Trebouxia lynnae sp. nov. (Former Trebouxia sp. TR9): Biology and Biogeography of an Epitome Lichen Symbiotic Microalga
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Cultivation of Phycobionts
2.2. DNA Extraction, Amplification, and Sequencing
2.3. Phycobiont Phylogenetic Analysis
2.4. Microscopic Analyses of Phycobionts Grown in Culture
2.5. Isotopic Discrimination
3. Results
- Family Trebouxiaceae Friedl
- Genus Trebouxia Puymaly
- Trebouxialynnae Barreno sp. nov.
3.1. Phycobiont Phylogenetic Analysis
3.2. Geographical Occurrence
3.3. Morphological and Ultrastructural Characterization of Trebouxia lynnae sp. nov. in Culture
3.4. Isotopic Discrimination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawksworth, D.L.; Grube, M. Lichens redefined as complex ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Lendemer, J.C. A call to reconceptualize lichen symbioses. Trends Ecol. Evol. 2022, 37, 582–589. [Google Scholar] [CrossRef]
- Kranner, I.; Pichler, G.; Grube, M. The lichen market place. New Phytol. 2022, 234, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Honegger, R. Lichen-forming fungi and their photobionts. In The Mycota V: Plant Relationships, 2nd ed.; Deising, H.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 307–333. [Google Scholar]
- Leavitt, S.D.; Kraichak, E.; Nelsen, M.P.; Altermann, S.; Divakar, P.K.; Alors, D.; Esslinger, T.L.; Crespo, A.; Lumbsch, T. Fungal specificity and selectivity for algae play a major role in determining lichen partnerships across diverse ecogeographic regions in the lichen-forming family Parmeliaceae (Ascomycota). Mol. Ecol. 2015, 24, 3779–3797. [Google Scholar] [CrossRef]
- Muggia, L.; Nelsen, M.P.; Kirika, P.M.; Barreno, E.; Beck, A.; Lindgren, H.; Lumbsch, T.; Leavitt, S.D.; Trebouxia Working Group. Formally described species woefully underrepresent phylogenetic diversity in the common lichen photobiont genus Trebouxia (Trebouxiophyceae, Chlorophyta): An impetus for developing an integrated taxonomy. Mol. Phyl. Evol. 2020, 149, 106821. [Google Scholar] [CrossRef]
- Nelsen, M.P.; Lücking, R.; Boyce, C.K.; Lumbsch, H.T.; Ree, R.H. The macroevolutionary dynamics of symbiotic and phenotypic diversification in lichens. Proc. Natl. Acad. Sci. USA 2020, 117, 21495–21503. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, M.P.; Leavitt, S.D.; Heller, K.; Muggia, L.; Lumbsch, H.T. Contrasting patterns of climatic niche divergence in Trebouxia—A Clade of lichen-forming algae. Front. Microbiol. 2022, 13, 791546. [Google Scholar] [CrossRef]
- Xu, M.; De Boer, H.; Olafsdottir, E.S.; Omarsdottir, S.; Heidmarsson, S. Phylogenetic diversity of the lichenized algal genus Trebouxia (Trebouxiophyceae, Chlorophyta): A new lineage and novel insights from fungal-algal association patterns of Icelandic Cetrarioid lichens (Parmeliaceae, Ascomycota). Bot. J. Linn. Soc. 2020, 194, 460–468. [Google Scholar] [CrossRef]
- De Carolis, R.; Cometto, A.; Moya, P.; Barreno, E.; Grube, M.; Tretiach, M.; Leavitt, S.D.; Muggia, L. Photobiont diversity in lichen symbioses from extreme environments. Front. Microbiol. 2022, 13, 809804. [Google Scholar] [CrossRef] [PubMed]
- Kosecka, M.; Kukwa, M.; Jabłonska, A.; Flakus, A.; Rodríguez-Flakus, P.; Ptach, Ł.; Guzow-Krzeminska, B. Phylogeny and ecology of Trebouxia photobionts from Bolivian lichens. Front. Microbiol. 2022, 13, 779784. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, I.D.; Mazur, E.; Miadlikowska, J.; Flakus, A.; Rodríguez-Flakus, P.; Pardo-De la Hoz, C.J.; Cieslak, E.; Sliwa, L.; Lutzoni, F. Turnover of Lecanoroid mycobionts and their Trebouxia photobionts along an elevation gradient in Bolivia highlights the role of environment in structuring the lichen symbiosis. Front. Microbiol. 2021, 12, 774839. [Google Scholar] [CrossRef]
- Friedl, T. Comparative ultrastructure of pyrenoids in Trebouxia (Microthamniales, Chlorophyta). Plant Syst. Evol. 1989, 164, 145–159. [Google Scholar] [CrossRef]
- Muggia, L.; Leavitt, S.; Barreno, E. The hidden diversity of lichenized Trebouxiophyceae (Chlorophyta). Phycologia 2018, 57, 503–524. [Google Scholar] [CrossRef] [Green Version]
- Bordenave, C.D.; Muggia, L.; Chiva, S.; Leavitt, S.D.; Carrasco, P.; Barreno, E. Chloroplast morphology and pyrenoid ultrastructural analyses reappraise the diversity of the lichen phycobiont genus Trebouxia (Chlorophyta). Algal Res. 2022, 61, 102561. [Google Scholar] [CrossRef]
- Garrido Benavent, I.; Chiva, S.; Bordenave, C.D.; Molins, A.; Barreno, E. Trebouxia maresiae (Trebouxiophyceae, Chlorophyta), a new lichenized species of microalga found in coastal environments. Cryptogam. Algol. 2022, 43, 135–145. [Google Scholar] [CrossRef]
