A Simple Fluorescent Cholesterol Labeling Method to Cryoprotect and Detect Plasma Lipoprotein-X
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Synthetic Lp-X
2.2. Lipoprotein Labeling with Fluorescent Lipids/Trehalose
2.3. Agarose Gel Electrophoresis
2.4. Plasma Samples
3. Results
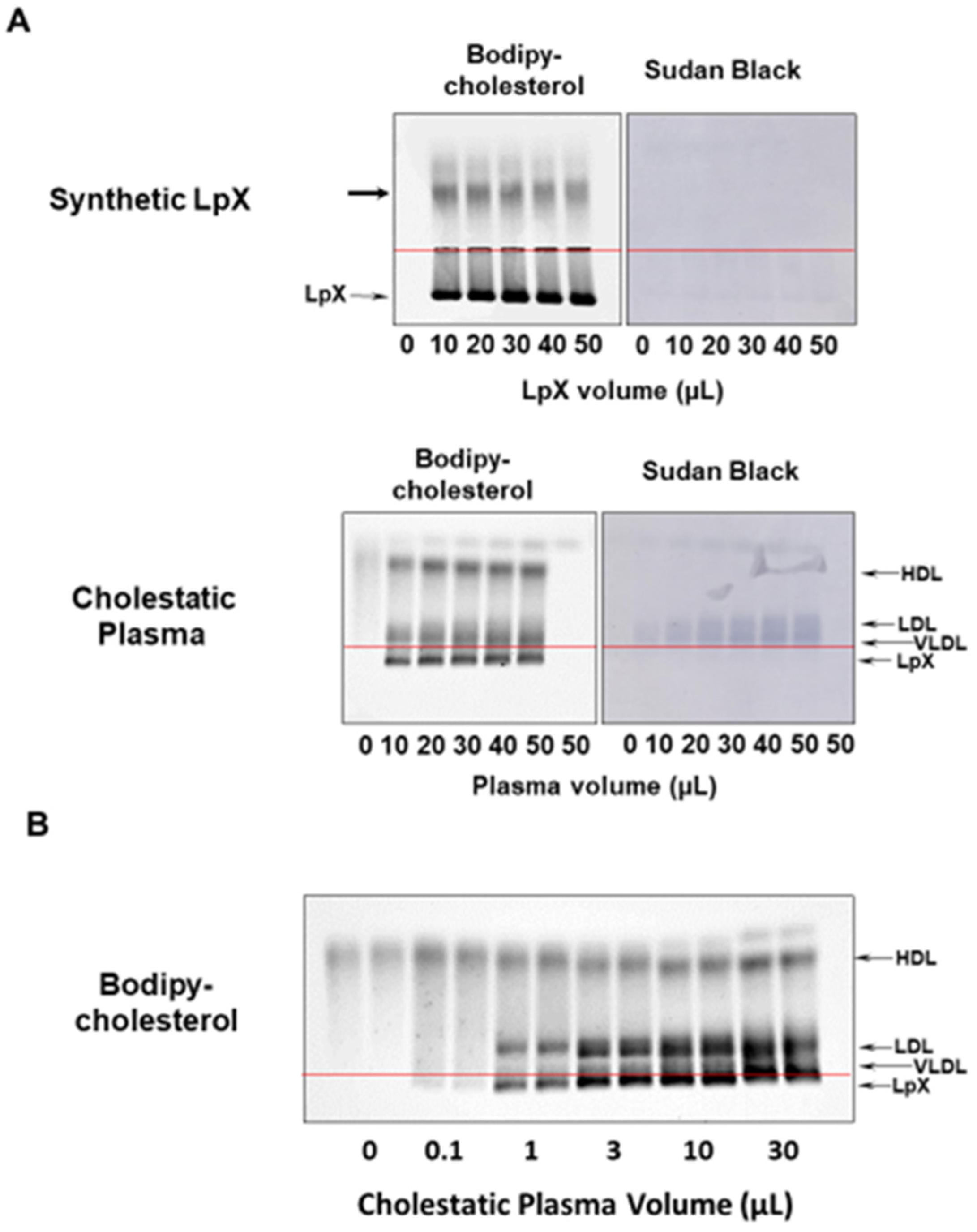
3.1. BODIPY-Cholesterol Complexed to Fatty-Acid-Free BSA Labeled Synthetic and Human Cholestatic Plasma LpX
3.2. BODIPY-Cholesterol Labeling of Lipoproteins in Normal and Cholestatic Plasma Containing LpX Was Unaltered by Trehalose
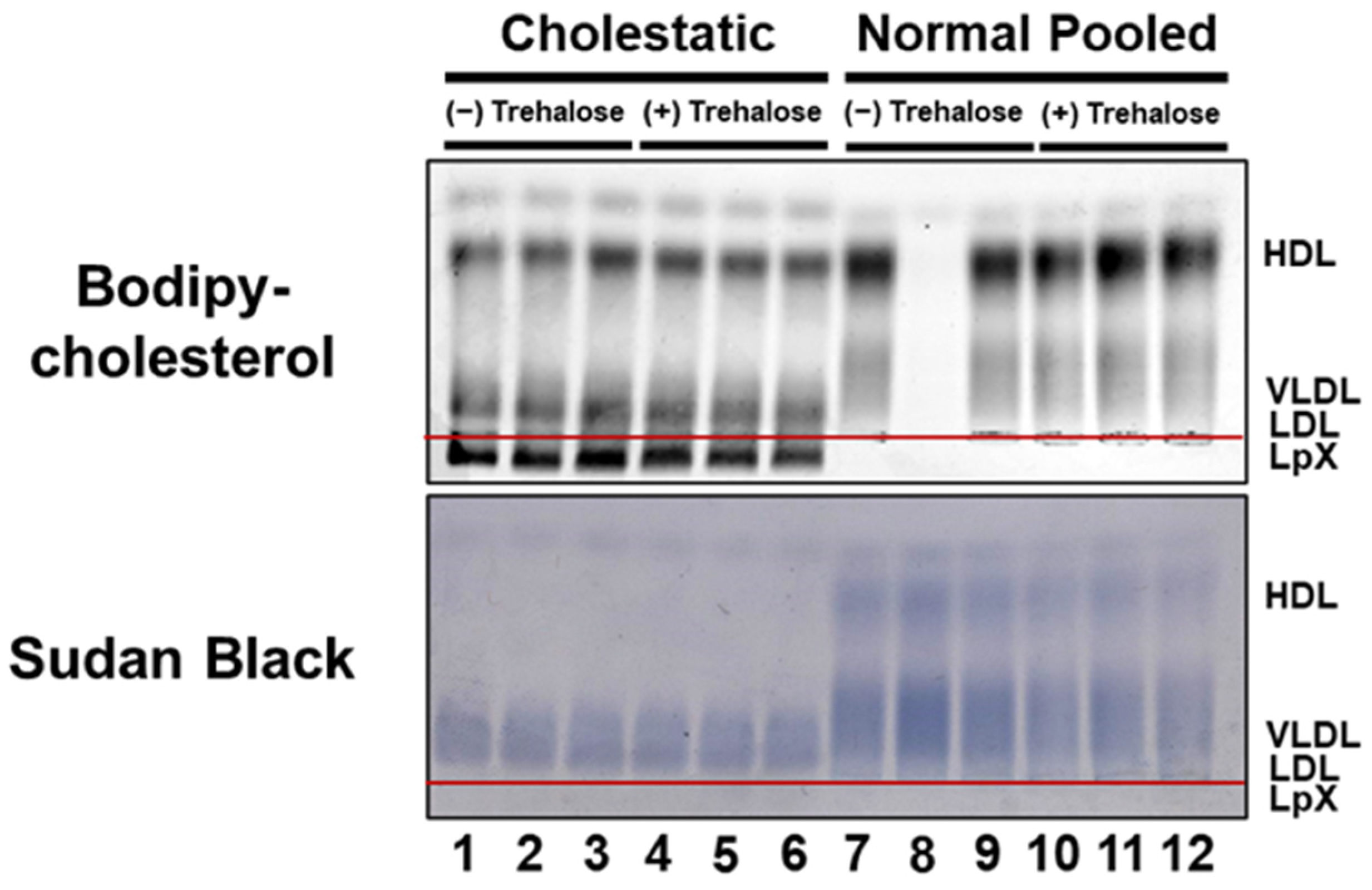
3.3. Combined Trehalose and BODIPY-Cholesterol Fatty-Acid-Free BSA Treatment Stabilized Cholestatic LpX during Freeze/Thawing
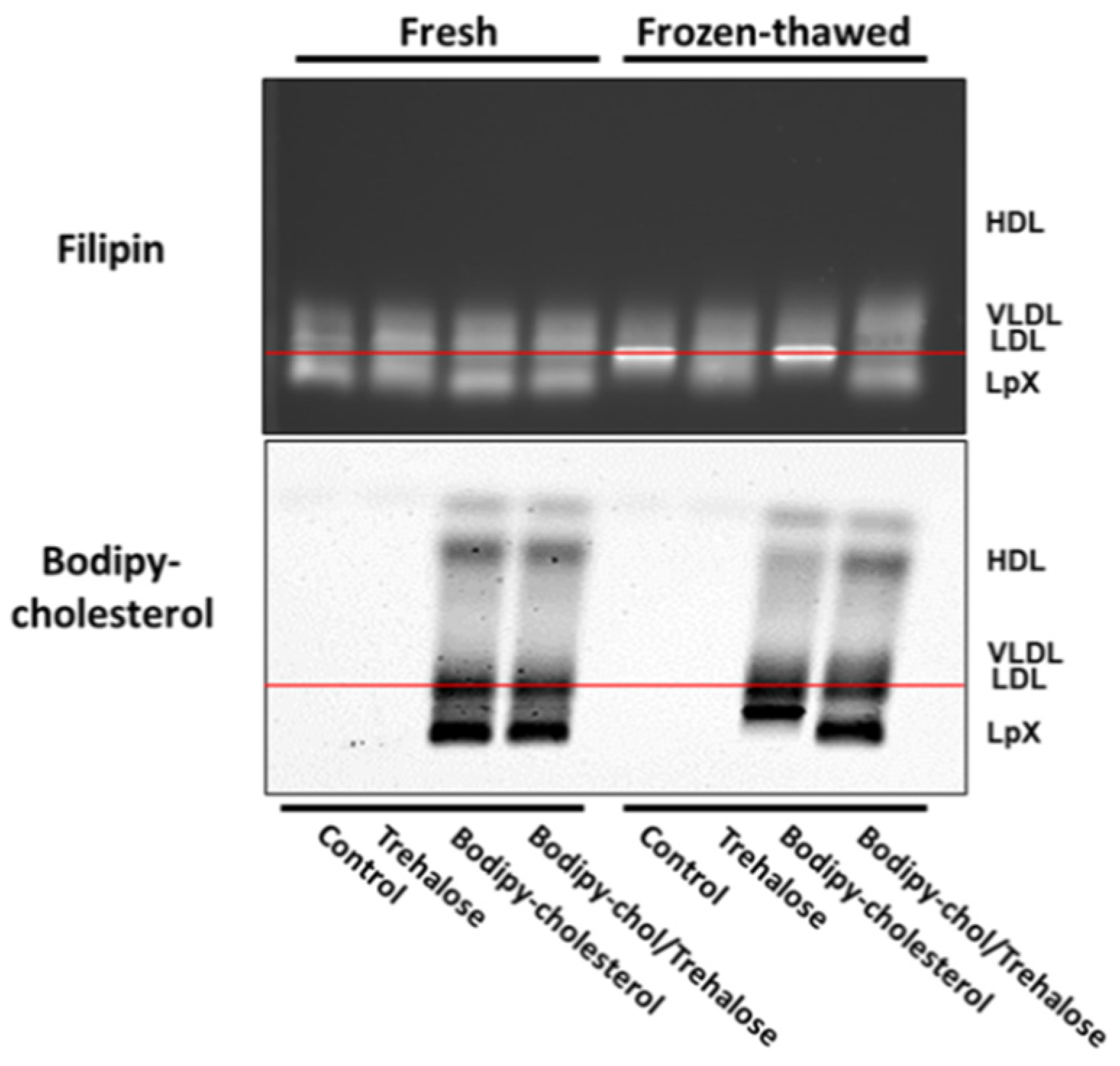
3.4. Combined Trehalose Treatment and Fatty-Acid-Free BSA-Mediated Labeling with BODIPY-Cholesterol, Lissaminerhodamine Phosphatidylethanolamine, or Both Stabilized Cholestatic LpX
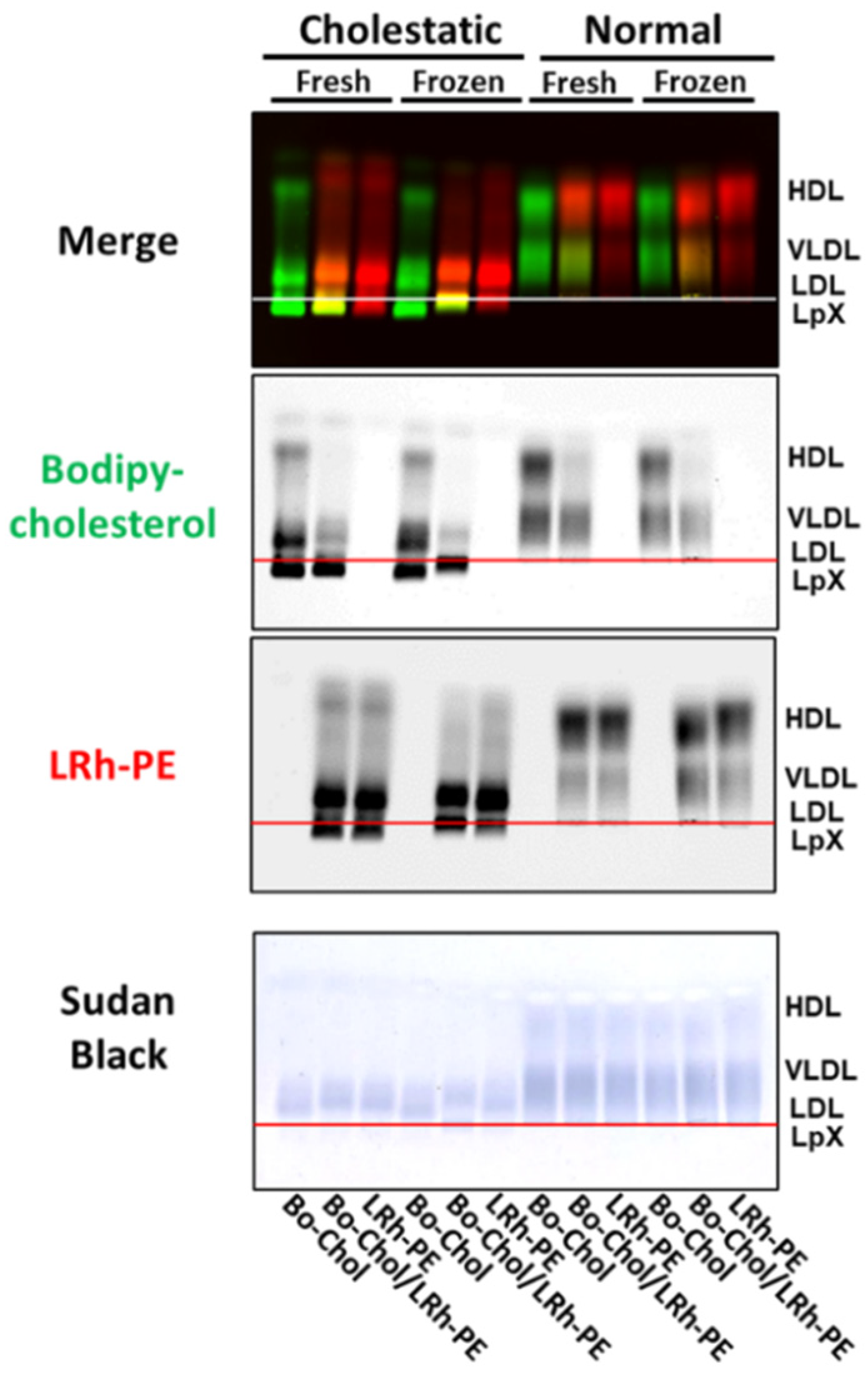
3.5. Combined Trehalose and BODIPY-Cholesterol Fatty-Acid-Free BSA Treatment Stabilized FLD LpX

