Current Understanding on the Genetic Basis of Key Metabolic Disorders: A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Diabetes Mellitus (DM)
3. Familial Hypercholesterolemia (FH)
4. Gaucher Disease (GD)
5. Mucopolysaccharidosis Type II (MPS II) (Hunter Syndrome)
6. Krabbe Disease (KD)
7. Metachromatic Leukodystrophy
8. Mitochondrial Encephalopathy with Lactic Acidosis and Stroke-like Episodes (MELAS) Syndrome
9. Niemann-Pick Disease (NPD)
10. Phenylketonuria (PKU)
11. Hereditary Porphyrias
12. Familial Hypertriglyceridemia
13. Galactosemia
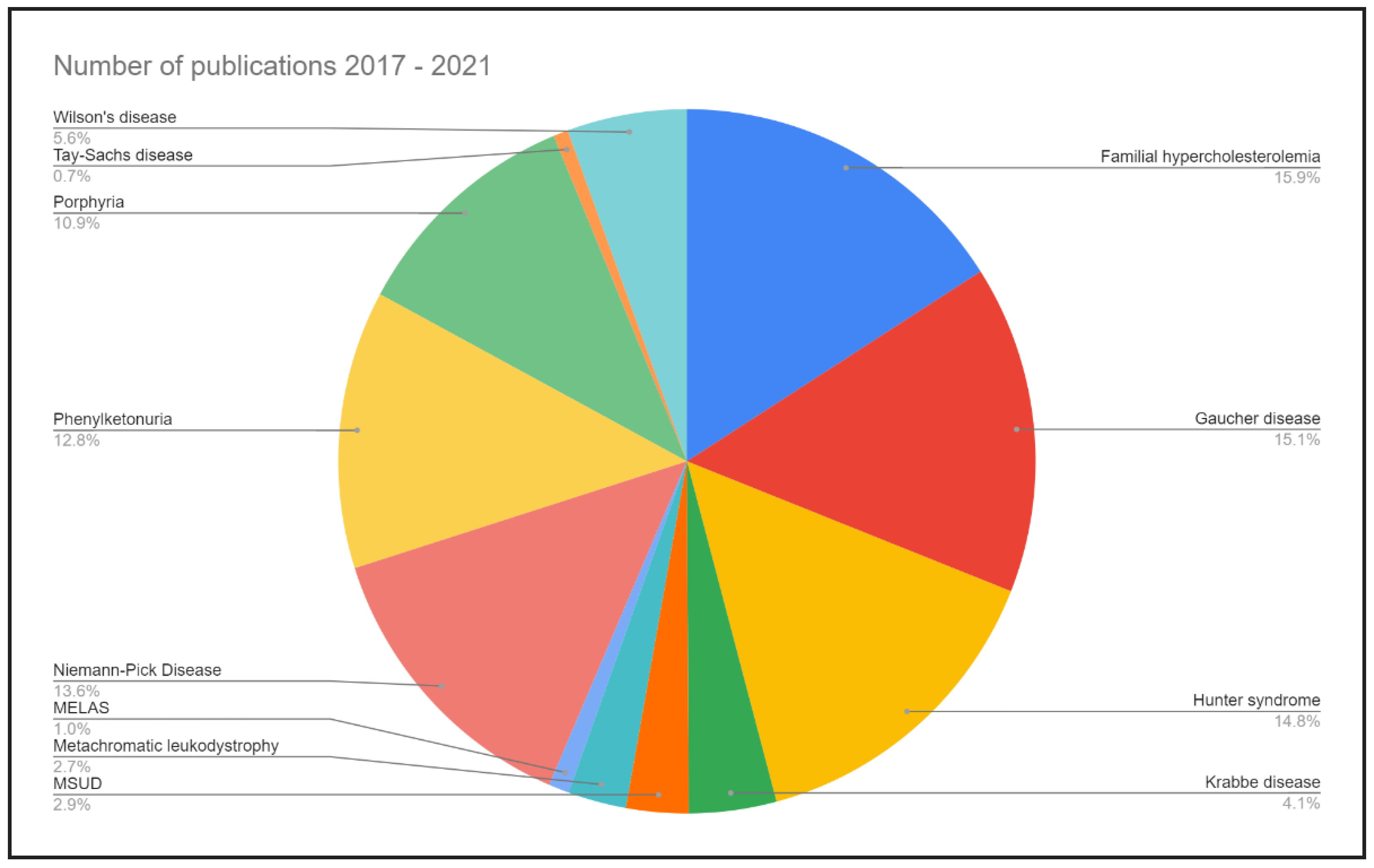
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Du, A.; Liu, H.; Wang, Z.; Hu, J. Systematic Analysis of the Global, Regional and National Burden of Cardiovascular Diseases from 1990 to 2017. J. Epidemiol. Glob. Health 2021, 12, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Mapa-Tassou, C.; Katte, J.-C.; Maadjhou, C.M.; Mbanya, J.C. Economic Impact of Diabetes in Africa. Curr. Diabetes Rep. 2019, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Narayan, K.M.V.; Eggleston, K. Economic Impact of Diabetes in South Asia: The Magnitude of the Problem. Curr. Diabetes Rep. 2019, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Urakami, T.; Kuwabara, R.; Yoshida, K. Economic Impact of Diabetes in Japan. Curr. Diabetes Rep. 2019, 19, 2. [Google Scholar] [CrossRef]
- Ademi, Z.; Marquina, C.; Zomer, E.; Bailey, C.; Owen, A.; Pang, J.; Norman, R.; Watts, G.F.; Liew, D. The economic impact of familial hypercholesterolemia on productivity. J. Clin. Lipidol. 2020, 14, 799–806.e3. [Google Scholar] [CrossRef]
- Hendrieckx, C.; Ivory, N.; Singh, H.; Frier, B.M.; Speight, J. Impact of severe hypoglycaemia on psychological outcomes in adults with Type 2 diabetes: A systematic review. Diabet. Med. 2019, 36, 1082–1091. [Google Scholar] [CrossRef]
- Dennick, K.; Sturt, J.; Speight, J. What is diabetes distress and how can we measure it? A narrative review and conceptual model. J. Diabetes Its Complicat. 2017, 31, 898–911. [Google Scholar] [CrossRef]
- Grzymski, J.J.; Elhanan, G.; Rosado, J.A.M.; Smith, E.; Schlauch, K.A.; Read, R.; Rowan, C.; Slotnick, N.; Dabe, S.; Metcalf, W.J.; et al. Population genetic screening efficiently identifies carriers of autosomal dominant diseases. Nat. Med. 2020, 26, 1235–1239. [Google Scholar] [CrossRef]
- Sharp, S.A.; Rich, S.S.; Wood, A.R.; Jones, S.E.; Beaumont, R.N.; Harrison, J.W.; Schneider, D.A.; Locke, J.M.; Tyrrell, J.; Weedon, M.N.; et al. Development and Standardization of an Improved Type 1 Diabetes Genetic Risk Score for Use in Newborn Screening and Incident Diagnosis. Diabetes Care 2019, 42, 200–207. [Google Scholar] [CrossRef]
- Roman, T.S.; Crowley, S.B.; Roche, M.I.; Foreman, A.K.M.; O’Daniel, J.M.; Seifert, B.A.; Lee, K.; Brandt, A.; Gustafson, C.; DeCristo, D.M.; et al. Genomic Sequencing for Newborn Screening: Results of the NC NEXUS Project. Am. J. Hum. Genet. 2020, 107, 596–611. [Google Scholar] [CrossRef]
- Milluzzo, A.; Maugeri, A.; Barchitta, M.; Sciacca, L.; Agodi, A. Epigenetic Mechanisms in Type 2 Diabetes Retinopathy: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 10502. [Google Scholar] [CrossRef] [PubMed]
- Do, W.L.; Gohar, J.; McCullough, L.E.; Galaviz, K.I.; Conneely, K.N.; Narayan, K.M.V. Examining the association between adiposity and DNA methylation: A systematic review and meta-analysis. Obes. Rev. 2021, 22, e13319. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, V.S.; Reddy, M.A.; Natarajan, R. Emerging Role of Long Non-Coding RNAs in Diabetic Vascular Complications. Front. Endocrinol. 2021, 12, 665811. [Google Scholar] [CrossRef]
- Ibarra, P.E.; García-Solís, P.; Solís-Sáinz, J.C.; Cruz-Hernández, A. Expression of miRNA in obesity and insulin resistance: A review. Endokrynol. Polska 2021, 72, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Bhawal, R.; Fu, Q.; Anderson, E.T.; Gibson, G.E.; Zhang, S. Serum Metabolomic and Lipidomic Profiling Reveals Novel Biomarkers of Efficacy for Benfotiamine in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 13188. [Google Scholar] [CrossRef]
- Kumar, A.; Welch, N.; Mishra, S.; Bellar, A.; Silva, R.N.; Li, L.; Singh, S.S.; Sharkoff, M.; Kerr, A.; Chelluboyina, A.K.; et al. Metabolic reprogramming during hyperammonemia targets mitochondrial function and postmitotic senescence. JCI Insight 2021, 6, 154089. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.C.; Kwitek, A.E. Multi-Omic Approaches to Identify Genetic Factors in Metabolic Syndrome. Compr. Physiol. 2021, 12, 3045–3084. [Google Scholar] [CrossRef]
- Manivannan, M.; Jogalekar, M.P.; Kavitha, M.S.; Maran, B.A.V.; Gangadaran, P. A mini-review on the effects of COVID-19 on younger individuals. Exp. Biol. Med. 2020, 246, 293–297. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Smith, M.D.; Kim, S.; Sotirchos, E.S.; Kornberg, M.D.; Douglas, M.; Nourbakhsh, B.; Graves, J.; Rattan, R.; Poisson, L.; et al. Multi-omic evaluation of metabolic alterations in multiple sclerosis identifies shifts in aromatic amino acid metabolism. Cell Rep. Med. 2021, 2, 100424. [Google Scholar] [CrossRef]
- Ghosh, S.; Pramanik, S. Structural diversity, functional aspects and future therapeutic applications of human gut microbiome. Arch. Microbiol. 2021, 203, 5281–5308. [Google Scholar] [CrossRef]
- Gwak, H.-J.; Lee, S.J.; Rho, M. Application of computational approaches to analyze metagenomic data. J. Microbiol. 2021, 59, 233–241. [Google Scholar] [CrossRef]
- Tierney, B.T.; Tan, Y.; Kostic, A.D.; Patel, C.J. Gene-level metagenomic architectures across diseases yield high-resolution microbiome diagnostic indicators. Nat. Commun. 2021, 12, 2907. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, M.O.; Åberg, F.; Männistö, V.; Havulinna, A.S.; Méric, G.; Liu, Y.; Loomba, R.; Vázquez-Baeza, Y.; Tripathi, A.; Valsta, L.M.; et al. Links between gut microbiome composition and fatty liver disease in a large population sample. Gut Microbes 2021, 13, 1888673. [Google Scholar] [CrossRef] [PubMed]
- Dinakis, E.; Nakai, M.; Gill, P.A.; Yiallourou, S.; Sata, Y.; Muir, J.; Carrington, M.; Head, G.A.; Kaye, D.M.; Marques, F.Z. The Gut Microbiota and Their Metabolites in Human Arterial Stiffness. Heart Lung Circ. 2021, 30, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Roth-Schulze, A.J.; Penno, M.A.S.; Ngui, K.M.; Oakey, H.; Bandala-Sanchez, E.; Smith, A.D.; Allnutt, T.R.; Thomson, R.L.; Vuillermin, P.J.; Craig, M.E.; et al. Type 1 diabetes in pregnancy is associated with distinct changes in the composition and function of the gut microbiome. Microbiome 2021, 9, 167. [Google Scholar] [CrossRef]
- Umirah, F.; Neoh, C.F.; Ramasamy, K.; Lim, S.M. Differential gut microbiota composition between type 2 diabetes mellitus patients and healthy controls: A systematic review. Diabetes Res. Clin. Pract. 2021, 173, 108689. [Google Scholar] [CrossRef]
