Biomarkers of Frailty: miRNAs as Common Signatures of Impairment in Cognitive and Physical Domains
Abstract
:Simple Summary
Abstract
1. Introduction
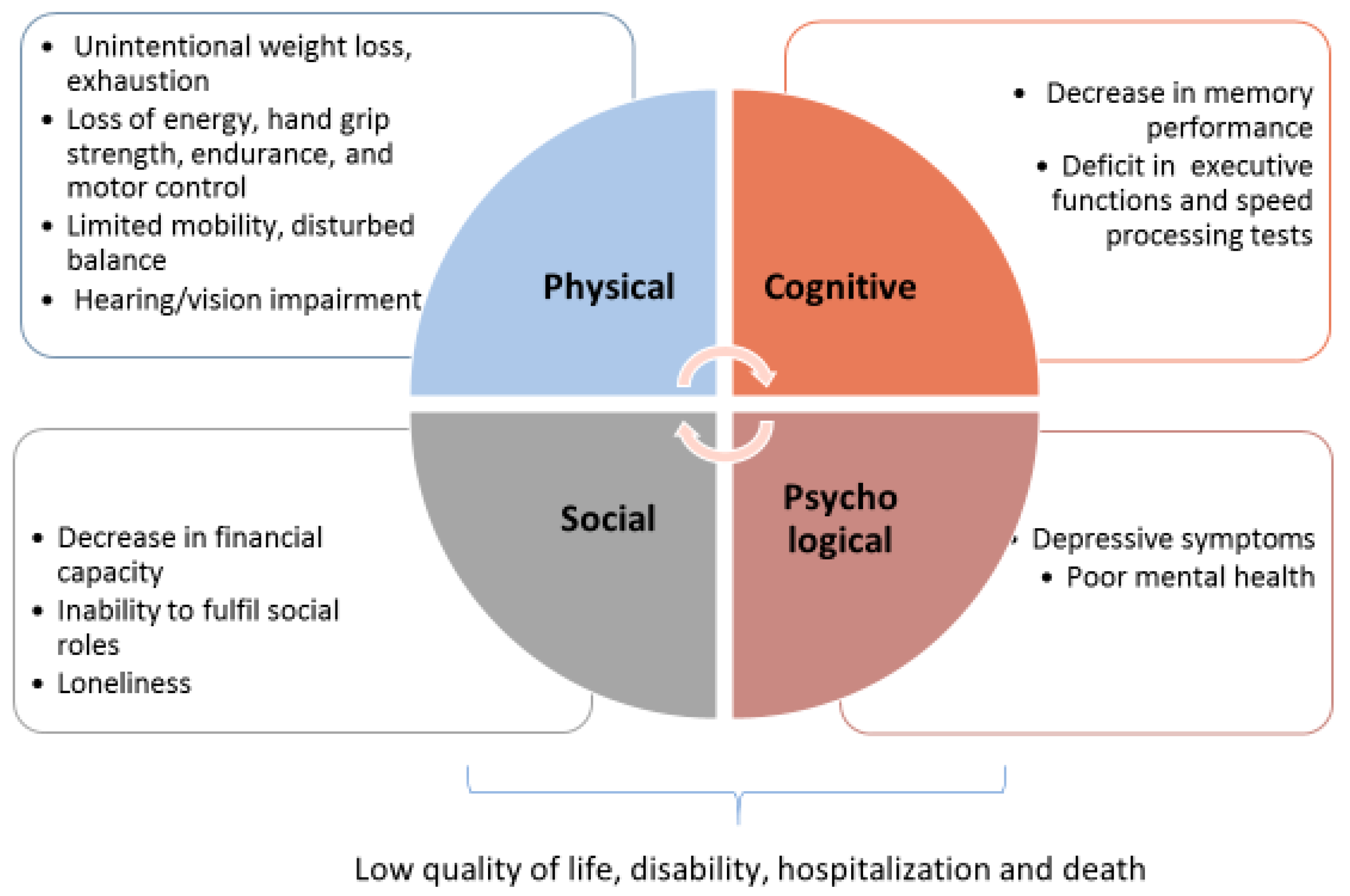
1.1. The Frailty Domains
- The physical domain
- Physical frailty was reported by Maxwell and Wang [8] as “characterized by gradual loss of energy, strength, endurance, and motor control”. It is defined basically considering ≥4 of 8 criteria, reflecting the screening of muscle health and functional status: unintentional weight loss, exhaustion, strength, perceived health, walking, balance, hearing and vision impairments [19]. A series of socio-demographic, lifestyle, and health-related factors have been shown to be associated with physical frailty, such as age, female sex, cardiovascular diseases, multimorbidity, BMI, and smoking.
- The cognitive domain
- Cognitive impairment in absence of dementia is considered a relevant domain of frailty. Cognitive impairment is defined as <10th percentile on global cognitive functioning, detected with cognitive tests, such as the MMSE (Mini Mental State Examination) score [20] and the cognitive abilities screening instrument (CASI) [21].
- The psychological and social domains
- Psychological frailty is defined on the base of two criteria such as depressive symptoms and mental health, and is measured by Geriatric Depression Scale and Mental Health Inventory 5 (MHI-5) [22]. A higher psychological frailty risk is associated with the female sex, low educational level, smoking, a short sleep duration and multi-morbidity, while being married, a long sleep duration and being physically active are normally associated with a lower risk of being psychologically frail.
- Social frailty is measured as ≥2 of 3 criteria among loneliness, social support and social participation.
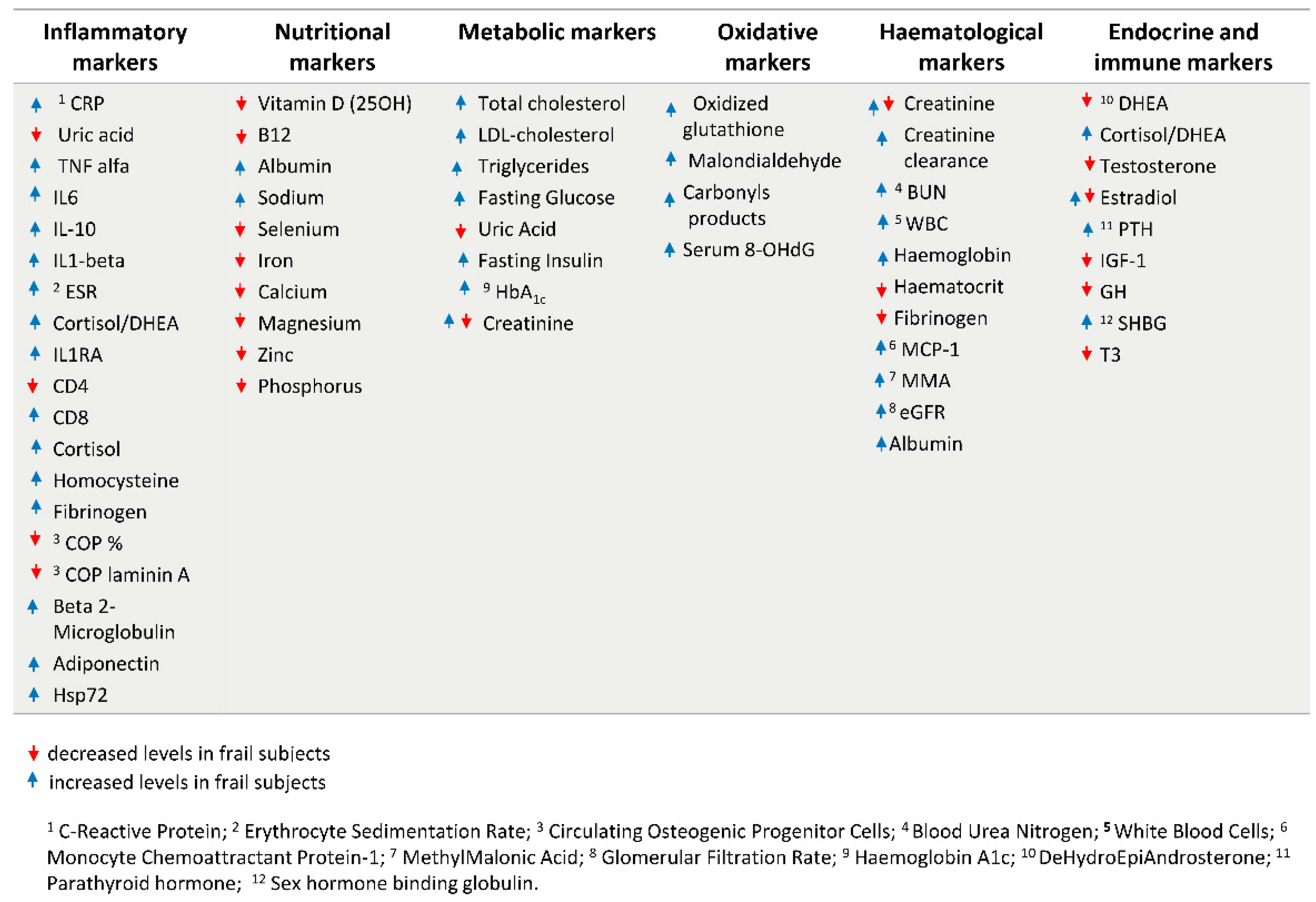
1.2. Current Biomarkers of Frailty
2. Search for Novel Biomarkers of Frailty: The Role of miRNAs
3. MiRNAs as Biomarkers of the Physical Domain of Frailty
4. MiRNAs as Biomarkers of the Cognitive Domain of Frailty
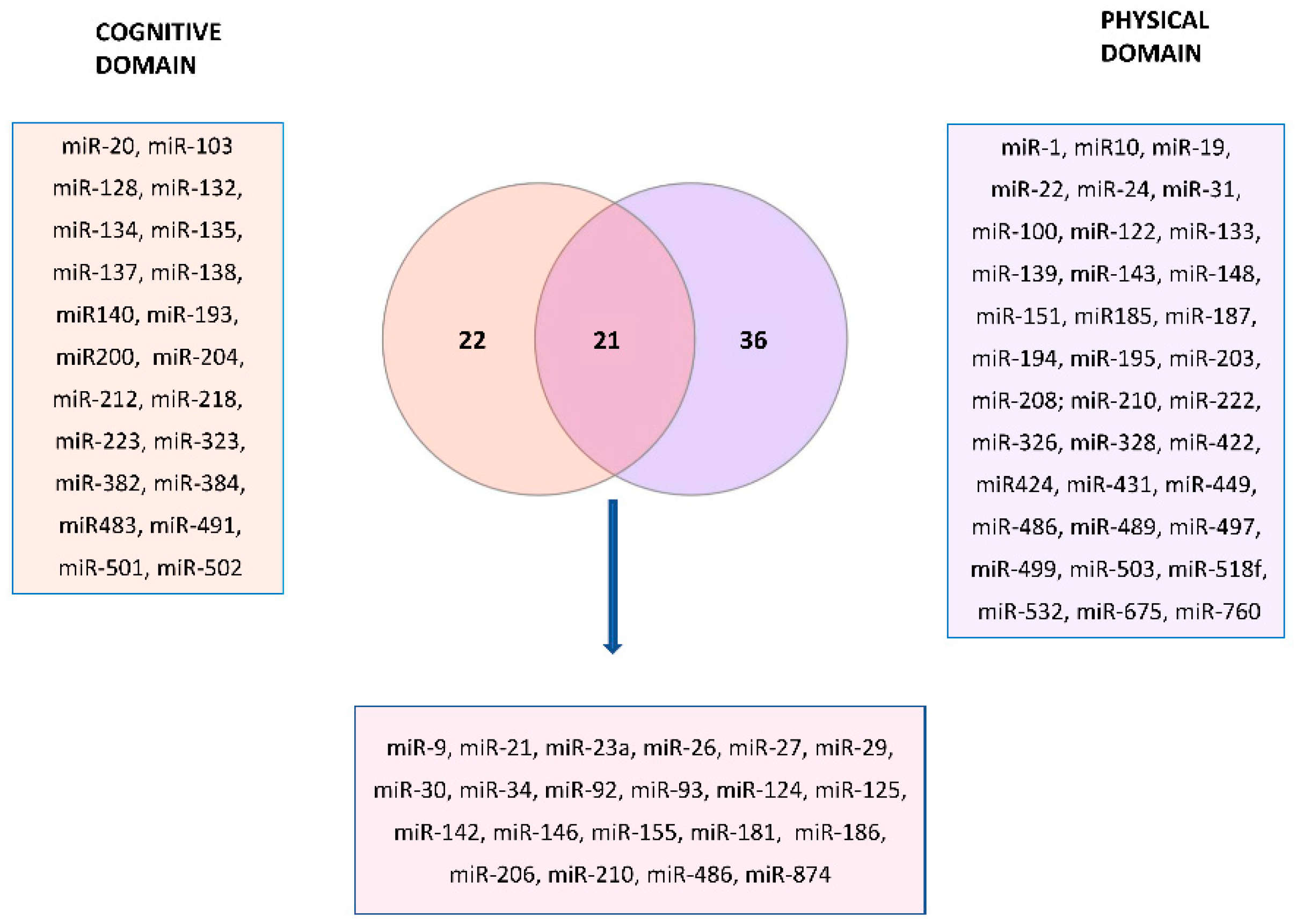
5. In Silico Analysis of Shared miRNAs between Cognitive and Physical Domains
6. Conclusions and Final Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abate, M.; Di Iorio, A.; Di Renzo, D.; Paganelli, R.; Saggini, R.; Abate, G. Frailty in the elderly: The physical dimension. Eura Medicophys. 2007, 43, 407–415. [Google Scholar] [PubMed]
- Gordon, A.L.; Masud, T.; Gladman, J.R. Now that we have a definition for physical frailty, what shape should frailty medicine take? Age Ageing 2014, 43, 8–9. [Google Scholar] [CrossRef] [Green Version]
- Sathyan, S.; Verghese, J. Genetics of frailty: A longevity perspective. Trans. Res. 2020, 221, 83–96. [Google Scholar] [CrossRef]
- Abellan van Kan, G.; Rolland, Y.M.; Morley, J.E.; Vellas, B. Frailty: Toward a clinical definition. J. Am. Med. Dir. Assoc. 2008, 9, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Ernsth Bravell, M.; Westerlind, B.; Midlöv, P.; Östgren, C.J.; Borgquist, L.; Lannering, C.; Mölstad, S. How to assess frailty and the need for care? Report from the Study of Health and Drugs in the Elderly (SHADES) in community dwellings in Sweden. Arch. Gerontol. Geriatr. 2011, 53, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Mohandas, A.; Reifsnyder, J.; Jacobs, M.; Fox, T. Current and future directions in frailty research. Popul. Health Manag. 2011, 14, 277–283. [Google Scholar] [CrossRef]
- Manthorpe, J.; Iliffe, S. The many meanings of frailty: Is there a shared understanding? Nurs. Resid. Care 2015, 17, 575–576. [Google Scholar] [CrossRef]
- Maxwell, C.A.; Wang, J. Understanding Frailty: A Nurse’s Guide. Nurs. Clin. N. Am. 2017, 52, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Lozupone, M.; Solfrizzi, V.; Sardone, R.; Dibello, V.; Di Lena, L.; D’Urso, F.; Stallone, R.; Petruzzi, M.; Giannelli, G.; et al. Different Cognitive Frailty Models and Health- and Cognitive-related Outcomes in Older Age: From Epidemiology to Prevention. J. Alzheimers Dis. 2018, 62, 993–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin. Geriatr. Med. 2011, 27, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Gobbens, R.J.J.; Luijkx, K.G.; van Assen, M.A.L.M. Explaining quality of life of older people in the Netherlands using a multidimensional assessment of frailty. Qual. Life Res. 2013, 22, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.L.; Boter, H.; Buskens, E.; Slaets, J.P. Measurement properties of the Groningen Frailty Indicator in home-dwelling and institutionalized elderly people. J. Am. Med. Dir. Assoc. 2012, 13, 546–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieland, D.; Hirth, V. Comprehensive geriatric assessment. Cancer Control. 2003, 10, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Gottesman, I.I.; Gould, T.D. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am. J. Psychiatry 2003, 160, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Cannon, T.D.; Keller, M.C. Endophenotypes in the genetic analyses of mental disorders. Annu Rev. Clin. Psychol. 2006, 2, 267–290. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Lindenberg, A.; Weinberger, D.R. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat. Rev. Neurosci. 2006, 7, 818–827. [Google Scholar] [CrossRef]
- van Oostrom, S.H.; van der, A.D.L.; Rietman, M.L.; Picavet, H.S.J.; Lette, M.; Verschuren, W.M.M.; de Bruin, S.R.; Spijkerman, A.M.W. A four-domain approach of frailty explored in the Doetinchem Cohort Study. BMC Geriatr. 2017, 17, 196. [Google Scholar] [CrossRef] [Green Version]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Teng, E.L.; Hasegawa, K.; Homma, A.; Imai, Y.; Larson, E.; Graves, A.; Sugimoto, K.; Yamaguchi, T.; Sasaki, H.; Chiu, D. The Cognitive Abilities Screening Instrument (CASI): A practical test for cross-cultural epidemiological studies of dementia. Int. Psychogeriatr. 1994, 6, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Coyne, J.C. Screening for Depression in Clinical Practice: An Evidence-Based Guide; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Ruan, Q.; Yu, Z.; Chen, M.; Bao, Z.; Li, J.; He, W. Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing Res. Rev. 2015, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grande, G.; Haaksma, M.L.; Rizzuto, D.; Melis, R.J.F.; Marengoni, A.; Onder, G.; Welmer, A.K.; Fratiglioni, L.; Vetrano, D.L. Co-occurrence of cognitive impairment and physical frailty, and incidence of dementia: Systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2019, 107, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Schneider, J.A.; Leurgans, S.; Bennett, D.A. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology 2008, 71, 499–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiwara, Y.; Shinkai, S.; Kumagai, S.; Amano, H.; Yoshida, Y.; Yoshida, H.; Kim, H.; Suzuki, T.; Ishizaki, T.; Haga, H.; et al. Longitudinal changes in higher-level functional capacity of an older population living in a Japanese urban community. Arch. Gerontol. Geriatr. 2003, 36, 141–153. [Google Scholar] [CrossRef]
