Observation of Palatal Wound Healing Process Following Various Degrees of Mucoperiosteal and Bone Trauma in a Young Rat Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
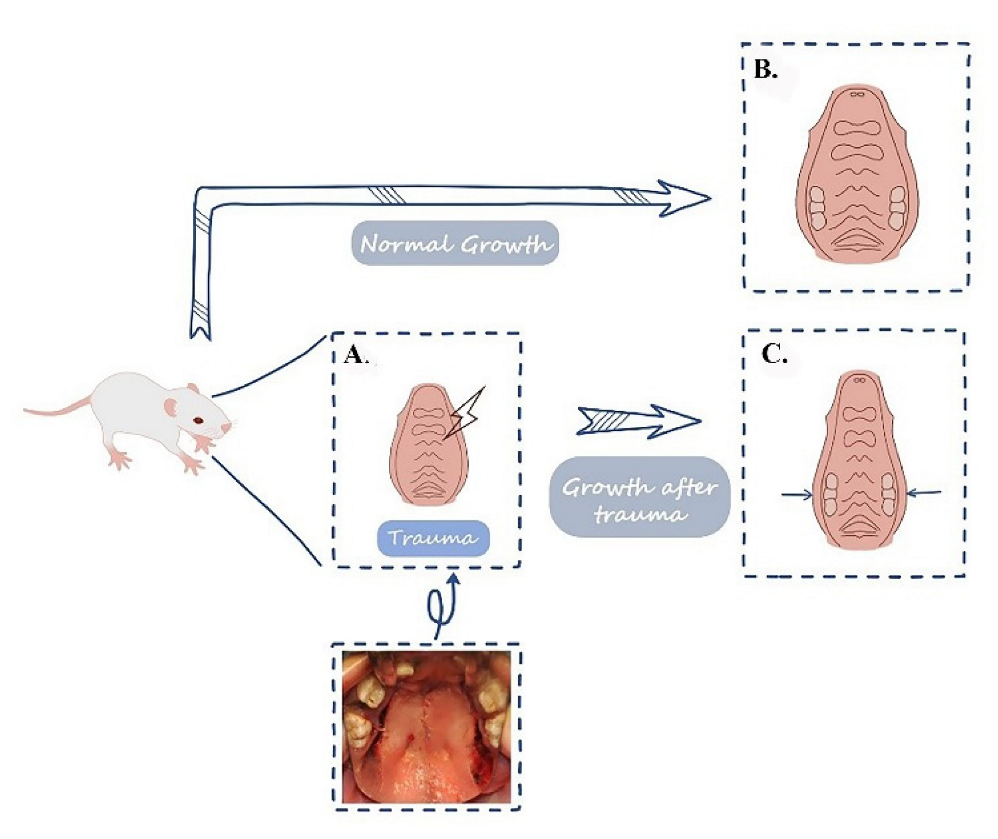
2.1. Animal Model Establishment
2.2. Various Degrees of Palatal Traumas
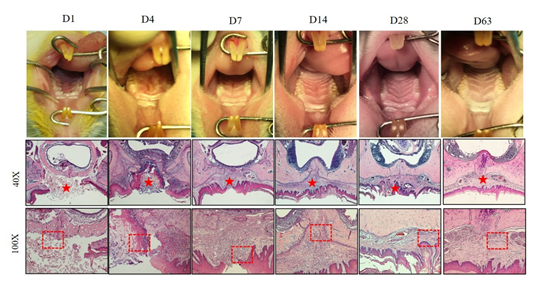
2.3. Sample Harvesting
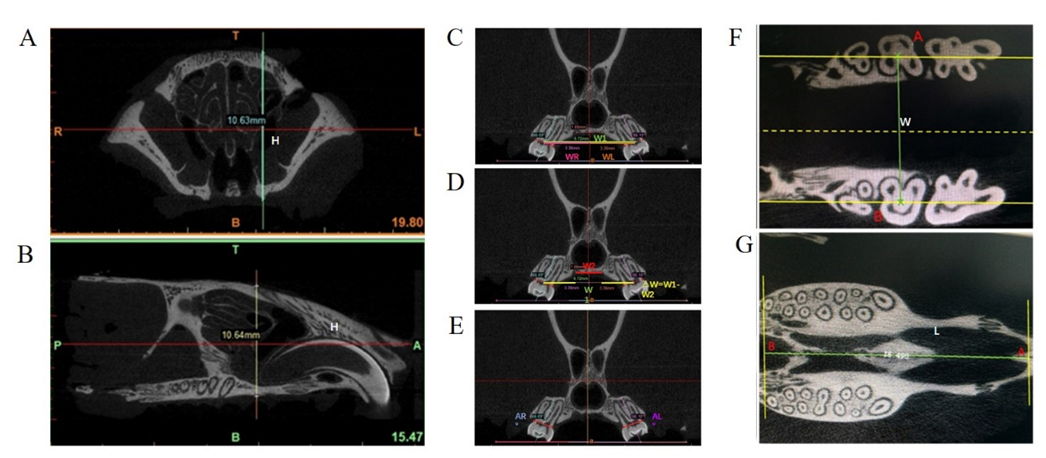
2.4. Micro-CT Scanning and Measurement of the Maxillofacial Growth
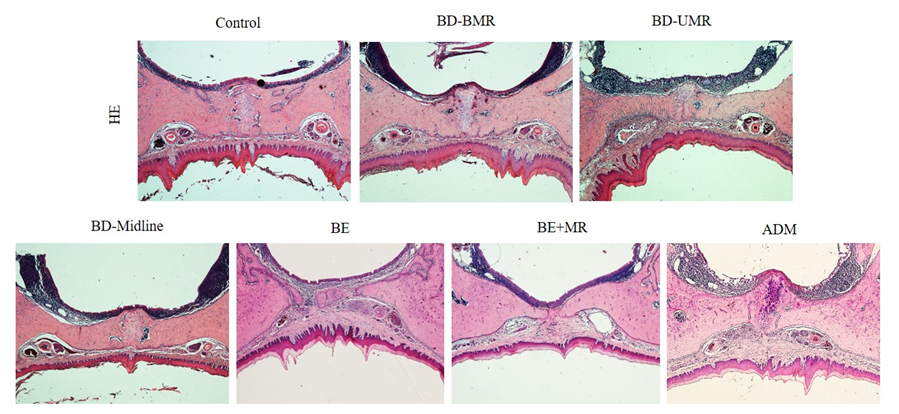
2.5. Histological Analysis of the Palate
2.6. Statistical Analyses
3. Results
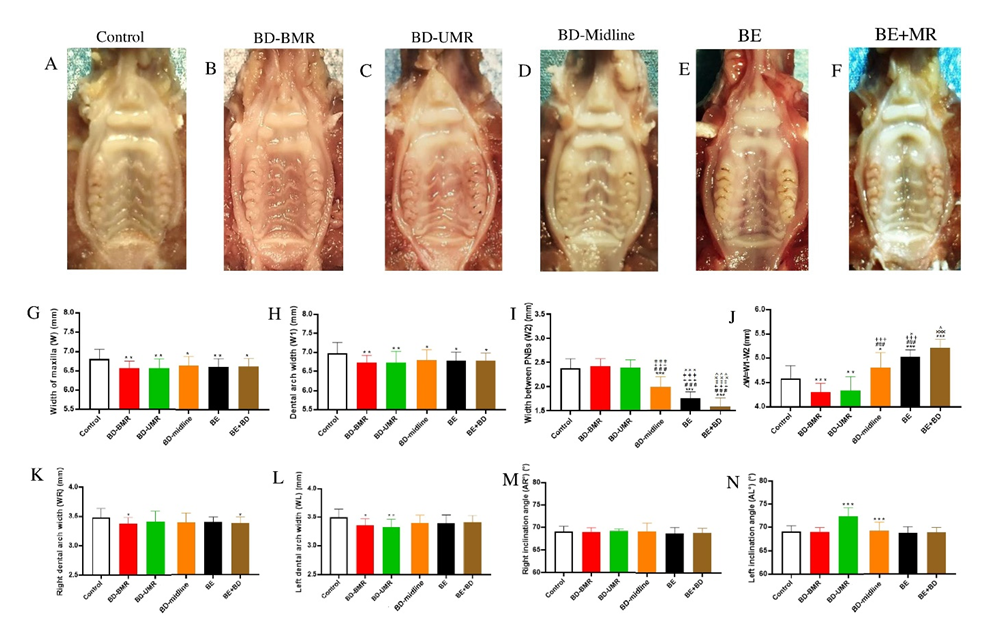
3.1. The Impact of Different Degrees of Palatal Trauma on the Maxillofacial Growth
3.1.1. Width of the Maxilla (W) and Dental Arch Width (W1)
3.1.2. Width between PNBs (W2) and ΔW = W1−W2
3.1.3. Right Dental Arch Width (WR), Left Dental Arch Width (WL), Right Inclination Angle (AR°), and Left Inclination Angle (AL°)
3.2. The Application of ADM to Treat the Bone Denudation Caused by Mucoperiosteum Removal on the Midline of the Palate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, K.; Deng, M.; Naluai-Cecchini, T.; Glass, I.A.; Cox, T.C. Differences in Oral Structure and Tissue Interactions during Mouse vs. Human Palatogenesis: Implications for the Translation of Findings from Mice. Front. Physiol. 2017, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.O.; Jiang, R. Palatogenesis: Morphogenetic and molecular mechanisms of secondary palate development. Development 2012, 139, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, H.; Wen, J.; Zhou, J.; Liu, Y.; Cheng, B.; Chen, X.; Wei, J. Nomograms forecasting long-term overall and cancer-specific survival of patients with oral squamous cell carcinoma. Cancer Med. 2018, 7, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.W.H.; van Lanschot, C.G.F.; Aaboubout, Y.; de Ridder, M.; Hegt, V.N.; Barroso, E.M.; Meeuwis, C.A.; Sewnaik, A.; Hardillo, J.A.; Monserez, D.; et al. Intraoperative Assessment of the Resection Specimen Facilitates Achievement of Adequate Margins in Oral Carcinoma. Front. Oncol. 2020, 10, 614593. [Google Scholar] [CrossRef]
