Drivers of the Ectoparasite Community and Co-Infection Patterns in Rural and Urban Burrowing Owls
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
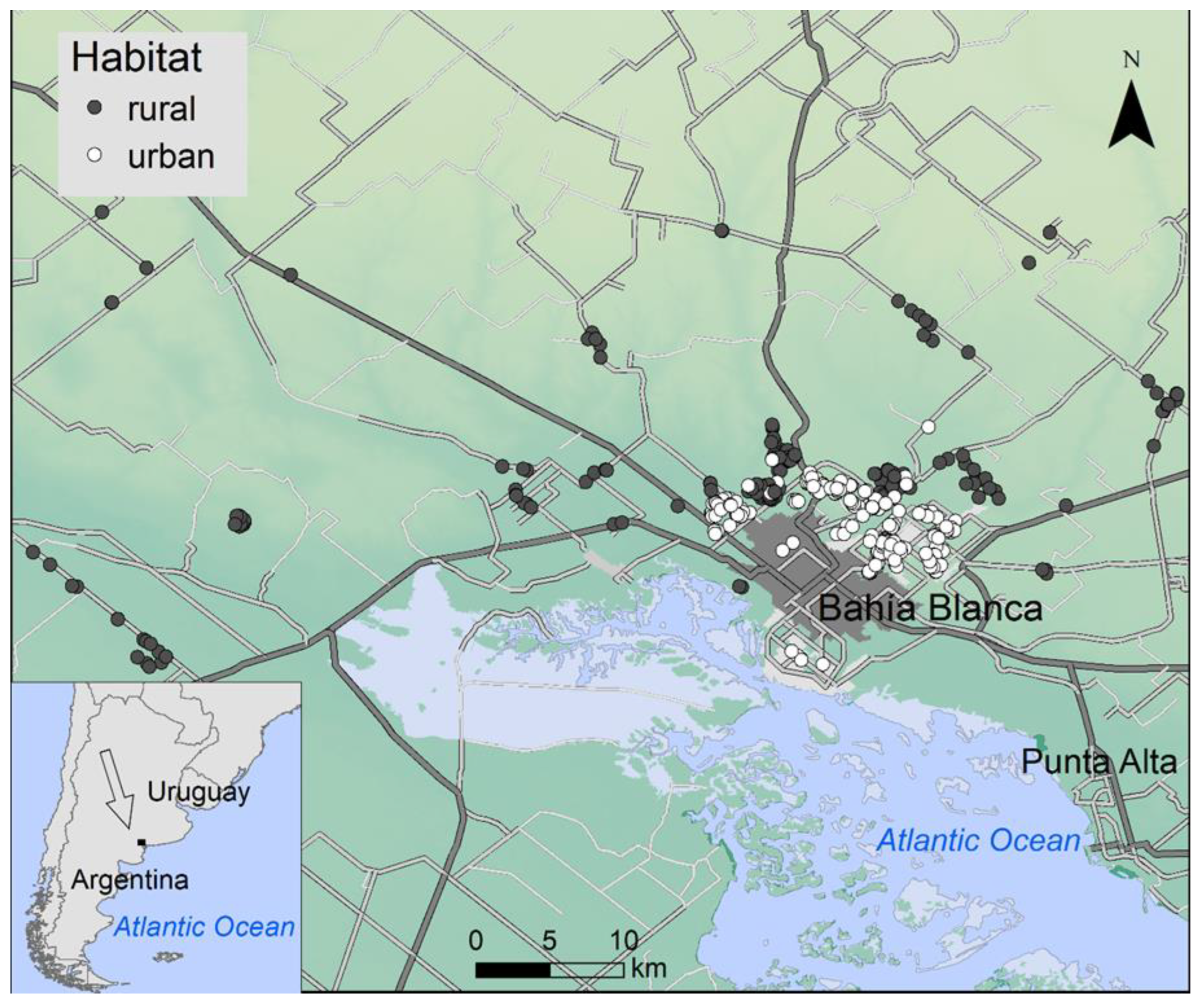
2.1. Study Area and Species
2.2. Sample Collection
2.3. Statistical Analysis
3. Results
3.1. Ectoparasite Community
3.2. Co-Infection Pattern
4. Discussion
4.1. Ectoparasite Community and Co-Infection Pattern
4.2. Individual and Environmental Factors Explaining Infection Pattern
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krasnov, B.R.; Poulin, R.; Mouillot, D. Scale-dependence of phylogenetic signal in ecological traits of ectoparasites. Ecography 2011, 34, 114–122. [Google Scholar] [CrossRef]
- Dáttilo, W.; Barrozo-Chávez, N.; Lira-Noriega, A.; Guevara, R.; Villalobos, F.; Santiago-Alarcon, D.; Neves, F.S.; Izzo, T.; Ribeiro, S.P. Species-level drivers of mammalian ectoparasite faunas. J. Anim. Ecol. 2020, 89, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, A.; Bagge, A.M.; Valtonen, E.T. Interspecific and intraspecific variations in the monogenean communities of fish: A question of the study scale? Parasitology 2007, 134, 1237–1242. [Google Scholar] [CrossRef]
- Pedersen, A.B.; Fenton, A. Emphasizing the ecology in parasite community ecology. Trends Ecol. Evol. 2007, 22, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Veiga, J. Determinants of the Host-Parasite Relationship in a System Formed by a Cavity-Nesting Bird and its Ectoparasites in an Arid Ecosystem. Ph.D. Dissertation, Granada University, Granada, Spain, 2020. [Google Scholar]
- Poulin, R. Evolutionary Ecology of Parasites: From Individuals to Communities; Chapman and Hall: London, UK, 1998. [Google Scholar]
- Morand, S. Wormy world: Comparative tests of theoretical hypotheses on parasite species richness. In Evolutionary Biology of Host-Parasite Relationships: Theory Meets Reality; Poulin, R., Morand, S., Skorping, A., Eds.; Elsevier Science: Amsterdam, The Netherlands; New York, NY, USA, 2000; pp. 63–79. [Google Scholar]
- Poulin, R.; Morand, S. Parasite Biodiversity; Smithsonian Books: Washington, DC, USA, 2004. [Google Scholar]
- Freed, L.A.; Medeiros, M.C.; Bodner, G.R. Explosive increase in ectoparasites in Hawaiian forest birds. J. Parasitol. 2008, 94, 1009–1021. [Google Scholar] [CrossRef]
- Chakraborty, D.; Reddy, M.; Tiwari, S.; Umapathy, G. Land use change increases wildlife parasite diversity in Anamalai Hills, Western Ghats, India. Sci Rep. 2019, 9, 11975. [Google Scholar] [CrossRef]
- Millins, C.; Dickinson, E.R.; Isakovic, P.; Gilbert, L.; Wojciechoswka, A.; Paterson, V.; Tao, F.; Jahn, M.; Kilbride, E.; Birtles, R.; et al. Landscape structure affects the prevalence and distribution of a tick-borne zoonotic pathogen. Parasit. Vectors 2018, 11, 621. [Google Scholar] [CrossRef]
