Interplay of Seasonality, Major and Trace Elements: Impacts on the Polychaete Diopatra neapolitana
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Sampling and Measurement of Physic-Chemical Parameters
2.2. Samples Analysis
2.2.1. Environmental Parameters
2.2.2. Quantification of Major and Trace Elements in Sediments and Tissue Samples
2.2.3. Biochemical Parameters
2.2.4. Metabolism-Related Parameters
2.2.5. Antioxidant Defences
2.2.6. Oxidative Damage Endpoints
2.3. Statistical Data Analysis
3. Results
3.1. Physical and Chemical Characteristics of Sediment Samples
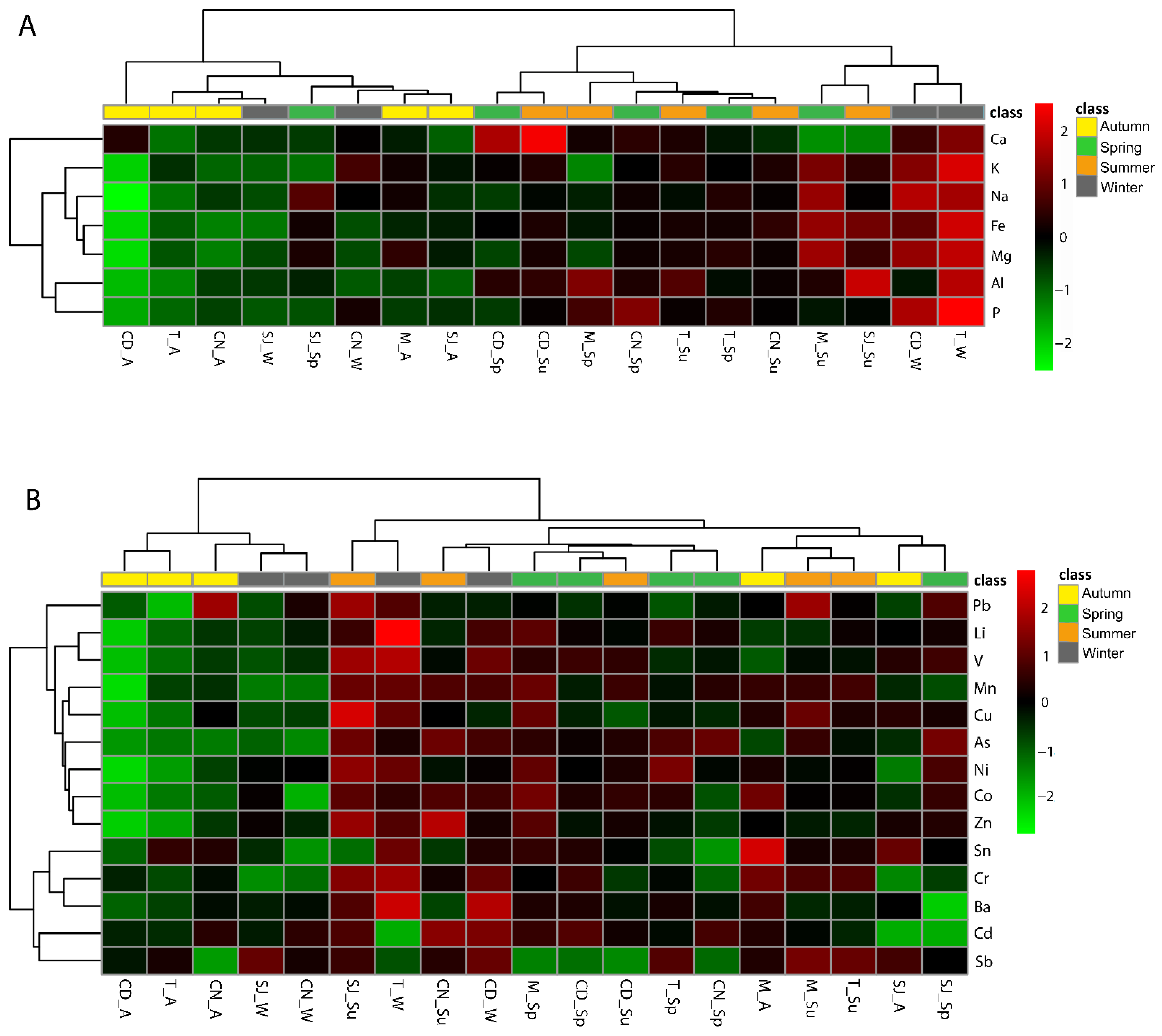
3.2. Elements Concentration in Sediments
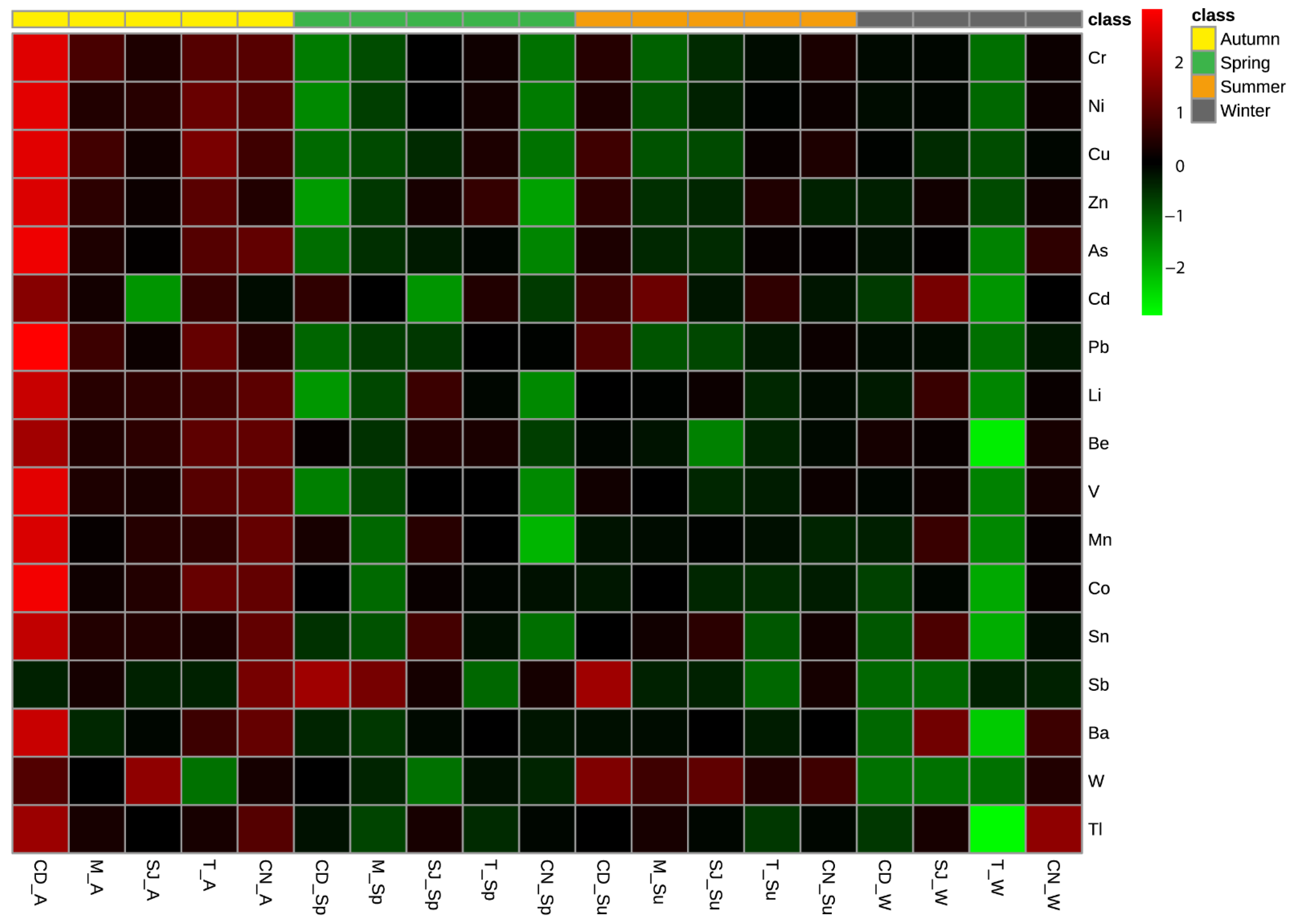
3.3. Bioaccumulation of Elements in Polychaetes
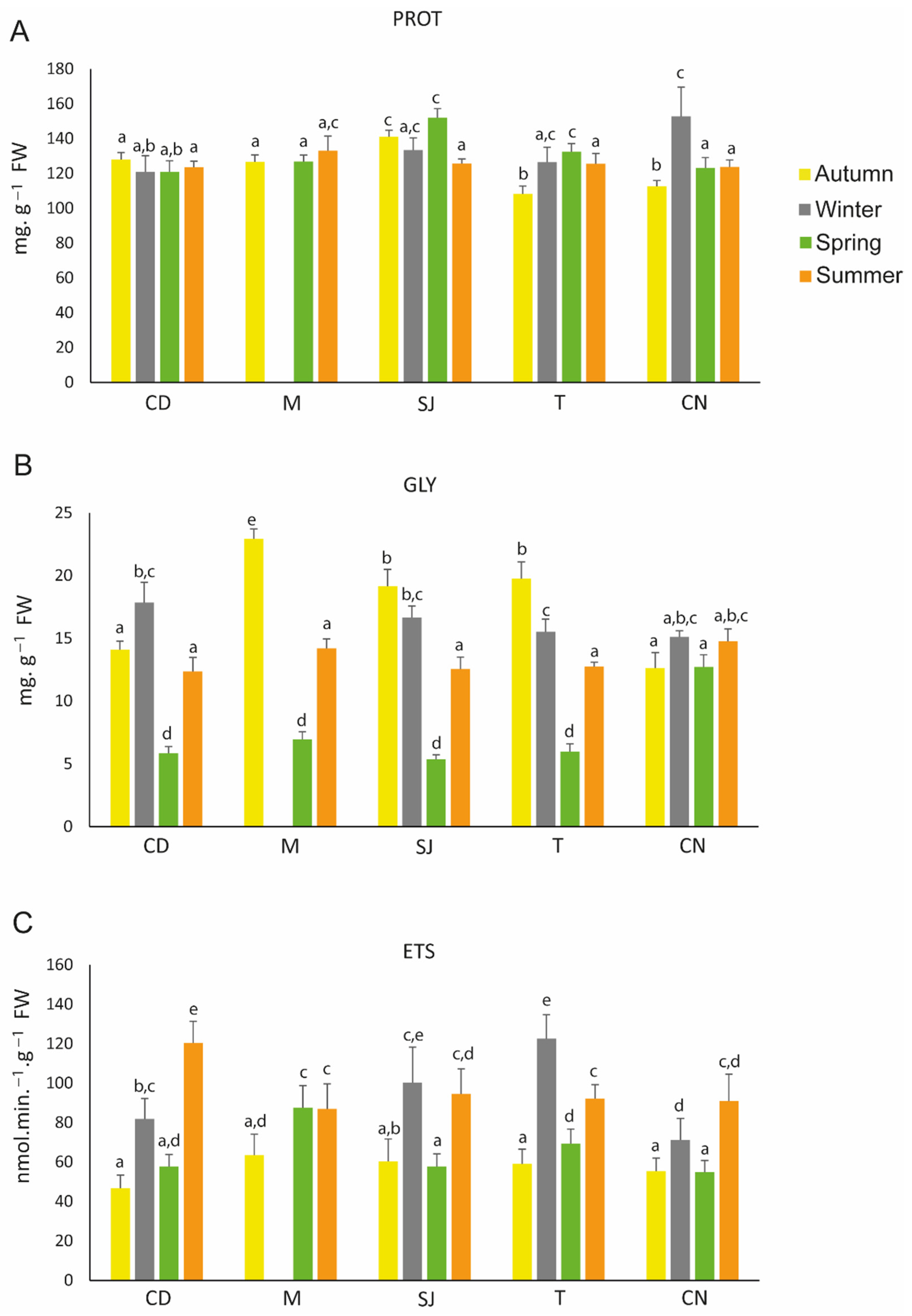
3.4. Metabolism-Related Parameters
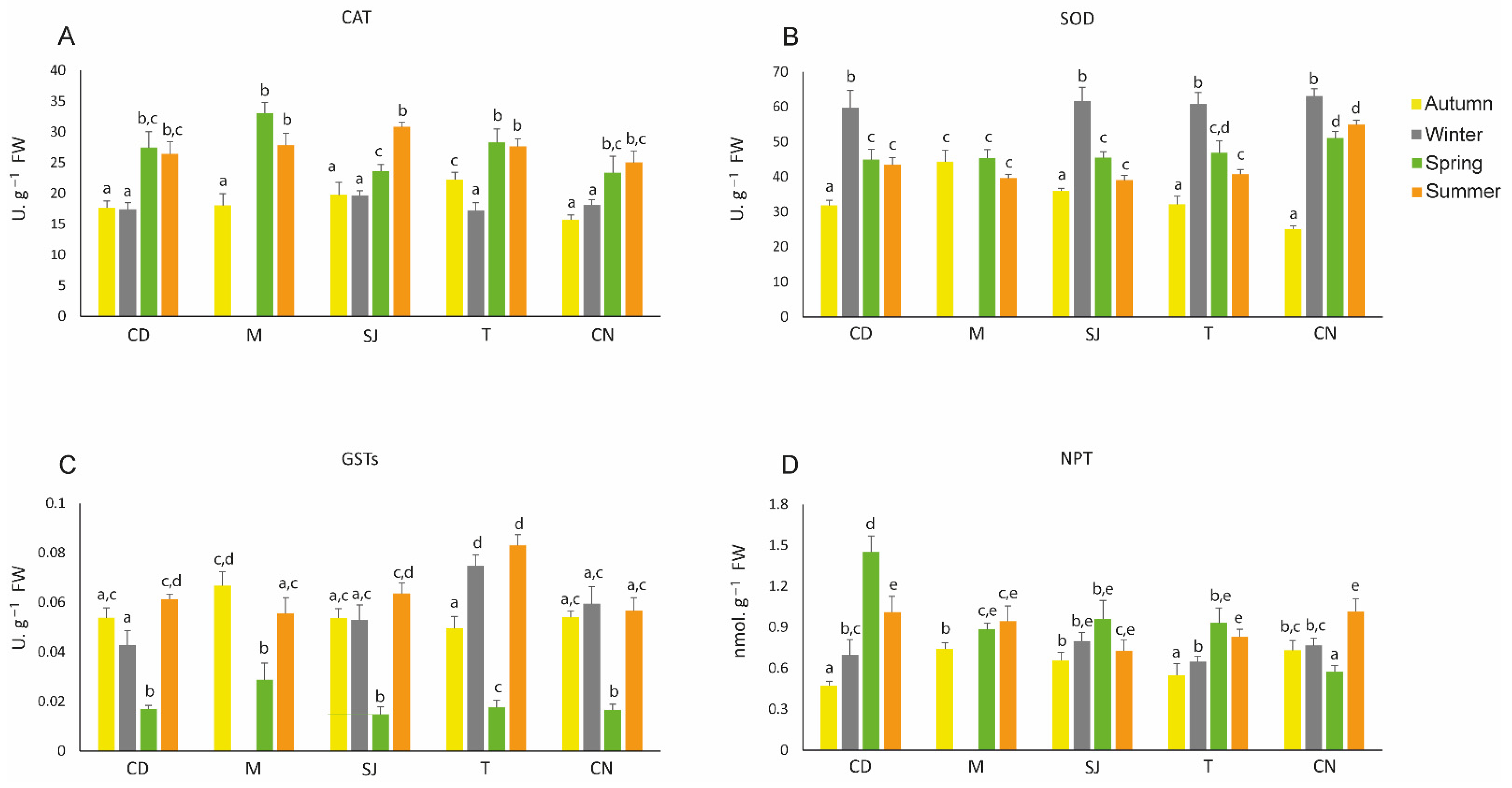
3.5. Antioxidant Defences
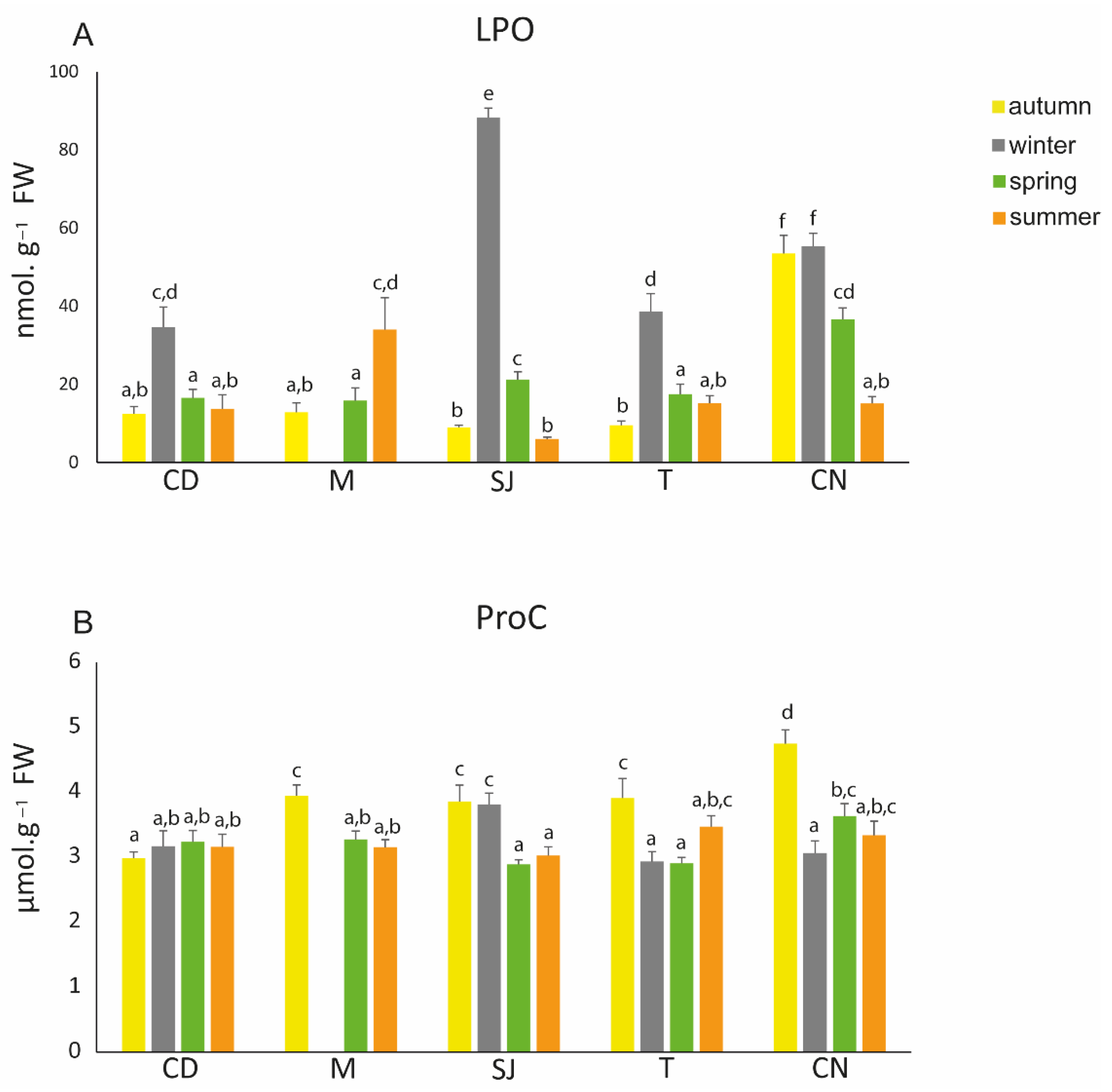
3.6. Oxidative Damage Endpoints