- Makizako, H.; Shimada, H.; Doi, T.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Makino, K.; Lee, S. Social Frailty Leads to the Development of Physical Frailty among Physically Non-Frail Adults: A Four-Year Follow-Up Longitudinal Cohort Study. Int. J. Environ. Res. Public. Health 2018, 15, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.J.; Peng, L.N.; Lin, C.H.; Chen, R.C.; Lin, S.Z.; Loh, C.H.; Kao, S.L.; Hung, T.S.; Chang, C.Y.; Huang, C.F.; et al. Effects of incorporating multidomain interventions into integrated primary care on quality of life: A randomised controlled trial. Lancet 2021, 2, e712–e723. [Google Scholar] [CrossRef]
- Whalley, L.J.; Murray, A.D.; Staff, R.T.; Starr, J.M.; Deary, I.J.; Fox, H.C.; Lemmon, H.; Duthie, S.J.; Collins, A.R.; Crawford, J.R. How the 1932 and 1947 mental surveys of Aberdeen schoolchildren provide a framework to explore the childhood origins of late onset disease and disability. Maturitas 2011, 69, 365–372. [Google Scholar] [CrossRef]
- Evans, W.J.; Paolisso, G.; Abbatecola, A.M.; Corsonello, A.; Bustacchini, S.; Strollo, F.; Lattanzio, F. Frailty and muscle metabolism dysregulation in the elderly. Biogerontology. 2010, 11, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Cohen, A.A.; Xue, Q.L.; Walston, J.; Bandeen-Roche, K.; Varadhan, R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat. Aging 2021, 1, 36–46. [Google Scholar] [CrossRef]
- Kwak, D.; Thompson, L.V. Frailty: Past, present, and future? Sports Med. Health Sci. 2021, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Choi, S.R.; Choi, M.J.; Kim, S.G.; Lee, Y.K.; Noh, J.W.; Kim, H.J.; Song, Y.R. Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin. Nutr. 2014, 33, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, P.; Alyousefi, N.; Abdul-Rahman, P.S.; Kamaruzzaman, S.B.; Chin, A.V.; Tan, M.P. A systematic review of studies comparing potential biochemical biomarkers of frailty with frailty assessments? Eur. Geriatr. Med. 2017, 8, 397–407. [Google Scholar] [CrossRef]
- Sargent, L.; Nalls, M.; Starkweather, A.; Hobgood, S.; Thompson, H.; Amella, E.J.; Singleton, A. Shared biological pathways for frailty and cognitive impairment: A systematic review. Ageing Res. Rev. 2018, 47, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Saedi, A.A.; Feehan, J.; Phu, S.; Duque, G. Current and emerging biomarkers of frailty in the elderly. Clin. Interv. Aging 2019, 14, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supriya, R.; Singh, K.P.; Gao, Y.; Li, F.; Dutheil, F.; Baker, J.S. A Multifactorial Approach for Sarcopenia Assessment: A Literature Review. Biology 2021, 10, 1354. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Borras, C.; Gomez-Cabrera, M.C. A free radical theory of frailty. Free Radic. Biol. Med. 2018, 124, 358–363. [Google Scholar] [CrossRef]
- Serviddio, G.; Romano, A.D.; Greco, A.; Rollo, T.; Bellanti, F.; Altomare, E.; Vendemiale, G. Frailty syndrome is associated with altered circulating redox balance and increased markers of oxidative stress. Int. J. Immunopathol. Pharmacol. 2009, 22, 819–827. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Fukuo, K.; Suzuki, K.; Yoshino, G.; Kazumi, T. Relationships of systemic oxidative stress to body fat distribution, adipokines and inflammatory markers in healthy middle-aged women. Endocr. J. 2009, 56, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Ju, S.Y.; Lee, J.Y.; Kim, D.H. Low 25-hydroxyvitamin D levels and the risk of frailty syndrome: A systematic review and dose-response meta-analysis. BMC Geriatr. 2018, 18, 206. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, B.; Liang, C.; Li, Y.; Song, Y.H. Cytokine Signaling in Skeletal Muscle Wasting. Trends Endocrinol. Metab. 2016, 27, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Kameda, M.; Teruya, T.; Yanagida, M.; Kondoh, H. Reduced uremic metabolites are prominent feature of sarcopenia, distinct from antioxidative markers for frailty. Aging 2021, 13, 20915–20934. [Google Scholar] [CrossRef] [PubMed]
- Swiecicka, A.; Eendebak, R.J.A.H.; Lunt, M.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Forti, G.; Giwercman, A.; Han, T.S.; Slowikowska-Hilczer, J.; et al. European Male Ageing Study Group. Reproductive Hormone Levels Predict Changes in Frailty Status in Community-Dwelling Older Men: European Male Ageing Study Prospective Data. J. Clin. Endocrinol. Metab. 2018, 103, 701–709. [Google Scholar] [CrossRef]
- Carcaillon, L.; García-García, F.J.; Tresguerres, J.A.; Gutiérrez Avila, G.; Kireev, R.; Rodríguez-Mañas, L. Higher levels of endogenous estradiol are associated with frailty in postmenopausal women from the toledo study for healthy aging. J. Clin. Endocrinol. Metab. 2012, 97, 2898–2906. [Google Scholar] [CrossRef] [Green Version]
- Picca, A.; Coelho-Junior, H.J.; Cesari, M.; Marini, F.; Miccheli, A.; Gervasoni, J.; Bossola, M.; Landi, F.; Bernabei, R.; Marzetti, E.; et al. The metabolomics side of frailty: Toward personalized medicine for the aged. Exp. Gerontol. 2019, 126, 110692. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R. Biomarkers of frailty: Moving the field forward. Exp. Gerontol. 2020, 133, 110868. [Google Scholar] [CrossRef] [PubMed]