- Que, Y.; Cao, M.; He, J.; Zhang, Q.; Chen, Q.; Yan, C.; Lin, A.; Yang, L.; Wu, Z.; Zhu, D.; et al. Gut Bacterial Characteristics of Patients with Type 2 Diabetes Mellitus and the Application Potential. Front. Immunol. 2021, 12, 722206. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lu, B.; Gong, J.; Li, L.; Chen, G.; Zhang, J.; Chen, Y.; Tian, X.; Han, B.; Guo, Y.; et al. Chickpea Extract Ameliorates Metabolic Syndrome Symptoms via Restoring Intestinal Ecology and Metabolic Profile in Type 2 Diabetic Rats. Mol. Nutr. Food Res. 2021, 65, 2100007. [Google Scholar] [CrossRef]
- Cox, D.B.T.; Gootenberg, J.S.; Abudayyeh, O.O.; Franklin, B.; Kellner, M.J.; Joung, J.; Zhang, F. RNA editing with CRISPR-Cas13. Science 2017, 358, 1019–1027. [Google Scholar] [CrossRef]
- Abudayyeh, O.O.; Gootenberg, J.S.; Essletzbichler, P.; Han, S.; Joung, J.; Belanto, J.J.; Verdine, V.; Cox, D.B.T.; Kellner, M.J.; Regev, A.; et al. RNA targeting with CRISPR–Cas13. Nature 2017, 550, 280–284. [Google Scholar] [CrossRef]
- Tsagkaraki, E.; Nicoloro, S.M.; DeSouza, T.; Solivan-Rivera, J.; Desai, A.; Lifshitz, L.M.; Shen, Y.; Kelly, M.; Guilherme, A.; Henriques, F.; et al. CRISPR-enhanced human adipocyte browning as cell therapy for metabolic disease. Nat. Commun. 2021, 12, 6931. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Bao, C.; Xie, Y.; Guo, H.; Liu, Y.; Li, J.; Liu, R.; Li, P.; Bai, J.; Yan, Y.; et al. Targeted core-shell nanoparticles for precise CTCF gene insert in treatment of metastatic breast cancer. Bioact. Mater. 2022, 11, 1–14. [Google Scholar] [CrossRef]
- Modell, A.E.; Lim, D.; Nguyen, T.M.; Sreekanth, V.; Choudhary, A. CRISPR-based therapeutics: Current challenges and future applications. Trends Pharmacol. Sci. 2021, 43, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Manjón, A.G.; Linder, S.; Teunissen, H.; Friskes, A.; Zwart, W.; de Wit, E.; Medema, R.H. Unexpected gene activation following CRISPR-Cas9-mediated genome editing. EMBO Rep. 2021, 23, e53902. [Google Scholar] [CrossRef] [PubMed]
- Rehm, H.L.; Berg, J.S.; Brooks, L.D.; Bustamante, C.D.; Evans, J.P.; Landrum, M.J.; Ledbetter, D.H.; Maglott, D.R.; Martin, C.L.; Nussbaum, R.L.; et al. ClinGen—The Clinical Genome Resource. N. Engl. J. Med. 2015, 372, 2235–2242. [Google Scholar] [CrossRef]
- Chua, K.-P.; Kimmel, L.E.; Conti, R.M. Spending for Orphan Indications Among Top-Selling Orphan Drugs Approved to Treat Common Diseases. Health Aff. 2021, 40, 453–460. [Google Scholar] [CrossRef]
- Simoens, S. Pricing and reimbursement of orphan drugs: The need for more transparency. Orphanet J. Rare Dis. 2011, 6, 42. [Google Scholar] [CrossRef]
- Edwards, K.F.; Liebman, J.F. How Often are Orphan Drugs Orphaned by the Thermochemical Community? Curr. Med. Chem. 2020, 27, 23–31. [Google Scholar] [CrossRef]
- Simoens, S.; Cassiman, D.; Dooms, M.; Picavet, E. Orphan Drugs for Rare Diseases. Drugs 2012, 72, 1437–1443. [Google Scholar] [CrossRef]
- Koçkaya, G.; Atalay, S.; Oğuzhan, G.; Kurnaz, M.; Ökçün, S.; Gedik, S.; Şaylan, M.; Şencan, N. Analysis of patient access to orphan drugs in Turkey. Orphanet J. Rare Dis. 2021, 16, 68. [Google Scholar] [CrossRef]
- Lopata, E.; Terrone, C.; Gopalan, A.; Ladikos, N.; Richardson, T. Meeting the affordability challenges posed by orphan drugs: A survey of payers, providers, and employers. J. Manag. Care Spéc. Pharm. 2021, 27, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Monguio, R.; Spargo, T.; Seoane-Vazquez, E. Ethical imperatives of timely access to orphan drugs: Is possible to reconcile economic incentives and patients’ health needs? Orphanet J. Rare Dis. 2017, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Wang, Y.; Pan, X.; Zhang, L.; Huang, R.; Chen, X.; Hu, J.; Xu, Y.; Jin, S. The availability and affordability of orphan drugs for rare diseases in China. Orphanet J. Rare Dis. 2016, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Moraes, F.C.; Pichon, C.; Letourneur, D.; Chaubet, F. miRNA Delivery by Nanosystems: State of the Art and Perspectives. Pharmaceutics 2021, 13, 1901. [Google Scholar] [CrossRef]
- Borkotoky, S.; Murali, A. The highly efficient T7 RNA polymerase: A wonder macromolecule in biological realm. Int. J. Biol. Macromol. 2018, 118, 49–56. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Y.; Zhang, Z.; Tang, B.; Zhou, Z.; Chen, H. Nanoparticle-Based RNAi Therapeutics Targeting Cancer Stem Cells: Update and Prospective. Pharmaceutics 2021, 13, 2116. [Google Scholar] [CrossRef]
- Crooke, S.T.; Witztum, J.L.; Bennett, C.F.; Baker, B.F. RNA-Targeted Therapeutics. Cell Metab. 2019, 29, 501. [Google Scholar] [CrossRef]
- Crooke, S.T.; Baker, B.F.; Crooke, R.M.; Liang, X.-H. Antisense technology: An overview and prospectus. Nat. Rev. Drug Discov. 2021, 20, 427–453. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Nguyen, L.H.; Odisho, A.S.; Maxey, B.S.; Pruitt, J.W.; Girma, B.; Cornett, E.M.; Kaye, A.M.; Kaye, A.D. The Antisense Oligonucleotide Nusinersen for Treatment of Spinal Muscular Atrophy. Orthop. Rev. 2021, 13, 24934. [Google Scholar] [CrossRef]
- Ivanova, M.M.; Dao, J.; Kasaci, N.; Adewale, B.; Nazari, S.; Noll, L.; Fikry, J.; Sanati, A.H.; Goker-Alpan, O. Cellular and biochemical response to chaperone versus substrate reduction therapies in neuropathic Gaucher disease. PLoS ONE 2021, 16, e0247211. [Google Scholar] [CrossRef]
- Peng, Y.; Liou, B.; Lin, Y.; Fannin, V.; Zhang, W.; Feldman, R.A.; Setchell, K.D.R.; Grabowski, G.A.; Sun, Y. Substrate Reduction Therapy Reverses Mitochondrial, mTOR, and Autophagy Alterations in a Cell Model of Gaucher Disease. Cells 2021, 10, 2286. [Google Scholar] [CrossRef] [PubMed]
- Guérard, N.; Oder, D.; Nordbeck, P.; Zwingelstein, C.; Morand, O.; Welford, R.W.; Dingemanse, J.; Wanner, C. Lucerastat, an Iminosugar for Substrate Reduction Therapy: Tolerability, Pharmacodynamics, and Pharmacokinetics in Patients with Fabry Disease on Enzyme Replacement. Clin. Pharmacol. Ther. 2017, 103, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Pearson-Stuttard, J.; Selvin, E.; Gregg, E.W. Interpreting global trends in type 2 diabetes complications and mortality. Diabetologia 2021, 65, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, P.; Lin, F. Mapping global research trends in diabetes and COVID-19 outbreak in the past year: A bibliometric analysis. Ann. Palliat. Med. 2022, 11, 1241–1252. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P.-F. Diabetes and gut microbiota. World J. Diabetes 2021, 12, 1693–1703. [Google Scholar] [CrossRef]
- Ichikawa, M.; Yamakawa, T.; Sakamoto, R.; Takahashi, K.; Suzuki, J.; Matsuura-Shinoda, M.; Shigematsu, E.; Tanaka, S.; Kaneshiro, M.; Asakura, T.; et al. A cross-sectional study of the relationship between quality of life and sleep quality in Japanese patients with type 1 diabetes mellitus. Endocr. J. 2022, 69, 399–406. [Google Scholar] [CrossRef]
- Ojo, O.; Wang, X.-H.; Ojo, O.O.; Adegboye, A.R.A. The Effects of Almonds on Gut Microbiota, Glycometabolism, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2021, 13, 3377. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. A review on gut microbiota: A central factor in the pathophysiology of obesity. Lipids Health Dis. 2021, 20, 65. [Google Scholar] [CrossRef]