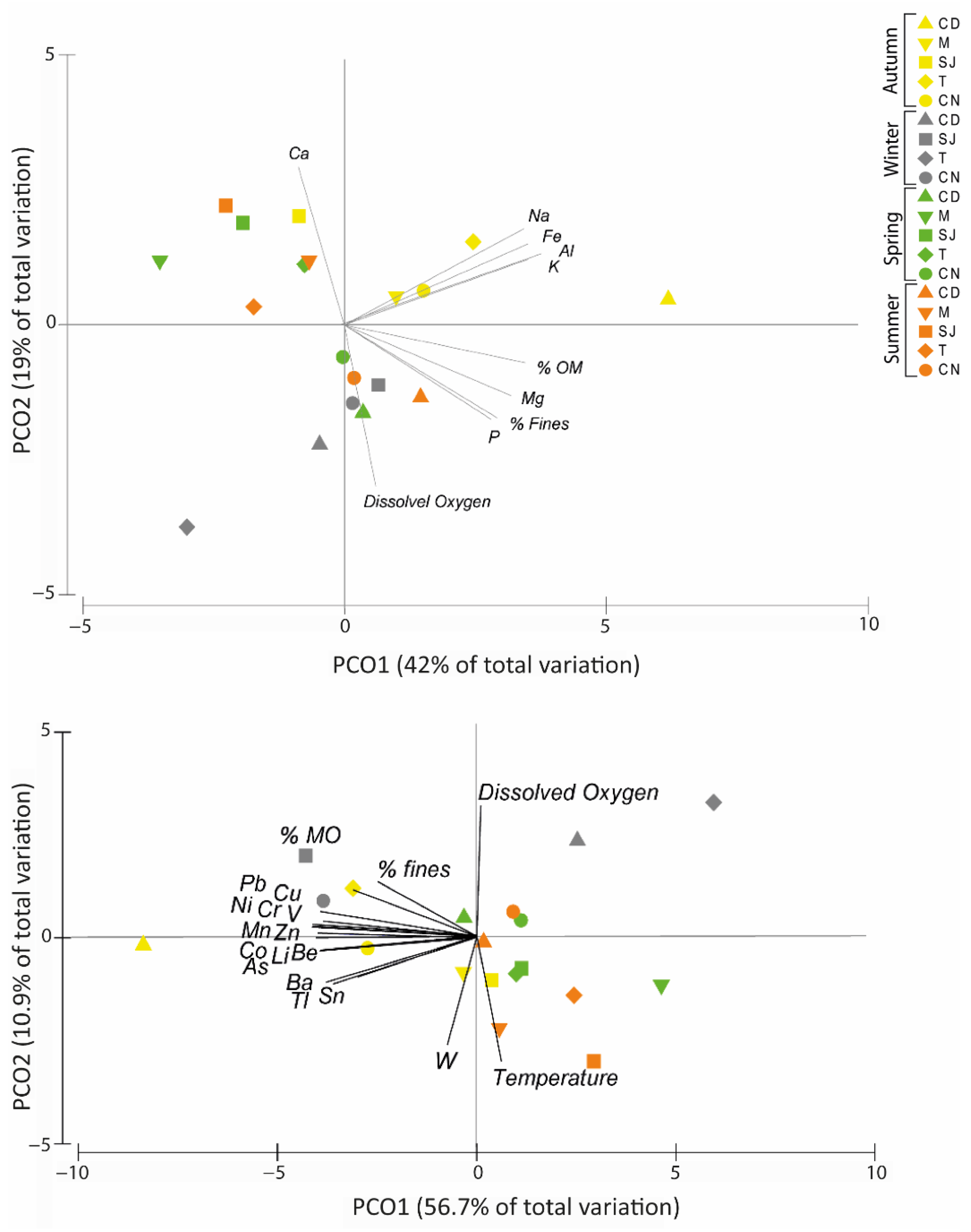
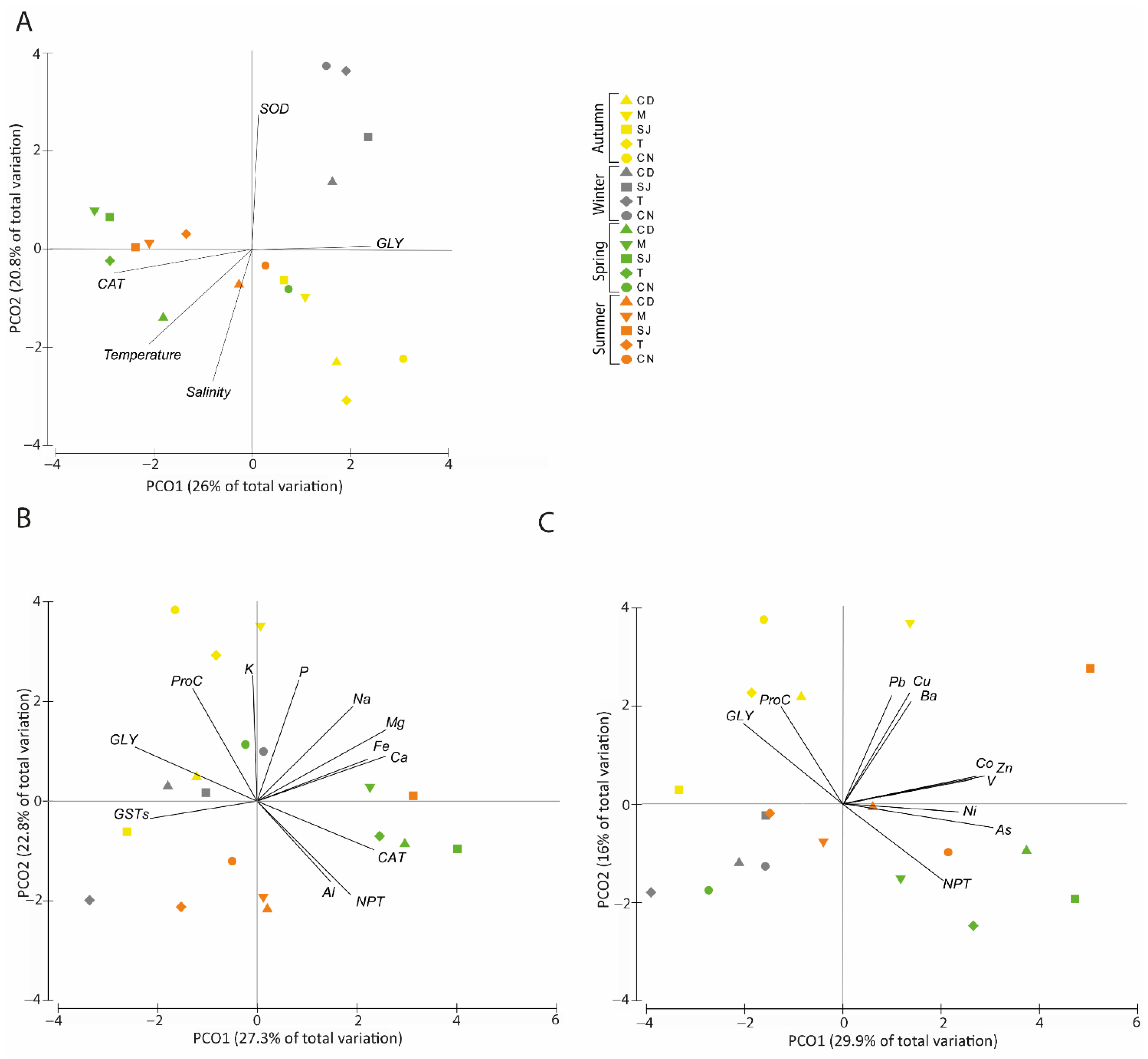
3.7. Multivariate Analysis as a Tool to Summarise Information
3.7.1. Elements and Physical–Chemical Data
3.7.2. Biochemical Parameters and Physical–Chemical Data
3.7.3. Elements and Biochemical Parameters Correlation
Major Elements
Trace Elements
4. Discussion
4.1. Physical and Chemical Characteristics of Sediment Samples
4.2. Polychaete Bioaccumulation
4.3. Biochemical Responses
4.3.1. Metabolism-Related Parameters
4.3.2. Antioxidant Defences
4.3.3. Oxidative Damage Endpoints
4.4. Elements and Biochemical Parameters Correlation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, M.; Oliveira, A.; Queiroga, H.; Brotas, V.; Fortunato, A.B.; Zhang, Y.J. Modelação Ecológica do Ecossistema Planctónico da Ria de Aveiro. Actas Jorn. Ria Aveiro 2011, 248. Available online: http://repositorio.lnec.pt:8080/xmlui/handle/123456789/1003140 (accessed on 2 March 2022).
