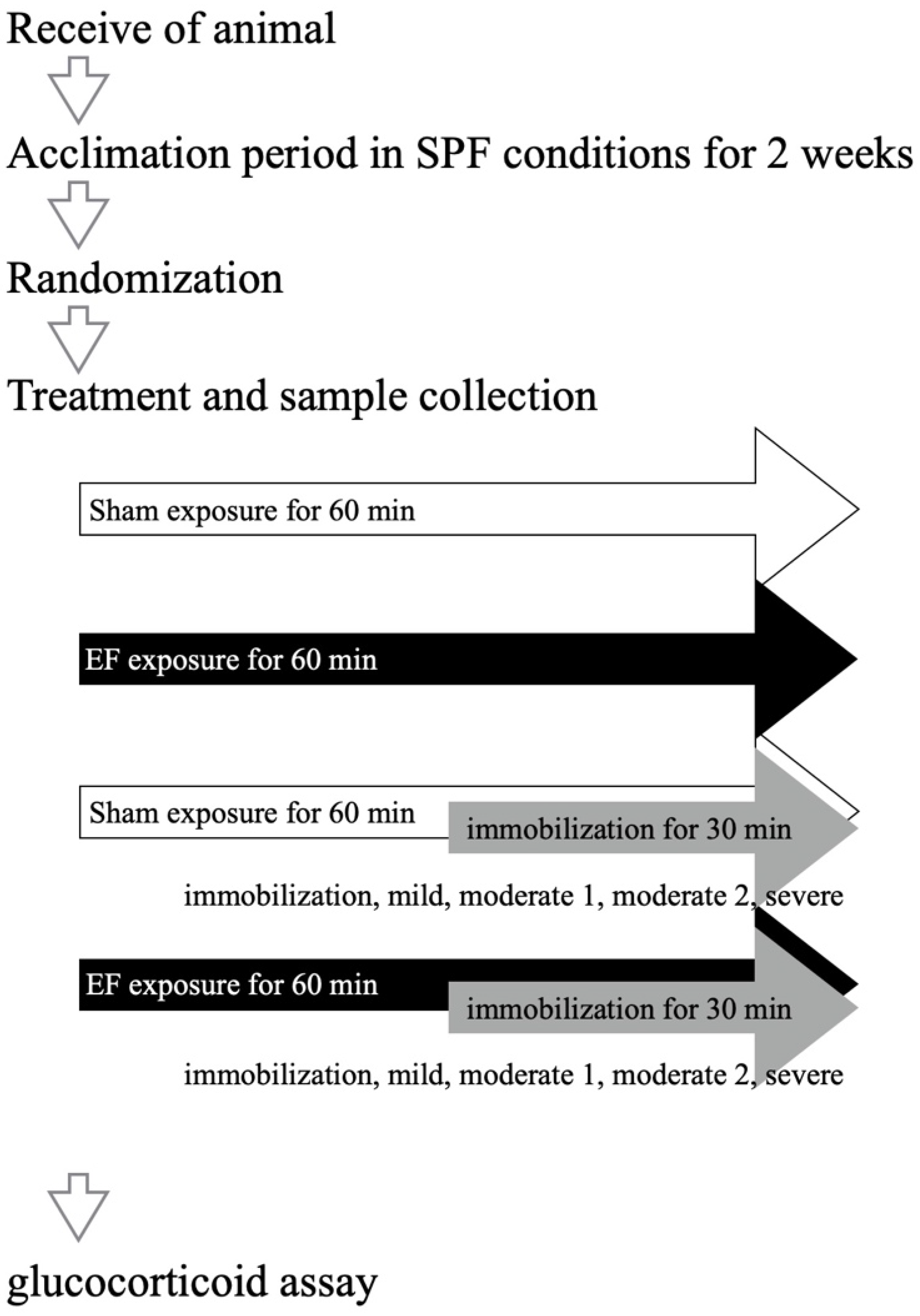
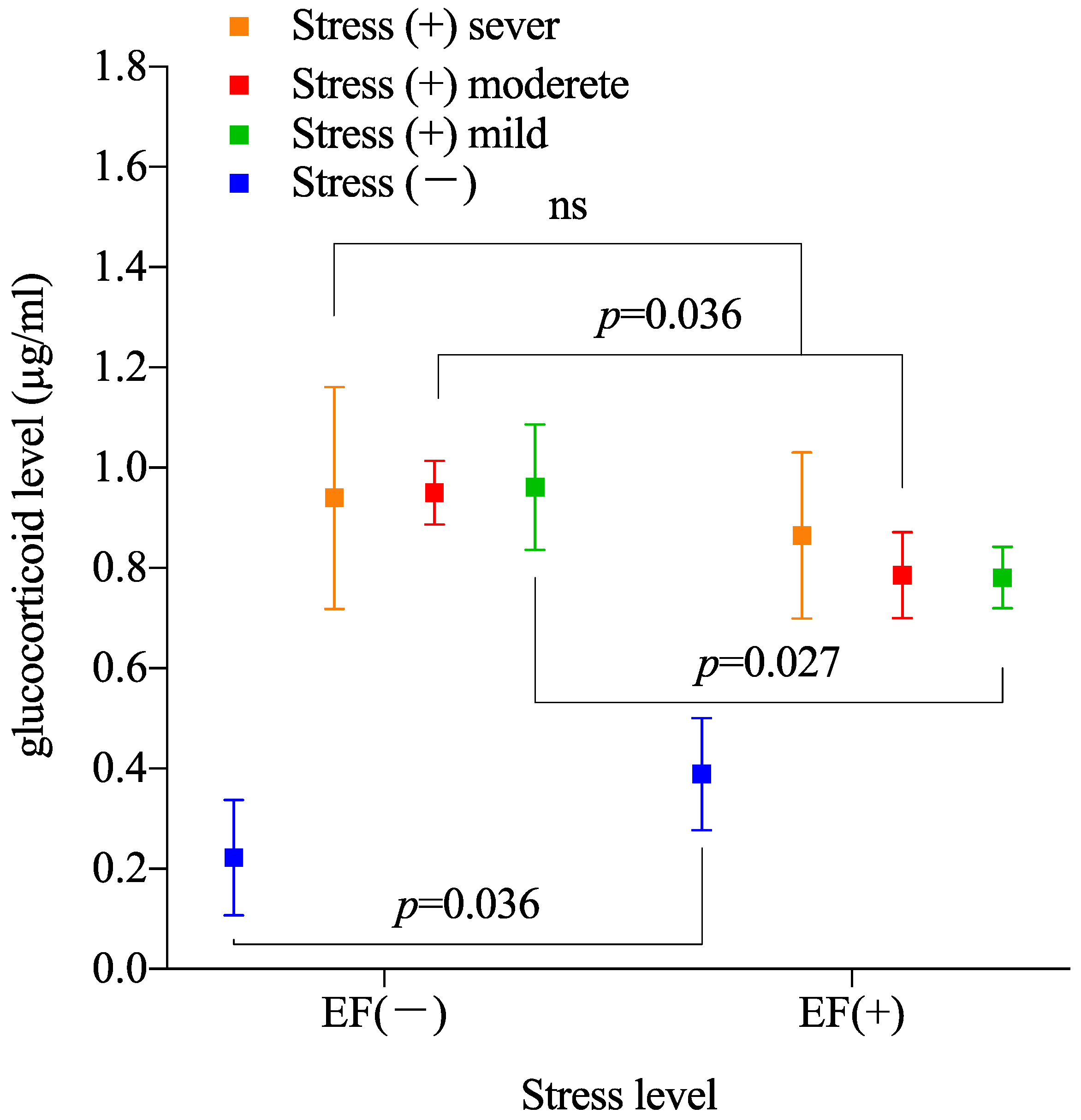
2.5. Immobilization Degree and EF Suppression of Stress Response
2.6. Statistical Analysis
3. Results
3.1. Effect of Mouse Posture on EF-Induced Suppression of Stress Response
3.2. Effect of Stress Degree on Suppressive Effect of EF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Gaps in Knowledge Relevant to the “Guidelines for Limiting Exposure to Time-Varying Electric and Magnetic Fields (1 Hz–100 kHz)”. Health Phys. 2020, 118, 533–542. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Principles for Non-ionizing Radiation Protection. Health Phys. 2020, 118, 477–482. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for Limiting Exposure to Time-Varying Electric and Magnetic Fields (1 Hz to 100 kHz). Health Phys. 2010, 99, 818–836. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Extremely Low Frequency Fields Environmental Health Criteria Monograph; WHO: Geneva, Switzerland, 2007; Volume 238, Available online: https://www.who.int/publications/i/item/9789241572385 (accessed on 9 July 2022).