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahsan, L.; Ossoli, A.F.; Freeman, L.; Vaisman, B.; Amar, M.J.; Shamburek, R.D.; Remaley, A.T. Chapter 7-Role of Lecithin: Cholesterol Acyltransferase in HDL Metabolism and Atherosclerosis. In The HDL Handbook, 2nd ed.; Komoda, T., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 159–194. [Google Scholar]
- Ossoli, A.; Neufeld, E.B.; Thacker, S.G.; Vaisman, B.; Pryor, M.; Freeman, L.A.; Brantner, C.A.; Baranova, I.; Francone, N.O.; Demosky, S.J., Jr.; et al. Lipoprotein X Causes Renal Disease in lcat Deficiency. PLoS ONE 2016, 11, e0150083. [Google Scholar] [CrossRef] [PubMed]
- Santamarina-Fojo, S.; Hoeg, J.M.; Assmann, G.; Bryan Brewer, H. Lecithin Cholesterol Acyltransferase Deficiency and Fish Eye Disease. In The Online Metabolic and Molecular Bases of Inherited Disease; Valle, D.L., Antonarakis, S., Ballabio, A., Beaudet, A.L., Mitchell, G.A., Eds.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Soros, P.; Bottcher, J.; Maschek, H.; Selberg, O.; Muller, M.J. Lipoprotein-X in Patients with Cirrhosis: Its Relationship to Cholestasis and Hypercholesterolemia. Hepatology 1998, 28, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Phatlhane, D.V.; Zemlin, A.E. Severe Hypercholesterolemia Mediated by Lipoprotein x in A Patient with Cholestasis. Ann. Hepatol. 