- Gasulla, F.; Guéra, A.; Barreno, E. A simple and rapid method for isolating lichen photobionts. Symbiosis 2010, 51, 175–179. [Google Scholar] [CrossRef]
- Muggia, L.; Leavitt, S.; Barreno, E. Report of the meeting of the Trebouxia-working group. Int. Lichenol. Newsl. 2016, 49, 35–37. [Google Scholar]
- Molins, A.; Moya, P.; García-Breijo, F.J.; Reig-Armiñana, J.; Barreno, E. A multi-tool approach to assess microalgal diversity in lichens: Isolation, Sanger sequencing, HTS and ultrastructural correlations. Lichenologist 2018, 50, 123–138. [Google Scholar] [CrossRef] [Green Version]
- Muggia, L.; Grube, M.; Tretiach, M. Genetic diversity and photobiont associations in selected taxa of the Tephromela atra group (Lecanorales, lichenised Ascomycota). Mycol. Prog. 2008, 7, 147–160. [Google Scholar] [CrossRef]
- Muggia, L.; Vancurová, L.; Škaloud, P.; Peksa, O.; Wedin, M.; Grube, M. The symbiotic playground of lichen thalli–a highly flexible photobiont association in rock-inhabiting lichens. FEMS Microbiol. Ecol. 2013, 85, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casano, L.M.; del Campo, E.M.; García-Breijo, F.J.; Reig-Armiñana, J.; Gasulla, F.; del Hoyo, A.; Guéra, A.; Barreno, E. Two Trebouxia algae with different physiological performances are ever-present in lichen thalli of Ramalina farinacea. Coexistence versus competition? Environ. Microbiol. 2011, 13, 806–818. [Google Scholar] [CrossRef]
- del Campo, E.M.; Català, S.; Casano, L.M.; Gimeno, J.; del Hoyo, A.; Martínez-Alberola, F.; Grube, M.; Barreno, E. The genetic structure of the cosmopolitan three-partner lichen Ramalina farinacea evidences the concerted diversification of symbionts. FEMS Microbiol. Ecol. 2013, 83, 310–323. [Google Scholar] [CrossRef] [Green Version]
- Dal Grande, F.; Alors, D.; Divakar, P.K.; Bálint, M.; Crespo, A.; Schmitt, I. Insights into intrathalline genetic diversity of the Cosmopolitan lichen symbiotic green alga Trebouxia decolorans Ahmadjian using microsatellite markers. Mol. Phylogenet. Evol. 2014, 72, 54–60. [Google Scholar] [CrossRef]
- Catalá, S.; del Campo, E.M.; Barreno, E.; García-Breijo, F.J.; Reig-Armiñana, J.; Casano, L.M. Coordinated ultrastructural and phylogenomic analyses shed light on the hidden phycobiont diversity of Trebouxia microalgae in Ramalina fraxinea. Mol. Phylogenet. Evol. 2016, 94, 765–777. [Google Scholar] [CrossRef]
- Molins, A.; Moya, P.; García-Breijo, F.J.; Reig-Armiñana, J.; Barreno, E. Molecular and morphological diversity of Trebouxia microalgae in sphaerothallioid Circinaria spp. lichens. J. Phycol. 2018, 54, 494–504. [Google Scholar] [CrossRef]
- del Campo, E.; Casano, L.M.; del Hoyo, A.; Martínez-Alberola, F.; Barreno, E. A rapid and cost-efficient DMSO-based method for isolating DNA from cultured lichen phycobionts. Taxon 2010, 59, 588–591. [Google Scholar] [CrossRef]
- del Campo, E.; Gimeno, J.; Casano, L.M.; Gasulla, F.; García-Breijo, F.; Reig-Armiñana, J.; Barreno, E. South European populations of Ramalina farinacea (L.) Ach. share different Trebouxia algae. Bibl. Lichenol. 2010, 105, 247–256. [Google Scholar]
- Blázquez, M.; Hernández-Moreno, L.S.; Gasulla, F.; Pérez-Vargas, I.; Pérez-Ortega, S. The role of photobionts as drivers of diversification in an island radiation of lichen-forming fungi. Front. Microbiol. 2021, 12, 784182. [Google Scholar] [CrossRef]
- Moya, P.; Molins, A.; Chiva, S.; Bastida, J.; Barreno, E. Symbiotic microalgal diversity within lichenicolous lichens and crustose hosts on Iberian Peninsula gypsum biocrusts. Sci. Rep. UK 2020, 10, 14060. [Google Scholar] [CrossRef]
- Moya, P.; Molins, A.; Martínez-Alberola, F.; Muggia, L.; Barreno, E. Unexpected associated microalgal diversity in the lichen Ramalina farinacea is uncovered by pyrosequencing analyses. PLoS ONE 2017, 12, e0175091. [Google Scholar]
- Molins, A.; Moya, P.; Muggia, L.; Barreno, E. Thallus growth stage and geographic origin shape microalgal diversity in Ramalina farinacea lichen holobionts. J. Phycol. 2021, 57, 975–987. [Google Scholar] [CrossRef]
- del Hoyo, A.; Álvarez, R.; del Campo, E.; Gasulla, F.; Barreno, E.; Casano, L.M. Oxidative stress induces distinct physiological responses in the two Trebouxia phycobionts of the lichen Ramalina farinacea. Ann. Bot. 2011, 107, 109–118. [Google Scholar] [CrossRef]
- Catalá, M.; Gasulla, F.; García-Breijo, F.; Reig-Armiñana, J.; Barreno, E. Fungal-associated NO is involved in the regulation of oxidative stress during rehydration in lichen symbiosis. BMC Microbiol. 2010, 297, 297. [Google Scholar] [CrossRef] [Green Version]