- Ramzy, A.; Thompson, D.M.; Ward-Hartstonge, K.A.; Ivison, S.; Cook, L.; Garcia, R.V.; Loyal, J.; Kim, P.T.; Warnock, G.L.; Levings, M.K.; et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell 2021, 28, 2047–2061.e5. [Google Scholar] [CrossRef]
- Sintov, E.; Gerace, D.; Melton, D.A. A human ESC line for efficient CRISPR editing of pluripotent stem cells. Stem Cell Res. 2021, 57, 102591. [Google Scholar] [CrossRef]
- Goyal, A.; Gupta, Y.; Kalaivani, M.; Praveen, P.A.; Ambekar, S.; Tandon, N. SARS-CoV-2 Seroprevalence in Individuals With Type 1 and Type 2 Diabetes Compared With Controls. Endocr. Pract. 2021, 28, 191–198. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, C.; Peng, J.; Li, Z.; Li, F.; Wang, J.; Hu, A.; Peng, M.; Huang, K.; Fan, D.; et al. COVID-19 induces new-onset insulin resistance and lipid metabolic dysregulation via regulation of secreted metabolic factors. Signal Transduct. Target. Ther. 2021, 6, 427. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, C.; Lotta, L.A. Genomic insights into the causes of type 2 diabetes. Lancet 2018, 391, 2463–2474. [Google Scholar] [CrossRef]
- Kennedy, A.E.; Ozbek, U.; Dorak, M.T. What has GWAS done for HLA and disease associations? Int. J. Immunogenetics 2017, 44, 195–211. [Google Scholar] [CrossRef]
- Barrett, J.C.; Clayton, D.G.; Concannon, P.; Akolkar, B.; Cooper, J.D.; Erlich, H.A.; Julier, C.; Morahan, G.; Nerup, J.; Nierras, C.; et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 2009, 41, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.Z.; Santos, R.D. Past, Present, and Future of Familial Hypercholesterolemia Management. Methodist DeBakey Cardiovasc. J. 2021, 17, 28–35. [Google Scholar] [CrossRef]
- Jiang, W.-Z.; Zhang, L.-W.; He, C.-H.; Ruan, M.-H.; Ji, Y.; Yu, J.-R.; Zhou, H.-W. Progress on familial hypercholesterolemia. Hereditas 2021, 43, 1011–1022. [Google Scholar] [CrossRef]
- Jones, L.K.; Brownson, R.C.; Williams, M.S. Applying implementation science to improve care for familial hypercholesterolemia. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 141–151. [Google Scholar] [CrossRef]
- Rizos, C.V.; Skoumas, I.; Rallidis, L.; Skalidis, E.; Tziomalos, K.; Garoufi, A.; Anagnostis, P.; Sfikas, G.; Kotsis, V.; Doumas, M.; et al. LDL cholesterol target achievement in heterozygous familial hypercholesterolemia patients according to 2019 ESC/EAS lipid guidelines: Implications for newer lipid-lowering treatments. Int. J. Cardiol. 2021, 345, 119–124. [Google Scholar] [CrossRef]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. Monoclonal Antibodies in the Management of Familial Hypercholesterolemia: Focus on PCSK9 and ANGPTL3 Inhibitors. Curr. Atheroscler. Rep. 2021, 23, 79. [Google Scholar] [CrossRef] [PubMed]
- Di Taranto, M.D.; Giacobbe, C.; Fortunato, G. Familial hypercholesterolemia: A complex genetic disease with variable phenotypes. Eur. J. Med Genet. 2019, 63, 103831. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Akioyamen, L.E.; Aljenedil, S.; Rivière, J.-B.; Ruel, I.; Genest, J. Genetic testing for familial hypercholesterolemia: Impact on diagnosis, treatment and cardiovascular risk. Eur. J. Prev. Cardiol. 2019, 26, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Volta, A.; Hovingh, G.K.; Grefhorst, A. Genetics of familial hypercholesterolemia. Curr. Opin. Lipidol. 2018, 29, 80–86. [Google Scholar] [CrossRef]
- Shiu, E.Y.; Leung, N.H.L.; Cowling, B.J. Controversy around airborne versus droplet transmission of respiratory viruses. Curr. Opin. Infect. Dis. 2019, 32, 372–379. [Google Scholar] [CrossRef]
- Tada, H.; Okada, H.; Nomura, A.; Yashiro, S.; Nohara, A.; Ishigaki, Y.; Takamura, M.; Kawashiri, M.-A. Rare and Deleterious Mutations in ABCG5/ABCG8 Genes Contribute to Mimicking and Worsening of Familial Hypercholesterolemia Phenotype. Circ. J. 2019, 83, 1917–1924. [Google Scholar] [CrossRef]
- Nguyen, Y.; Stirnemann, J.; Belmatoug, N. Gaucher disease: A review. Rev. Med. Interne 2019, 40, 313–322. [Google Scholar] [CrossRef]
- Stirnemann, J.; Belmatoug, N.; Camou, F.; Serratrice, C.; Froissart, R.; Caillaud, C.; Levade, T.; Astudillo, L.; Serratrice, J.; Brassier, A.; et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017, 18, 441. [Google Scholar] [CrossRef]
- Brady, R.O. Enzyme Replacement for Lysosomal Diseases. Annu. Rev. Med. 2006, 57, 283–296. [Google Scholar] [CrossRef]
- Mistry, P.K.; Lopez, G.; Schiffmann, R.; Barton, N.W.; Weinreb, N.J.; Sidransky, E. Gaucher disease: Progress and ongoing challenges. Mol. Genet. Metab. 2016, 120, 8–21. [Google Scholar] [CrossRef]
- Gary, S.; Ryan, E.; Steward, A.; Sidransky, E. Recent advances in the diagnosis and management of Gaucher disease. Expert Rev. Endocrinol. Metab. 2018, 13, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.S.; LaMarca, M.E.; Scott, C.R.; Sidransky, E. Gaucher disease: Mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum. Mutat. 2008, 29, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Mullin, S.; Hughes, D.; Mehta, A.; Schapira, A.H.V. Neurological effects of glucocerebrosidase gene mutations. Eur. J. Neurol. 2018, 26, 388-e29. [Google Scholar] [CrossRef] [PubMed]
- Malekkou, A.; Sevastou, I.; Mavrikiou, G.; Georgiou, T.; Vilageliu, L.; Moraitou, M.; Michelakakis, H.; Prokopiou, C.; Drousiotou, A. A novel mutation deep within intron 7 of the GBA gene causes Gaucher disease. Mol. Genet. Genom. Med. 2020, 8, e1090. [Google Scholar] [CrossRef]
- Mozafari, H.; Taghikhani, M.; Rahimi, Z.; Vaisi-Raygani, A.; Ansari, S.; Khatami, S.; Alaei, M.; Saghiri, R. Analysis of glucocerebrosidase gene mutations in Iranian patients with Gaucher disease: Identification of 6 novel mutations. Iran. J. Child Neurol. 2020, 15, 139–166. [Google Scholar] [CrossRef]
- D’Avanzo, F.; Rigon, L.; Zanetti, A.; Tomanin, R. Mucopolysaccharidosis Type II: One Hundred Years of Research, Diagnosis, and Treatment. Int. J. Mol. Sci. 2020, 21, 1258. [Google Scholar] [CrossRef]
- Dias, B.M.C.; Lanza, F.C.; Msc, J.D.S.; Aranda, C.S.; Solé, D.; Martins, A.M.; Wandalsen, G.F. Mucopolysaccharidosis patients have reduced functional capacity. Pediatr. Pulmonol. 2021, 57, 538–543. [Google Scholar] [CrossRef]
- Giugliani, R.; Martins, A.M.; Okuyama, T.; Eto, Y.; Sakai, N.; Nakamura, K.; Morimoto, H.; Minami, K.; Yamamoto, T.; Yamaoka, M.; et al. Enzyme Replacement Therapy with Pabinafusp Alfa for Neuronopathic Mucopolysaccharidosis II: An Integrated Analysis of Preclinical and Clinical Data. Int. J. Mol. Sci. 2021, 22, 10938. [Google Scholar] [CrossRef]
- Ohira, M.; Kikuchi, E.; Mizuta, S.; Yoshida, N.; Onodera, M.; Nakanishi, M.; Okuyama, T.; Mashima, R. Production of therapeutic iduronate-2-sulfatase enzyme with a novel single-stranded RNA virus vector. Genes Cells 2021, 26, 891–904. [Google Scholar] [CrossRef]
- Jain, M.; De Jesus, O. Krabbe Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wenger, D.A.; Luzi, P.; Rafi, M.A. Advances in the Diagnosis and Treatment of Krabbe Disease. Int. J. Neonatal Screen. 2021, 7, 57. [Google Scholar] [CrossRef]