- Zafari, S.; Backes, C.; Leidinger, P.; Meese, E.; Keller, A. Regulatory microRNA networks: Complex patterns of target pathways for disease-related and housekeeping microRNAs. Genom. Proteom. Bioinform. 2015, 13, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Hammond, S.M. An overview of microRNAs. Adv. Drug. Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Sheng, P.; Fields, C.; Aadland, K.; Wei, T.; Kolaczkowski, O.; Gu, T.; Kolaczkowski, B.; Xie, M. Dicer cleaves 5’-extended microRNA precursors originating from RNA polymerase II transcription start sites. Nucleic Acids Res. 2018, 46, 5737–5752. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wang, D. The Pattern of microRNA Binding Site Distribution. Genes 2017, 8, 296. [Google Scholar] [CrossRef] [Green Version]
- Moretti, F.; Thermann, R.; Hentze, M.W. Mechanism of translational regulation by miR-2 from sites in the 5′ untranslated region or the open reading frame. RNA 2010, 16, 2493–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denzler, R.; McGeary, S.E.; Title, A.C.; Agarwal, V.; Bartel, D.P.; Stoffel, M. Impact of MicroRNA Levels, Target-Site Complementarity, and Cooperativity on Competing Endogenous RNA-Regulated Gene Expression. Mol. Cell 2016, 64, 565–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohel, M.H. Extracellular/Circulating MicroRNAs: Release Mechanisms, Functions and Challenges. Achiev. Life Sci. 2016, 10, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Bayraktar, R.; Van Roosbroeck, K.; Calin, G.A. Cell-to-cell communication: microRNAs as hormones. Mol. Oncol. 2017, 11, 1673–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.J.; Suh, Y. Circulating miRNAs in Ageing and Ageing-Related Diseases. J. Genet. Genom. 2014, 41, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 2016, 410, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenetics 2018, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- ElSharawy, A.; Keller, A.; Flachsbart, F.; Wendschlag, A.; Jacobs, G.; Kefer, N.; Brefort, T.; Leidinger, P.; Backes, C.; Meese, E.; et al. Genome-wide miRNA signatures of human longevity. Aging Cell 2012, 11, 607–616. [Google Scholar] [CrossRef]
- Olivieri, F.; Capri, M.; Bonafè, M.; Morsiani, C.; Jung, H.J.; Spazzafumo, L.; Viña, J.; Suh, Y. Circulating miRNAs and miRNA shuttles as biomarkers: Perspective trajectories of healthy and unhealthy aging. Mech. Ageing Dev. 2017, 165, 162–170. [Google Scholar] [CrossRef] [Green Version]
- Serna, E.; Gambini, J.; Borras, C.; Abdelaziz, K.M.; Belenguer, A.; Sanchis, P.; Avellana, J.A.; Rodriguez-Mañas, L.; Viña, J. Centenarians, but not octogenarians, up-regulate the expression of microRNAs. Sci. Rep. 2012, 2, 961. [Google Scholar] [CrossRef] [Green Version]
- Noren Hooten, N.; Fitzpatrick, M.; Wood, W.H., 3rd; De, S.; Ejiogu, N.; Zhang, Y.; Mattison, J.A.; Becker, K.G.; Zonderman, A.B.; Evans, M.K. Age-related changes in microRNA levels in serum. Aging 2013, 5, 725–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith-Vikos, T.; Slack, F.J. MicroRNAs and their roles in aging. J. Cell Sci. 2012, 125, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.J.; Lee, K.P.; Kwon, K.S.; Suh, Y. MicroRNAs in Skeletal Muscle Aging: Current Issues and Perspectives. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Woldemichael, B.T.; Mansuy, I.M. Micro-RNAs in cognition and cognitive disorders: Potential for novel biomarkers and therapeutics. Biochem. Pharmacol. 2016, 104, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Verjan, J.C.; Ramírez-Aldana, R.; Pérez-Zepeda, M.U.; Quiroz-Baez, R.; Luna-López, A.; Gutierrez Robledo, L.M. Systems biology and network pharmacology of frailty reveal novel epigenetic targets and mechanisms. Sci. Rep. 2019, 9, 10593. [Google Scholar] [CrossRef]
- Rusanova, I.; Diaz-Casado, M.E.; Fernández-Ortiz, M.; Aranda-Martínez, P.; Guerra-Librero, A.; García-García, F.J.; Escames, G.; Mañas, L.; Acuña-Castroviejo, D. Analysis of Plasma MicroRNAs as Predictors and Biomarkers of Aging and Frailty in Humans. Oxid. Med. Cell. Longev. 2018, 2018, 7671850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ipson, B.R.; Fletcher, M.B.; Espinoza, S.E.; Fisher, A.L. Identifying Exosome-Derived MicroRNAs as Candidate Biomarkers of Frailty. J. Frailty Aging 2018, 7, 100–103. [Google Scholar] [CrossRef]
- Carini, G.; Musazzi, L.; Bolzetta, F.; Cester, A.; Fiorentini, C.; Ieraci, A.; Maggi, S.; Popoli, M.; Veronese, N.; Barbon, A. The Potential Role of miRNAs in Cognitive Frailty. Front. Aging Neurosci. 2021, 13, 763110. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, S.Y.; Bae, Y.S. Upregulation of miR-760 and miR-186 is associated with replicative senescence in human lung fibroblast cells. Mol. Cells 2014, 37, 620–627. [Google Scholar] [CrossRef] [Green Version]
- Morton, S.U.; Sefton, C.R.; Zhang, H.; Dai, M.; Turner, D.L.; Uhler, M.D.; Agrawal, P.B. microRNA-mRNA profile of skeletal muscle differentiation and relevance to congenital myotonic dystrophy. Int. J. Mol. Sci. 2021, 22, 2692. [Google Scholar] [CrossRef]
- Wang, X.H. MicroRNA in myogenesis and muscle atrophy. Curr. Opinion Clin. Nutr. Metab. Care 2013, 16, 258–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, A.P.; Lamon, S.; Boon, H.; Wada, S.; Güller, I.; Brown, E.L.; Chibalin, A.V.; Zierath, J.R.; Snow, R.J.; Stepto, N.; et al. Regulation of miRNAs in human skeletal muscle following acute endurance exercise and short-term endurance training. J. Physiol. 2013, 591, 4637–4653. [Google Scholar] [CrossRef] [PubMed]
- Coolen, M.; Katz, S.; Bally-Cuif, L. miR-9: A versatile regulator of neurogenesis. Front. Cell Neurosci. 2013, 7, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Gu, Z.; Ni, P.; Qiao, Y.; Chen, C.; Liu, X.; Lin, J.; Chen, N.; Fan, Q. NF-kappaB P50/P65 hetero-dimer mediates differential regulation of CD166/ALCAM expression via interaction with micoRNA-9 after serum deprivation, providing evidence for a novel negative auto-regulatory loop. Nucleic Acids Res. 2011, 39, 6440–6455. [Google Scholar] [CrossRef] [Green Version]
- Weilner, S.; Skalicky, S.; Salzer, B.; Keider, V.; Wagner, M.; Hildner, F.; Gabriel, C.; Dovjak, P.; Pietschmann, P.; Grillari-Voglauer, R.; et al. Differentially circulating miRNAs after recent osteoporotic fractures can influence osteogenic differentiation. Bone 2015, 79, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanai, K.; Kaneko, S.; Ishii, H.; Aomatsu, A.; Ito, K.; Hirai, K.; Ookawara, S.; Ishibashi, K.; Morishita, Y. MicroRNAs in Sarcopenia: A Systematic Review. Front. Med. 2020, 7, 180. [Google Scholar] [CrossRef]
