Succession of the Bacterial Communities and Functional Characteristics in Sheep Manure Composting
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Aerobic Composting and Sampling
2.2. Physicochemical Parameters Analysis
2.3. DNA Extraction and 16S rRNA Gene Sequencing
2.4. Bioinformatics and Data Statistical Analysis
3. Results and Discussion
3.1. Changes of Physicochemical Properties during Composting
3.2. Alpha Diversity of Microbial Community during the Composting Process
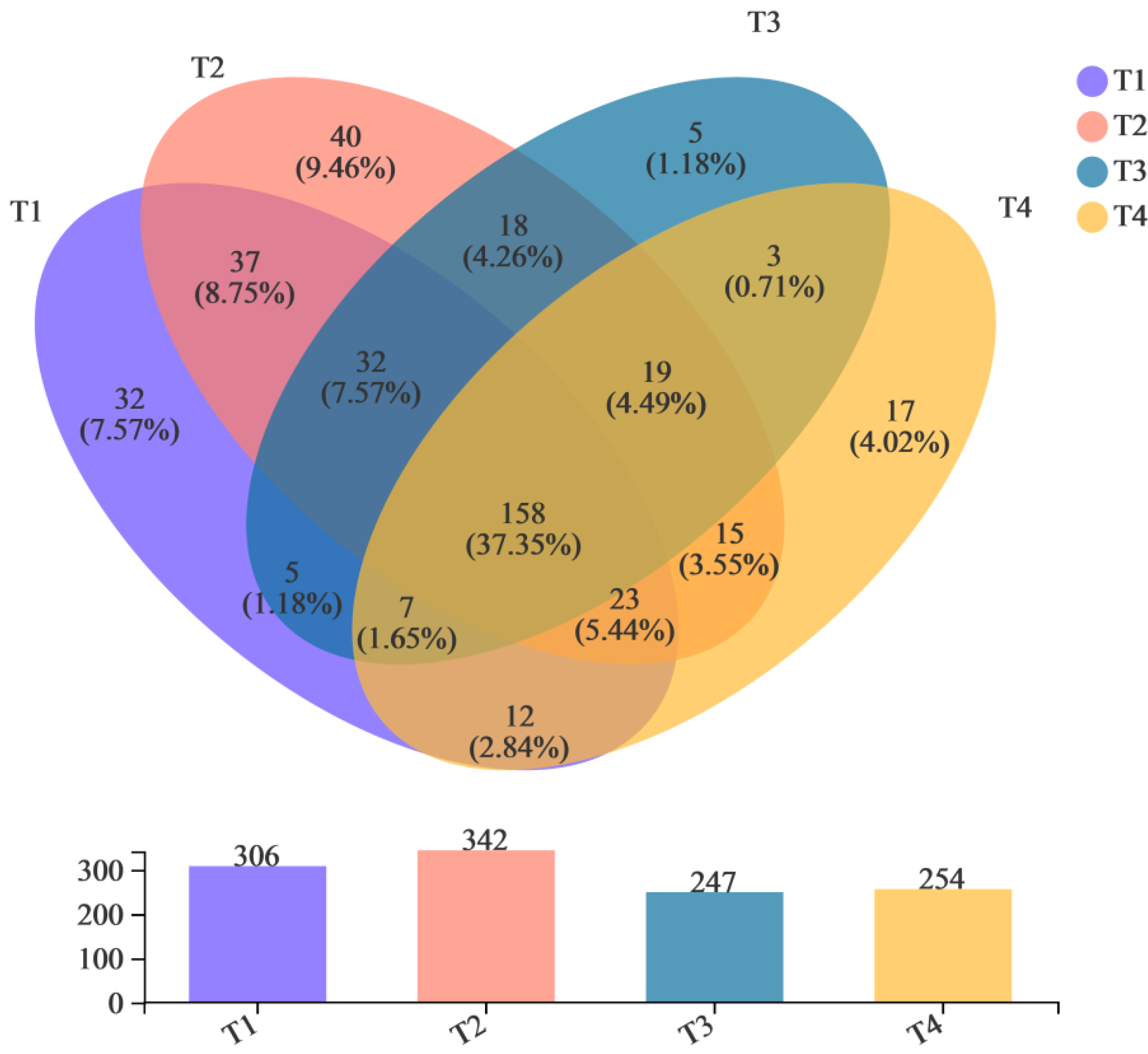
3.3. Bacterial Community Succession during the Composting Process
3.4. Correlation between Relative Abundance of Bacterial Genera and Physicochemical Factors
3.5. Bacterial Function Predictions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Zhou, Y.; Qin, S.; Kumar Awasth, S.; Liu, T.; Liu, H.; Zhang, Z.; Kumar Awasthi, M. Distribution of heavy metal resistant bacterial community succession in cow manure biochar amended sheep manure compost. Bioresour. Technol. 2021, 335, 125282. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Duan, Y.; Awasthi, S.K.; Liu, T.; Zhang, Z.; Kim, S.H.; Pandey, A. Effect of biochar on emission, maturity and bacterial dynamics during sheep manure compositing. Renew. Energy 2020, 152, 421–429. [Google Scholar] [CrossRef]
- Bao, Y.; Feng, Y.; Qiu, C.; Zhang, J.; Wang, Y.; Lin, X. Organic matter- and temperature-driven deterministic assembly processes govern bacterial community composition and functionality during manure composting. Waste Manag. 2021, 131, 31–40. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Q.; Chen, X.; He, Y.; Li, R.; Li, J.; Zhang, Z. Pathways and mechanisms of nitrogen transformation during co-composting of pig manure and diatomite. Bioresour. Technol. 2021, 329, 124914. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Sarsaiya, S.; Patel, A.; Juneja, A.; Singh, R.P.; Yan, B.; Awasthi, S.K.; Jain, A.; Liu, T.; Duan, Y. Refining biomass residues for sustainable energy and bio-products: An assessment of technology, its importance, and strategic applications in circular bio-economy. Renew. Sustain. Energy Rev. 2020, 127, 109876. [Google Scholar] [CrossRef]
- Chen, X.; Liu, R.; Hao, J.; Li, D.; Wei, Z.; Teng, R.; Sun, B. Protein and carbohydrate drive microbial responses in diverse ways during different animal manures composting. Bioresour. Technol. 2019, 271, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Gou, C.; Wang, Y.; Zhang, X.; Lou, Y.; Gao, Y. Inoculation with a psychrotrophic-thermophilic complex microbial agent accelerates onset and promotes maturity of dairy manure-rice straw composting under cold climate conditions. Bioresour. Technol. 2017, 243, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhao, Y.; Lu, Q.; Cao, Z.; Wei, Z. Organophosphorus-degrading bacterial community during composting from different sources and their roles in phosphorus transformation. Bioresour. Technol. 2018, 264, 277–284. [Google Scholar] [CrossRef]