- Serafim, A.; Company, R.; Lopes, B.; Pereira, C.; Cravo, A.; Fonseca, V.F.; França, S.; Bebianno, M.J.; Cabral, H.N. Evaluation of Sediment Toxicity in Different Portuguese Estuaries: Ecological Impact of Metals and Polycyclic Aromatic Hydrocarbons. Estuarine Coast. Shelf Sci. 2013, 130, 30–41. [Google Scholar] [CrossRef]
- Abreu, S.N.; Pereira, E.; Vale, C.; Duarte, A.C. Accumulation of Mercury in Sea Bass from a Contaminated Lagoon (Ria de Aveiro, Portugal). Mar. Pollut. Bulletin 2000, 40, 293–297. [Google Scholar] [CrossRef]
- Jonkers, N.; Sousa, A.; Galante-Oliveira, S.; Barroso, C.M.; Kohler, H.-P.E.; Giger, W. Occurrence and Sources of Selected Phenolic Endocrine Disruptors in Ria De Aveiro, Portugal. Environ. Sci. Pollut. Res. 2010, 17, 834–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, J.F.; Dias, J.M.; Cardoso, A.C.; Silva, C.I.V. The Water Quality of The Ria De Aveiro Lagoon, Portugal: From the Observations to The Implementation of a Numerical Model. Mar. Environ. Res. 2005, 60, 594–628. [Google Scholar] [CrossRef] [PubMed]
- Riba, I.; García-Luque, E.; Blasco, J.; DelValls, T.A. “Bioavailability of Heavy Metals Bound to Estuarine Sediments as a Function of Ph and Salinity Values. Chem. Speciat. Bioavailab 2003, 15, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Marisa, I.; Matozzo, V.; Martucci, A.; Franceschinis, E.; Brianese, N.; Marin, M.G. Bioaccumulation and Effects of Titanium Dioxide Nanoparticles and Bulk in The Clam Ruditapes Philippinarum. Mar. Environ. Res. 2018, 136, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Thit, A.; Dybowska, A.; Købler, C.; Kennaway, G.; Selck, H. Influence of Copper Oxide Nanoparticle Shape on Bioaccumulation, Cellular Internalization and Effects in The Estuarine Sediment-Dwelling Polychaete, Nereis Diversicolor. Mar. Environ. Res. 2015, 111, 89–98. [Google Scholar] [CrossRef]
- Bellas, J.; Albentosa, M.; Vidal-Liñán, L.; Besada, V.; Franco, M.A.; Fumega, J.; González-Quijano, A.; Viñas, L.; Beiras, R. Combined Use of Chemical, Biochemical and Physiological Variables in Mussels for The Assessment of Marine Pollution Along The N-NW Spanish Coast. Mar. Environ. Res. 2014, 96, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Figueira, E.; Cardoso, P.; Freitas, R. Ruditapes Decussatus and Ruditapes Philippinarum Exposed to Cadmium: Toxicological Effects and Bioaccumulation Patterns. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 156, 80–86. [Google Scholar] [CrossRef]
- Figueira, E.; Lima, A.; Branco, D.; Quintino, V.; Rodrigues, A.M.; Freitas, R. Health Concerns of Consuming Cockles (Cerastoderma Edule L.) From A Low Contaminated Coastal System. Environ. Int. 2011, 37, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Kennish, M.J. Environmental Threats and Environmental Future of Estuaries. Environ. Conserv. 2002, 29, 78–107. [Google Scholar] [CrossRef]
- Pires, A.; Velez, C.; Figueira, E.; Soares, A.M.V.M.; Freitas, R. Effects of Sediment Contamination on Physiological and Biochemical Responses of The Polychaete Diopatra Neapolitana, An Exploited Natural Resource. Mar. Pollut. Bull. 2017, 119, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Solé, M.; Kopecka-Pilarczyk, J.; Blasco, J. Pollution Biomarkers in Two Estuarine Invertebrates, Nereis Diversicolor and Scrobicularia Plana, From A Marsh Ecosystem in SW Spain. Environ. Int. 2009, 35, 523–531. [Google Scholar] [CrossRef]
- Ferreira-Cravo, M.; Ventura-Lima, J.; Sandrini, J.Z.; Amado, L.L.; Geracitano, L.A.; Rebelo, M.; Bianchini, A.; Monserrat, J.M. Antioxidant Responses in Different Body Regions of the Polychaeta Laeonereis Acuta (Nereididae) Exposed to Copper. Ecotoxicol. Environ. Saf. 2009, 72, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Mauri, M.; Baraldi, E.; Simonini, R. Effects of Zinc Exposure on the Polychaete Dinophilus Gyrociliatus: A Life-Table Response Experiment. Aquat. Toxicol. 2003, 65, 93–100. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, A.; Zhou, Y.; Liu, H.; Yang, D. The Influence of Cadmium on the Antioxidant Enzyme Activities in Polychaete Perinereis Aibuhitensis Grube (Annelida: Polychaeta). Chin. J. Oceanol. Limnol. 2010, 28, 849–855. [Google Scholar] [CrossRef]
- Bouraoui, Z.; Ghedira, J.; Boussetta, H. Biomarkers Responses in Different Body Regions of the Polychaeta Hediste Diversicolor (Nereidae, Polychaete) Exposed to Copper. J. Integr. Coast. Zo. Manag. 2015, 15, 371–376. [Google Scholar] [CrossRef]
- Wallace, W.G.; Luoma, S.N. Subcellular Compartmentalization of Cd and Zn in Two Bivalves. Ii. Significance Of Trophically Available Metal (Tam). Mar. Ecol. Prog. Ser. 2003, 249, 183–197. [Google Scholar] [CrossRef]
- Bhuiyan, K.A.; Rodríguez, B.M.; Pires, A.; Riba, I.; Dellvals, A.; Freitas, R.; Conradi, M. Experimental Evidence of Uncertain Future of The Keystone Ragworm Hediste Diversicolor (O.F. Müller, 1776) Under Climate Change Conditions. Sci. Total Environ. 2021, 750, 142031. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.; Pires, A.; Velez, C.; Martins, R.; Figueira, E.; Soares, A.M.V.M.; Freitas, R. Seasonal and Spatial Alterations in Macrofaunal Communities and in Nephtys Cirrosa (Polychaeta) Oxidative Stress Under a Salinity Gradient: A Comparative Field Monitoring Approach. Ecol. Indic. 2019, 96, 192–201. [Google Scholar] [CrossRef]
- Dean, H.K. The use of Polychaetes (Annelida) as Indicator Species of Marine Pollution: A Review. Rev. De Biol. Tropical 2008, 56, 11–38. [Google Scholar]
- Rodrigues, A.M.; Quintino, V.; Sampaio, L.; Freitas, R.; Neves, R. Benthic Biodiversity Patterns in Ria De Aveiro, Western Portugal: Environmental-Biological Relationships. Estuar. Coast. Shelf Sci. 2011, 95, 338–348. [Google Scholar] [CrossRef]
- Parameswaran, V. Distribution of Diopatra Neapolitana Delle Chiaje (Polychaeta) in the South-West Coast of India. Indian J. Geo-Mar. Sci. 1973, 2, 62–63. [Google Scholar]