- Zhang, G.; He, M.; Wu, P.; Zhang, X.; Zhou, K.; Li, T.; Zhang, T.; Xie, K.; Dai, G.; Wang, J. MicroRNA-27b-3p Targets the Myostatin Gene to Regulate Myoblast Proliferation and Is Involved in Myoblast Differentiation. Cells 2021, 10, 423. [Google Scholar] [CrossRef]
- Borja-Gonzalez, M.; Casas-Martinez, J.C.; McDonagh, B.; Goljanek-Whysall, K. Inflamma-miR-21 Negatively Regulates Myogenesis during Ageing. Antioxidants 2020, 9, 345. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Li, H.; Li, T.; Fan, J.; Zhao, R.C.; Weng, X. MicroRNA expression profile of dexamethasone-induced human bone marrow-derived mesenchymal stem cells during osteogenic differentiation. J. Cell Biochem. 2014, 115, 1683–1691. [Google Scholar] [CrossRef]
- Seeliger, C.; Karpinski, K.; Haug, A.T.; Vester, H.; Schmitt, A.; Bauer, J.S.; van Griensven, M. Five freely circulating miRNAs and bone tissue miRNAs are associated with osteoporotic fractures. J. Bone Miner. Res. 2014, 29, 1718–1728. [Google Scholar] [CrossRef]
- Panach, L.; Mifsut, D.; Tarín, J.J.; Cano, A.; García-Pérez, M.A. Serum Circulating MicroRNAs as Biomarkers of Osteoporotic Fracture. Calcif. Tissue Int. 2015, 97, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Dey, B.K.; Gagan, J.; Yan, Z.; Dutta, A. miR-26a is required for skeletal muscle differentiation and regeneration in mice. Genes Dev. 2012, 26, 2180–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Pan, Y.; Xie, C.; Zhang, Y. miR-34a exerts as a key regulator in the dedifferentiation of osteosarcoma via PAI-1-Sox2 axis. Cell Death Dis. 2018, 9, 777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chan, M.C.; Yu, Y.; Bei, Y.; Chen, P.; Zhou, Q.; Cheng, L.; Chen, L.; Ziegler, O.; Rowe, G.C.; et al. miR-29b contributes to multiple types of muscle atrophy. Nat. Commun. 2017, 8, 15201. [Google Scholar] [CrossRef] [Green Version]
- Van Pelt, D.W.; Vechetti, I.J., Jr.; Lawrence, M.M.; Van Pelt, K.L.; Patel, P.; Miller, B.F.; Butterfield, T.A.; Dupont-Versteegden, E.E. Serum extracellular vesicle miR-203a-3p content is associated with skeletal muscle mass and protein turnover during disuse atrophy and regrowth. Am. J. Physiol. Cell Physiol. 2020, 319, C419–C431. [Google Scholar] [CrossRef]
- Wang, Z.C.; Wang, Z.Z.; Ma, H.J.; Wang, C.C.; Wang, H.T. Attenuation of the hypoxia-induced miR-34a protects cardiomyocytes through maintenance of glucose metabolism. Biochem. Biophys. Res. Commun. 2018, 498, 375–381. [Google Scholar] [CrossRef]
- Iannone, F.; Montesanto, A.; Cione, E.; Crocco, P.; Caroleo, M.C.; Dato, S.; Rose, G.; Passarino, G. Expression Patterns of Muscle-Specific miR-133b and miR-206 Correlate with Nutritional Status and Sarcopenia. Nutrients 2020, 12, 297. [Google Scholar] [CrossRef] [Green Version]
- He, N.; Zhang, Y.L.; Zhang, Y.; Feng, B.; Zheng, Z.; Wang, D.; Zhang, S.; Guo, Q.; Ye, H. Circulating MicroRNAs in Plasma Decrease in Response to Sarcopenia in the Elderly. Front. Genet. 2020, 11, 167. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, L.; Moore, B.T.; Peng, X.H.; Fang, X.; Lappe, J.M.; Recker, R.R.; Xiao, P. MiR-133a in human circulating monocytes: A potential biomarker associated with postmenopausal osteoporosis. PLoS ONE 2012, 7, e34641. [Google Scholar] [CrossRef]
- Dobrowolny, G.; Martone, J.; Lepore, E.; Casola, I.; Petrucci, A.; Inghilleri, M.; Morlando, M.; Colantoni, A.; Scicchitano, B.M.; Calvo, A.; et al. A longitudinal study defined circulating microRNAs as reliable biomarkers for disease prognosis and progression in ALS human patients. Cell Death Discov. 2021, 7, 4. [Google Scholar] [CrossRef]
- Feng, Y.; Wan, P.; Yin, L.; Lou, X. The Inhibition of MicroRNA-139-5p Promoted Osteoporosis of Bone Marrow-Derived Mesenchymal Stem Cells by Targeting Wnt/Beta-Catenin Signaling Pathway by NOTCH1. J. Microbiol. Biotechnol. 2020, 30, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ye, Y.; Zhang, W.; Wang, J.; Chen, A.; Guo, F. miR 142 3p promotes osteoblast differentiation by modulating Wnt signaling. Mol. Med. Rep. 2013, 7, 689–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margolis, L.M.; Lessard, S.J.; Ezzyat, Y.; Fielding, R.A.; Rivas, D.A. Circulating MicroRNA Are Predictive of Aging and Acute Adaptive Response to Resistance Exercise in Men. J. Geront. A Biol Sci Med Sci. 2017, 72, 1319–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Deng, J.; Qiu, Y.; Gao, J.; Li, J.; Guan, L.; Lee, H.; Zhou, Q.; Xiao, J. Non-coding RNA basis of muscle atrophy. Mol. Ther. Nucleic Acids 2021, 26, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Onodera, Y.; Teramura, T.; Takehara, T.; Itokazu, M.; Mori, T.; Fukuda, K. Inflammation-associated miR-155 activates differentiation of muscular satellite cells. PLoS ONE 2018, 13, e0204860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, M.J.; McCarthy, J.J.; Sinha, M.; Spratt, H.M.; Volpi, E.; Esser, K.A.; Rasmussen, B.B. Aging and microRNA expression in human skeletal muscle: A microarray and bioinformatics analysis. Physiol. Genom. 2011, 43, 595–603. [Google Scholar] [CrossRef] [Green Version]
- Antoniou, A.; Mastroyiannopoulos, N.P.; Uney, J.B.; Phylactou, L.A. miR-186 inhibits muscle cell differentiation through myogenin regulation. J. Biol. Chem. 2014, 289, 3923–3935. [Google Scholar] [CrossRef] [Green Version]
- Garmilla-Ezquerra, P.; Sañudo, C.; Delgado-Calle, J.; Pérez-Nuñez, M.I.; Sumillera, M.; Riancho, J.A. Analysis of the bone microRNome in osteoporotic fractures. Calcif. Tissue Int. 2015, 96, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhang, D.; Pan, N.; Sun, N.; Wang, Q.; Fan, J.; Zhou, P.; Zhu, W.; Jiang, L. Identification of miR-194-5p as a potential biomarker for postmenopausal osteoporosis. Peer J. 2015, 3, e971. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Yamamoto, T.; Sehara-Fujisawa, A. miR-195/497 induce postnatal quiescence of skeletal muscle stem cells. Nat. Commun. 2014, 5, 4597. [Google Scholar] [CrossRef]
- Okugawa, Y.; Toiyama, Y.; Hur, K.; Yamamoto, A.; Yin, C.; Ide, S.; Kitajima, T.; Fujikawa, H.; Yasuda, H.; Koike, Y.; et al. Circulating miR-203 derived from metastatic tissues promotes myopenia in colorectal cancer patients. J. Cachexia Sarcopenia Muscle 2019, 10, 536–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, S.; Sun, B.; Yin, X.; Guo, X.; Chao, D.; Zhang, C.; Zhang, C.Y.; Chen, X.; Ma, J. Time-course responses of circulating microRNAs to three resistance training protocols in healthy young men. Sci. Rep. 2017, 7, 2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Moore, B.T.; Wang, Y.; Peng, X.H.; Lappe, J.M.; Recker, R.R.; Xiao, P. MiR-422a as a potential cellular microRNA biomarker for postmenopausal osteoporosis. PLoS ONE 2014, 9, e97098. [Google Scholar] [CrossRef] [Green Version]