- Ghabash, G.; Wilkes, J.; Barney, B.J.; Bonkowsky, J.L. Hospitalization Burden and Incidence of Krabbe Disease. J. Child Neurol. 2021, 37, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Nasir, G.; Chopra, R.; Elwood, F.; Ahmed, S.S. Krabbe Disease: Prospects of Finding a Cure Using AAV Gene Therapy. Front. Med. 2021, 8, 760236. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, P.R.; Gass, J.M.; Vairo, F.; Farnham, K.M.; Atwal, H.K.; Macklin, S.; Klee, E.W.; Atwal, P.S. Maple syrup urine disease: Mechanisms and management. Appl. Clin. Genet. 2017, 10, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jakher, Y.; Ahrens-Nicklas, R.C. Brain Branched-Chain Amino Acids in Maple Syrup Urine Disease: Implications for Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 7490. [Google Scholar] [CrossRef]
- Homaei, S.C.; Barone, H.; Kleppe, R.; Betari, N.; Reif, A.; Haavik, J. ADHD symptoms in neurometabolic diseases: Underlying mechanisms and clinical implications. Neurosci. Biobehav. Rev. 2021, 132, 838–856. [Google Scholar] [CrossRef]
- Van Calcar, S.; Sowa, M.; Rohr, F.; Beazer, J.; Setlock, T.; Weihe, T.; Pendyal, S.; Wallace, L.; Hansen, J.; Stembridge, A.; et al. Nutrition management guideline for very-long chain acyl-CoA dehydrogenase deficiency (VLCAD): An evidence- and consensus-based approach. Mol. Genet. Metab. 2020, 131, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.; Vachharajani, N.; Chang, S.-H.; Park, Y.; Khan, A.S.; Chapman, W.C.; Doyle, M.M. Domino liver transplants: Where do we stand after a quarter-century? A US national analysis. HPB 2022, 24, 1026–1034. [Google Scholar] [CrossRef]
- Raghu, V.K.; Carr-Boyd, P.D.; Squires, J.E.; Vockley, J.; Goldaracena, N.; Mazariegos, G.V. Domino transplantation for pediatric liver recipients: Obstacles, challenges, and successes. Pediatr. Transplant. 2021, 25, e14114. [Google Scholar] [CrossRef]
- Lamichhane, A.; Rocha Cabrero, F. Metachromatic Leukodystrophy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Finsterer, J. Mitochondrial metabolic stroke: Phenotype and genetics of stroke-like episodes. J. Neurol. Sci. 2019, 400, 135–141. [Google Scholar] [CrossRef]
- Garone, C.; D’Souza, A.R.; Dallabona, C.; Lodi, T.; Rebelo-Guiomar, P.; Rorbach, J.; Donati, M.A.; Procopio, E.; Montomoli, M.; Guerrini, R.; et al. Defective mitochondrial rRNA methyltransferase MRM2 causes MELAS-like clinical syndrome. Hum. Mol. Genet. 2017, 26, 4257–4266. [Google Scholar] [CrossRef]
- Barcelos, I.; Shadiack, E.; Ganetzky, R.D.; Falk, M.J. Mitochondrial medicine therapies: Rationale, evidence, and dosing guidelines. Curr. Opin. Pediatr. 2020, 32, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, H.; Azhar, W. Niemann-Pick Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Pallottini, V.; Pfrieger, F.W. Understanding and Treating Niemann–Pick Type C Disease: Models Matter. Int. J. Mol. Sci. 2020, 21, 8979. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.; Fawaz, M.V.; Azaria, R.D.; Hollon, T.; Liu, E.A.; Kunkel, T.J.; Halseth, T.A.; Krus, K.; Ming, R.; Morin, E.E.; et al. Synthetic high-density lipoprotein nanoparticles for the treatment of Niemann–Pick diseases. BMC Med. 2019, 17, 200. [Google Scholar] [CrossRef]
- Van Wegberg, A.M.J.; Macdonald, A.; Ahring, K.; BéLanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, A.; Oussalah, A.; Jeannesson, E.; Guéant, J.-L.; François, F. La phénylcétonurie. Med. Sci. 2020, 36, 725–734. [Google Scholar] [CrossRef]
- Jameson, E.; Remmington, T. Dietary interventions for phenylketonuria. Cochrane Database Syst. Rev. 2020, 2021, CD001304. [Google Scholar] [CrossRef]
- Verduci, E.; Carbone, M.; Borghi, E.; Ottaviano, E.; Burlina, A.; Biasucci, G. Nutrition, Microbiota and Role of Gut-Brain Axis in Subjects with Phenylketonuria (PKU): A Review. Nutrients 2020, 12, 3319. [Google Scholar] [CrossRef] [PubMed]
- Grisch-Chan, H.M.; Schwank, G.; Harding, C.O.; Thöny, B. State-of-the-Art 2019 on Gene Therapy for Phenylketonuria. Hum. Gene Ther. 2019, 30, 1274–1283. [Google Scholar] [CrossRef]
- Stein, P.E.; Badminton, M.N.; Rees, D.C. Update review of the acute porphyrias. Br. J. Haematol. 2016, 176, 527–538. [Google Scholar] [CrossRef]
- Di Pierro, E.; Granata, F. Nutrients and Porphyria: An Intriguing Crosstalk. Int. J. Mol. Sci. 2020, 21, 3462. [Google Scholar] [CrossRef]
- Brandão, P.R.D.P.; Titze-De-Almeida, S.S.; Titze-De-Almeida, R. Leading RNA Interference Therapeutics Part 2: Silencing Delta-Aminolevulinic Acid Synthase 1, with a Focus on Givosiran. Mol. Diagn. Ther. 2019, 24, 61–68. [Google Scholar] [CrossRef]
- Balwani, M.; Sardh, E.; Ventura, P.; Peiró, P.A.; Rees, D.C.; Stölzel, U.; Bissell, D.M.; Bonkovsky, H.L.; Windyga, J.; Anderson, K.E.; et al. Phase 3 Trial of RNAi Therapeutic Givosiran for Acute Intermittent Porphyria. N. Engl. J. Med. 2020, 382, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Ramani, P.K.; Parayil Sankaran, B. Tay-Sachs Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Solovyeva, V.V.; Shaimardanova, A.A.; Chulpanova, D.S.; Kitaeva, K.V.; Chakrabarti, L.; Rizvanov, A.A. New Approaches to Tay-Sachs Disease Therapy. Front. Physiol. 2018, 9, 1663. [Google Scholar] [CrossRef] [PubMed]
- Guindi, M. Wilson disease. Semin. Diagn. Pathol. 2019, 36, 415–422. [Google Scholar] [CrossRef]
- Tarantino, G.; Porcu, C.; Arciello, M.; Andreozzi, P.; Balsano, C. Prediction of carotid intima-media thickness in obese patients with low prevalence of comorbidities by serum copper bioavailability. J. Gastroenterol. Hepatol. 2018, 33, 1511–1517. [Google Scholar] [CrossRef]
- Członkowska, A.; Litwin, T.; Dusek, P.; Ferenci, P.; Lutsenko, S.; Medici, V.; Rybakowski, J.K.; Weiss, K.H.; Schilsky, M.L. Wilson disease. Nat. Rev. Dis. Primers 2018, 4, 21. [Google Scholar] [CrossRef]
- Goyal, A.; Cusick, A.S.; Reilly, E. Familial Hypertriglyceridemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wang, S.; Cheng, Y.; Shi, Y.; Zhao, W.; Gao, L.; Fang, L.; Jin, X.; Han, X.; Sun, Q.; Li, G.; et al. Identification and Characterization of Two Novel Compounds: Heterozygous Variants of Lipoprotein Lipase in Two Pedigrees with Type I Hyperlipoproteinemia. Front. Endocrinol. 2022, 13, 874608. [Google Scholar] [CrossRef]
- Al-Waili, K.; Al-Rasadi, K.; Al-Bulushi, M.; Habais, M.; Al-Mujaini, A.; Al-Yaarubi, S.; Rimbert, A.; Zadjali, R.; Khaniabadi, P.M.; Al-Barwani, H.; et al. The Genetic Spectrum of Familial Hypertriglyceridemia in Oman. Front. Genet. 2022, 13, 886182. [Google Scholar] [CrossRef]
- Guo, D.; Zheng, Y.; Gan, Z.; Guo, Y.; Jiang, S.; Yang, F.; Xiong, F.; Zheng, H. A Heterozygous LMF1 Gene Mutation (c.1523C>T), Combined with an LPL Gene Mutation (c.590G>A), Aggravates the Clinical Symptoms in Hypertriglyceridemia. Front. Genet. 2022, 13, 814295. [Google Scholar] [CrossRef]
- Garay-García, K.; Gaete, P.V.; Mendivil, C.O. Severe hypertriglyceridemia secondary to splice-site and missense variants in LMF1 in three patients from Ecuador. J. Clin. Lipidol. 2022, 16, 277–280. [Google Scholar] [CrossRef]