- Davies, A.; Davies, A.; Wren, Y.; Deacon, S.; Cobb, A.R.M.; Chummun, S. Exploring the Relationship Between Palatal Cleft Type and Width with the Use of Relieving Incisions in Primary Repair. Cleft Palate Craniofacial J. 2022, 59, 659–668. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yabuki, Y.; Kokubo, K.; Yasumura, K.; Hirakawa, T.; Fukawa, T.; Yamamoto, K. A predictor of a postoperative fistula after double-opposing Z-plasty in bilateral cleft lip and palate patients. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2022, 75, 1931–1936. [Google Scholar] [CrossRef]
- Othieno, F.; Tatum, S.A. Prevention and management of oronasal fistulas. Curr. Opin. Otolaryngol. Head Neck Surg. 2021, 29, 320–326. [Google Scholar] [CrossRef]
- Shetty, V.; Sreekumar, C.; Patteta, N.K.; Bahl, D.; Sailer, H.F. Correlation between surgical protocols for palatoplasty and midfacial growth in cleft lip and palate patients: A long-term, single centre study. J. Cranioaxillofac. Surg. 2021, 49, 1010–1019. [Google Scholar] [CrossRef]
- Shetty, V.; Patteta, N.K.; Yadav, A.; Bahl, D.; Sailer, H.F. Does the Timing of 1-Stage Palatoplasty with Radical Muscle Dissection Effect Long-Term Midface Growth? A Single-Center Retrospective Analysis. Cleft Palate Craniofacial J. 2022, 59, 239–245. [Google Scholar] [CrossRef]
- Kattan, A.E.; Abdulghafour, M.; Ahmed, B.A.; Gelidan, A.G.; Alhumsi, T.R. The use of acellular dermal matrix in palatoplasty to decrease the rate of postoperative oronasal fistula. JPMA J. Pak. Med. Assoc. 2022, 72, 337–341. [Google Scholar]
- Boháč, M.; Danišovič, Ľ.; Koller, J.; Dragúňová, J.; Varga, I. What happens to an acellular dermal matrix after implantation in the human body? A histological and electron microscopic study. Eur. J. Histochem. EJH 2018, 62, 2873. [Google Scholar] [CrossRef]