2015, 14, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Kattah, L.; Gómez, A.; Gutiérrez, S.; Puerto, K.; Moreno-Pallares, E.D.; Jaramillo, A.; Mendivil, C.O. Hypercholesterolemia due to Lipoprotein X: Case Report and Thematic Review. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 1179551419878687. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Baker, A.L.; Chow, M.J.; Hay, R.V. Hyperviscosity Syndrome in A Hypercholesterolemic Patient with Primary Biliary Cirrhosis. Gastroenterology 1990, 98, 1351–1357. [Google Scholar] [CrossRef]
- Suzuki, L.; Hirayama, S.; Fukui, M.; Sasaki, M.; Hiroi, S.; Ayaori, M.; Terai, S.; Tozuka, M.; Watada, H.; Miida, T. Lipoprotein-x in Cholestatic Patients Causes Xanthomas and Promotes Foam Cell Formation in Human Macrophages. J. Clin. Lipidol. 2017, 11, 110–118. [Google Scholar] [CrossRef]
- Griffin, E.; Breckenridge, W.C.; Kuksis, A.; Bryan, M.H.; Angel, A. Appearance and Characterization of Lipoprotein X during Continuous Intralipid Infusions in The Neonate. J. Clin. Investig. 1979, 64, 1703–1712. [Google Scholar] [CrossRef]