- Wehe, T.; Fiege, D. Annotated Checklist of the Polychaete Species of the Seas Surrounding the Arabian Peninsula: Red Sea, Gulf of Aden, Arabian Sea, Gulf of Oman, Arabian Gulf. Fauna Arab. 2002, 19, 7–238. [Google Scholar]
- Cunha, T.; Hall, A.; Queiroga, H. Estimation of the Diopatra Neapolitan Annual Harvest Resulting from Digging Activity in Canal de Mira, Ria de Aveiro. Fish. Res. 2005, 76, 56–66. [Google Scholar] [CrossRef]
- Moreira, S.; Lima, I.; Ribeiro, R.; Guilhermino, L. Effects of Estuarine Sediment Contamination on Feeding and on Key Physiological Functions of the Polychaete Hediste Diversicolor: Laboratory and in Situ Assays. Aquat. Toxicol. 2006, 78, 186–201. [Google Scholar] [CrossRef]
- Méndez, N.; Lacorte, S.; Barata, C. Effects of The Pharmaceutical Fluoxetine in Spiked-Sediments on Feeding Activity And Growth Of The Polychaete Capitella Teleta. Mar. Environ. Res. 2013, 89, 76–82. [Google Scholar] [CrossRef]
- Daǧli, E.; Ergen, Z.; Çinar, M.E. One-Year Observation on the Population Structure of Diopatra Neapolitana Delle Chiaje (Polychaeta: Onuphidae) in Izmir Bay (Aegean Sea, Eastern Mediterranean). Mar. Ecol. 2005, 26, 265–272. [Google Scholar] [CrossRef]
- Wethey, D.S.; Woodin, S.A.; Hilbish, T.J.; Jones, S.J.; Lima, F.P.; Brannock, P.M. Response of Intertidal Populations to Climate: Effects of Extreme Events Versus Long Term Change. J. Exp. Mar. Bio. Ecol. 2011, 400, 132–144. [Google Scholar] [CrossRef]
- Pires, A.; Quintino, V.; Gentil, F.; Freitas, R.; Rodrigues, A.M. Reproductive Biology of a Brooding Diopatra Species: Diopatra Marocensis. Estuar. Coast. Shelf Sci. 2012, 110, 85–92. [Google Scholar] [CrossRef]
- Pires, A.; Gentil, F.; Quintino, V.; Rodrigues, A.M. Reproductive Biology of Diopatra Neapolitana (Annelida, Onuphidae), an Exploited Natural Resource in Ria de Aveiro (Northwestern Portugal). Mar. Ecol. 2012, 33, 56–65. [Google Scholar] [CrossRef]
- Bailey-Brock, J.H. Ecology of the Tube-Building Polychaete Diopatra Leuckarti Kinberg, 1865 (Onuphidae) in Hawaii: Community Structure, and Sediment Stabilizing Properties. Zool. J. Linn. Society 1984, 80, 191–199. [Google Scholar] [CrossRef]
- Thomsen, M.S.; McGlathery, K. Facilitation of Macroalgae by The Sedimentary Tube Forming Polychaete Diopatra Cuprea Estuar. Coast. Shelf Sci. 2005, 62, 63–73. [Google Scholar] [CrossRef]
- Carregosa, V.; Velez, C.; Soares, A.M.V.M.; Figueira, E.; Freitas, R. Physiological and Biochemical Responses of Three Veneridae Clams Exposed to Salinity Changes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 177–178, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Almeida, Â.; Pires, A.; Velez, C.; Calisto, V.; Schneider, R.J.; Esteves, V.I.; Wrona, F.J.; Figueira, E.; Soares, A.M.V.M. The Effects of Carbamazepine on Macroinvertebrate Species: Comparing Bivalves and Polychaetes Biochemical Responses. Water Res. 2015, 85, 137–147. [Google Scholar] [CrossRef]
- Freitas, R.; Pires, A.; Velez, C.; Almeida, Â.; Moreira, A.; Wrona, F.J.; Soares, A.M.V.M.; Figueira, E. Effects of Seawater Acidification on Diopatra Neapolitana (Polychaete, Onuphidae): Biochemical and Regenerative Capacity Responses. Ecol. Indic. 2016, 60, 152–161. [Google Scholar] [CrossRef]
- Pires, A.; Figueira, E.; Moreira, A.; Soares, A.M.V.M.; Freitas, R. The Effects of Water Acidification, Temperature and Salinity on The Regenerative Capacity of The Polychaete Diopatra Neapolitana. Mar. Environ. Res. 2015, 106, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Pires, A.; Velez, C.; Almeida, Â.; Wrona, F.J.; Soares, A.M.V.M.; Figueira, E. The Effects of Salinity Changes on the Polychaete Diopatra Neapolitana: Impacts on Regenerative Capacity and Biochemical Markers. Aquat. Toxicol. 2015, 163, 167–176. [Google Scholar] [CrossRef]
- Hook, S.E.; Gallagher, E.P.; Batley, G.E. The Role of Biomarkers in the Assessment of Aquatic Ecosystem Health. Integr. Environ. Assess. Manag. 2014, 10, 327–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cossu, C.; Doyotte, A.; Jacquin, M.C.; Babut, M.; Exinger, A.; Vasseur, P. Glutathione Reductase, Selenium-Dependent Glutathione Peroxidase, Glutathione Levels, And Lipid Peroxidation in Freshwater Bivalves, Unio Tumidus, As Biomarkers Of Aquatic Contamination In Field Studies. Ecotoxicol. Environ. Saf. 1997, 38, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Zomer Sandrini, J.; Regoli, F.; Fattorini, D.; Notti, A.; Inácio, A.F.; Linde-Arias, A.F.; Laurino, J.; Bainy, A.C.D.; Marins, L.F.F.; Monserrat, J.M. Short-Term Responses to Cadmium Exposure in the Estuarine Polychaete Laeonereis Acuta (Polychaeta, Nereididae): Subcellular Distribution and Oxidative Stress Generation. Environ. Toxicol. Chem. 2006, 25, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Coppola, F.; De Marchi, L.; Codella, V.; Pretti, C.; Chiellini, F.; Morelli, A.; Polese, G.; Soares, A.M.V.M.; Figueira, E. The Influence of Arsenic on the Toxicity of Carbon Nanoparticles in Bivalves. J. Hazard. Mater. 2018, 358, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Chora, S.; McDonagh, B.; Sheehan, D.; Starita-Geribaldi, M.; Roméo, M.; Bebianno, M.J. Ubiquitination and Carbonylation as Markers of Oxidative-Stress in Ruditapes Decussatus. Mar. Environ. Res. 2008, 66, 95–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppola, F.; Pires, A.; Velez, C.; Soares, A.M.V.M.; Pereira, E.; Figueira, E.; Freitas, R. Biochemical and Physiological Alterations Induced in Diopatra Neapolitana After a Long-Term Exposure to Arsenic. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 189, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.M.; Lopes, J.F.; Dekeyser, I. Tidal Propagation in Ria de Aveiro lagoon, Portugal. Phys. Chem. Earth Part B Hydrol. Ocean. Atmos. 2000, 25, 369–374. [Google Scholar] [CrossRef]
- Quintino, V.; Rodrigues, A.; Gentil, F. Assessment of Macrozoobenthic Communities in the Lagoon of Obidos, Western Coast of Portugal. Sci. Mar. (Barc.) 1989, 53, 645–654. [Google Scholar]