- Connolly, M.; Paul, R.; Farre-Garros, R.; Natanek, S.A.; Bloch, S.; Lee, J.; Lorenzo, J.P.; Patel, H.; Cooper, C.; Sayer, A.A.; et al. miR-424-5p reduces ribosomal RNA and protein synthesis in muscle wasting. J. Cachexia Sarcopenia Muscle 2018, 9, 400–416. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Shin, Y.J.; Panda, A.C.; Abdelmohsen, K.; Kim, J.Y.; Lee, S.M.; Bahn, Y.J.; Choi, J.Y.; Kwon, E.S.; Baek, S.J.; et al. miR-431 promotes differentiation and regeneration of old skeletal muscle by targeting Smad4. Genes Dev. 2015, 29, 1605–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, T.H.; Quach, N.L.; Charville, G.W.; Liu, L.; Park, L.; Edalati, A.; Yoo, B.; Hoang, P.; Rando, T.A. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature 2012, 482, 524–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, S.; Dey, B.K.; Dutta, A. MiR-322/424 and -503 are induced during muscle differentiation and promote cell cycle quiescence and differentiation by down-regulation of Cdc25A. Mol. Biol. Cell. 2010, 21, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wei, S.; Xu, F.; Cai, X.; Wang, H.; Ding, R. MicroRNA-532-5p is implicated in the regulation of osteoporosis by forkhead box O1 and osteoblast differentiation. BMC Musculoskelet Disord. 2020, 21, 296. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Lee, J.Y.; Donaldson, A.V.; Natanek, S.A.; Vaidyanathan, S.; Man, W.D.; Hopkinson, N.S.; Sayer, A.A.; Patel, H.P.; Cooper, C.; et al. Increased expression of H19/miR-675 is associated with a low fat-free mass index in patients with COPD. J. Cachexia Sarcopenia Muscle 2016, 7, 330–344. [Google Scholar] [CrossRef] [Green Version]
- Mei, L.; Li, M.; Zhang, T. MicroRNA miR-874-3p inhibits osteoporosis by targeting leptin (LEP). Bioengineered 2021, 12, 11756–11767. [Google Scholar] [CrossRef]
- Brown, D.M.; Goljanek-Whysall, K. microRNAs: Modulators of the underlying pathophysiology of sarcopenia? Ageing Res. Rev. 2015, 24, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Nakasa, T.; Ishikawa, M.; Shi, M.; Shibuya, H.; Adachi, N.; Ochi, M. Acceleration of muscle regeneration by local injection of muscle-specific microRNAs in rat skeletal muscle injury model. J. Cell. Mol. Med. 2010, 14, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Cao, J.J.; Margolis, L.M.; Sauter, E.R.; Whigham, L.D.; McClung, J.P.; Rood, J.C.; Carbone, J.W.; Combs, G.F., Jr.; Young, A.J. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: A randomized controlled trial. FASEB J. 2013, 27, 3837–3847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camera, D.M.; Ong, J.N.; Coffey, V.G.; Hawley, J.A. Selective Modulation of MicroRNA Expression with Protein Ingestion Following Concurrent Resistance and Endurance Exercise in Human Skeletal Muscle. Front. Physiol. 2016, 7, 87. [Google Scholar] [CrossRef] [Green Version]
- Olivieri, F.; Prattichizzo, F.; Giuliani, A.; Matacchione, G.; Rippo, M.R.; Sabbatinelli, J.; Bonafè, M. miR-21 and miR-146a: The microRNAs of inflammaging and age-related diseases. Ageing Res. Rev. 2021, 70, 101374. [Google Scholar] [CrossRef] [PubMed]
- Dimassi, S.; Karkeni, E.; Laurant, P.; Tabka, Z.; Landrier, J.F.; Riva, C. Microparticle miRNAs as Biomarkers of Vascular Function and Inflammation Response to Aerobic Exercise in Obesity? Obesity 2018, 26, 1584–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorillo, A.A.; Heier, C.R.; Novak, J.S.; Tully, C.B.; Brown, K.J.; Uaesoontrachoon, K.; Vila, M.C.; Ngheim, P.P.; Bello, L.; Kornegay, J.N.; et al. TNF-α-Induced microRNAs Control Dystrophin Expression in Becker Muscular Dystrophy. Cell. Rep. 2015, 12, 1678–1690. [Google Scholar] [CrossRef]
- Fiorillo, A.A.; Tully, C.B.; Damsker, J.M.; Nagaraju, K.; Hoffman, E.P.; Heier, C.R. Muscle miRNAome shows suppression of chronic inflammatory miRNAs with both prednisone and vamorolone. Physiol. Genom. 2018, 50, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Juźwik, C.A.; S Drake, S.; Zhang, Y.; Paradis-Isler, N.; Sylvester, A.; Amar-Zifkin, A.; Douglas, C.; Morquette, B.; Moore, C.S.; Fournier, A.E. microRNA dysregulation in neurodegenerative diseases: A systematic review. Prog. Neurobiol. 2019, 182, 101664. [Google Scholar] [CrossRef]
- Liu, N.; Bezprozvannaya, S.; Shelton, J.M.; Frisard, M.I.; Hulver, M.W.; McMillan, R.P.; Wu, Y.; Voelker, K.A.; Grange, R.W.; Richardson, J.A.; et al. Mice lacking microRNA 133a develop dynamin 2–dependent centronuclear myopathy. J. Clin. Investig. 2011, 121, 3258–3268. [Google Scholar] [CrossRef] [Green Version]
- Morsiani, C.; Terlecki-Zaniewicz, L.; Skalicky, S.; Bacalini, M.G.; Collura, S.; Conte, M.; Sevini, F.; Garagnani, P.; Salvioli, S.; Hackl, M.; et al. Circulating miR-19a-3p and miR-19b-3p characterize the human aging process and their isomiRs associate with healthy status at extreme ages. Aging Cell 2021, 20, e13409. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Kouwaki, T.; Oshiumi, H. Aging-Associated Extracellular Vesicles Contain Immune Regulatory microRNAs Alleviating Hyperinflammatory State and Immune Dysfunction in the Elderly. Iscience 2020, 23, 101520. [Google Scholar] [CrossRef]
- Purohit, P.K.; Saini, N. Mitochondrial microRNA (MitomiRs) in cancer and complex mitochondrial diseases: Current status and future perspectives. Cell. Mol. Life Sci. 2021, 78, 1405–1421. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Coffinier, C.; Choe, Y.; Beigneux, A.P.; Davies, B.S.; Yang, S.H.; Barnes, R.H., 2nd; Hong, J.; Sun, T.; Pleasure, S.J.; et al. Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA. Proc. Natl. Acad. Sci. USA 2012, 109, E423–E431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, M.; Yamada, H.; Munetsuna, E.; Yamazaki, M.; Hatta, T.; Iwahara, A.; Ohashi, K.; Ishikawa, H.; Tsuboi, Y.; Inoue, T.; et al. Associations of serum microRNA-20a, -27a, and -103a with cognitive function in a Japanese population: The Yakumo study. Arch. Gerontol. Geriatr. 2019, 82, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Bian, Z. MicroRNA-21 Is a Versatile Regulator and Potential Treatment Target in Central Nervous System Disorders. Front. Mol. Neurosci. 2022, 15, 842288. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.B.; Mufson, E.J.; Counts, S.E. Evidence for a neuroprotective microRNA pathway in amnestic mild cognitive impairment. Front. Neurosci. 2015, 9, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Absalon, S.; Kochanek, D.M.; Raghavan, V.; Krichevsky, A.M. MiR-26b, upregulated in Alzheimer’s disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons. J. Neurosci. 2013, 33, 14645–14659. [Google Scholar] [CrossRef]