- Joukhadar, R.; Chiu, K. Severe Hypercholesterolemia in Patients with Graft-Vs-Host Disease Affecting The Liver after Stem Cell Transplantation. Endocr. Pract. 2012, 18, 90–97. [Google Scholar] [CrossRef]
- Turchin, A.; Wiebe, D.A.; Seely, E.W.; Graham, T.; Longo, W.; Soiffer, R. Severe Hypercholesterolemia Mediated by Lipoprotein X in Patients with Chronic Graft-Versus-Host Disease of The Liver. Bone Marrow Transplant. 2005, 35, 85–89. [Google Scholar] [CrossRef][Green Version]
- Freeman, L.A.; Shamburek, R.D.; Sampson, M.L.; Neufeld, E.B.; Sato, M.; Karathanasis, S.K.; Remaley, A.T. Plasma Lipoprotein-X Quantification on Filipin-Stained Gels: Monitoring Recombinant LCAT Treatment Ex Vivo. J. Lipid Res. 2019, 60, 1050–1057. [Google Scholar] [CrossRef]
- Corradini, D.; Strekalova, E.G.; Stanley, H.E.; Gallo, P. Microscopic Mechanism of Protein Cryopreservation in An Aqueous Solution with Trehalose. Sci. Rep. 2013, 3, 1218. [Google Scholar] [CrossRef]