- Hichri, I.; Boscari, A.; Meilhoc, E.; Catalá, M.; Barreno, E.; Bruand, C.; Lanfranco, L.; Brouquisse, R. Nitric oxide: A multi-task player in plant-microorganism symbioses. In Gasotransmitters in Plants. Signalling and Communication in Plants Series; Lamattina, L., García-Mata, C., Eds.; Springer Nature: Cham, Switzerland, 2016; pp. 239–268. [Google Scholar]
- Expósito, J.R.; Martín San Román, S.; Barreno, E.; Reig-Armiñana, J.; Garcia-Breijo, F.; Catalá, M. Inhibition of NO biosynthetic activities during rehydration of Ramalina farinacea lichen thalli provokes increases in lipid peroxidation. Plants 2019, 8, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Expósito, J.R.; Coello, A.J.; Barreno, E.; Casano, L.M.; Catalá, M. Endogenous NO is involved in dissimilar responses to rehydration and Pb (NO3)2 in Ramalina farinacea thalli and its isolated phycobionts. Microb. Ecol. 2020, 79, 604–616. [Google Scholar] [CrossRef]
- Expósito, J.R.; Barreno, E.; Catalá, M. Role of NO in lichens. In Nitric Oxide in Plant Biology; Singh, V.P., Singh, S., Tripathi, D.K., Romero-Puertas, M.C., Sandalio, L.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 18, pp. 407–429. [Google Scholar]
- Hinojosa-Vidal, E.; Marco, F.; Martínez-Alberola, F.; Escaray, F.J.; García-Breijo, F.J.; Reig-Armiñana, J.; Barreno, E. Characterization of the responses to saline stress in the symbiotic green microalga Trebouxia sp. TR9. Planta 2018, 248, 1473–1486. [Google Scholar] [CrossRef]
- Pérez-Rodrigo, M.; Moya, P.; Marco, F.; Carrasco, P.; Barreno, E.A. Symbiotic Trebouxia sp. TR9, Asterochloris erici and free-living Chlorella vulgaris green microalgae respond differentially to osmotic and saline stresses. In Proceedings of the IAL9 Program & Abstracts Book, Universidade Federal de Sergipe, Brasil, International Association for Lichenology 9th Symposium “Unlocking the inner Lichen” Virtual-IAL9, Bonito, Brasil, 1–6 August 2021; pp. 136–137. Available online: https://doity.com.br/ial9/blog/ial-program-book (accessed on 1 August 2022).
- Catalá, M.; Gasulla, F.; Pradas Del Real, A.E.; García-Breijo, F.; Reig-Armiñana, J.; Barreno, E. The organic air pollutant cumene hydroperoxide interferes with NO antioxidant role in rehydrating lichen. Environ. Pollut. 2013, 179, 277–284. [Google Scholar] [CrossRef]
- Domínguez-Morueco, N.; Moreno, H.; Barreno, E.; Catalá, M. Preliminary assessment of microalgae isolated from lichens as testing species for environmental monitoring: Lichen phycobionts present high sensitivity to environmental micropollutants. Ecotox. Environ. Safe. 2014, 99, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, R.; del Hoyo, A.; Díaz-Rodríguez, C.; Coello, A.J.; del Campo, E.M.; Catalá, M.; Barreno, E.; Casano, L.M. Lichen rehydration in heavy metal-polluted environments: Pb modulates the oxidative response of both Ramalina farinacea thalli and its isolated microalgae. Microb. Ecol. 2015, 69, 698–709. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, R.; del Hoyo, A.; García-Breijo, F.; Reig-Armiñana, J.; del Campo, E.M.; Guéra, A.; Barreno, E.; Casano, L.M. Different strategies to achieve Pb-tolerance by the two Trebouxia algae coexisting in the lichen Ramalina farinacea. J. Plant Physiol. 2012, 169, 1797–1806. [Google Scholar] [CrossRef]
- Casano, L.M.; Braga, M.R.; Álvarez, R.; del Campo, E.M.; Barreno, E. Differences in the cell walls and extracellular polymers of the two Trebouxia microalgae coexisting in the lichen Ramalina farinacea are consistent with their distinct capacity to immobilize extracellular Pb. Plant Sci. 2015, 236, 195–204. [Google Scholar] [CrossRef]
- Gasulla, F.; del Campo, E.M.; Casano, L.M.; Guéra, A. Advances in understanding desiccation tolerance of lichens and lichen-forming algae. Plants 2021, 10, 807. [Google Scholar] [CrossRef]
- Martínez-Alberola, F. Caracterización Genómica del Microalga Trebouxia sp. TR9 Aislada del Liquen Ramalina farinacea (L.) Ach. Mediante Secuenciación Masiva. Ph. D. Thesis, Universitat de València, Burjassot, Spain, 2015. Available online: http://roderic.uv.es/handle/10550/48824 (accessed on 1 August 2022).
- Pérez-Rodrigo, M.; Montero-Pau, J.; Marco, F.; Moya, P.; Carrasco, P.; Barreno, E. The nuclear genome of the lichen symbiont microalga Trebouxia sp.TR9 (Trebouxiophyceae, Chlorophyta): New assembly and annotation. In Proceedings of the IAL9 Program & Abstracts Book, Universidade Federal de Sergipe, Brasil, International Association for Lichenology 9th Symposium “Unlocking the inner Lichen” Virtual-IAL9, Bonito, Brasil, 1–6 August 2021; Poster 187313. pp. 187–188. Available online: https://www.bib.irb.hr/1139857/download/1139857.FULL_BOOK.pdf (accessed on 1 August 2022).