- Doeglas, D.J. Grain-Size Indices, Classification and Environment. Sedimentology 1968, 10, 83–100. [Google Scholar] [CrossRef]
- Byers, S.C.; Mills, E.L.; Stewart, P.L. A Comparison of Methods of Determining Organic Carbon in Marine Sediments, with Suggestions for a Standard method. Hydrobiologia 1978, 58, 43–47. [Google Scholar] [CrossRef]
- Wallace, W.; Lee, B.; Luoma, S. Subcellular Compartmentalization of Cd and Zn in Two Bivalves. I. Significance of Metal-Sensitive Fractions (MSF) and Biologically Detoxified Metal (BDM). Mar. Ecol. Prog. Ser. 2003, 249, 183–197. [Google Scholar] [CrossRef]
- Cheng, Z.; Man, Y.B.; Nie, X.P.; Wong, M.H. Trophic Relationships and Health Risk Assessments of Trace Metals in The Aquaculture Pond Ecosystem of Pearl River Delta, China. Chemosphere 2013, 90, 2142–2148. [Google Scholar] [CrossRef]
- Robinson, H.W. The Biuret Reaction in The Determination of Serum Proteins. J. Chem. Inf. Model 2017, 53, 1689–1699. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- King, F.D.; Packard, T.T. Respiration and The Activity of the Respiratory Electron Transport System in Marine Zooplankton. Limnol. Oceanogr. 1975, 20, 849–854. [Google Scholar] [CrossRef]
- De Coen, W.M.; Janssen, C.R. The Use of Biomarkers in Daphnia Magna Toxicity Testing. IV. Cellular Energy Allocation: A new Methodology to Assess the Energy Budget of Toxicant-Stressed Daphnia Populations. J. Aquat. Ecosyst. Stress Recovery 1997, 6, 43–55. [Google Scholar] [CrossRef]
- Johansson, L.H.; Håkan Borg, L.A. A Spectrophotometric Method for Determination of Catalase Activity in Small Tissue Samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of Total, Protein-Bound, And Nonprotein Sulfhydryl Groups in Tissue with Ellman’s Reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Parvez, S.; Sayeed, I.; Pandey, S.; Ahmad, A.; Bin-Hafeez, B.; Haque, R.; Ahmad, I.; Raisuddin, S. Modulatory Effect of Copper on Nonenzymatic Antioxidants in Freshwater Fish Channa Punctatus (Bloch.). Biol. Trace Elem. Res. 2003, 93, 237–248. [Google Scholar] [CrossRef]
- Oliveira, M.; Ahmad, I.; Maria, V.L.; Pacheco, M.; Santos, M.A. Antioxidant Responses Versus DNA Damage and Lipid Peroxidation in Golden Grey Mullet Liver: A Field Study at Ria De Aveiro (Portugal). Arch. Environ. Contam. Toxicol. 2010, 59, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. In Methods in enzymology; Academic Press: Cambridge, MA, USA, 1978; Volume 52, pp. 302–310. [Google Scholar] [CrossRef]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-Dinitrophenylhydrazine Spectrophotometric Assay for Quantification of Carbonyls in Oxidized Proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Udechukwu, M.C.; Yiridoe, C.; Gibson, A.; Gong, M. Antioxidant Mechanism of Potato Protein Hydrolysates Against In Vitro Oxidation of Reduced Glutathione. J. Funct. Foods 2016, 20, 195–203. [Google Scholar] [CrossRef]
- Tornero, V.; Arias, A.M.; Blasco, J. Trace Element Contamination in the Guadalquivir River Estuary Ten Years After the Aznalcóllar Mine Spill. Mar. Pollut. Bull. 2014, 86, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Pires, A.; Quintino, V.; Rodrigues, A.M.; Figueira, E. Subcellular Partitioning of Elements and Availability for Trophic Transfer: Comparison Between the Bivalve Cerastoderma Edule and the Polychaete Diopatra Neapolitana. Estuar. Coast. Shelf Sci. 2012, 99, 21–30. [Google Scholar] [CrossRef]
- Siscar, R.; Torreblanca, A.; Palanques, A.; Solé, M. Metal Concentrations and Detoxification Mechanisms in Solea Solea and Solea Senegalensis from NW Mediterranean Fishing Grounds. Mar. Pollut. Bull. 2013, 77, 90–99. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, Toxicity and Oxidative Stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Song, Y.; Yuan, P.; Cui, W.; Qiu, G. The Remediation of Heavy Metals Contaminated Sediment. J. Hazard. Mater. 2009, 161, 633–640. [Google Scholar] [CrossRef]
- Guieu, C.; Martin, J.M. The Level and Fate of Metals in the Danube River Plume. Estuar. Coast. Shelf Sci. 2002, 54, 501–512. [Google Scholar] [CrossRef]
- Castro, R.; Pereira, S.; Lima, A.; Corticeiro, S.; Válega, M.; Pereira, E.; Duarte, A.; Figueira, E. Accumulation, Distribution and Cellular Partitioning of Mercury in Several Halophytes of a Contaminated Salt Marsh. Chemosphere 2009, 76, 1348–1355. [Google Scholar] [CrossRef]
- Velez, C.; Pires, A.; Sampaio, L.; Cardoso, P.; Moreira, A.; Leandro, S.; Figueira, E.; Soares, A.M.V.M.; Freitas, R. The Use of Cerastoderma Glaucum as a Sentinel and Bioindicator Species: Take-Home Message. Ecol. Indic. 2016, 62, 228–241. [Google Scholar] [CrossRef]
- Costa, S.; Lopes, J.; Coppola, F.; Correia, S.; Henriques, B.; Leite, C.; Soares, A.M.V.M.; Zengjie, J.; Pereira, E.; Chiesa, S.; et al. Bioaccumulation and Biochemical Patterns of Ruditapes Philippinarum Clams: Responses to Seasonality and Low Contamination Levels. Estuar. Coast. Shelf Sci. 2020, 243, 106883. [Google Scholar] [CrossRef]
- Ribeiro, D.R.G.; Faccin, H.; Molin, T.R.D.; de Carvalho, L.M.; Amado, L.L. Metal and Metalloid Distribution in Different Environmental Compartments of The Middle Xingu River in the Amazon, Brazil. Sci. Total Environ. 2017, 605–606, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.; Lucentini, L.; Freitas, R.; Marzano, F.N.; Breda, S.; Figueira, E.; Cail-Mily, N.; Herbert, R.; Soares, A.M.V.M.; Argesse, E. Mapping the Stranger: Genetic Diversity of Manila Clam in European Coastal Lagoons. Bull. Jpn. Fish. Res. Educ. Agency 2016, 42, 55–65. [Google Scholar]
- Elliott, M.; Cutts, N.D.; Trono, A. A typology of Marine and Estuarine Hazards and Risks as Vectors of Change: A Review for Vulnerable Coasts and Their Management. Ocean. Coast. Manag. 2014, 93, 88–99. [Google Scholar] [CrossRef]