- Cao, G.; Song, T.; Shen, Y.; Jin, Q.; Feng, W.; Fan, L.; Cai, W. Diversity of Bacterial and Fungal Communities in Wheat Straw Compost for Agaricus bisporus Cultivation. HortScience 2019, 54, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Xu, X.; Qiu, X.; Zhang, J. Biochar influences the succession of microbial communities and the metabolic functions during rice straw composting with pig manure. Bioresour. Technol. 2019, 272, 10–18. [Google Scholar] [CrossRef]
- Mao, H.; Lv, Z.; Sun, H.; Li, R.; Zhai, B.; Wang, Z.; Awasthi, M.K.; Wang, Q.; Zhou, L. Improvement of biochar and bacterial powder addition on gaseous emission and bacterial community in pig manure compost. Bioresour. Technol. 2018, 258, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Neher, D.A.; Weicht, T.R.; Bates, S.T.; Leff, J.W.; Fierer, N. Changes in bacterial and fungal communities across compost recipes, preparation methods, and composting times. PLoS ONE 2013, 8, e79512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello, A.; Han, Y.; Zhu, H.; Deng, L.; Yang, W.; Meng, Q.; Sun, Y.; Egbeagu, U.U.; Sheng, S.; Wu, X. Microbial community composition, co-occurrence network pattern and nitrogen transformation genera response to biochar addition in cattle manure-maize straw composting. Sci. Total. Environ. 2020, 721, 137759. [Google Scholar] [CrossRef]
- Qiao, C.; Ryan Penton, C.; Liu, C.; Shen, Z.; Ou, Y.; Liu, Z.; Xu, X.; Li, R.; Shen, Q. Key extracellular enzymes triggered high-efficiency composting associated with bacterial community succession. Bioresour. Technol. 2019, 288, 121576. [Google Scholar] [CrossRef]
- Kong, W.; Sun, B.; Zhang, J.; Zhang, Y.; Gu, L.; Bao, L.; Liu, S. Metagenomic analysis revealed the succession of microbiota and metabolic function in corncob composting for preparation of cultivation medium for Pleurotus ostreatus. Bioresour. Technol. 2020, 306, 123156. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Xia, J.; Chen, Y. Effect of microbial inoculation on physicochemical properties and bacterial community structure of citrus peel composting. Bioresour. Technol. 2019, 291, 121843. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-Q.; Zhu, X.-H.; Wu, J.; Guo, D.-Y.; Zhang, L.-H.; Feng, Y. Dynamics of microbial diversity during the composting of agricultural straw. J. Integr. Agric. 2021, 20, 1121–1136. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.C.; Zhang, H.H.; Shi, H.L.; Hu, T.; Ngo, H.H. Characteristics of nitrogen transformation and microbial community in an aerobic composting reactor under two typical temperatures. Bioresour. Technol. 2013, 137, 270–277. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Chen, H.; Wang, Q.; Liu, T.; Duan, Y.; Awasthi, S.K.; Ren, X.; Tu, Z.; Li, J.; Zhao, J. Succession of bacteria diversity in the poultry manure composted mixed with clay: Studies upon its dynamics and associations with physicochemical and gaseous parameters. Bioresour. Technol. 2018, 267, 618–625. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, Y.; Fan, Y.; Zhang, F.; Tan, W.; He, X.; Xi, B. Roles of bacterial community in the transformation of dissolved organic matter for the stability and safety of material during sludge composting. Bioresour. Technol. 2018, 267, 378–385. [Google Scholar] [CrossRef]