- Martínez-Alberola, F.; Barreno, E.; Casano, L.M.; Gasulla, F.; Molins, A.; del Campo, E.M. Dynamic evolution of mitochondrial genomes in Trebouxiophyceae including the first completely assembled mtDNA from a lichen-forming microalga. Sci. Rep. 2019, 9, 8209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Alberola, F.; Barreno, E.; Casano, L.M.; Gasulla, F.; Molins, A.; Moya, P.; González-Hourcade, M.; del Campo, E.M. The chloroplast genome of the lichen-symbiont microalga Trebouxia sp. TR9 (Trebouxiophyceae, Chlorophyta) shows short inverted repeats with a single gene and loss of the RPS4 gene, which is encoded by the nucleus. J. Phycol. 2020, 56, 170–184. [Google Scholar] [CrossRef]
- Lange, O.L.; Kilian, E.; Ziegler, H. Water vapor uptake and photosynthesis of lichens: Performance differences in species with green and blue-green algae as phycobionts. Oecologia 1986, 71, 104–110. [Google Scholar] [CrossRef]
- Lange, O.L.; Green, T.G.A.; Ziegler, H. Water status related photosynthesis and carbon isotope discrimination in species of the lichen genus Pseudocyphellaria with green or blue-green photobionts and in photosymbiodemes. Oecologia 1988, 75, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Lange, O.L.; Meyer, A.; Zellner, H.; Heber, U. Photosynthesis and water relations of lichen soil crusts: Field measurements in the coastal fog zone of the Namib Desert. Funct. Ecol. 1994, 8, 253–264. [Google Scholar] [CrossRef]
- Smith, E.C.; Griffiths, H. The occurrence of the chloroplast pyrenoid is correlated with the activity of a CO2-concentrating mechanism and carbon isotope discrimination in lichens and bryophytes. Planta 1996, 198, 6–16. [Google Scholar] [CrossRef]
- Beardall, J.; Giordano, M. Ecological implications of microalgal and cyanobacterial CO2 concentrating mechanisms, and their regulation. Funct. Plant Biol. 2002, 29, 335–347. [Google Scholar] [CrossRef]
- Young, E.B.; Beardall, J. Modulation of photosynthesis and inorganic carbon acquisition in a marine microalga by nitrogen, iron, and light availability. Can. J. Bot. 2005, 83, 917–928. [Google Scholar] [CrossRef]
- Brandenburg, K.M.; Rost, B.; Van de Waal, D.B.; Hoins, M.; Sluijs, A. Physiological control on carbon isotope fractionation in marine phytoplankton. Biogeosciences 2022, 19, 3305–3315. [Google Scholar] [CrossRef]
- Hikosaka, K.; Terashima, I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ. 1995, 18, 605–618. [Google Scholar] [CrossRef]
- Ögren, E.; Sundin, U. Photosynthetic responses to variable light: A comparison of species from contrasting habitats. Oecologia 1996, 106, 18–27. [Google Scholar] [CrossRef]
- Beardall, J.; Griffiths, H.; Raven, J.A. Carbon isotope discrimination and the CO2 accumulating mechanism in Chlorella emersonii. J. Exp. Bot. 1982, 33, 729–737. [Google Scholar] [CrossRef]
- Raven, J.A.; Johnston, A.M. Mechanisms of inorganic-carbon acquisition in marine phytoplankton and their implications for the use of other resources. Limnol. Oceanogr. 1991, 36, 1701–1714. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.C.; Griffiths, H. A pyrenoid-based carbon-concentrating mechanism is present in terrestrial bryophytes of the class Anthocerotae. Planta 1996, 200, 203–212. [Google Scholar] [CrossRef]