- Mitani, Y.; Matsugi, A.; Okano, H.; Nedachi, T.; Hara, H. Effect of Exposure to a High-Voltage Alternating Current Electric Field on Muscle Extensibility. J. Jpn. Soc. Balneol. Climatol. Phys. Med. 2015, 78, 244–252. [Google Scholar]
- Mattsson, M.O.; Simkó, M. Emerging Medical Applications Based on Non-ionizing Electromagnetic Fields from 0 Hz to 10 THz. Med. Devices 2019, 12, 347–368. [Google Scholar] [CrossRef]
- Ohtsuki, T.; Nabeta, T.; Nakanishi, H.; Kawahata, H.; Ogihara, T.; Morishita, R.; Aoki, M. Electric Field Exposure Improves Subjective Symptoms Related to Sleeplessness in College Students: A Pilot Study of Electric Field Therapy for Sleep Disorder. IEMAMC 2017, 17, 37–48. [Google Scholar] [CrossRef][Green Version]
- Shinba, T.; Takahashi, K.; Kanetake, S.; Nedachi, T.; Yamaneki, M.; Doge, F.; Hori, T.; Harakawa, S.; Miki, M.; Hara, H.; et al. A Pilot Study on Electric Field Therapy for Chronic Pain with No Obvious Underlying Diseases. Jpn. Soc. Integrat. Med. 2012, 5, 68–72. [Google Scholar]
- Ito, F.; Ohsaki, K.; Takahashi, K.; Hara, H. The Effects of Electric Field Therapeutic Device (Healthtron) on the Stiffness in the Neck and Shoulder Area: Changes in Subjective Symptoms, Blood Circulation and the Autonomic Nervous System. J. Jpn. Soc. Balneol. Climatol. Phys. Med. 2005, 68, 110–121. Available online: http://jstage.jst.go.jp/article/onki1962/68/2/68_2_110/_article/-char/ja/ (accessed on 9 July 2022).