- Kirschner, R.E.; Cabling, D.S.; Slemp, A.E.; Siddiqi, F.; LaRossa, D.D.; Losee, J.E. Repair of oronasal fistulae with acellular dermal matrices. Plast. Reconstr. Surg. 2006, 118, 1431–1440. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Chen, Y.; Zhang, Y. Conditional deletion of Bmp2 in cranial neural crest cells recapitulates Pierre Robin sequence in mice. Cell Tissue Res. 2019, 376, 199–210. [Google Scholar] [CrossRef]
- Meng, T.; Shi, B.; Huang, X.; Zheng, Q.; Wang, Y.; Wu, M.; Lu, Y.; Li, S. Roles of different areas of palatine bone denudation on growth and development of the maxilla and dental arch: An experimental study. J. Craniofacial Surg. 2007, 18, 391–398. [Google Scholar] [CrossRef][Green Version]
- Wong, L.S.; Lu, T.C.; Hang, D.T.D.; Chen, P.K. The Impact of Facial Growth in Unilateral Cleft Lip and Palate Treated with 2 Different Protocols. Ann. Plast. Surg. 2020, 84, 541–544. [Google Scholar] [CrossRef]
- Smith, A.; Ray, M.; Chaiet, S. Primary Palate Trauma in Patients Presenting to US Emergency Departments, 2006–2010. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 244–251. [Google Scholar] [CrossRef]
- Friede, H.; Johanson, B. A follow-up study of cleft children treated with vomer flap as part of a three-stage soft tissue surgical procedure. Facial morphology and dental occlusion. Scand. J. Plast. Reconstr. Surg. 1977, 11, 45–57. [Google Scholar]
- Marshall, C.D.; Hu, M.S.; Leavitt, T.; Barnes, L.A.; Lorenz, H.P.; Longaker, M.T. Cutaneous Scarring: Basic Science, Current Treatments, and Future Directions. Adv. Wound Care 2018, 7, 29–45. [Google Scholar] [CrossRef]
- Srivastava, A.; DeSagun, E.Z.; Jennings, L.J.; Sethi, S.; Phuangsab, A.; Hanumadass, M.; Reyes, H.M.; Walter, R.J. Use of porcine acellular dermal matrix as a dermal substitute in rats. Ann. Surg. 2001, 233, 400–408. [Google Scholar] [CrossRef]
- Chaushu, L.; Atzil, S.; Vered, M.; Chaushu, G.; Matalon, S.; Weinberg, E. Age-Related Palatal Wound Healing: An Experimental In Vivo Study. Biology 2021, 10, 240. [Google Scholar] [CrossRef]