- Freitas, R.; Costa, E.; Velez, C.; Santos, J.; Lima, A.; Oliveira, C.; Rodrigues, A.M.; Quintino, V.; Figueira, E. Looking for Suitable Biomarkers in Benthic Macroinvertebrates Inhabiting Coastal Areas with Low Metal Contamination: Comparison Between the Bivalve Cerastoderma Edule and the Polychaete Diopatra Neapolitana. Ecotoxicol. Environ. Saf. 2012, 75, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Poirier, L.; Berthet, B.; Amiard, J.-C.; Jeantet, A.-Y.; Amiard-Triquet, C. A Suitable Model for The Biomonitoring of Trace Metal Bioavailabilities in Estuarine Sediments: The Annelid Polychaete Nereis Diversicolor. J. Mar. Biol. Assoc. U. K. 2006, 86, 71–82. [Google Scholar] [CrossRef]
- Helland, S.; Christian Nejstgaard, J.; Jørgen Fyhn, H.; Egge, J.K.; Båmstedt, U. Effects of Starvation, Season, And Diet on The Free Amino Acid and Protein Content of Calanus Finmarchicus Females. Mar. Biol. 2003, 143, 297–306. [Google Scholar] [CrossRef]
- Coppola, F.; Almeida, Â.; Henriques, B.; Soares, A.M.V.M.; Figueira, E.; Pereira, E.; Freitas, R. Biochemical Responses and Accumulation Patterns of Mytilus Galloprovincialis Exposed to Thermal Stress and Arsenic Contamination. Ecotoxicol. Environ. Saf. 2018, 147, 54–962. [Google Scholar] [CrossRef] [PubMed]
- Holloway, G.J.; Sibly, R.M.; Povey, S.R. Evolution in Toxin-Stressed Environments. Funct. Ecol. 1990, 289–294. [Google Scholar] [CrossRef]
- Freitas, R.; Almeida, Â.; Calisto, V.; Velez, C.; Moreira, A.; Schneider, R.J.; Esteves, V.I.; Wrona, F.J.; Figueira, E.; Soares, A.M.V.M. The Impacts of Pharmaceutical Drugs Under Ocean Acidification: New Data on Single and Combined Long-Term Effects of Carbamazepine on Scrobicularia Plana. Sci. Total Environ. 2016, 541, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, L.; von Fumetti, S.; Nagel, P. Temperature Effects on the Feeding and Electron Transport System (ETS) Activity of Gammarus Fossarum. Aquat. Ecol. 2015, 49, 71–80. [Google Scholar] [CrossRef]
- Simčič, T.; Pajk, F.; Jaklič, M.; Brancelj, A.; Vrezec, A. The Thermal Tolerance of Crayfish Could Be Estimated from Respiratory Electron Transport System Activity. J. Therm. Biol. 2014, 41, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Morosetti, B.; Freitas, R.; Pereira, E.; Hamza, H.; Andrade, M.; Coppola, F.; Maggioni, D.; Torrec, C.D. Will Temperature Rise Change the Biochemical Alterations Induced in Mytilus Galloprovincialis by Cerium Oxide Nanoparticles and Mercury? Environ. Res. 2020, 188, 109778. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, L.; Pretti, C.; Gabriel, B.; Marques, P.A.A.P.; Freitas, R.; Neto, V. An Overview of Graphene Materials: Properties, Applications and Toxicity on Aquatic Environments. Sci. Total Environ. 2018, 631–632, 1440–1456. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M. Energy-Limited Tolerance to Stress as a Conceptual Framework to Integrate the Effects of Multiple Stressors. Integr. Comp. Biol. 2013, 53, 597–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aebi, H. Catalase In Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Andrade, M.; De Marchi, L.; Soares, A.M.V.M.; Rocha, R.J.M.; Figueira, E.; Freitas, R. Are the Effects Induced by Increased Temperature Enhanced in Mytilus Galloprovincialis Submitted to Air Exposure? Sci. Total Environ. 2019, 647, 431–440. [Google Scholar] [CrossRef]
- Gomes, T.; Gonzalez-Rey, M.; Rodríguez-Romero, A.; Trombini, C.; Riba, I.; Blasco, J.; Bebiano, M.J. Biomarkers in Nereis Diversicolor (Polychaeta: Nereididae) as Management Tools for Environmental Assessment on the Southwest Iberian Coast. Sci. Mar. 2013, 77, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Bocchetti, R.; Fattorini, D.; Gambi, M.C.; Regoli, F. Trace Metal Concentrations and Susceptibility to Oxidative Stress in the Polychaete Sabella Spallanzanii (Gmelin) (Sabellidae): Potential Role of Antioxidants in Revealing Stressful Environmental Conditions in the Mediterranean. Arch. Environ. Contam. Toxicol. 2004, 46, 353–361. [Google Scholar] [CrossRef]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free Radicals, Antioxidants, and Nutrition. Nutrition. 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein Carbonylation as a Major Hallmark of Oxidative Damage: Update of Analytical Strategies. Mass Spectrom. Rev. 2014, 33, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cavallo, E.; Guarnizo-Méndez, J.; Yépez-Terrill, A.; Cárdenas-Rivero, A.; Díaz-Castillo, F.; Méndez-Cuadro, D. Protein Carbonylation Is a Mediator in Larvicidal Mechanisms of Tabernaemontana Cymosa Ethanolic Extract. J. King Saud Univ. Sci. 2019, 31, 464–471. [Google Scholar] [CrossRef]
- Soto-Jiménez, M.F. Transferencia De Elementos Traza En Tramas Tróficas Acuáticas. Hidrobiológica. 2011, 21, 239–248. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giménez, V.; Cardoso, P.; Sá, C.; Patinha, C.; Ferreira da Silva, E.; Figueira, E.; Pires, A. Interplay of Seasonality, Major and Trace Elements: Impacts on the Polychaete Diopatra neapolitana. Biology 2022, 11, 1153. https://doi.org/10.3390/biology11081153
Giménez V, Cardoso P, Sá C, Patinha C, Ferreira da Silva E, Figueira E, Pires A. Interplay of Seasonality, Major and Trace Elements: Impacts on the Polychaete Diopatra neapolitana. Biology. 2022; 11(8):1153. https://doi.org/10.3390/biology11081153
Chicago/Turabian StyleGiménez, Valéria, Paulo Cardoso, Carina Sá, Carla Patinha, Eduardo Ferreira da Silva, Etelvina Figueira, and Adília Pires. 2022. "Interplay of Seasonality, Major and Trace Elements: Impacts on the Polychaete Diopatra neapolitana" Biology 11, no. 8: 1153. https://doi.org/10.3390/biology11081153
APA StyleGiménez, V., Cardoso, P., Sá, C., Patinha, C., Ferreira da Silva, E., Figueira, E., & Pires, A. (2022). Interplay of Seasonality, Major and Trace Elements: Impacts on the Polychaete Diopatra neapolitana. Biology, 11(8), 1153. https://doi.org/10.3390/biology11081153