- Shinba, T.; Nedachi, T.; Harakawa, S. Extremely Low-Frequency Electric Field Exposure Increases Theta Power of EEG in Both Eyes-Open and Eyes-Closed Resting Conditions in Healthy Male Subjects. IEEJ Trans. Electr. Electron. Eng. 2021, 16, 592–599. [Google Scholar] [CrossRef]
- Nedachi, T.; Haketa, K.; Harakawa, S.; Miura, N.; Wakame, K. Effect of Combining Sleep-Promoting Food Intake and Electric Field Application on Sleep in Healthy Participants: A Pilot Study. Funct. Foods Health Dis. 2021, 11, 659. [Google Scholar] [CrossRef]
- Coskun, O.; Comlekci, S. Effect of ELF Electric Field on Some on Biochemistry Characters in the Rat Serum. Toxicol. Ind. Health. 2011, 27, 329–333. [Google Scholar] [CrossRef]
- Akpinar, D.; Ozturk, N.; Ozen, S.; Agar, A.; Yargicoglu, P. The Effect of Different Strengths of Extremely Low-Frequency Electric Fields on Antioxidant Status, Lipid Peroxidation, and Visual Evoked Potentials. Electromagn. Biol. Med. 2012, 31, 436–448. [Google Scholar] [CrossRef]
- Di, G.; Gu, X.; Lin, Q.; Wu, S.; Kim, H.B. A Comparative Study on Effects of Static Electric Field and Power Frequency Electric Field on Hematology in Mice. Ecotoxicol. Environ. Saf. 2018, 166, 109–115. [Google Scholar] [CrossRef]
- Gok, D.K.; Akpinar, D.; Hidisoglu, E.; Ozen, S.; Agar, A.; Yargicoglu, P. The Developmental Effects of Extremely Low Frequency Electric Fields on Visual and Somatosensory Evoked Potentials in Adult Rats. Electromagn. Biol. Med. 2016, 35, 65–74. [Google Scholar] [CrossRef]
- Weigel, R.J.; Jaffe, R.A.; Lundstrom, D.L.; Forsythe, W.C.; Anderson, L.E. Stimulation of Cutaneous Mechanoreceptors by 60-Hz Electric Fields. Bioelectromagnetics 1987, 8, 337–350. [Google Scholar] [CrossRef]
- Weigel, R.J.; Lundstrom, D.L. Effect of Relative Humidity on the Movement of Rat Vibrissae in a 60-Hz Electric Field. Bioelectromagnetics 1987, 8, 107–110. [Google Scholar] [CrossRef]
- Romo, R.; Hernández, A.; Zainos, A.; Brody, C.; Salinas, E. Exploring the Cortical Evidence of a Sensory-Discrimination Process. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 1039–1051. [Google Scholar] [CrossRef][Green Version]
- Romo, R.; Hernández, A.; Zainos, A.; Brody, C.D.; Lemus, L. Sensing without Touching: Psychophysical Performance Based on Cortical Microstimulation. Neuron 2000, 26, 273–278. [Google Scholar] [CrossRef]
- Romo, R.; Hernández, A.; Zainos, A.; Salinas, E. Somatosensory Discrimination Based on Cortical Microstimulation. Nature 1998, 392, 387–390. [Google Scholar] [CrossRef]
- Kato, M.; Ohta, S.; Shimizu, K.; Tsuchida, Y.; Matsumoto, G. Detection-Threshold of 50-Hz Electric Fields by Human Subjects. Bioelectromagnetics 1989, 10, 319–327. [Google Scholar] [CrossRef]
- Reilly, J.P. Neuroelectric Mechanisms Applied to Low Frequency Electric and Magnetic Field Exposure Guidelines–part I: Sinusoidal Waveforms. Health Phys. 2002, 83, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Harakawa, S.; Hori, T.; Inoue, N.; Okano, H.; Nedachi, T.; Suzuki, H. Effect of Extensive Electric Field Therapy in Bone Density. Jpn. Soc. Integrat. Med. 2014, 7, 60–66. [Google Scholar]
- Harakawa, S.; Inoue, N.; Hori, T.; Tochio, K.; Kariya, T.; Takahashi, K.; Doge, F.; Suzuki, H.; Nagasawa, H. Effects of a 50-Hz Electric Field on Plasma Lipid Peroxide Level and Antioxidant Activity in Rats. Bioelectromagnetics 2005, 26, 589–594. [Google Scholar] [CrossRef]