- Palmqvist, K. Photosynthetic CO2-use efficiency in lichens and their isolated photobionts: The possible role of a CO2-concentrating mechanism. Planta 1993, 191, 48–56. [Google Scholar] [CrossRef]
- Palmqvist, K.; Samuelsson, G.; Badger, M.R. Photobiont-related differences in carbon acquisition among green-algal lichens. Planta 1994, 195, 70–79. [Google Scholar] [CrossRef]
- Bold, H.C. Themorphology of Chlamydomonas chlamydogama sp. nov. Bull. Torrey Bot. Club 1949, 76, 101–108. [Google Scholar] [CrossRef]
- Bischoff, H.W.; Bold, H.C. Physiological studies: IV. In Some Soil Algae from Enchanted Rock and Related Algal Species; University of Texas: Austin, TX, USA, 1963; p. 6318. [Google Scholar]
- Kroken, S.; Taylor, J.W. Phylogenetic species, reproductive mode, and specificity of the green alga Trebouxia forming lichens with the fungal genus Letharia. Bryol. 2000, 103, 645–660. [Google Scholar] [CrossRef]
- Nelsen, M.P.; Plata, E.R.; Andrew, C.J.; Lücking, R.; Lumbsch, H.T. Phylogenetic diversity of Trentepohlialean algae associated with lichen-forming fungi 1. J. Phycol. 2011, 47, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, H.; Velmala, S.; Högnabba, F.; Goward, T.; Holien, H.; Myllys, L. High fungal selectivity for algal symbionts in the genus Bryoria. Lichenologist 2014, 46, 681–695. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2008, 9, 286–298. [Google Scholar] [CrossRef] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop, New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Rambaut, A. FigTree, 1.4.2 Software; Institute of Evolutionary Biology: Edinburgh, UK, 2014. [Google Scholar]
- Moya, P.; Chiva, S.; Molins, A.; Jadrná, I.; Škaloud, P.; Peksa, O.; Barreno, E. Myrmecia israelensis as the primary symbiotic microalga in squamulose lichens growing in European and Canary Islands terricolous communities. Fottea 2018, 18, 72–85. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gartner, G. Die Gattung Trebouxia Puymaly (Chlorellales, Chlorophyceae). Arch. Hydrobiol. Suppl. 1985, 71, 495–548. [Google Scholar]
- Archibald, P.A. Trebouxia de Puymaly (Chlorophyceae, Chlorococcales) and Pseudotrebouxia gen. nov. (Chlorophyceae, Chlorosarcinales). Phycologia 1975, 14, 125–137. [Google Scholar] [CrossRef]
- Friedl, T.; Gärtner, G. Trebouxia (Pleurastrales, Chlorophyta) as a phycobiont in the lichen genus Diploschistes. Archiv. Protistenkd. 1988, 135, 147–158. [Google Scholar] [CrossRef]
- Voytsekhovich, A.; Beck, A. Lichen photobionts of the rocky outcrops of Karadag massif (Crimean Peninsula). Symbiosis 2016, 68, 9–24. [Google Scholar] [CrossRef]
- Ahmadjian, V. Some new and interesting species of Trebouxia, a genus of lichenized algae. Am. J. Bot. 1960, 47, 677–683. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Penas, Á.; del Río, S.; Díaz González, T.E.; Rivas-Sáenz, S. Bioclimatology of the Iberian Peninsula and the Balearic Islands. In The Vegetation of the Iberian Peninsula; Loidi, J., Ed.; Springer: Cham, Switzerland, 2017; Volume 12, pp. 29–80. [Google Scholar]