- Pérez, J.M.; Meneguz, P.G.; Dematteis, A.; Rossi, L.; Serrano, E. Parasites and conservation biology: The ‘ibex-ecosystem’. Biodivers. Conserv. 2006, 15, 2033–2047. [Google Scholar] [CrossRef]
- Bandilla, M.; Valtonen, E.T.; Suomalainen, L.R.; Aphalo, P.J.; Hakalahti, A.T. A link between ectoparasite infection and susceptibilityto bacterial disease in rainbow trout. Int. J. Parasitol. 2006, 36, 987–991. [Google Scholar] [CrossRef]
- Dobson, R.J.; Barnes, E.H. Interaction between Ostertagia circumcincta and Haemonchus contortus infection in young lambs. Int. J. Parasitol. 1995, 25, 495–501. [Google Scholar] [CrossRef]
- Johnson, P.T.; Buller, I.D. Parasite competition hidden by correlated co-infection: Using surveys and experiments to understand parasite interactions. Ecology 2011, 92, 535–541. [Google Scholar] [CrossRef]
- Cox, F.E.G. Concomitant infections, parasites and immune responses. Parasitology 2001, 122, S23–S28. [Google Scholar] [CrossRef]
- Lello, J.; Norman, R.A.; Boag, B.; Hudson, P.J.; Fenton, A. Pathogen interactions, population cycles, and phase shifts. Am. Nat. 2008, 171, 176–182. [Google Scholar] [CrossRef]
- Ng, Y.L.; Hamdan, N.E.S.; Tuen, A.A.; Mohd-Azlan, J.; Chong, Y.L. Co-infections of ectoparasite species in synanthropic rodents of western Sarawak, Malaysian Borneo. Trop. Biomed. 2017, 34, 723–731. [Google Scholar]
- Hoffmann, S.; Horak, I.G.; Bennett, N.C.; Lutermann, H. Evidence for interspecific interactions in the ectoparasite infracommunity of a wild mammal. Parasit. Vectors 2016, 9, 58. [Google Scholar] [CrossRef]
- Karvonen, A.; Sepälä, O.; Valtonen, E.T. Host immunization shapes interspecies associations in trematode parasites. J. Anim. Ecol. 2009, 78, 945–952. [Google Scholar] [CrossRef]
- Jolles, A.E.; Ezenwa, V.O.; Etienne, R.S.; Turner, W.C.; Olff, H. Interactions between macroparasites and microparasites drive infection patterns in free-ranging African buffalo. Ecology 2008, 89, 2239–2250. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Faeth, S.H.; Bang, C.; Saari, S. Urban biodiversity: Patterns and mechanisms. Ann. N. Y. Acad. Sci. 2011, 1223, 69–81. [Google Scholar] [CrossRef]
- Sol, D.; González-Lagos, C.; Moreira, D.; Maspons, J.; Lapiedra, O. Urbanisation tolerance and the loss of avian diversity. Ecol. Lett. 2014, 17, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Aronson, M.F.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. Royal Soc. B 2014, 281, 20133330. [Google Scholar] [CrossRef] [PubMed]