- Botta, L.S.; Delforno, T.P.; Rabelo, C.A.B.S.; Silva, E.L.; Varesche, M.B.A. Microbial community analyses by high-throughput sequencing of rumen microorganisms fermenting office paper in mesophilic and thermophilic lysimeters. Process Saf. Environ. Prot. 2020, 136, 182–193. [Google Scholar] [CrossRef]
- Tao, Z.; Chen, C.; Yang, Q.; Zhong, Z.; Wan, Y.; Chen, S.; Yao, F.; Pi, Z.; Li, X.; Wang, D. Understanding the impact of allicin for organic matter release and microorganism community in anaerobic co-digestion of food waste and waste activated sludge. Sci. Total. Environ. 2021, 776, 145598. [Google Scholar] [CrossRef]
- Zhou, G.; Qiu, X.; Chen, L.; Zhang, C.; Ma, D.; Zhang, J. Succession of organics metabolic function of bacterial community in response to addition of earthworm casts and zeolite in maize straw composting. Bioresour. Technol. 2019, 280, 229–238. [Google Scholar] [CrossRef]
- Qin, R.; Su, C.; Mo, T.; Liao, L.; Zhu, F.; Chen, Y.; Chen, M. Effect of excess sludge and food waste feeding ratio on the nutrient fractions, and bacterial and fungal community during aerobic co-composting. Bioresour. Technol. 2021, 320, 124339. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Wang, S.P.; Suna, Z.Y.; Wang, S.T.; Shuai, W.L.; She, C.H.; Tang, Y.Q. Performance and microbial community dynamics during rice straw composting using urea or protein hydrolysate as a nitrogen source: Acomparative study. Waste Manag. 2021, 135, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Y.; Zhang, J.; Zhong, X.Z.; Tan, L.; Tang, Y.Q.; Kida, K. Production of nitrate-rich compost from the solid fraction of dairy manure by a lab-scale composting system. Waste Manag. 2016, 51, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Ji, M.; Chen, A.; Zhang, B.; Shi, J.; Liu, L.; Li, X.; Sun, J. Evaluating the impact of rice husk on successions of bacterial and fungal communities during cow manure composting. Environ. Technol. Innov. 2021, 24, 102084. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wei, J.K.; Wang, Q.H.; Yang, R.; Gao, X.J.; Sang, Y.X.; Cai, P.P.; Zhang, G.Q.; Chen, Q.J. Lignocellulose utilization and bacterial communities of millet straw based mushroom (Agaricus bisporus) production. Sci. Rep. 2019, 9, 1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Jain, M.S.; Jambhulkar, R.; Kalamdhad, A.S. Biochar amendment for batch composting of nitrogen rich organic waste: Effect on degradation kinetics, composting physics and nutritional properties. Bioresour. Technol. 2018, 253, 204–213. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, Y.; Yang, H.; Zhu, L.; Cai, B.; Luo, S.; Cao, J.; Wei, Z. Transformation of organic nitrogen fractions with different molecular weights during different organic wastes composting. Bioresour. Technol. 2018, 262, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.J.; Meng, H.B.; Zhao, L.X.; Li, G.X.; Zhou, H.B.; Cheng, H.S.; Ding, J.T.; Zhang, X.; Wang, J. Analysis of composting standards at home and abroad and its enlightenment to China. Trans. Chin. Soc. Agric. Eng. 2019, 35, 6. [Google Scholar]
- Zhang, J.Z.; Zhong, X.Z.; Huang, Y.L.; Tan, L.; Tang, Y.Q. Maturity and microbial community structure dynamics during simulated windrow composting of solid fraction of dairy manure. Chin. J. Appl. Environ. Biol. 2016, 22, 6. [Google Scholar]
- Cao, Y.; Huang, H.Y.; Xu, Y.D.; Wu, H.S. Chemical and Biological Changes During Early Stage of Composting of Different Animal Wastes. J. Agro-Environ. Sci. 2015, 34, 2207. [Google Scholar]
- Dong, C.M.; Deng, X.K.; Qin, H.L.; Zhao, Y.; Liu, X.Y.; Ruan, Y.Z. High-Temperature Composting of Mixtures of Chicken Manure and Coconut Husk Different in C/N Rati. J. Ecol. Rural. Environ. 2015, 31, 5. [Google Scholar]
- Bai, L.; Deng, Y.; Li, J.; Ji, M.; Ruan, W. Role of the proportion of cattle manure and biogas residue on the degradation of lignocellulose and humification during composting. Bioresour. Technol. 2020, 307, 122941. [Google Scholar] [CrossRef]
- Zhao, X.L.; Luo, Y.L.; Yu, X.; Jia, H.T.; Li, J.J.; Liu, H.P. Impact of temperature and straw adding ratio on contents of nutrients in cow dung aerobic compost. Chin. J. Environ. Eng. 2014, 8, 7. [Google Scholar]
- Keng, Z.X.; Chong, S.; Ng, C.G.; Ridzuan, N.I.; Hanson, S.; Pan, G.T.; Lau, P.L.; Supramaniam, C.V.; Singh, A.; Chin, C.F. Community-scale composting for food waste: A life-cycle assessment-supported case study. J. Clean. Prod. 2020, 261, 121220. [Google Scholar] [CrossRef]
- Scaglia, B.; Pognani, M.; Adani, F. Evaluation of hormone-like activity of the dissolved organic matter fraction (DOM) of compost and digestate. Sci. Total Environ. 2015, 514, 314–321. [Google Scholar] [CrossRef]
- Yu, Z.J.; Wang, Y.Y.; Zhang, L.G.; Chang, J.; Gao, H.J.; Sun, Y.D. Physical and chemical maturity indexes and Fourier transform infrared (FTIR) spectroscopy of animal manures during composting. Chin. J. Appl. Ecol. 2016, 27, 7. [Google Scholar]
- Zhang, M.; Luo, J.; Yan, S.; Chen, W.; Liu, X.; Zhang, Z. Changes in bacterial communities during two agricultural solid wastes’ co-composting processes. Ann. Microbiol. 2018, 68, 743–754. [Google Scholar] [CrossRef]