- Luna-Castillo, K.P.; Olivares-Ochoa, X.C.; Hernández-Ruiz, R.G.; Llamas-Covarrubias, I.M.; Rodríguez-Reyes, S.C.; Betancourt-Núñez, A.; Vizmanos, B.; Martínez-López, E.; Muñoz-Valle, J.F.; Márquez-Sandoval, F.; et al. The Effect of Dietary Interventions on Hypertriglyceridemia: From Public Health to Molecular Nutrition Evidence. Nutrients 2022, 14, 1104. [Google Scholar] [CrossRef] [PubMed]
- Oh, R.C.; Trivette, E.T.; Westerfield, K.L. Management of hypertriglyceridemia: Common questions and answers. Am. Fam. Physician 2020, 102, 347–354. [Google Scholar]
- Chait, A. Hypertriglyceridemia. Endocrinol. Metab. Clin. N. Am. 2022, 51, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Pulipati, V.P.; Brinton, E.A.; Hatipoglu, B. Management of Mild-to-Moderate Hypertriglyceridemia. Endocr. Pract. 2022; in press. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Maki, K.C.; Bays, H.E.; Aguilera, F.; Gould, G.; Hegele, R.A.; Moriarty, P.M.; Robinson, J.G.; Shi, P.; Tur, J.F.; et al. Effectiveness of a Novel ω-3 Krill Oil Agent in Patients with Severe Hypertriglyceridemia. JAMA Netw. Open 2022, 5, e2141898. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk. JAMA 2020, 324, 2268. [Google Scholar] [CrossRef]
- Kim, K.; Ginsberg, H.N.; Choi, S.H. New, Novel Lipid-Lowering Agents for Reducing Cardiovascular Risk: Beyond Statins. Diabetes Metab. J. 2022, 46, 517–532. [Google Scholar] [CrossRef]
- Khoury, E.; Croteau, L.; Lauzière, A.; Gaudet, D. Lessons learned from the evinacumab trials in the treatment of homozygous familial hypercholesterolemia. Futur. Cardiol. 2022, 18, 507–518. [Google Scholar] [CrossRef]
- Kaplon, H.; Chenoweth, A.; Crescioli, S.; Reichert, J.M. Antibodies to watch in 2022. mAbs 2022, 14, 2014296. [Google Scholar] [CrossRef]
- Gupta, A.; Balakrishnan, B.; Karki, S.; Slayton, M.; Jash, S.; Banerjee, S.; Grahn, T.H.M.; Jambunathan, S.; Disney, S.; Hussein, H.; et al. Human CIDEC transgene improves lipid metabolism and protects against high-fat diet-induced glucose intolerance in mice. J. Biol. Chem. 2022, 102347. [Google Scholar] [CrossRef]
- Li, L.; Ma, L.; Sun, M.; Jiao, J.; Zhang, Y.; Tang, Y.; Yang, N.; Kong, Y. High-Throughput Sequencing Reveals the Loss-of-Function Mutations in GALT Cause Recessive Classical Galactosemia. Front. Pediatr. 2020, 8, 443. [Google Scholar] [CrossRef]
- S, U.K.; Kumar, T.; R, S.; C, G.P.D.; Zayed, H. An extensive computational approach to analyze and characterize the functional mutations in the galactose-1-phosphate uridyl transferase (GALT) protein responsible for classical galactosemia. Comput. Biol. Med. 2019, 117, 103583. [Google Scholar] [CrossRef]
- Waisbren, S.E.; Tran, C.; Demirbas, D.; Gubbels, C.S.; Hsiao, M.; Daesety, V.; Berry, G.T. Transient developmental delays in infants with Duarte-2 variant galactosemia. Mol. Genet. Metab. 2021, 134, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Crespo, C.; Eiroa, H.; Otegui, M.I.; Bonetto, M.C.; Chertkoff, L.; Gravina, L.P. Molecular analysis of GALT gene in Argentinian population: Correlation with enzyme activity and characterization of a novel Duarte-like allele. Mol. Genet. Metab. Rep. 2020, 25, 100695. [Google Scholar] [CrossRef]
- Ohlsson, A.; Hunt, M.; Wedell, A.; Von Döbeln, U. Heterogeneity of disease-causing variants in the Swedish galactosemia population: Identification of 16 novel GALT variants. J. Inherit. Metab. Dis. 2019, 42, 1008–1018. [Google Scholar] [CrossRef]
- Papachristoforou, R.; Petrou, P.P.; Sawyer, H.; Williams, M.; Drousiotou, A. Classic galactosaemia in the Greek Cypriot population: An epidemiological and molecular study. Ann. Hum. Genet. 2019, 83, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Jo, K.I.; Ko, D.-H.; Lee, D.H.; Song, J.; Jin, D.-K.; Ki, C.-S.; Lee, S.-Y.; Kim, J.-W.; Lee, Y.-W.; et al. Novel GALTvariations and mutation spectrum in the Korean population with decreased galactose-1-phosphate uridyltransferase activity. BMC Med Genet. 2014, 15, 94. [Google Scholar] [CrossRef]
- Özgül, R.K.; Güzel-Ozantürk, A.; Dundar, H.; Yücel-Yılmaz, D.; Coskun, T.; Sivri, S.; Aydoǧdu, S.; Tokatlı, A.; Dursun, A.; Aydoğdu, S.; et al. Galactosemia in the Turkish population with a high frequency of Q188R mutation and distribution of Duarte-1 and Duarte-2 variations. J. Hum. Genet. 2013, 58, 675–678. [Google Scholar] [CrossRef]
- Rokaitė, R.; Traberg, R.; Dženkaitis, M.; Kučinskienė, R.; Labanauskas, L. Two Lithuanian Cases of Classical Galactosemia with a Literature Review: A Novel GALT Gene Mutation Identified. Medicina 2020, 56, 559. [Google Scholar] [CrossRef]
- Singh, R.; Thapa, B.R.; Kaur, G.; Prasad, R. Frequency Distribution of Q188R, N314D, Duarte 1, and Duarte 2 GALT Variant Alleles in an Indian Galactosemia Population. Biochem. Genet. 2012, 50, 871–880. [Google Scholar] [CrossRef]
- Succoio, M.; Sacchettini, R.; Rossi, A.; Parenti, G.; Ruoppolo, M. Galactosemia: Biochemistry, Molecular Genetics, Newborn Screening, and Treatment. Biomolecules 2022, 12, 968. [Google Scholar] [CrossRef]
- McCorvie, T.J.; Gleason, T.J.; Fridovich-Keil, J.L.; Timson, D.J. Misfolding of galactose 1-phosphate uridylyltransferase can result in type I galactosemia. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2013, 1832, 1279–1293. [Google Scholar] [CrossRef]
- Banford, S.; McCorvie, T.; Pey, A.; Timson, D. Galactosemia: Towards Pharmacological Chaperones. J. Pers. Med. 2021, 11, 106. [Google Scholar] [CrossRef]
- Delnoy, B.; Coelho, A.; Rubio-Gozalbo, M. Current and Future Treatments for Classic Galactosemia. J. Pers. Med. 2021, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Daenzer, J.M.I.; Rasmussen, S.A.; Patel, S.; McKenna, J.; Fridovich-Keil, J.L. Neonatal GALT gene replacement offers metabolic and phenotypic correction through early adulthood in a rat model of classic galactosemia. J. Inherit. Metab. Dis. 2021, 45, 203–214. [Google Scholar] [CrossRef]
- Brophy, M.L.; Stansfield, J.C.; Ahn, Y.; Cheng, S.H.; Murphy, J.E.; Bell, R.D. AAV-mediated expression of galactose-1-phosphate uridyltransferase corrects defects of galactose metabolism in classic galactosemia patient fibroblasts. J. Inherit. Metab. Dis. 2021, 45, 481–492. [Google Scholar] [CrossRef]
- Ramírez-Hernández, M.A.; Figuera, L.E.; Rizo-de la Torre, L.C.; Mendoza-Ruvalcaba, M.T.M.-T.S.C.; Arnaud-López, L.; García-Ortiz, J.E.; Zúñiga-González, G.M.; Puebla-Pérez, A.M.; Gómez-Meda, B.C.; Gallegos-Arreola, M.P. Mutational spec-trum of the iduronate-2-sulfatase gene in Mexican patients with Hunter syndrome. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5115–5127. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, Y.; Miura, A.; Yamazaki, N.; So, T.; Kosuga, M.; Yanagi, K.; Kaname, T.; Yamagata, T.; Sakuraba, H.; Okuyama, T. A cDNA analysis disclosed the discordance of genotype-phenotype correlation in a patient with attenuated MPS II and a 76-base deletion in the gene for iduronate-2-sulfatase. Mol. Genet. Metab. Rep. 2020, 25, 100692. [Google Scholar] [CrossRef]