- Rebolo-Ifrán, N.; Tella, J.L.; Carrete, M. Urban conservation hotspots: Predation release allows the grassland-specialist burrowing owl to perform better in the city. Sci. Rep. 2017, 7, 3527. [Google Scholar] [CrossRef] [PubMed]
- Lepczyk, C.A.; Aronson, M.F.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S. Biodiversity in the city: Fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation. BioScience 2017, 67, 799–807. [Google Scholar] [CrossRef]
- Møller, A.P.; Diaz, M.; Flensted-Jensen, E.; Grim, T.; Ibáñez-Álamo, J.D.; Jokimäki, J.; Mänd, R.; Markó, G.; Tryjanowski, P. High urban population density of birds reflects their timing of urbanization. Oecologia 2012, 170, 867–875. [Google Scholar] [CrossRef]
- Thomas, J.P.; Jung, T.S. Life in a northern town: Rural villages in the boreal forest are islands of habitat for an endangered bat. Ecosphere 2019, 10, e02563. [Google Scholar] [CrossRef]
- Shwartz, A.; Strubbe, D.; Butler, C.J.; Matthysen, E.; Kark, S. The effect of enemy-release and climate conditions on invasive birds: A regional test using the rose-ringed parakeet (Psittacula krameri) as a case study. Divers. Distrib. 2009, 15, 310–318. [Google Scholar] [CrossRef]
- Oro, D.; Genovart, M.; Tavecchia, G.; Fowler, M.S.; Martínez-Abraín, A. Ecological and evolutionary implications of food subsidies from humans. Ecol. Lett. 2013, 16, 1501–1514. [Google Scholar] [CrossRef]
- de Araujo, G.M.; Peres, C.A.; Baccaro, F.B.; Guerta, R.S. Urban waste disposal explains the distribution of Black Vultures (Coragyps atratus) in an Amazonian metropolis: Management implications for birdstrikes and urban planning. PeerJ 2018, 6, e5491. [Google Scholar] [CrossRef]
- Stracey, C.M.; Robinson, S.K. Are urban habitats ecological traps for a native songbird? Season-long productivity, apparent survival, and site fidelity in urban and rural habitats. J. Avian Biol. 2012, 43, 50–60. [Google Scholar] [CrossRef]
- Rebolo-Ifrán, N.; Carrete, M.; Sanz-Aguilar, A.; Rodríguez-Martínez, S.; Cabezas, S.; Marchant, T.A.; Bortolotti, G.R.; Tella, J.L. Links between fear of humans, stress and survival support a non-random distribution of birds among urban and rural habitats. Sci. Rep. 2015, 5, 13723. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.; Palma, A.; Sanz-Aguilar, A.; Tella, J.L.; Carrete, M. Sex, personality and conspecific density influence natal dispersal with lifetime fitness consequences in urban and rural burrowing owls. PLoS ONE 2020, 15, e0226089. [Google Scholar] [CrossRef]
- Suárez-Rodríguez, M.; López-Rull, I.; Macías Garcia, C. Incorporation of cigarette butts into nests reduces nest ectoparasite load in urban birds: New ingredients for an old recipe? Biol. Lett. 2013, 9, 20120931. [Google Scholar] [CrossRef]