- Takahashi, K.; Doge, F.; Yoshioka, M. Prolonged Ca2+ Transients in ATP-Stimulated Endothelial Cells Exposed to 50-Hz Electric Fields. Cell Biol. Int. 2005, 29, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kuroki, M.; Doge, F.; Sawasaki, Y.; Yoshioka, M. Effects of Low-Frequency Electric Fields on the Intracellular Ca2+ Response Induced in Human Vascular Endothelial Cells by Vasoactive Substances. Electromagn. Biol. Med. 2002, 21, 279–286. [Google Scholar] [CrossRef]
- Harakawa, S.; Nedachi, T.; Suzuki, H. Extremely Low-Frequency Electric Field Suppresses Not Only Induced Stress Response but Also Stress-Related Tissue Damage in Mice. Sci. Rep. 2020, 10, 20930. [Google Scholar] [CrossRef]
- Harakawa, S.; Nedachi, T.; Shinba, T.; Suzuki, H. Stress-Reducing Effect of a 50-Hz Electric Field in Mice after Repeated Immobilizations, Electric Field Shields, and Polarization of the Electrodes. Biology 2022, 11, 323. [Google Scholar] [CrossRef]
- Kariya, T.; Hori, T.; Harakawa, S.; Inoue, N.; Nagasawa, H. Exposure to 50-Hz Electric Fields on Stress Response Initiated by Infection with the Protozoan Parasite, Toxoplasma gondii, in Mice. J. Protozool. Res. 2006, 16, 51–59. [Google Scholar]
- Imaki, T.; Nahan, J.L.; Rivier, C.; Sawchenko, P.E.; Vale, W. Differential Regulation of Corticotropin-Releasing Factor mRNA in Rat Brain Regions by Glucocorticoids and Stress. J. Neurosci. 1991, 11, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. The General Adaptation Syndrome and the Diseases of Adaptation. J. Clin. Endocrinol. Metab. 1946, 6, 117–230. [Google Scholar] [CrossRef]
- Nicolaides, N.C.; Kyratzi, E.; Lamprokostopoulou, A.; Chrousos, G.P.; Charmandari, E. Stress, the Stress System and the Role of Glucocorticoids. Neuroimmunomodulation 2015, 22, 6–19. [Google Scholar] [CrossRef]
- Hori, T.; Inoue, N.; Suzuki, H.; Harakawa, S. Exposure to 50-Hz Electric Fields Reduces Stress-Induced Glucocorticoid Levels in BALB/C Mice in a kV/m- and Duration-Dependent Manner. Bioelectromagnetics 2015, 36, 302–308. [Google Scholar] [CrossRef]
- Hori, T.; Inoue, N.; Suzuki, H.; Harakawa, S. Configuration-Dependent Variability of the Effect of an Electric Field on the Plasma Glucocorticoid Level in Immobilized Mice. Bioelectromagnetics 2017, 38, 265–271. [Google Scholar] [CrossRef]
- Harakawa, S.; Hori, T.; Nedachi, T.; Suzuki, H. Gender and Age Differences in the Suppressive Effect of a 50-Hz Electric Field on the Immobilization-Induced Increase of Plasma Glucocorticoid in Mice. Bioelectromagnetics 2020, 41, 156–163. [Google Scholar] [CrossRef]
- Kirby, E.D.; Geraghty, A.C.; Ubuka, T.; Bentley, G.E.; Kaufer, D. Stress Increases Putative Gonadotropin Inhibitory Hormone and Decreases Luteinizing Hormone in Male Rats. Proc. Natl Acad. Sci. USA 2009, 106, 11324–11329. [Google Scholar] [CrossRef]
- Bahlouli, W.; Breton, J.; Lelouard, M.; L’Huillier, C.; Tirelle, P.; Salameh, E.; Amamou, A.; Atmani, K.; Goichon, A.; Bôle-Feysot, C.; et al. Stress-Induced Intestinal Barrier Dysfunction Is Exacerbated during Diet-Induced Obesity. J. Nutr. Biochem. 2020, 81, 108382. [Google Scholar] [CrossRef]