- Wang, K.; Mao, H.; Li, X. Functional characteristics and influence factors of microbial community in sewage sludge composting with inorganic bulking agent. Bioresour. Technol. 2018, 249, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.Z.; Li, X.X.; Zeng, Y.; Wang, S.P.; Sun, Z.Y.; Tang, Y.Q. Dynamic change of bacterial community during dairy manure composting process revealed by high-throughput sequencing and advanced bioinformatics tools. Bioresour. Technol. 2020, 306, 123091. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mao, H.; Wang, Z.; Tian, Y. Succession of organics metabolic function of bacterial community in swine manure composting. J. Hazard. Mater. 2018, 360, 471–480. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Wang, Z.; Chen, G.; Wang, L. Dynamic changes of the dominant functioning microbial community in the compost of a 90-m(3) aerobic solid state fermentor revealed by integrated meta-omics. Bioresour. Technol. 2016, 203, 1–10. [Google Scholar] [CrossRef]
- Bao, Y.; Dolfing, J.; Guo, Z.; Chen, R.; Wu, M.; Li, Z.; Lin, X.; Feng, Y. Important ecophysiological roles of non-dominant Actinobacteria in plant residue decomposition, especially in less fertile soils. Microbiome 2021, 9, 84. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, J.A.; Suarez-Estrella, F.; Vargas-Garcia, M.C.; Lopez, M.J.; Jurado, M.M.; Moreno, J. Dynamics of bacterial microbiota during lignocellulosic waste composting: Studies upon its structure, functionality and biodiversity. Bioresour. Technol. 2015, 175, 406–416. [Google Scholar] [CrossRef]
- Tortosa, G.; Castellano-Hinojosa, A.; Correa-Galeote, D.; Bedmar, E.J. Evolution of bacterial diversity during two-phase olive mill waste (“alperujo”) composting by 16S rRNA gene pyrosequencing. Bioresour. Technol. 2017, 224, 101–111. [Google Scholar] [CrossRef]
- Wei, H.; Wang, L.; Hassan, M.; Xie, B. Succession of the functional microbial communities and the metabolic functions in maize straw composting process. Bioresour. Technol. 2018, 256, 333–341. [Google Scholar] [CrossRef]
- Cahyani, V.R.; Matsuya, K.; Asakawa, S.; Kimura, M. Succession and phylogenetic composition of bacterial communities responsible for the composting process of rice straw estimated by PCR-DGGE analysis. Soil Sci. Plant Nutr. 2003, 49, 619–630. [Google Scholar] [CrossRef]
- Yao, S.T.; Deng, Y.; Fan, Y.J. Effects of simulated warming on the composition and diversity of soil prokaryotic communities in Alpine grasslands. Acta Agrestia Sin. 2021, 29, 7. [Google Scholar]
- Guo, Y.X.; Chen, Q.J.; Qin, Y.; Yang, Y.R.; Yang, Q.Z.; Wang, Y.X.; Cheng, Z.A.; Cao, N.; Zhang, G.Q. Succession of the microbial communities and function prediction during short-term peach sawdust-based composting. Bioresour. Technol. 2021, 332, 125079. [Google Scholar] [CrossRef]
- Yang, Y.; Awasthi, M.K.; Bao, H.; Bie, J.; Lei, S.; Lv, J. Exploring the microbial mechanisms of organic matter transformation during pig manure composting amended with bean dregs and biochar. Bioresour. Technol. 2020, 313, 123647. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, Q.; Niu, Q.; Meng, Q.; Yan, H.; Wang, S.; Li, Q. The degradation of organic matter coupled with the functional characteristics of microbial community during composting with different surfactants. Bioresour. Technol. 2021, 321, 124446. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, Y.; Qi, H.; Zhao, X.; Yang, T.; Du, Y.; Zhang, H.; Wei, Z. Identifying the key factors that affect the formation of humic substance during different materials composting. Bioresour. Technol. 2017, 244, 1193–1196. [Google Scholar] [CrossRef]