- Wemer, L.; Hegemann, A.; Isaksson, C.; Nebel, C.; Kleindorfer, S.; Gamauf, A.; Adrion, M.; Sumasgutner, P. Reduced ectoparasite load, body mass and blood haemolysis in Eurasian kestrels (Falco tinnunculus) along an urban–rural gradient. Sci. Nat. 2021, 108, 42. [Google Scholar] [CrossRef]
- Bauerová, P.; Krajzingrová, T.; Těšický, M.; Velová, H.; Hraníček, J.; Musil, S.; Svobodová, J.; Albrecht, T.; Vinkler, M. Longitudinally monitored lifetime changes in blood heavy metal concentrations and their health effects in urban birds. Sci. Total Environ. 2020, 723, 138002. [Google Scholar] [CrossRef]
- Phillips, J.N.; Gentry, K.E.; Luther, D.A.; Derryberry, E.P. Surviving in the city: Higher apparent survival for urban birds but worse condition on noisy territories. Ecosphere 2018, 9, e02440. [Google Scholar] [CrossRef]
- Carrete, M.; Tella, J.L. Inter-individual variability in fear of humans and relative brain size of the species are related to contemporary urban invasion in birds. PLoS ONE 2011, 6, e18859. [Google Scholar] [CrossRef]
- Palma, A.; Blas, J.; Tella, J.L.; Cabezas, S.; Marchant, T.A.; Carrete, M. Differences in adrenocortical responses between rural and urban burrowing owls: Poorly-known underlying mechanisms and their implications for conservation. Conserv. Physiol. 2020, 8, coaa054. [Google Scholar] [CrossRef]
- Werner, C.S.; Nunn, C.L. Effect of urban habitat use on parasitism in mammals: A meta-analysis. Proc. R. Soc. B 2020, 287, 20200397. [Google Scholar] [CrossRef]
- Delgado-V., C.A.; French, K. Parasite–bird interactions in urban areas: Current evidence and emerging questions. Landsc. Urban Plan. 2012, 105, 5–14. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, S.; Carrete, M.; Roques, S.; Rebolo-Ifrán, N.; Tella, J.L. High urban breeding densities do not disrupt genetic monogamy in a bird species. PLoS ONE 2014, 9, e91314. [Google Scholar] [CrossRef] [PubMed]
- Collias, N.E.; Collias, E.C. Nest Building Behavior in Birds; Princeton University Press: Princeton, NJ, USA, 1984. [Google Scholar]
- Machicote, M.; Branch, L.; Villarreal, D. Burrowing owls and burrowing mammals: Are ecosystem engineers interchangeable as facilitators? Oikos 2004, 106, 527–535. [Google Scholar] [CrossRef]
- Luna, Á.; Lois, N.A.; Rodríguez-Martinez, S.; Palma, A.; Sanz-Aguilar, A.; Tella, J.L.; Carrete, M. Urban life promotes delayed dispersal and family living in a non-social bird species. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- York, M.M.; Rosenberg, D.K.; Sturm, K.K. Diet and food-niche breadth of burrowing owls (Athene cunicularia) in the Imperial Valley, California. West. N. Am. Nat. 2002, 62, 280–287. [Google Scholar]