- Moya, P.; Molins, A.; Škaloud, P.; Divakar, P.K.; Chiva, S.; Dumitru, C.; Crespo, A.; Barreno, E. Biodiversity Patterns and Ecological Preferences of the Photobionts Associated with the Lichen-Forming Genus Parmelia. Front. Microbiol. 2021, 12, 765310. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Heuser, J.E.; Roth, R.; Goodenough, U. Eisosome ultrastructure and evolution in fungi, microalgae, and lichens. Eukaryot. Cell 2015, 14, 1017–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melkonian, M. Flagellar apparatus ultrastructure in relation to green algal classification. In Systematics of the Green Algae; Irvine, D.E.G., John, D.M., Eds.; Academic Press: London, UK; Orlando, FL, USA, 1984; pp. 73–120. [Google Scholar]
- Škaloud, P.; Steinová, J.; Řídká, T.; Vančurová, L.; Peksa, O. Assembling the challenging puzzle of algal biodiversity: Species delimitation within the genus Asterochloris (Trebouxiophyceae, Chlorophyta). J. Phycol. 2015, 51, 507–527. [Google Scholar] [CrossRef]
- Chiva, S.; Dumitru, C.; Bordenave, C.D.; Barreno, E. Watanabea green microalgae (Trebouxiophyceae) inhabiting lichen holobiomes: Watanabea lichenicola sp. nova. Phycol. Res. 2021, 69, 226–236. [Google Scholar] [CrossRef]
- Muggia, L.; Kopun, T.; Grube, M. Effects of growth media on the diversity of culturable fungi from lichens. Molecules 2017, 22, 824. [Google Scholar] [CrossRef] [Green Version]
- Moya, P.; Chiva, S.; Molins, A.; Garrido-Benavent, I.; Barreno, E. Unravelling the symbiotic microalgal diversity in Buellia zoharyi (lichenized Ascomycota) from the Iberian Peninsula and Balearic Islands using DNA metabarcoding. Diversity 2021, 13, 220. [Google Scholar] [CrossRef]
- Helms, G.; Friedl, T.; Rambold, G.; Mayrhofer, H. Identification of photobionts from the lichen family Physciaceae using algal-specific ITS rDNA sequencing. Lichenologist 2001, 33, 73–86. [Google Scholar] [CrossRef]
- Beck, A. Selektivität der Symbionten Schwermetalltoleranter Flechten. Ph.D. Thesis, Ludwig-Maximilians-Universität München, Munich, Germany, 2002. [Google Scholar]
- Arakawa-Kobayashi, S.; Kanaseki, T. A study of lipid secretion from the lichen symbionts, ascomycetous fungus Myelochroa leucotyliza and green alga Trebouxia sp. J. Struct. Biol. 2004, 146, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.; Goodenough, U. Lichen 1. Solo fungal and algal partners. Algal Res. 2021, 58, 102334. [Google Scholar] [CrossRef]
- Arakawa, S.; Kanaseki, T.; Wagner, R.; Goodenough, U. Ultrastructure of the foliose lichen Myelochroa leucotyliza and its solo fungal and algal (Trebouxia sp.) partners. Algal Res. 2022, 62, 102571. [Google Scholar] [CrossRef]
- González-Hourcade, M.; Braga, M.R.; del Campo, E.M.; Ascaso, C.; Patiño, C.; Casano, L.M. Ultrastructural and biochemical analyses reveal cell wall remodelling in lichen-forming microalgae submitted to cyclic desiccation–rehydration. Ann. Bot. 2020, 125, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, U.; Wagner, R.; Roth, R. Lichen 4. The Algal Layer. Algal Res. 2021, 58, 102355. [Google Scholar] [CrossRef]