- Marttila, S.; Rovio, S.; Mishra, P.P.; Seppälä, I.; Lyytikäinen, L.P.; Juonala, M.; Waldenberger, M.; Oksala, N.; Ala-Korpela, M.; Harville, E.; et al. Adulthood blood levels of hsa-miR-29b-3p associate with preterm birth and adult metabolic and cognitive health. Sci. Rep. 2021, 11, 9203. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, S.; Laskowska-Kaszub, K.; Dębski, K.J.; Wojsiat, J.; Dąbrowski, M.; Gabryelewicz, T.; Kuźnicki, J.; Wojda, U. Profile of 6 microRNA in blood plasma distinguish early stage Alzheimer’s disease patients from non-demented subjects. Oncotarget. 2017, 8, 16122–16143. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Jaber, V.R.; LeBeauf, A.; Sharfman, N.M.; Lukiw, W.J. microRNA-34a (miRNA-34a) Mediated Down-Regulation of the Post-synaptic Cytoskeletal Element SHANK3 in Sporadic Alzheimer’s Disease (AD). Front. Neurol. 2019, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, M.M.J.; Krauskopf, J.; Ramaekers, J.G.; Kleinjans, J.C.S.; Prickaerts, J.; Briedé, J.J. Circulating microRNAs as potential biomarkers for psychiatric and neurodegenerative disorders. Prog. Neurobiol. 2020, 185, 101732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, P.; Zhou, C.; Zhang, X.; Ma, F.; Xu, Y.; Hamblin, M.H.; Yin, K.J. Regulatory microRNAs and vascular cognitive impairment and dementia. CNS Neurosci. Ther. 2020, 26, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Dong, X.; Zheng, D.; Nao, J. MiR-124 and the Underlying Therapeutic Promise of Neurodegenerative Disorders. Front. Pharmacol. 2020, 10, 1555. [Google Scholar] [CrossRef] [PubMed]
- Banzhaf-Strathmann, J.; Benito, E.; May, S.; Arzberger, T.; Tahirovic, S.; Kretzschmar, H.; Fischer, A.; Edbauer, D. MicroRNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer’s disease. EMBO J. 2014, 33, 1667–16680. [Google Scholar] [CrossRef] [Green Version]
- Sheinerman, K.S.; Tsivinsky, V.G.; Crawford, F.; Mullan, M.J.; Abdullah, L.; Umansky, S.R. Plasma microRNA biomarkers for detection of mild cognitive impairment. Aging 2012, 4, 590–605. [Google Scholar] [CrossRef] [Green Version]
- Hadar, A.; Milanesi, E.; Walczak, M.; Puzianowska-Kuźnicka, M.; Kuźnicki, J.; Squassina, A.; Niola, P.; Chillotti, C.; Attems, J.; Gozes, I.; et al. SIRT1, miR-132 and miR-212 link human longevity to Alzheimer’s Disease. Sci. Rep. 2018, 8, 8465. [Google Scholar] [CrossRef]
- Xie, B.; Zhou, H.; Zhang, R.; Song, M.; Yu, L.; Wang, L.; Liu, Z.; Zhang, Q.; Cui, D.; Wang, X.; et al. Serum miR-206 and miR-132 as Potential Circulating Biomarkers for Mild Cognitive Impairment. J. Alzheimers Dis. 2015, 45, 721–731. [Google Scholar] [CrossRef]
- Cha, D.J.; Mengel, D.; Mustapic, M.; Liu, W.; Selkoe, D.J.; Kapogiannis, D.; Galasko, D.; Rissman, R.A.; Bennett, D.A.; Walsh, D.M. miR-212 and miR-132 Are Downregulated in Neurally Derived Plasma Exosomes of Alzheimer’s Patients. Front. Neurosci. 2019, 13, 1208. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.M.; Jia, R.H.; Wei, D.; Cui, W.Y.; Jiang, W. MiR-134 blockade prevents status epilepticus like-activity and is neuroprotective in cultured hippocampal neurons. Neurosci. Lett. 2014, 572, 20–25. [Google Scholar] [CrossRef]
- Konovalova, J.; Gerasymchuk, D.; Arroyo, S.N.; Kluske, S.; Mastroianni, F.; Pereyra, A.V.; Domanskyi, A. Human-Specific Regulation of Neurotrophic Factors MANF and CDNF by microRNAs. Int. J. Mol. Sci. 2021, 22, 9691. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.T.; Liu, C.G.; Gao, S.C.; Zhang, Y.; Wang, P.C. The Serum Exosome Derived MicroRNA-135a, -193b, and -384 Were Potential Alzheimer’s Disease Biomarkers. Biomed. Environ. Sci. 2018, 31, 87–96. [Google Scholar] [PubMed]
- Siegert, S.; Seo, J.; Kwon, E.J.; Rudenko, A.; Cho, S.; Wang, W.; Flood, Z.; Martorell, A.J.; Ericsson, M.; Mungenast, A.E.; et al. The schizophrenia risk gene product miR-137 alters presynaptic plasticity. Nat. Neurosci. 2015, 18, 1008–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schröder, J.; Ansaloni, S.; Schilling, M.; Liu, T.; Radke, J.; Jaedicke, M.; Schjeide, B.M.; Mashychev, A.; Tegeler, C.; Radbruch, H.; et al. MicroRNA-138 is a potential regulator of memory performance in humans. Front. Hum. Neurosci. 2014, 8, 501. [Google Scholar] [PubMed] [Green Version]
- Gullett, J.M.; Chen, Z.; O’Shea, A.; Akbar, M.; Bian, J.; Rani, A.; Porges, E.C.; Foster, T.C.; Woods, A.J.; Modave, F.; et al. MicroRNA predicts cognitive performance in healthy older adults. Neurobiol. Aging. 2020, 95, 186–194. [Google Scholar] [CrossRef]
- Wei, W.; Wang, Z.Y.; Ma, L.N.; Zhang, T.T.; Cao, Y.; Li, H. MicroRNAs in Alzheimer’s Disease: Function and Potential Applications as Diagnostic Biomarkers. Front. Mol. Neurosci. 2020, 13, 160. [Google Scholar] [CrossRef]
- Ansari, A.; Maffioletti, E.; Milanesi, E.; Marizzoni, M.; Frisoni, G.B.; Blin, O.; Richardson, J.C.; Bordet, R.; Forloni, G.; Gennarelli, M.; et al. miR-146a and miR-181a are involved in the progression of mild cognitive impairment to Alzheimer’s disease. Neurobiol. Aging. 2019, 82, 102–109. [Google Scholar] [CrossRef]
- Satoh, J.; Kino, Y.; Niida, S. MicroRNA-Seq Data Analysis Pipeline to Identify Blood Biomarkers for Alzheimer’s Disease from Public Data. Biomark. Insights 2015, 10, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.G.; Song, J.; Zhang, Y.Q.; Wang, P.C. MicroRNA-193b is a regulator of amyloid precursor protein in the blood and cerebrospinal fluid derived exosomal microRNA-193b is a biomarker of Alzheimer’s disease. Mol. Med. Rep. 2014, 10, 2395–2400. [Google Scholar] [CrossRef] [Green Version]