- Jin, P.; Hao, J.-W.; Chen, K.; Dong, C.-S.; Yang, Y.-B.; Mo, Z.-H. A 3′ splice site mutation of IDS gene in a Chinese family with mucopolysaccharidosis type II. Gene 2013, 528, 236–240. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Zhang, X.; Wang, Y.; Qiu, W.; Ye, J.; Han, L.; Gao, X.; Gu, X. Analysis of the IDS Gene in 38 Patients with Hunter Syndrome: The c.879G>A (p.Gln293Gln) Synonymous Variation in a Female Create Exonic Splicing. PLoS ONE 2011, 6, e22951. [Google Scholar] [CrossRef]
- Olsen, T.C.; Eiken, H.G.; Knappskog, P.M.; Kase, B.F.; Månsson, J.-E.; Boman, H.; Pold, J. Mutations in the iduronate-2-sulfatase gene in five Norwegians with Hunter syndrome. Qual. Life Res. 1996, 97, 198–203. [Google Scholar] [CrossRef]
- Timms, K.M.; Lu, F.; Shen, Y.; Pierson, C.A.; Muzny, D.M.; Gu, Y.; Nelson, D.L.; A Gibbs, R. 130 kb of DNA sequence reveals two new genes and a regional duplication distal to the human iduronate-2-sulfate sulfatase locus. Genome Res. 1995, 5, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Lualdi, S.; Pittis, M.G.; Regis, S.; Parini, R.; Allegri, A.E.; Furlan, F.; Bembi, B.; Filocamo, M. Multiple cryptic splice sites can be activated by IDS point mutations generating misspliced transcripts. Klin. Wochenschr. 2006, 84, 692–700. [Google Scholar] [CrossRef]
- Lobo-Menendez, F.; Bowman, L.H.; Dewey, M.J. Inverted Gcg/CGC trinucleotide microsatellites in the 5’-region of Mus IDS mRNA: Recurrent induction of aberrant reverse transcripts. Mol. Biol. Rep. 2004, 31, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Cudry, S.; Froissart, R.; Bouton, O.; Maire, I.; Bozon, D. The 2.1-, 5.4- and 5.7-kb transcripts of the IDS gene are generated by different polyadenylation signals. Biochim. Biophys. Acta 1999, 1447, 35–42. [Google Scholar] [CrossRef]
- Malmgren, H.; Carlberg, B.-M.; Pettersson, U.; Bondeson, M.-L. Identification of an Alternative Transcript from the Human Iduronate-2-sulfatase (IDS) Gene. Genomics 1995, 29, 291–293. [Google Scholar] [CrossRef]
- Ghabash, G.; Wilkes, J.; Bonkowsky, J.L. National U.S. Patient and Transplant Data for Krabbe Disease. Front. Pediatr. 2021, 9, 764626. [Google Scholar] [CrossRef] [PubMed]
- Iacono, S.; Del Giudice, E.; Leon, A.; La Bella, V.; Spataro, R. A novel compound heterozygous mutation in GALC associated with adult-onset Krabbe disease: Case report and literature review. Neurogenetics 2022, 23, 1–9. [Google Scholar] [CrossRef]
- Nicita, F.; Stregapede, F.; Deodato, F.; Pizzi, S.; Martinelli, S.; Pagliara, D.; Aiello, C.; Cumbo, F.; Piemonte, F.; D’Amico, J.; et al. “Atypical” Krabbe disease in two siblings harboring biallelic GALC mutations including a deep intronic variant. Eur. J. Hum. Genet. 2022, 30, 984–988. [Google Scholar] [CrossRef]
- He, Z.; Pang, X.; Bai, J.; Wang, H.; Feng, F.; Du, R.; Huang, X. A novel GALC gene mutation associated with adult-onset Krabbe disease: A case report. Neurocase, 2022; online ahead of print. [Google Scholar] [CrossRef]
- D, T.K.; Jain, N.; S, U.K.; Jena, P.P.; Ramamoorthy, S.; C, G.P.D.; Zayed, H. Molecular dynamics simulations to decipher the structural and functional consequences of pathogenic missense mutations in the galactosylceramidase (GALC) protein causing Krabbe’s disease. J. Biomol. Struct. Dyn. 2020, 39, 1795–1810. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Lian, Y.; Li, Y.; Du, L.; Xie, N.; Wang, C. A new compound heterozygous mutation in adult-onset Krabbe disease. Int. J. Neurosci. 2020, 130, 1267–1271. [Google Scholar] [CrossRef]
- Saavedra-Matiz, C.A.; Luzi, P.; Nichols, M.; Orsini, J.J.; Caggana, M.; Wenger, D.A. Expression of individual mutations and haplotypes in the galactocerebrosidase gene identified by the newborn screening program in New York State and in confirmed cases of Krabbe’s disease. J. Neurosci. Res. 2016, 94, 1076–1083. [Google Scholar] [CrossRef]
- Gucev, Z.; Tasic, V. Compound Galactosylceramidase Gene (GALC) Heterozygosity in a Boy with Infantile Krabbe Disease (KD). PRILOZI 2015, 36, 99–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cesani, M.; Lorioli, L.; Grossi, S.; Amico, G.; Fumagalli, F.; Spiga, I.; Filocamo, M.; Biffi, A. Mutation Update of ARSA and PSAP Genes Causing Metachromatic Leukodystrophy. Hum. Mutat. 2015, 37, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.L.; Matta, D.; Gupta, N.; Kabra, M.; Ranganath, P.; Aggarwal, S.; Phadke, S.R.; Datar, C.; Gowrishankar, K.; Kamate, M.; et al. Spectrum of ARSA variations in Asian Indian patients with Arylsulfatase A deficient metachromatic leukodystrophy. J. Hum. Genet. 2019, 64, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Manshadi, M.D.; Kamalidehghan, B.; Aryani, O.; Khalili, E.; Dadgar, S.; Tondar, M.; Ahmadipour, F.; Meng, G.Y.; Houshmand, M. Four novel ARSA gene mutations with pathogenic impacts on metachromatic leukodystrophy: A bioinformatics approach to predict pathogenic mutations. Ther. Clin. Risk Manag. 2017, 13, 725–731. [Google Scholar] [CrossRef]
- Froukh, T. First Record Mutations in the Genes ASPA and ARSA Causing Leukodystrophy in Jordan. BioMed Res. Int. 2019, 2019, 7235914. [Google Scholar] [CrossRef]
- Lee, J.S.; Kanai, K.; Suzuki, M.; Kim, W.S.; Yoo, H.S.; Fu, Y.; Kim, D.-K.; Jung, B.C.; Choi, M.; Oh, K.W.; et al. Arylsulfatase A, a genetic modifier of Parkinson’s disease, is an α-synuclein chaperone. Brain 2019, 142, 2845–2859. [Google Scholar] [CrossRef]
- Aslan, M.; Kirik, S.; Özgör, B.; Güngör, S. Two siblings with metachromatic leukodystrophy caused by a novel identified homozygous mutation in the ARSA gene. J. Pediatr. Endocrinol. Metab. 2018, 31, 1047–1051. [Google Scholar] [CrossRef]
- Özkan, A.; Özkara, H.A. Metachromatic leukodystrophy: Biochemical characterization of two (p.307Glu→Lys, p.318Trp→Cys) arylsulfatase A mutations. Intractable Rare Dis. Res. 2016, 5, 280–283. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Y.; Zheng, D.; Yan, A.; Tu, X.; Lin, J.; Lan, F. Whole-exome sequencing identifies compound heterozygous mutations in ARSA of two siblings presented with atypical onset of metachromatic leukodystrophy from a Chinese pedigree. Clin. Chim. Acta 2016, 460, 135–137. [Google Scholar] [CrossRef]
- Siri, L.; Rossi, A.; Lanza, F.; Mazzotti, R.; Costa, A.; Stroppiano, M.; Gaiero, A.; Cohen, A.; Biancheri, R.; Filocamo, M. A novel homozygous splicing mutation in PSAP gene causes metachromatic leukodystrophy in two Moroccan brothers. Neurogenetics 2014, 15, 101–106. [Google Scholar] [CrossRef]
- Madaan, P.; Jauhari, P.; Chakrabarty, B.; Kumar, A.; Gulati, S. Saposin B–Deficient Metachromatic Leukodystrophy Mimicking Acute Flaccid Paralysis. Neuropediatrics 2019, 50, 318–321. [Google Scholar] [CrossRef]
- Kolnikova, M.; Jungova, P.; Skopkova, M.; Foltan, T.; Gasperikova, D.; Mattosova, S.; Chandoga, J. Late Infantile Metachromatic Leukodystrophy Due to Novel Pathogenic Variants in the PSAP Gene. J. Mol. Neurosci. 2019, 67, 559–563. [Google Scholar] [CrossRef]