- Carniel, F.C.; Zanelli, D.; Bertuzzi, S.; Tretiach, M. Desiccation tolerance and lichenization: A case study with the aeroterrestrial microalga Trebouxia sp. (Chlorophyta). Planta 2015, 242, 493–505. [Google Scholar] [CrossRef] [Green Version]
- Carniel, F.C.; Gerdol, M.; Montagner, A.; Banchi, E.; De Moro, G.; Manfrin, C.; Muggia, L.; Pallavicini, A.; Tretiach, M. New features of desiccation tolerance in the lichen photobiont Trebouxia gelatinosa are revealed by a transcriptomic approach. Plant Mol. Biol. 2016, 91, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Melkonian, M.; Berns, B. Zoospore ultrastructure in the green alga Friedmannia israelensis: An absolute configuration analysis. Protoplasma 1983, 114, 67–84. [Google Scholar] [CrossRef]
- Melkonian, M.; Peveling, E. Zoospore ultrastructure in species of Trebouxia and Pseudotrebouxia (Chlorophyta). Plant Syst. Evol. 1988, 158, 183–210. [Google Scholar] [CrossRef]
- Máguas, C.; Griffiths, H.; Broadmeadow, M.S.J. Gas exchange and carbon isotope discrimination in lichens: Evidence for interactions between CO2-concentrating mechanisms and diffusion limitation. Planta 1995, 196, 95–102. [Google Scholar] [CrossRef]
- Lee, Y.I.; Lim, H.S.; Yoon, H.I. Carbon and nitrogen isotope composition of vegetation on King George Island, maritime Antarctic. Polar Biol. 2009, 32, 1607–1615. [Google Scholar] [CrossRef]
- Beck, A.; Mayr, C. Nitrogen and carbon isotope variability in the green-algal lichen Xanthoria parietina and their implications on mycobiont–photobiont interactions. Ecol. Evol. 2012, 2, 3132–3144. [Google Scholar] [CrossRef] [PubMed]
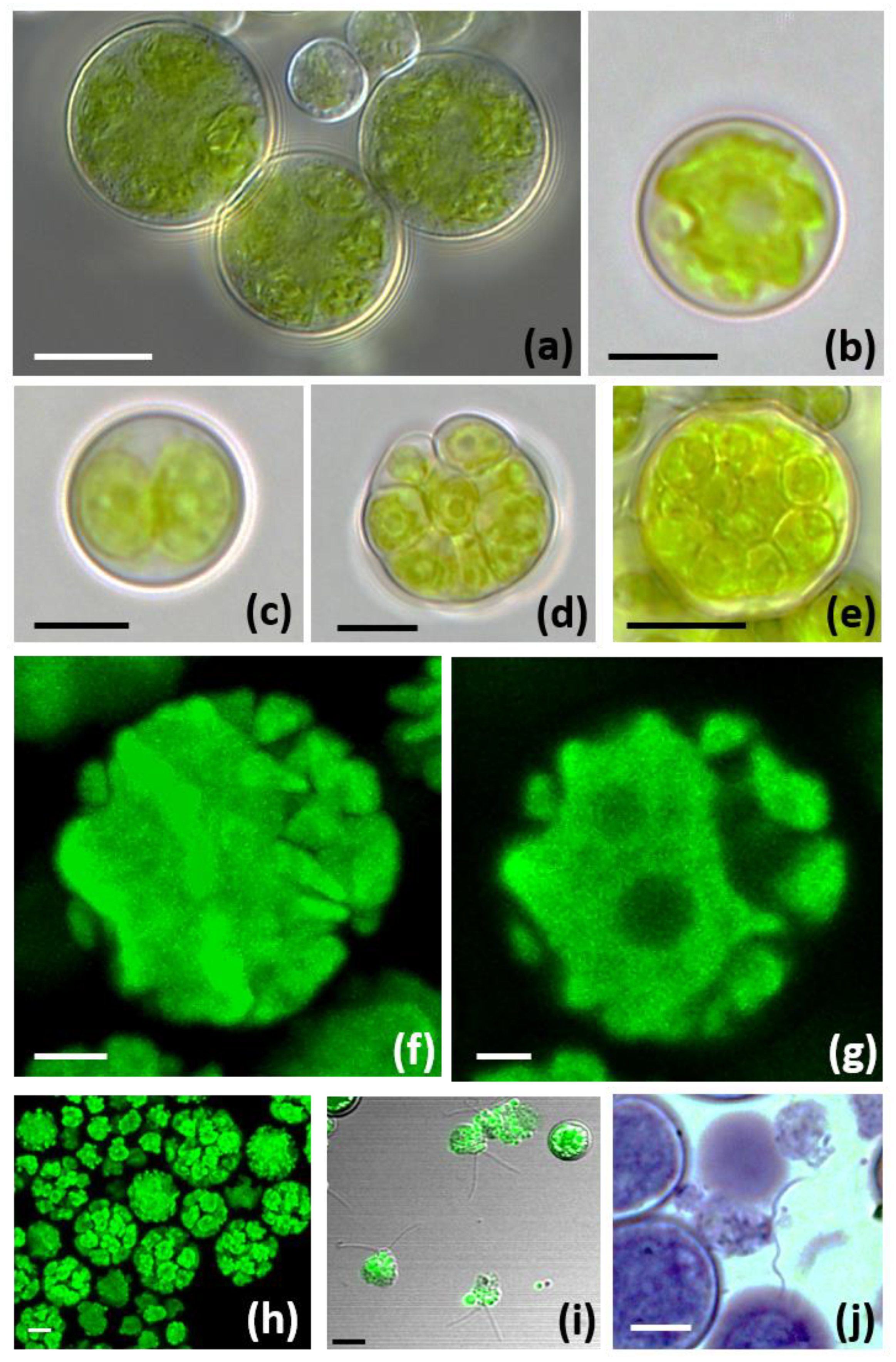
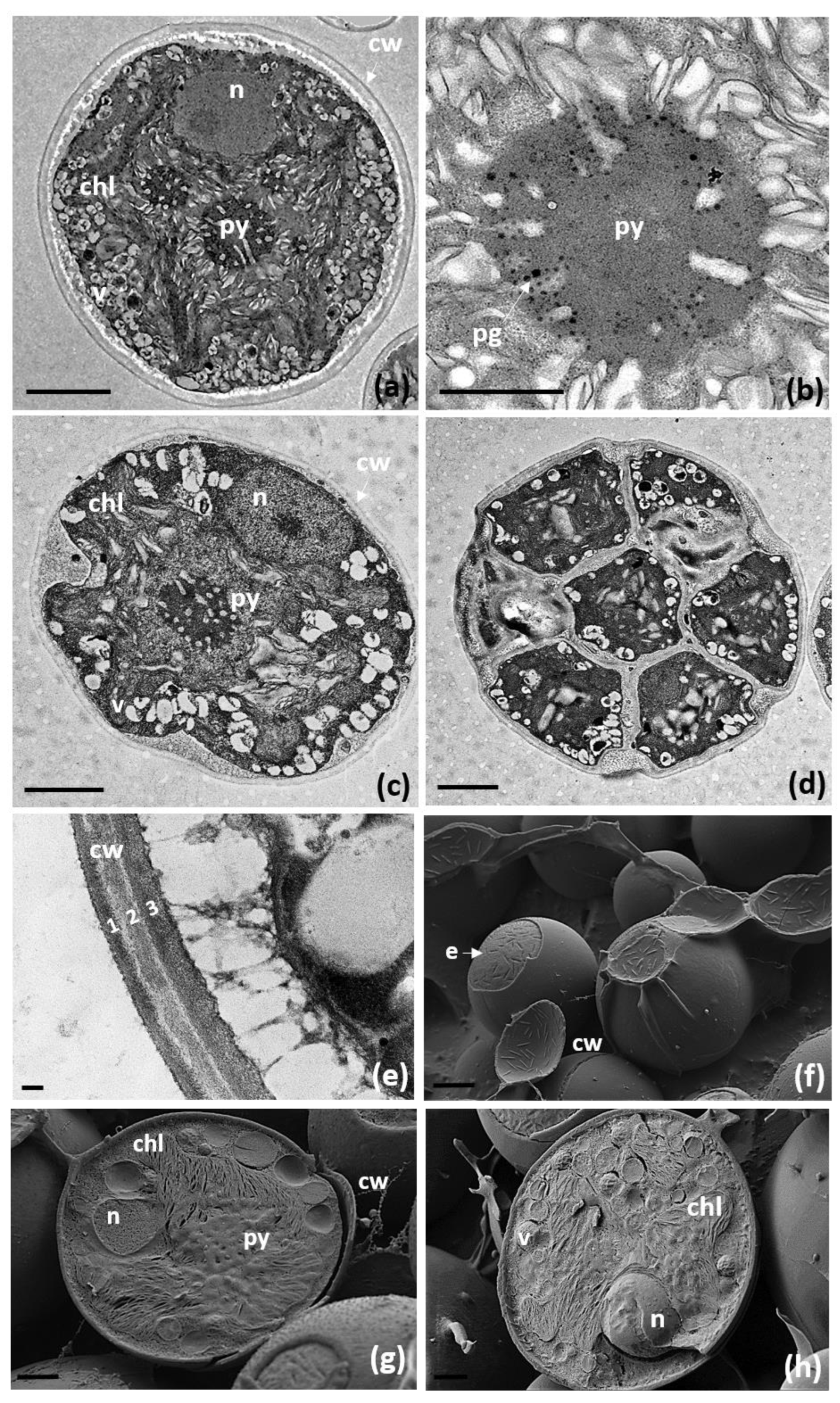
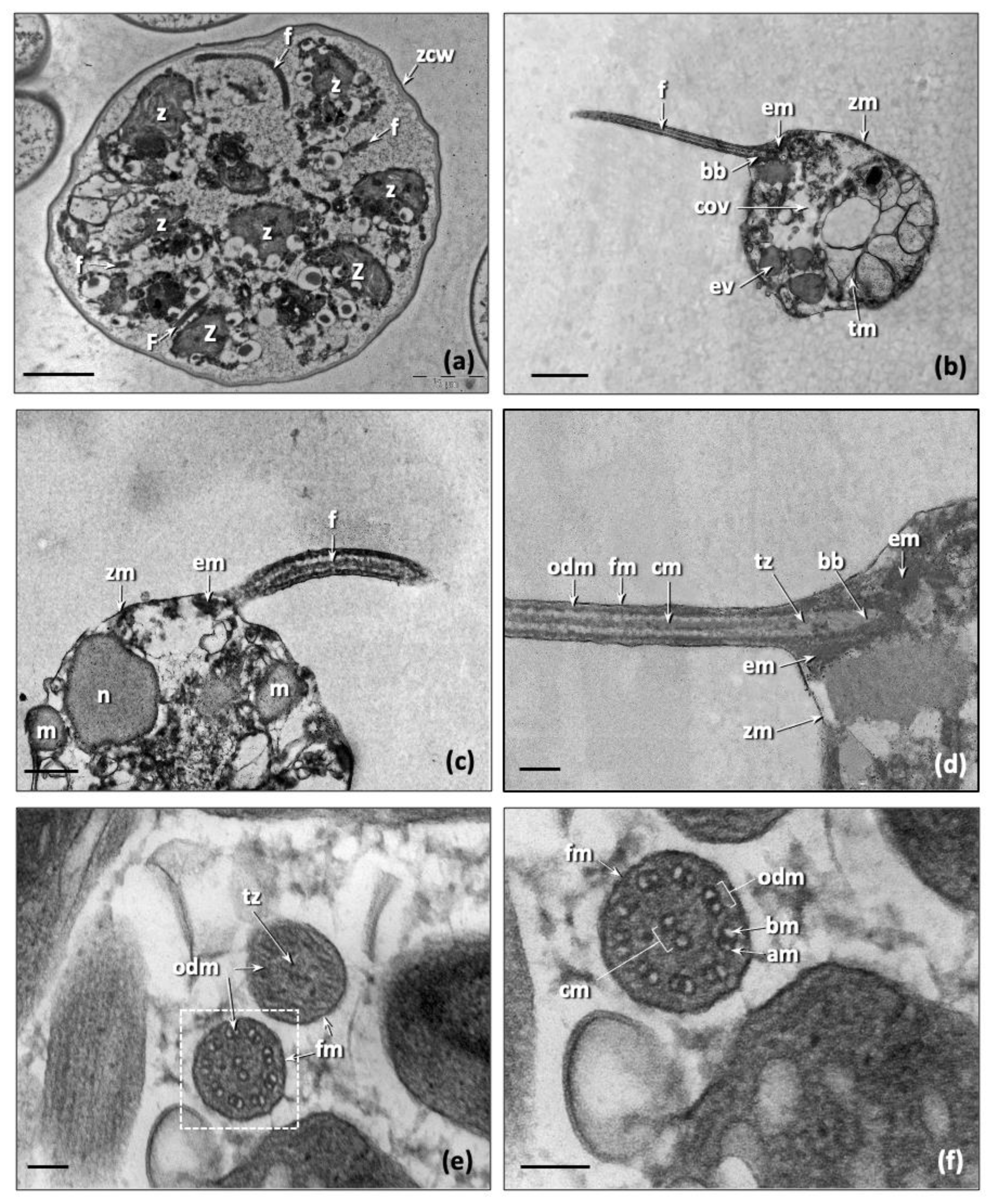

| Trebouxia Species | Shape and Size of Mature Cells | Chloroplast-Type | Pyrenoid-Type |
|---|---|---|---|
| Trebouxia aggregata | Vegetative spherical cells 9–14 μm in diameter [80] Vegetative spherical cells 8–16 (24) μm in diameter [81] | Crenulata-type with slightly branched lobes | Gigantea-type, single pyrenoid |
| Trebouxia arboricola | Vegetative spherical cells 13–15 μm in diameter [80] | Crenulata-type with slightly branched lobes | Gigantea-type, single pyrenoid |
| Trebouxia asymmetrica | Vegetative cells are often ovoid or ellipsoidal, 19 × 14 μm maximum size [82] | Shallowly lobed-type with flat lobe terminations | Gigantea-type, multiple pyrenoids |
| Trebouxia crenulata | Vegetative spherical cells 10–16 (20) μm in diameter [80] Vegetative spherical cells 5–18 (24) μm in diameter [81] | Crenulata-type with branched, tree-like lobes | Crenulata-type, single pyrenoid |
| Trebouxia cretacea | Vegetative spherical cells 15–20 (30) μm in diameter, but also ovoid and ellipsoid cells 20–22 (–30) × 15 μm [83] | Crenulata-type with small unbranched lobes | Gigantea-type, multiple pyrenoids |