- Kaalund, S.S.; Venø, M.T.; Bak, M.; Møller, R.S.; Laursen, H.; Madsen, F.; Broholm, H.; Quistorff, B.; Uldall, P.; Tommerup, N.; et al. Aberrant expression of miR-218 and miR-204 in human mesial temporal lobe epilepsy and hippocampal sclerosis-convergence on axonal guidance. Epilepsia 2014, 55, 2017–2027. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.C.; Yeh, T.H.; Chen, R.S.; Chen, H.C.; Huang, Y.Z.; Weng, Y.H.; Cheng, Y.C.; Liu, Y.C.; Cheng, A.J.; Lu, Y.C.; et al. Upregulated Expression of MicroRNA-204-5p Leads to the Death of Dopaminergic Cells by Targeting DYRK1A-Mediated Apoptotic Signaling Cascade. Front. Cell Neurosci. 2019, 13, 399. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Li, C.; Sun, A.; Wang, Y.; Zhou, S. Quantification of microRNA-210 in the cerebrospinal fluid and serum: Implications for Alzheimer’s disease. Exp. Ther. Med. 2015, 9, 1013–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, N.; Kikuchi, M.; Miyashita, A.; Hatsuta, H.; Saito, Y.; Kasuga, K.; Murayama, S.; Ikeuchi, T.; Kuwano, R. Serum microRNA miR-501-3p as a potential biomarker related to the progression of Alzheimer’s disease. Acta Neuropathol. Commun. 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuelezz, N.Z.; Nasr, F.E.; AbdulKader, M.A.; Bassiouny, A.R.; Zaky, A. MicroRNAs as Potential Orchestrators of Alzheimer’s Disease-Related Pathologies: Insights on Current Status and Future Possibilities. Front. Aging Neurosci. 2021, 13, 743573. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Patnaik, S.R.; Kotapati Raghupathy, R.; Al-Mubrad, T.M.; Craft, J.A.; Shu, X. Histological Characterization of the Dicer1 Mutant Zebrafish Retina. J. Ophthalmol. 2015, 2015, 309510. [Google Scholar] [CrossRef]
- Fiorenza, A.; Lopez-Atalaya, J.P.; Rovira, V.; Scandaglia, M.; Geijo-Barrientos, E.; Barco, A. Blocking miRNA Biogenesis in Adult Forebrain Neurons Enhances Seizure Susceptibility, Fear Memory, and Food Intake by Increasing Neuronal Responsiveness. Cereb. Cortex 2016, 26, 1619–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundermeier, T.R.; Sakami, S.; Sahu, B.; Howell, S.J.; Gao, S.; Dong, Z.; Golczak, M.; Maeda, A.; Palczewski, K. MicroRNA-processing Enzymes Are Essential for Survival and Function of Mature Retinal Pigmented Epithelial Cells in Mice. J. Biol. Chem. 2017, 292, 3366–3378. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.Y.; Hernandez-Rapp, J.; Jolivette, F.; Lecours, C.; Bisht, K.; Goupil, C.; Dorval, V.; Parsi, S.; Morin, F.; Planel, E.; et al. miR-132/212 deficiency impairs tau metabolism and promotes pathological aggregation in vivo. Hum. Mol. Genet. 2015, 24, 6721–6735. [Google Scholar] [CrossRef] [Green Version]
- Salta, E.; Sierksma, A.; Vanden Eynden, E.; De Strooper, B. miR-132 loss de-represses ITPKB and aggravates amyloid and TAU pathology in Alzheimer’s brain. EMBO Mol. Med. 2016, 8, 1005–1018. [Google Scholar] [CrossRef]
- Lau, P.; Bossers, K.; Janky, R.; Salta, E.; Frigerio, C.S.; Barbash, S.; Rothman, R.; Sierksma, A.S.; Thathiah, A.; Greenberg, D.; et al. Alteration of the microRNA network during the progression of Alzheimer’s disease. EMBO Mol. Med. 2013, 5, 1613–1634. [Google Scholar] [CrossRef] [Green Version]
- Soreq, H.; Wolf, Y. NeurimmiRs: microRNAs in the neuroimmune interface. Trends Mol. Med. 2011, 17, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Muthusami, S.; Vidya, B.; Shankar, E.M.; Vadivelu, J.; Ramachandran, I.; Stanley, J.A.; Selvamurugan, N. The Functional Significance of Endocrine-immune Interactions in Health and Disease. Curr. Protein. Pept. Sci. 2020, 21, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Licursi, V.; Conte, F.; Fiscon, G.; Paci, P. MIENTURNET: An interactive web tool for microRNA-target enrichment and networkbased analysis. BMC Bioinform. 2019, 20, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.H.; Hwang, W.; Ham, S.; Kim, E.; Altintas, O.; Park, S.; Son, H.G.; Lee, Y.; Lee, D.; Heo, W.D.; et al. A PTEN variant uncouples longevity from impaired fitness in Caenorhabditis elegans with reduced insulin/IGF-1 signaling. Nat. Commun. 2021, 12, 5631. [Google Scholar] [CrossRef]
- Kirstein, A.S.; Kehr, S.; Nebe, M.; Hanschkow, M.; Barth, L.; Lorenz, J.; Penke, M.; Breitfeld, J.; Le Duc, D.; Landgraf, K.; et al. PTEN regulates adipose progenitor cell growth, differentiation, and replicative aging. J. Biol. Chem. 2021, 297, 100968. [Google Scholar] [CrossRef]
- Li, Y.Z.; Di Cristofano, A.; Woo, M. Metabolic Role of PTEN in Insulin Signaling and Resistance. Cold Spring Harb. Perspect. Med. 2020, 10, a036137. [Google Scholar] [CrossRef]
- Ling, H.Y.; Hu, B.; Hu, X.B.; Zhong, J.; Feng, S.D.; Qin, L.; Liu, G.; Wen, G.B.; Liao, D.F. MiRNA-21 reverses high glucose and high insulin induced insulin resistance in 3T3-L1 adipocytes through targeting phosphatase and tensin homologue. Exp. Clin. Endocrinol. Diabetes 2012, 120, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Yuan, R.; Zhang, S.; Yu, J.; Huang, Y.; Lu, D.; Cheng, R.; Huang, S.; Ao, P.; Zheng, S.; Hood, L.; et al. Beyond cancer genes: Colorectal cancer as robust intrinsic states formed by molecular interactions. Open Biol. 2017, 7, 170169. [Google Scholar] [CrossRef] [Green Version]
- Iliopoulos, D.; Jaeger, S.A.; Hirsch, H.A.; Bulyk, M.L.; Struhl, K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol. Cell. 2010, 39, 493–506. [Google Scholar] [CrossRef] [Green Version]
- Nowak, D.G.; Cho, H.; Herzka, T.; Watrud, K.; DeMarco, D.V.; Wang, V.M.; Senturk, S.; Fellmann, C.; Ding, D.; Beinortas, T.; et al. MYC Drives Pten/Trp53-Deficient Proliferation and Metastasis due to IL6 Secretion and AKT Suppression via PHLPP2. Cancer Discov. 2015, 5, 636–651. [Google Scholar] [CrossRef] [Green Version]
- Yan, B.; Zhu, C.D.; Guo, J.T.; Zhao, L.H.; Zhao, J.L. miR-206 regulates the growth of the teleost tilapia (Oreochromis niloticus) through the modulation of IGF-1 gene expression. J. Exp. Biol. 2013, 216, 1265–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Gong, F.; Lu, Z.; Zhu, J.; Yu, Q. Downregulated MiR-206 expression promotes the proliferation and migration of macrophages by regulating IL-17A/REG3A pathway. Eur. J. Inflamm. 2020, 18, 2058739220917490. [Google Scholar] [CrossRef]
- Chai, X.; Si, H.; Song, J.; Chong, Y.; Wang, J.; Zhao, G. miR-486-5p Inhibits Inflammatory Response, Matrix Degradation and Apoptosis of Nucleus Pulposus Cells through Directly Targeting FOXO1 in Intervertebral Disc Degeneration. Cell Physiol. Biochem. 2019, 52, 109–118. [Google Scholar] [PubMed] [Green Version]