- Kuchař, L.; Ledvinová, J.; Hřebíček, M.; Myšková, H.; Dvořáková, L.; Berná, L.; Chrastina, P.; Asfaw, B.; Elleder, M.; Petermöller, M.; et al. Prosaposin deficiency and saposin B deficiency (activator-deficient metachromatic leukodystrophy): Report on two patients detected by analysis of urinary sphingolipids and carrying novel PSAP gene mutations. Am. J. Med Genet. Part A 2009, 149, 613–621. [Google Scholar] [CrossRef]
- Rafi, M.A.; Amini, S.; Zhang, X.L.; Wenger, D.A. Correction of sulfatide metabolism after transfer of prosaposin cDNA to cultured cells from a patient with SAP-1 deficiency. Am. J. Hum. Genet. 1992, 50, 1252–1258. [Google Scholar]
- Chakrabarty, S.; Govindaraj, P.; Sankaran, B.P.; Nagappa, M.; Kabekkodu, S.P.; Jayaram, P.; Mallya, S.; Deepha, S.; Ponmalar, J.N.J.; Arivinda, H.R.; et al. Contribution of nuclear and mitochondrial gene mutations in mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome. J. Neurol. 2021, 268, 2192–2207. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, X.T.; Xie, Z.Y.; Yuan, Y.; Wang, Z.X. Mitochondrial encephalopathy, lactic acidosis and stroke-like episodes/myoclonus epilepsy with ragged-red fibers/Leigh overlap syndrome caused by mitochondrial DNA 8344A > G mutation. BeiJing Da Xue Xue Bao 2020, 52, 851–855. [Google Scholar]
- Meira, B.; Roque, R.; Pinto, M.; Caetano, A. Late-onset presentation of POLG1-associated mitochondrial disease. BMJ Case Rep. 2019, 12, e228482. [Google Scholar] [CrossRef]
- Finsterer, J.; Scorza, F.A. Phenotypic spectrum of POLG1 mutations. Neurol. Sci. 2017, 39, 571–573. [Google Scholar] [CrossRef]
- Kang, H.; Zhou, M.; Xie, C.; Lu, K. A 2-bp deletion mutation in SMPD1 gene leading to lysosomal acid sphingomyelinase deficiency in a Chinese consanguineous pedigree. J. Pediatr. Endocrinol. Metab. 2022, 35, 1113–1116. [Google Scholar] [CrossRef]
- Pinto, C.; Sousa, D.; Ghilas, V.; Dardis, A.; Scarpa, M.; Macedo, M.F. Acid Sphingomyelinase Deficiency: A Clinical and Immunological Perspective. Int. J. Mol. Sci. 2021, 22, 12870. [Google Scholar] [CrossRef] [PubMed]
- Ancien, F.; Pucci, F.; Rooman, M. In Silico Analysis of the Molecular-Level Impact of SMPD1 Variants on Niemann-Pick Disease Severity. Int. J. Mol. Sci. 2021, 22, 4516. [Google Scholar] [CrossRef] [PubMed]
- Rhein, C.; Zoicas, I.; Marx, L.; Zeitler, S.; Hepp, T.; von Zimmermann, C.; Mühle, C.; Richter-Schmidinger, T.; Lenz, B.; Erim, Y.; et al. mRNA Expression of SMPD1 Encoding Acid Sphingomyelinase Decreases upon Antidepressant Treatment. Int. J. Mol. Sci. 2021, 22, 5700. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, F.N.; Duman, T.A. An update of the mutation spectrum of phenylalanine hydroxylase (PAH) gene in the population of Turkey. J. Pediatr. Endocrinol. Metab. 2022, 35, 663–668. [Google Scholar] [CrossRef]
- Jin, X.; Yan, Y.; Zhang, C.; Tai, Y.; An, L.; Yu, X.; Zhang, L.; Hao, S.; Cao, X.; Yin, C.; et al. Identification of novel deep intronic PAH gene variants in patients diagnosed with phenylketonuria. Hum. Mutat. 2021, 43, 56–66. [Google Scholar] [CrossRef]
- Alibakhshi, R.; Mohammadi, A.; Salari, N.; Khamooshian, S.; Kazeminia, M.; Moradi, K. Spectrum of PAH gene mutations in 1547 phenylketonuria patients from Iran: A comprehensive systematic review. Metab. Brain Dis. 2021, 36, 767–780. [Google Scholar] [CrossRef]
- Tresbach, R.H.; Sperb-Ludwig, F.; Ligabue-Braun, R.; Tonon, T.; Cardoso, M.T.D.O.; Heredia, R.S.; Rosa, M.T.A.D.S.; Martins, B.C.; Poubel, M.O.; Da Silva, L.C.S.; et al. Phenylketonuria Diagnosis by Massive Parallel Sequencing and Genotype-Phenotype Association in Brazilian Patients. Genes 2020, 12, 20. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, X.; Huang, C.; Xu, H.; Fu, C. Variants of the phenylalanine hydroxylase gene in neonates with phenylketonuria in Hainan, China. Scand. J. Clin. Lab. Investig. 2020, 80, 619–622. [Google Scholar] [CrossRef]
- Tao, Y.; Han, D.; Shen, H.; Li, X. Spectrum of PAH gene mutations and genotype-phenotype correlation in patients with phenylalanine hydroxylase deficiency from Shanxi province. Brain Dev. 2021, 43, 220–229. [Google Scholar] [CrossRef]
- Hillert, A.; Anikster, Y.; Belanger-Quintana, A.; Burlina, A.; Burton, B.K.; Carducci, C.; Chiesa, A.E.; Christodoulou, J.; Đorđević, M.; Desviat, L.R.; et al. The Genetic Landscape and Epidemiology of Phenylketonuria. Am. J. Hum. Genet. 2020, 107, 234–250. [Google Scholar] [CrossRef]
- Kuznetcova, I.; Gundorova, P.; Ryzhkova, O.; Polyakov, A. The study of the full spectrum of variants leading to hyperphenylalaninemia have revealed 10 new variants in the PAH gene. Metab. Brain Dis. 2019, 34, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Gundorova, P.; Stepanova, A.A.; Kuznetsova, I.A.; Kutsev, S.I.; Polyakov, A.V. Genotypes of 2579 patients with phenylketonuria reveal a high rate of BH4 non-responders in Russia. PLoS ONE 2019, 14, e0211048. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasap, P.; Ittiwut, C.; Srichomthong, C.; Sangsin, A.; Suphapeetiporn, K.; Shotelersuk, V. Massive parallel sequencing as a new diagnostic approach for phenylketonuria and tetrahydrobiopterin-deficiency in Thailand. BMC Med Genet. 2017, 18, 102. [Google Scholar] [CrossRef]
- Li, N.; Jia, H.; Liu, Z.; Tao, J.; Chen, S.; Li, X.; Deng, Y.; Jin, X.; Song, J.; Zhang, L.; et al. Molecular characterisation of phenylketonuria in a Chinese mainland population using next-generation sequencing. Sci. Rep. 2015, 5, 15769. [Google Scholar] [CrossRef] [PubMed]
- Item, C.B.; Farhadi, S.; Schanzer, A.; Greber-Platzer, S. DNA methylated alleles of the phenylalanine hydroxylase promoter remodeled at elevated phenylalanine levels in newborns with hyperphenylalaninemia. Clin. Biochem. 2017, 50, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, S.F.; Lyons-Weiler, J.; Spridik, K.; Biery, A.; Breck, J.; Vockley, J.; Yatsenko, S.; Sultana, T. Altered DNA methylation in PAH deficient phenylketonuria. Mol. Genet. Metab. 2015, 115, 72–77. [Google Scholar] [CrossRef]
- Aguilera, P.; Badenas, C.; Whatley, S.D.; To-Figueras, J. Late-onset cutaneous porphyria in a patient heterozygous for a uroporphyrinogen III synthase gene mutation. Br. J. Dermatol. 2016, 175, 1346–1350. [Google Scholar] [CrossRef]
- Bhusal, M.; Bhattarai, S.; Shah, M.; Khadka, A. Congenital erythropoietic porphyria: A case series of a rare uroporphyrinogen III synthase gene mutation in Nepalese patients. JAAD Case Rep. 2021, 10, 102–106. [Google Scholar] [CrossRef]
- Suzuki, H.; Namiki, T.; Enzan, N.; Tanaka, A.; Nakano, H. Novel mutation in the UROS gene causing congenital erythropoietic porphyria in an elderly Japanese female. J. Dermatol. 2022, 49, 16340. [Google Scholar] [CrossRef]
- Almanasra, A.; Havranek, B.; Islam, S.M. In-silico screening and microsecond molecular dynamics simulations to identify single point mutations that destabilize β-hexosaminidase A causing Tay-Sachs disease. Proteins Struct. Funct. Bioinform. 2021, 89, 1587–1601. [Google Scholar] [CrossRef]
- Bibi, F.; Ullah, A.; Bourinaris, T.; Efthymiou, S.; Kriouile, Y.; Sultan, T.; Haider, S.; Salpietro, V.; Houlden, H.; Raja, G.K. Tay-Sachs Disease: Two Novel Rare HEXA Mutations from Pakistan and Morocco. Klin. Padiatr. 2021, 233, 226–230. [Google Scholar] [CrossRef] [PubMed]