- Glafke, C.; Akhoondi, M.; Oldenhof, H.; Sieme, H.; Wolkers, W.F. Cryopreservation of Platelets Using Trehalose: The Role of Membrane Phase Behavior during Freezing. Biotechnol. Prog. 2012, 28, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, E.B.; Sato, M.; Gordon, S.M.; Durbhakula, V.; Francone, N.; Aponte, A.; Yilmaz, G.; Sviridov, D.; Sampson, M.; Tang, J.; et al. ApoA-I-Mediated Lipoprotein Remodeling Monitored with A Fluorescent Phospholipid. Biology 2019, 8, 53. [Google Scholar] [CrossRef]
- Lambruschini, C.; Relini, A.; Ridi, A.; Cordone, L.; Gliozzi, A. Trehalose Interacts with Phospholipid Polar Heads in Langmuir Monolayers. Langmuir 2000, 16, 5467–5470. [Google Scholar] [CrossRef]
- Andersen, H.D.; Wang, C.; Arleth, L.; Peters, G.H.; Westh, P. Reconciliation of Opposing Views on Membrane-Sugar Interactions. Proc. Natl. Acad. Sci. USA. 2011, 108, 1874–1878. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.P. Electrophoretic Separation of Plasma Lipoproteins in Agarose Gel. J. Lipid Res. 1968, 9, 693–700. [Google Scholar] [CrossRef]
- Ghosh, S.; Moss, D.B. Electroendosmosis Correction for Electrophoretic Mobility Determined in Cells. Anal. Biochem. 1974, 62, 365–370. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neufeld, E.B.; Freeman, L.A.; Durbhakula, V.; Sampson, M.L.; Shamburek, R.D.; Karathanasis, S.K.; Remaley, A.T. A Simple Fluorescent Cholesterol Labeling Method to Cryoprotect and Detect Plasma Lipoprotein-X. Biology 2022, 11, 1248. https://doi.org/10.3390/biology11081248
Neufeld EB, Freeman LA, Durbhakula V, Sampson ML, Shamburek RD, Karathanasis SK, Remaley AT. A Simple Fluorescent Cholesterol Labeling Method to Cryoprotect and Detect Plasma Lipoprotein-X. Biology. 2022; 11(8):1248. https://doi.org/10.3390/biology11081248
Chicago/Turabian StyleNeufeld, Edward B., Lita A. Freeman, Vinay Durbhakula, Maureen L. Sampson, Robert D. Shamburek, Sotirios K. Karathanasis, and Alan T. Remaley. 2022. "A Simple Fluorescent Cholesterol Labeling Method to Cryoprotect and Detect Plasma Lipoprotein-X" Biology 11, no. 8: 1248. https://doi.org/10.3390/biology11081248
APA StyleNeufeld, E. B., Freeman, L. A., Durbhakula, V., Sampson, M. L., Shamburek, R. D., Karathanasis, S. K., & Remaley, A. T. (2022). A Simple Fluorescent Cholesterol Labeling Method to Cryoprotect and Detect Plasma Lipoprotein-X. Biology, 11(8), 1248. https://doi.org/10.3390/biology11081248