- Kim, Y.J.; Hwang, S.H.; Lee, S.Y.; Shin, K.K.; Cho, H.H.; Bae, Y.C.; Jung, J.S. miR-486-5p induces replicative senescence of human adipose tissue-derived mesenchymal stem cells and its expression is controlled by high glucose. Stem Cells Dev. 2012, 21, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Niu, H.; Sha, G.; Zhang, Y.; Liu, P.; Li, Y. Serum SIRT1 Is Associated with Frailty and Adipokines in Older Adults. J. Nutr. Health Aging 2019, 23, 246–250. [Google Scholar] [CrossRef] [PubMed]

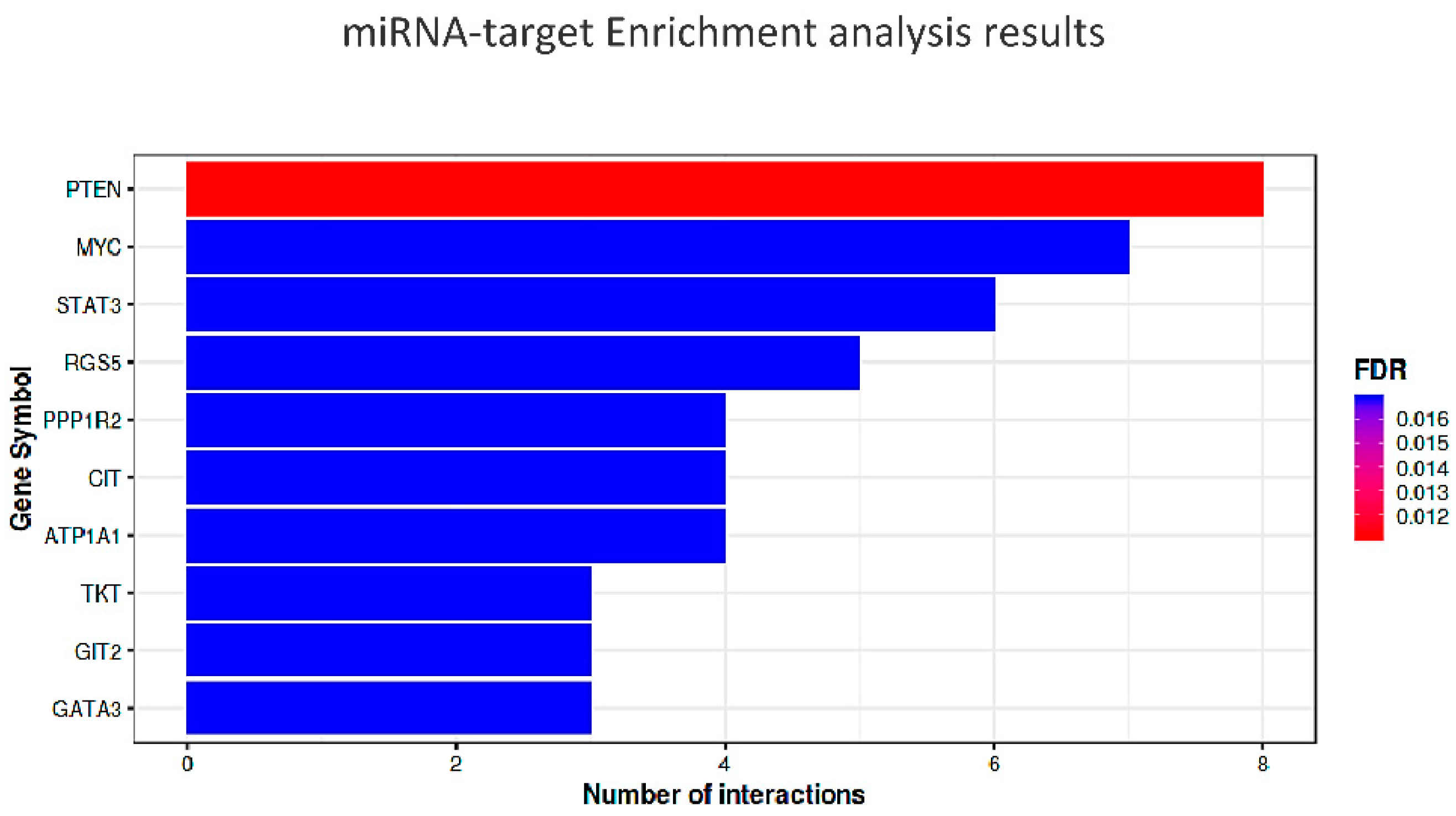
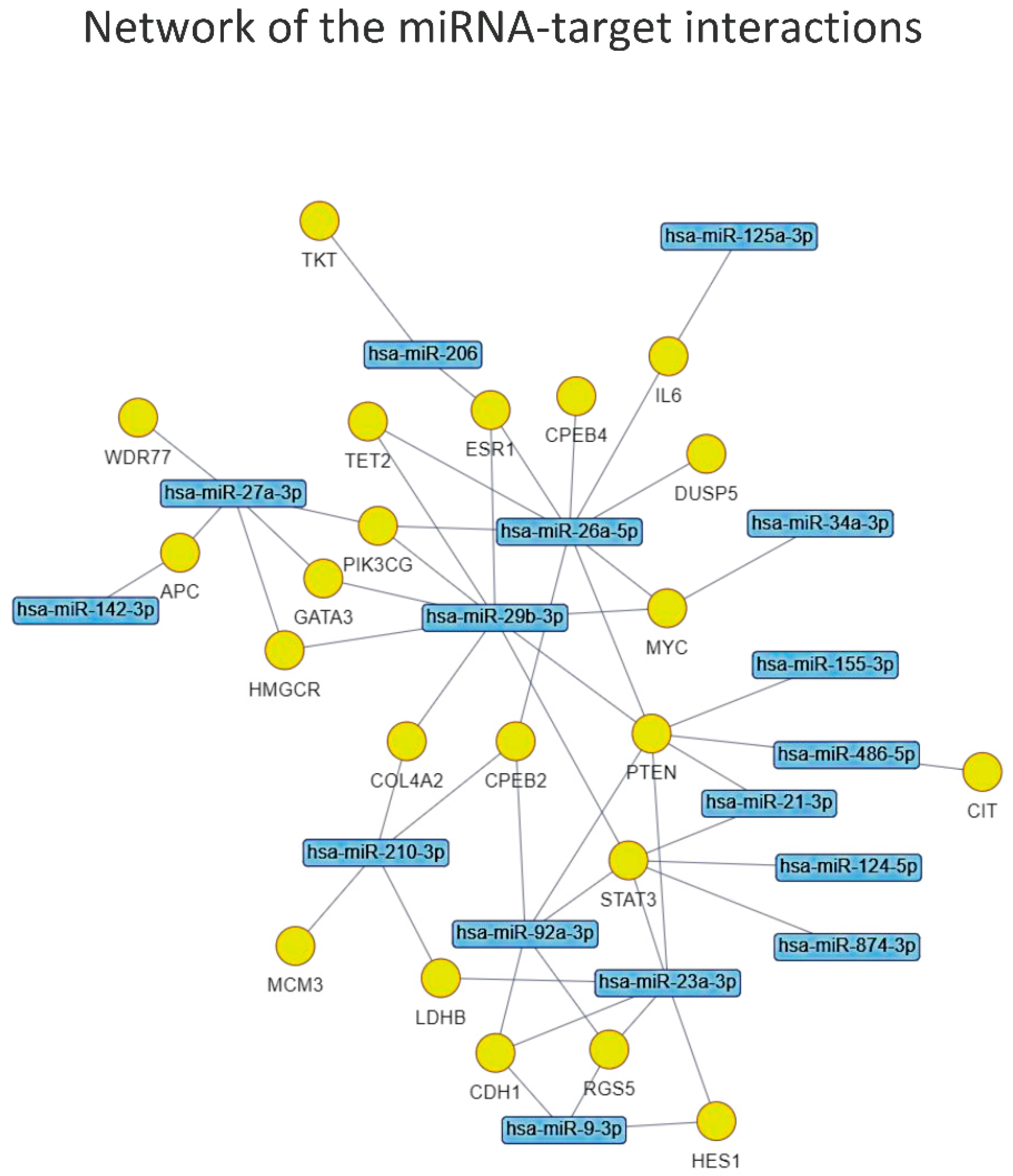
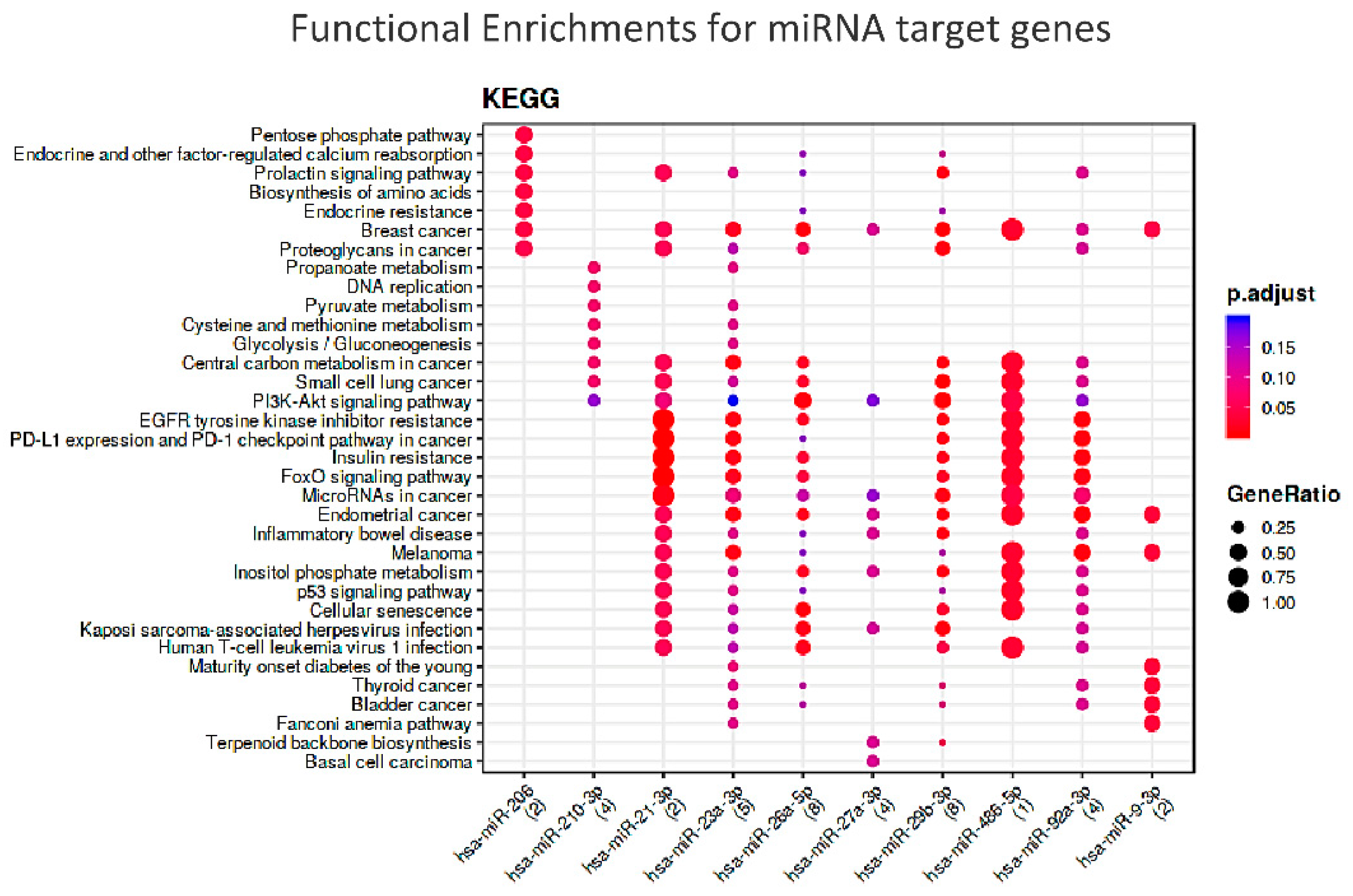
| miRNA-Target Enrichment Results | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | p-Value | FDR | O Rt | Interacting miRs | miR 1 | miR 2 | mioR 3 | miR 4 | miR 5 | miR 6 | miR 7 | miR 8 |
| PTEN | 2.75 × 10−6 | 0.011 | 0.126 | 8 | miR-26a | miR-29b | miR-23a | miR-92a | miR-155 | miR-34a | miR-486 | miR-21 |
| ATP1A1 | 3.02 × 10−5 | 0.016 | 0.048 | 4 | miR-93 | miR-92a | miR-26a | miR-155 | ||||
| CIT | 4.20 × 10−5 | 0.016 | 0.052 | 4 | miR-93 | miR-29b | miR-92a | miR-486 | ||||
| GATA3 | 1.59 × 10−5 | 0.016 | 0.018 | 3 | miR-92a | miR-29b | miR-27a | |||||
| GIT2 | 3.70 × 10−5 | 0.016 | 0.024 | 3 | miR-26a | miR-210 | miR-92a | |||||
| MYC | 2.84 × 10−5 | 0.016 | 0.140 | 7 | miR-26a | miR-30a | miR-92a | miR-23a | miR-125a | miR-34a | miR-29b | |
| PPP1R2 | 4.20 × 10−5 | 0.016 | 0.052 | 4 | miR-210 | miR-142 | miR-30a | miR-34a | ||||
| RGS5 | 3.13 × 10−5 | 0.016 | 0.079 | 5 | miR-142 | miR-92a | miR-124 | miR-9 | miR-23a | |||
| STAT3 | 2.64 × 10−5 | 0.016 | 0.107 | 6 | miR-92a | miR-874 | miR-21 | miR-23a | miR-124 | miR-29b | ||
| TKT | 3.70 × 10−5 | 0.016 | 0.024 | 3 | miR-92a | miR-26a | miR-206 | |||||
| CAMKV | 6.57 × 10−5 | 0.020 | 0.058 | 4 | miR-92a | miR-26a | miR-23a | miR-874 | ||||
| CCL8 | 6.22 × 10−5 | 0.020 | 0.008 | 2 | miR-23a | miR-92a | ||||||
| PDS5B | 6.22 × 10−5 | 0.020 | 0.008 | 2 | miR-27a | miR-92a | ||||||
| CTC1 | 7.08 × 10−5 | 0.020 | 0.127 | 6 | miR-93 | miR-92a | miR-26a | miR-29b | miR-181a | miR-874 | ||
| COL4A2 | 9.55 × 10−5 | 0.024 | 0.032 | 3 | miR-29b | miR-92a | miR-210 | |||||
| HES1 | 9.55 × 10−5 | 0.024 | 0.032 | 3 | miR-23a | miR-92a | miR-9 | |||||
| CPEB4 | 1.07 × 10−4 | 0.025 | 0.101 | 5 | miR-26a | miR-34a | miR-92a | miR-874 | miR-27a | |||
| KIAA1671 | 1.25 × 10−4 | 0.026 | 0.068 | 4 | miR-93 | miR-29b | miR-92a | miR-30a | ||||
| LDHB | 1.23 × 10−4 | 0.026 | 0.0350 | 3 | miR-186 | miR-23a | miR-210 | |||||
| CNOT1 | 1.94 × 10−4 | 0.029 | 0.040 | 3 | miR-93 | miR-92a | miR-23a | |||||
| CSAG1 | 1.85 × 10−4 | 0.029 | 0.012 | 2 | miR-186 | miR-93 | ||||||
| ESR1 | 2.04 × 10−4 | 0.029 | 0.116 | 5 | miR-206 | miR-29b | miR-26a | miR-142 | miR-874 | |||
| FUK | 1.85 × 10−4 | 0.029 | 0.012 | 2 | miR-93 | miR-92a | ||||||
| MCM3 | 1.56 × 10−4 | 0.029 | 0.037 | 3 | miR-93 | miR-92a | miR-210 | |||||
| P2RX7 | 2.15 × 10−4 | 0.029 | 0.078 | 4 | miR-9 | miR-146a | miR-186 | miR-125a | ||||
| PFDN2 | 1.56 × 10−4 | 0.029 | 0.037 | 3 | miR-93 | miR-210 | miR-92a | |||||
| PIK3CG | 2.15 × 10−4 | 0.029 | 0.078 | 4 | miR-29b | miR-27a | miR-142 | miR-26a | ||||
| PSMC3 | 1.85 × 10−4 | 0.029 | 0.01 | 2 | miR-92a | miR-23a | ||||||
| WDR77 | 1.94 × 10−4 | 0.029 | 0.076 | 4 | miR-27a | miR-93 | miR-186 | miR-125a | ||||
| PPARD | 2.38 × 10−4 | 0.031 | 0.043 | 3 | miR-92a | miR-29b | miR-30a | |||||
| KCTD5 | 2.89 × 10−4 | 0.036 | 0.084 | 4 | miR-92a | miR-26a | miR-125a | miR-34a | ||||
| TET2 | 2.87 × 10−4 | 0.036 | 0.045 | 3 | miR-92a | miR-29b | miR-26a | |||||
| APC | 3.43 × 10−4 | 0.037 | 0.048 | 3 | miR-210 | miR-27a | miR-142 | |||||
| CPEB2 | 3.47 × 10−4 | 0.037 | 0.088 | 4 | miR-210 | miR-26a | miR-92a | miR-142 | ||||
| FAU | 3.69 × 10−4 | 0.037 | 0.016 | 2 | miR-92a | miR-23a | ||||||
| GPD1L | 3.69 × 10−4 | 0.037 | 0.016 | 2 | miR-210 | miR-142 | ||||||
| IRAK1 | 3.43 × 10−4 | 0.037 | 0.048 | 3 | miR-93 | miR-92a | miR-142 | |||||
| PHB | 3.69 × 10−4 | 0.037 | 0.016 | 2 | miR-27a | miR-26a | ||||||
| SCAF8 | 3.69 × 10−4 | 0.037 | 0.016 | 2 | miR-29b | miR-92a | ||||||
| VMAC | 3.43 × 10−4 | 0.037 | 0.048 | 3 | miR-146a | miR-186 | miR-125a | |||||
| HMGCR | 4.05 × 10−4 | 0.039 | 0.051 | 3 | miR-92a | miR-29b | miR-27a | |||||
| HECTD1 | 4.74 × 10−4 | 0.043 | 0.053 | 3 | miR-210 | miR-142 | miR-92a | |||||
| TBC1D16 | 4.74 × 10−4 | 0.043 | 0.053 | 3 | miR-26a | miR-186 | miR-210 | |||||
| TUT1 | 4.74 × 10−4 | 0.043 | 0.053 | 3 | miR-93 | miR-92a | miR-26a | |||||
| ABCB9 | 6.12 × 10−4 | 0.046 | 0.020 | 2 | miR-210 | miR-26a | ||||||
| CDH1 | 6.34 × 10−4 | 0.046 | 0.059 | 3 | miR-92a | miR-23a | miR-9 | |||||
| DUSP5 | 5.51 × 10−4 | 0.046 | 0.056 | 3 | miR-27a | miR-92a | miR-26a | |||||
| IL6 | 6.34 × 10−4 | 0.046 | 0.059 | 3 | miR-142 | miR-26a | miR-125a | |||||
| INPP5A | 6.12 × 10−4 | 0.046 | 0.020 | 2 | miR-210 | miR-142 | ||||||
| KIF20A | 6.12 × 10−4 | 0.046 | 0.020 | 2 | miR-92a | miR-23a | ||||||
| NEK6 | 6.12 × 10−4 | 0.046 | 0.020 | 2 | miR-92a | miR-23a | ||||||
| PDE4B | 6.12 × 10−4 | 0.046 | 0.020 | 2 | miR-26a | miR-34a | ||||||
| SOCS6 | 6.34 × 10−4 | 0.046 | 0.059 | 3 | miR-23a | miR-27a | miR-142 | |||||
| UBE2R2 | 6.12 × 10−4 | 0.046 | 0.020 | 2 | miR-93 | miR-92a | ||||||
| ZNF618 | 6.34 × 10−4 | 0.046 | 0.059 | 3 | miR-21 | miR-27a | miR-210 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dato, S.; Crocco, P.; Iannone, F.; Passarino, G.; Rose, G. Biomarkers of Frailty: miRNAs as Common Signatures of Impairment in Cognitive and Physical Domains. Biology 2022, 11, 1151. https://doi.org/10.3390/biology11081151
Dato S, Crocco P, Iannone F, Passarino G, Rose G. Biomarkers of Frailty: miRNAs as Common Signatures of Impairment in Cognitive and Physical Domains. Biology. 2022; 11(8):1151. https://doi.org/10.3390/biology11081151
Chicago/Turabian StyleDato, Serena, Paolina Crocco, Francesca Iannone, Giuseppe Passarino, and Giuseppina Rose. 2022. "Biomarkers of Frailty: miRNAs as Common Signatures of Impairment in Cognitive and Physical Domains" Biology 11, no. 8: 1151. https://doi.org/10.3390/biology11081151
APA StyleDato, S., Crocco, P., Iannone, F., Passarino, G., & Rose, G. (2022). Biomarkers of Frailty: miRNAs as Common Signatures of Impairment in Cognitive and Physical Domains. Biology, 11(8), 1151. https://doi.org/10.3390/biology11081151