- Kong, Z.; Wang, X.; Wang, M.; Chai, L.; Wang, X.; Liu, D.; Shen, Q. Bacterial ecosystem functioning in organic matter biodegradation of different composting at the thermophilic phase. Bioresour. Technol. 2020, 317, 123990. [Google Scholar] [CrossRef]
- Toledo, M.; Gutiérrez, M.C.; Siles, J.A.; García-Olmo, J.; Martín, M.A. Chemometric analysis and NIR spectroscopy to evaluate odorous impact during the composting of different raw materials. J. Clean. Prod. 2017, 167, 154–162. [Google Scholar] [CrossRef]






| Samples | Temperature (℃) | Moisture (%) | TOC (% TS) | TN (% TS) | NO3−N (mg kg−1 TS) | NH4+-N (mg·kg−1 TS) | C/N | pH | GI (%) |
|---|---|---|---|---|---|---|---|---|---|
| T1 | 32.67 ± 1.46 d | 68.02 ± 1.62 a | 44.45 ± 0.66 a | 1.83 ± 0.036 bc | 0.28 ± 0.005 c | 0.52 ± 0.102 c | 24.3 ± 0.83 ab | 9.15 ± 0.06 d | 22.94 ± 2.1 c |
| T2 | 65.47 ± 1.2 a | 62.53 ± 2.11 b | 42.46 ± 0.59 b | 1.69 ± 0.015 d | 0.18 ± 0.009 d | 2.48 ± 0.103 a | 25.17 ± 0.5 a | 9.52 ± 0.06 a | 15.07 ± 0.71 d |
| T3 | 52.67 ± 1.39 b | 45.36 ± 0.75 c | 39.75 ± 0.63 c | 1.84 ± 0.03 b | 0.39 ± 0.015 b | 1.29 ± 0.151 b | 21.61 ± 0.3 c | 9.44 ± 0.02 b | 65.97 ± 2.52 b |
| T4 | 36.3 ± 0.78 c | 39.78 ± 1.88 d | 38.00 ± 0.7 d | 1.92 ± 0.036 a | 0.46 ± 0.016 a | 0.27 ± 0.054 d | 19.8 ± 0.66 d | 9.39 ± 0.01 bc | 76.6 ± 2.08 a |
| Samples | Simpson | Shannon | ACE | Chao1 | Coverage/% |
|---|---|---|---|---|---|
| T1 | 0.060 ± 0.012 bc | 3.889 ± 0.151 b | 722.143 ± 96.433 ab | 696.677 ± 36.616 ab | 99.19 ± 0.05 c |
| T2 | 0.033 ± 0.002 d | 4.311 ± 0.125 a | 774.075 ± 31.249 a | 767.328 ± 41.332 a | 99.13 ± 0.02 cd |
| T3 | 0.109 ± 0.011 a | 3.284 ± 0.125 cd | 680.684 ± 74.245 bc | 570.847 ± 65.211 c | 99.3 ± 0.07 bc |
| T4 | 0.094 ± 0.040 ab | 3.545 ± 0.324 bc | 608.342 ± 112.575 bcd | 544.9 ± 73.681 cd | 99.36 ± 0.086 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Li, J.; Che, Z.; Xue, L. Succession of the Bacterial Communities and Functional Characteristics in Sheep Manure Composting. Biology 2022, 11, 1181. https://doi.org/10.3390/biology11081181
Zhao X, Li J, Che Z, Xue L. Succession of the Bacterial Communities and Functional Characteristics in Sheep Manure Composting. Biology. 2022; 11(8):1181. https://doi.org/10.3390/biology11081181
Chicago/Turabian StyleZhao, Xu, Juan Li, Zongxian Che, and Lingui Xue. 2022. "Succession of the Bacterial Communities and Functional Characteristics in Sheep Manure Composting" Biology 11, no. 8: 1181. https://doi.org/10.3390/biology11081181
APA StyleZhao, X., Li, J., Che, Z., & Xue, L. (2022). Succession of the Bacterial Communities and Functional Characteristics in Sheep Manure Composting. Biology, 11(8), 1181. https://doi.org/10.3390/biology11081181