| Trebouxia decolorans | Vegetative spherical cells 10–13 (17) μm in diameter [80] Vegetative spherical cells 6–13 (20) μm in diameter [81] Vegetative spherical cells 19–25.5 (30) μm in diameter [84] | Deeply lobed-type | Decolorans-type, multiple pyrenoids |
| Trebouxia gigantea | Vegetative spherical cells 14–22 (27) μm in diameter [80] | Shallowly lobed-type with elongated lobes | Gigantea-type, single pyrenoid |
| Trebouxia incrustata | Vegetative spherical cells 10–14 (15) μm in diameter [80] Vegetative spherical cells 3–10 (22) μm in diameter [81] | Shallowly lobed-type with elongated lobes | Gigantea-type, single pyrenoid |
| Trebouxia jamesii | Vegetative spherical cells 10–15 (20) μm in diameter [80] | Shallowly lobed-type with elongated lobes | Impressa-type, single (or multiple) pyrenoid |
| Trebouxia lynnae | Vegetative spherical cells 7–12 (16) μm in diameter | Shallowly lobed-type with elongated lobes | Impressa-type, single (or multiple) pyrenoid |
| Trebouxia maresiae | Vegetative spherical cells 7–11 (15) μm in diameter [16] | Crenulata-type with branched tree-like lobes | Maresiae-type, single pyrenoid |
| Trebouxia showmanii | Vegetative spherical cells, often slightly ovoid or ellipsoidal, 11–16 (22) μm in diameter [80] | Crenulata-type with branched, tree-like lobes | Gigantea-type, single pyrenoid |
| Trebouxia solaris | Vegetative spherical cells 15–20 (22) μm in diameter [83] | Crenulata-type | Type not reported, single pyrenoid with starch grains or satellites |
| Trebouxia vagua | Vegetative spherical cells 15–20 μm in diameter, ovoid and ellipsoid cells 20–22 (28) × 13–18 μm [83] | Chloroplast with wide lobes in young cells and narrow lobes in old cells | Type not reported, single pyrenoid with starch grains or satellites |
| δ13C | δ15N | %C | %N | |
|---|---|---|---|---|
| Trebouxia lynnae | −16.96‰ | 6.93‰ | 47.55 | 6.41 |
| Trebouxia jamesii | −17.60‰ | 6.43‰ | 46.95 | 5.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreno, E.; Muggia, L.; Chiva, S.; Molins, A.; Bordenave, C.; García-Breijo, F.; Moya, P. Trebouxia lynnae sp. nov. (Former Trebouxia sp. TR9): Biology and Biogeography of an Epitome Lichen Symbiotic Microalga. Biology 2022, 11, 1196. https://doi.org/10.3390/biology11081196
Barreno E, Muggia L, Chiva S, Molins A, Bordenave C, García-Breijo F, Moya P. Trebouxia lynnae sp. nov. (Former Trebouxia sp. TR9): Biology and Biogeography of an Epitome Lichen Symbiotic Microalga. Biology. 2022; 11(8):1196. https://doi.org/10.3390/biology11081196
Chicago/Turabian StyleBarreno, Eva, Lucia Muggia, Salvador Chiva, Arantzazu Molins, César Bordenave, Francisco García-Breijo, and Patricia Moya. 2022. "Trebouxia lynnae sp. nov. (Former Trebouxia sp. TR9): Biology and Biogeography of an Epitome Lichen Symbiotic Microalga" Biology 11, no. 8: 1196. https://doi.org/10.3390/biology11081196
APA StyleBarreno, E., Muggia, L., Chiva, S., Molins, A., Bordenave, C., García-Breijo, F., & Moya, P. (2022). Trebouxia lynnae sp. nov. (Former Trebouxia sp. TR9): Biology and Biogeography of an Epitome Lichen Symbiotic Microalga. Biology, 11(8), 1196. https://doi.org/10.3390/biology11081196