- Ophof, R.; Maltha, J.C.; Von den Hoff, J.W.; Kuijpers-Jagtman, A.M. Histologic evaluation of skin-derived and collagen-based substrates implanted in palatal wounds. Wound Repair Regen. 2004, 12, 528–538. [Google Scholar] [CrossRef]
- De Virgilio, A.; Costantino, A.; Russo, E.; Ferreli, F.; Pellini, R.; Petruzzi, G.; Zocchi, J.; Spriano, G.; Mercante, G. Different Surgical Strategies in the Prevention of Frey Syndrome: A Systematic Review and Meta-analysis. Laryngoscope 2021, 131, 1761–1768. [Google Scholar] [CrossRef]
- Happe, A.; Debring, L.; Schmidt, A.; Fehmer, V.; Neugebauer, J. Immediate Implant Placement in Conjunction with Acellular Dermal Matrix or Connective Tissue Graft: A Randomized Controlled Clinical Volumetric Study. Int. J. Periodontics Restor. Dent. 2022, 42, 381–390. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, S.; Sakran, K.A.; Yin, J.; Lan, M.; Yang, C.; Wang, Y.; Zeng, N.; Huang, H.; Shi, B. Observation of Palatal Wound Healing Process Following Various Degrees of Mucoperiosteal and Bone Trauma in a Young Rat Model. Biology 2022, 11, 1142. https://doi.org/10.3390/biology11081142
Liu Y, Zhang S, Sakran KA, Yin J, Lan M, Yang C, Wang Y, Zeng N, Huang H, Shi B. Observation of Palatal Wound Healing Process Following Various Degrees of Mucoperiosteal and Bone Trauma in a Young Rat Model. Biology. 2022; 11(8):1142. https://doi.org/10.3390/biology11081142
Chicago/Turabian StyleLiu, Yingmeng, Shiming Zhang, Karim Ahmed Sakran, Jiayi Yin, Min Lan, Chao Yang, Yan Wang, Ni Zeng, Hanyao Huang, and Bing Shi. 2022. "Observation of Palatal Wound Healing Process Following Various Degrees of Mucoperiosteal and Bone Trauma in a Young Rat Model" Biology 11, no. 8: 1142. https://doi.org/10.3390/biology11081142
APA StyleLiu, Y., Zhang, S., Sakran, K. A., Yin, J., Lan, M., Yang, C., Wang, Y., Zeng, N., Huang, H., & Shi, B. (2022). Observation of Palatal Wound Healing Process Following Various Degrees of Mucoperiosteal and Bone Trauma in a Young Rat Model. Biology, 11(8), 1142. https://doi.org/10.3390/biology11081142