- İnci, A.; Ergin, F.B.C.; Biberoğlu, G.; Okur, I.; Ezgü, F.S.; Tümer, L. Two patients from Turkey with a novel variant in the GM2A gene and review of the literature. J. Pediatr. Endocrinol. Metab. 2021, 34, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ko, J.M.; Kim, M.S.; Kim, M.J.; Seong, M.; Yoo, T.; Lim, B.C.; Chae, J. Novel HEXA variants in Korean children with Tay–Sachs disease with regression of neurodevelopment from infancy. Mol. Genet. Genom. Med. 2021, 9, e1677. [Google Scholar] [CrossRef]
- Panzer, M.; Viveiros, A.; Schaefer, B.; Baumgartner, N.; Seppi, K.; Djamshidian, A.; Todorov, T.; Griffiths, W.J.H.; Schott, E.; Schuelke, M.; et al. Synonymous mutation in adenosine triphosphatase copper-transporting beta causes enhanced exon skipping in Wilson disease. Hepatol. Commun. 2022, 6, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Gul, B.; Firasat, S.; Tehreem, R.; Shan, T.; Afshan, K. Analysis of Wilson disease mutations in copper binding domain of ATP7B gene. PLoS ONE 2022, 17, e0269833. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; McCann, C.J.; Ralle, M.; Ray, K.; Ray, J.; Lutsenko, S.; Jayakanthan, S. Analysis of Wilson disease mutations revealed that interactions between different ATP7B mutants modify their properties. Sci. Rep. 2020, 10, 13487. [Google Scholar] [CrossRef]
- Guggilla, S.R.; Senagari, J.R.; Rao, P.; Madireddi, S. Spectrum of mutations in the ATP binding domain of ATP7B gene of Wilson Disease in a regional Indian cohort. Gene 2015, 569, 83–87. [Google Scholar] [CrossRef]
- Petrukhin, K.; Lutsenko, S.; Chernov, I.; Ross, B.M.; Kaplan, J.H.; Gilliam, T. Characterization of the Wilson disease gene encoding a P-type copper transporting ATPase: Genomic organization, alternative splicing, and structure/function predictions. Hum. Mol. Genet. 1994, 3, 1647–1656. [Google Scholar] [CrossRef]
- He, K.; Zhu, Z.; Chen, Y. Lipoprotein Lipase Gene Polymorphisms Are Associated with Myocardial Infarction Risk: A Meta-Analysis. Genet. Test. Mol. Biomarkers 2021, 25, 434–444. [Google Scholar] [CrossRef]
- Al-Bustan, S.A.; Al-Serri, A.; Alnaqeeb, M.; Annice, B.G.; Mojiminiyi, O. Genetic association of LPL rs1121923 and rs258 with plasma TG and VLDL levels. Sci. Rep. 2019, 9, 5572. [Google Scholar] [CrossRef]
- Schulpis, K.H.; Thodi, G.; Iakovou, K.; Chatzidaki, M.; Dotsikas, Y.; Molou, E.; Triantafylli, O.; Loukas, Y.L. Mutational analysis of GALT gene in Greek patients with galactosaemia: Identification of two novel mutations and clinical evaluation. Scand. J. Clin. Lab. Investig. 2017, 77, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Akar, M.; Celik, M.; Kasapkara, C.S.; Ozbek, M.N.; Aldudak, B.; Tuzun, H. Mutational analysis of the galactose-1-phosphate uridyltransferase (GALT) gene in southeast part of Turkey: A regional report. Genet. Couns. 2015, 26, 91–94. [Google Scholar] [PubMed]
- Viggiano, E.; Marabotti, A.; Burlina, A.; Cazzorla, C.; D’Apice, M.; Giordano, L.; Fasan, I.; Novelli, G.; Facchiano, A. Clinical and molecular spectra in galactosemic patients from neonatal screening in northeastern Italy: Structural and functional characterization of new variations in the galactose-1-phosphate uridyltransferase (GALT) gene. Gene 2015, 559, 112–118. [Google Scholar] [CrossRef]
- Rubio-Gozalbo, M.E.; Derks, B.; Das, A.M.; Meyer, U.; Möslinger, D.; Couce, M.L.; Empain, A.; Ficicioglu, C.; Palacios, N.J.; Pelegrin, M.M.D.L.S.D.; et al. Galactokinase deficiency: Lessons from the GalNet registry. Genet. Med. 2020, 23, 202–210. [Google Scholar] [CrossRef]
- Sneha, P.; Ebrahimi, E.A.; Ghazala, S.A.; D, T.K.; R, S.; C, G.P.D.; Zayed, H. Structural analysis of missense mutations in galactokinase 1 (GALK1) leading to galactosemia type-2. J. Cell. Biochem. 2018, 119, 7585–7598. [Google Scholar] [CrossRef]
- Park, H.-D.; Bang, Y.-L.; Park, K.U.; Kim, J.Q.; Jeong, B.-H.; Kim, Y.-S.; Song, Y.-H.; Song, J. Molecular and biochemical characterization of the GALK1 gene in Korean patients with galactokinase deficiency. Mol. Genet. Metab. 2007, 91, 234–238. [Google Scholar] [CrossRef]
| No | Metabolic Disorder | The Molecular Basis for the MD |
|---|---|---|
| 1 | Diabetes mellitus | Epigenetic mechanisms [11], long non-coding RNAs [13], microbiome [22,23,24,25,26,27,28,55,58] |
| 2 | Familial hypercholesterolemia (FH) | Genes encoding the LDL receptor (LDLR), apolipoprotein B (APOB) Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9), apolipoprotein E (APOE), signal-transducing adaptor family member 1 (STAP1) [72] |
| 3 | Gaucher disease | GBA gene [77,82,83,84] |
| 4 | Mucopolysaccharidosis type II | Iduronate-2-sulfatase (IDS) gene [152,153,154,155,156,157] IDS gene transcript regulation [158,159,160,161] |
| 5 | Krabbe disease (KD) | Galactosyl-Ceramidase (GALC) gene [162,163,164,165,166,167,168,169] |
| 6 | Metachromatic leukodystrophy | Arylsulfatase A (ARSA) gene [170,171,172,173,174,175,176,177,178], prosaposin (PSAP) gene [179,180,181,182] |
| 7 | Mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS) syndrome | Mitochondrial rRNA transferase gene [101,102], nuclear and mitochondrial genes associated with MELAS [183,184], nuclear DNA polymerase gamma (POLG1) gene [185,186] |
| 8 | Niemann-Pick disease | Acid sphingomyelinase (SMPD1) gene [187,188,189] transcript regulation [190] |
| 9 | Phenylketonuria | Phenylalanine hydroxylase (PAH) gene [191,192,193,194,195,196,197,198,199,200,201], epigenetic regulation [202,203] |
| 10 | Porphyria | Uroporphyrinogen III synthase (UROS) gene [204,205,206] |
| 11 | Tay-Sachs disease | Beta-hexosaminidase A (HEXA) gene [207,208,209,210] |
| 12 | Wilson disease | ATPase copper transporting beta (ATP7B) gene [211,212,213,214,215] |
| 13 | Familial hypertriglyceridemia | Lipoprotein lipase (LPL) gene [216,217] |
| 14 | Galactosemia | Galactose-1-phosphate uridylyltransferase (GALT1) gene [137,218,219,220], Galactokinase 1 (GALK1) gene [221,222,223] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, K.F.; Yong, W.T.L.; Bhuiyan, M.S.A.; Siddiquee, S.; Shah, M.D.; Venmathi Maran, B.A. Current Understanding on the Genetic Basis of Key Metabolic Disorders: A Review. Biology 2022, 11, 1308. https://doi.org/10.3390/biology11091308
Rodrigues KF, Yong WTL, Bhuiyan MSA, Siddiquee S, Shah MD, Venmathi Maran BA. Current Understanding on the Genetic Basis of Key Metabolic Disorders: A Review. Biology. 2022; 11(9):1308. https://doi.org/10.3390/biology11091308
Chicago/Turabian StyleRodrigues, Kenneth Francis, Wilson Thau Lym Yong, Md. Safiul Alam Bhuiyan, Shafiquzzaman Siddiquee, Muhammad Dawood Shah, and Balu Alagar Venmathi Maran. 2022. "Current Understanding on the Genetic Basis of Key Metabolic Disorders: A Review" Biology 11, no. 9: 1308. https://doi.org/10.3390/biology11091308
APA StyleRodrigues, K. F., Yong, W. T. L., Bhuiyan, M. S. A., Siddiquee, S., Shah, M. D., & Venmathi Maran, B. A. (2022). Current Understanding on the Genetic Basis of Key Metabolic Disorders: A Review. Biology, 11(9), 1308. https://doi.org/10.3390/biology11091308