- Lu, X.T.; Liu, Y.F.; Zhao, L.; Li, W.J.; Yang, R.X.; Yan, F.F.; Zhao, Y.X.; Jiang, F. Chronic Psychological Stress Induces Vascular Inflammation in Rabbits. Stress 2013, 16, 87–98. [Google Scholar] [CrossRef]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid Regulation of Inflammation and Its Functional Correlates: From HPA Axis to Glucocorticoid Receptor Dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55–63. [Google Scholar] [CrossRef]
- Hayley, S.; Kelly, O.; Anisman, H. Corticosterone Changes in Response to Stressors, Acute and Protracted Actions of Tumor Necrosis Factor-Alpha, and Lipopolysaccharide Treatments in Mice Lacking the Tumor Necrosis Factor-Alpha p55 Receptor Gene. Neuroimmunomodulation 2004, 11, 241–246. [Google Scholar] [CrossRef]
- Brattsand, R.; Linden, M. Cytokine Modulation by Glucocorticoids: Mechanisms and Actions in Cellular Studies. Aliment. Pharmacol. Ther. 1996, 10 (Suppl. S2), 81–90; discussion 91. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and Disorders of the Stress System. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Zenker, N.; Bernstein, D.E. The Estimation of Small Amounts of Corticosterone in Rat Plasma. J. Biol. Chem. 1958, 231, 695–701. [Google Scholar] [CrossRef]
- Kvetnansky, R.; Weise, V.K.; Thoa, N.B.; Kopin, I.J. Effects of Chronic Guanethidine Treatment and Adrenal Medullectomy on Plasma Levels of Catecholamines and Corticosterone in Forcibly Immobilized Rats. J. Pharmacol. Exp. Ther. 1979, 209, 287–291. [Google Scholar] [PubMed]
- Dieudonné, M. Electromagnetic Hypersensitivity: A Critical Review of Explanatory Hypotheses. Environ. Health 2020, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- House, R.V.; Ratajczak, H.V.; Gauger, J.R.; Johnson, T.R.; Thomas, P.T.; McCormick, D.L. Immune Function and Host Defense in Rodents Exposed to 60-Hz Magnetic Fields. Fundam. Appl. Toxicol. 1996, 34, 228–239. [Google Scholar] [CrossRef]
- Hori, T.; Yamsaard, T.; Ueta, Y.Y.; Harakawa, S.; Kaneko, E.; Miyamoto, A.; Xuan, X.; Toyoda, Y.; Suzuki, H. Exposure of C57BL/6J Male Mice to an Electric Field Improves Copulation Rates with Superovulated Females. J. Reprod. Dev. 2005, 51, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Hjeresen, D.L.; Kaune, W.T.; Decker, J.R.; Phillips, R.D. Effects of 60-Hz Electric Fields on Avoidance Behavior and Activity of Rats. Bioelectromagnetics 1980, 1, 299–312. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harakawa, S.; Hori, T.; Hiramoto, T.; Nedachi, T.; Shinba, T.; Suzuki, H. Suppression of Glucocorticoid Response in Stressed Mice Using 50 Hz Electric Field According to Immobilization Degree and Posture. Biology 2022, 11, 1336. https://doi.org/10.3390/biology11091336
Harakawa S, Hori T, Hiramoto T, Nedachi T, Shinba T, Suzuki H. Suppression of Glucocorticoid Response in Stressed Mice Using 50 Hz Electric Field According to Immobilization Degree and Posture. Biology. 2022; 11(9):1336. https://doi.org/10.3390/biology11091336
Chicago/Turabian StyleHarakawa, Shinji, Takuya Hori, Takao Hiramoto, Takaki Nedachi, Toshikazu Shinba, and Hiroshi Suzuki. 2022. "Suppression of Glucocorticoid Response in Stressed Mice Using 50 Hz Electric Field According to Immobilization Degree and Posture" Biology 11, no. 9: 1336. https://doi.org/10.3390/biology11091336
APA StyleHarakawa, S., Hori, T., Hiramoto, T., Nedachi, T., Shinba, T., & Suzuki, H. (2022). Suppression of Glucocorticoid Response in Stressed Mice Using 50 Hz Electric Field According to Immobilization Degree and Posture. Biology, 11(9), 1336. https://doi.org/10.3390/biology11091336