- Nabte, M.J.; Pardiñas, U.J.F.; Saba, S.L. The diet of the burrowing owl, Athene cunicularia, in the arid lands of northeastern Patagonia, Argentina. J. Arid Environ. 2008, 72, 1526–1530. [Google Scholar] [CrossRef]
- Romero-Vidal, P. Within-Population Trophic Specialization is Unrelated to Urbanization in Burrowing Owls. Master’s Thesis, Universidad Pablo de Olavide, Sevilla, Spain, 2017. [Google Scholar]
- Smith, B.W.; Belthoff, J.R. Identification of ectoparasites on burrowing owls in southwestern Idaho. J. Raptor Res. 2001, 35, 159–161. [Google Scholar]
- De Coster, G.; De Neve, L.; Martín-Gálvez, D.; Therry, L.; Lens, L. Variation in innate immunity in relation to ectoparasite load, age and season: A field experiment in great tits (Parus major). J. Exp. Biol. 2010, 17, 3012–3018. [Google Scholar] [CrossRef]
- Altizer, S.; Nunn, C.L.; Thrall, P.H.; Gittleman, J.L.; Antonovics, J.; Cunningham, A.A.; Dobson, A.P.; Ezenwa, V.; Jones, K.E.; Pedersen, A.B.; et al. Social organization and parasite risk in mammals: Integrating theory and empirical studies. Ann. Rev. Ecol. Evol. Syst. 2003, 34, 517–547. [Google Scholar] [CrossRef]
- Wigglesworth, V.B. Insect Physiology; Chapman and Hall: London, UK, 1984. [Google Scholar]
- Ehlers, J.; Poppert, S.; Ratovonamana, R.Y.; Ganzhorn, J.U.; Tappe, D.; Krüger, A. Ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop. 2019, 196, 83–92. [Google Scholar] [CrossRef]
- Klein, S.L. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004, 26, 247–264. [Google Scholar] [CrossRef]
- Owen, J.P.; Nelson, A.C.; Clayton, D.H. Ecological immunology of bird-ectoprasites systems. Trends Parasitol. 2010, 26, 530–539. [Google Scholar] [CrossRef]
- McCoy, K.D.; Boulinier, T.; Schjørring, S.; Michalakis, Y. Local adaptation of an ectoparasite Ixodes uriae to its seabird host. Evol. Ecol. Res. 2002, 4, 441–446. [Google Scholar]
- Carrete, M.; Tella, J.L. High individual consistency in fear of humans throughout the adult lifespan of rural and urban burrowing owls. Sci. Rep. 2013, 3, 3524. [Google Scholar] [CrossRef]
- Palma, R. Slide-mounting of lice: A detailed description of the Canada balsam technique. N. Z. Entomol. 1978, 6, 432–436. [Google Scholar] [CrossRef]
- Price, R.D.; Beer, J.R. The species of Colpocephalum (Mallophaga: Menoponidae) known to occur on the Strigiformes. J. Kansas Entomol. Soc. 1963, 36, 58–64. [Google Scholar]
- Clay, T. A Key to the genera of the Menoponidae (Amblycera: Mallophaga: Insecta). Bull. Br. Mus. (Nat. Hist) Entomol. 1969, 24, 3–26. [Google Scholar]
- Clayton, D.H.; Price, R.D. Taxonomy of the Strigiphilus cursitans group (Iscnhocera: Philopteridae), parasites of owls (Strigiformes). Ann. Entomol. Soc. Am. 1984, 77, 340–363. [Google Scholar] [CrossRef]
- Price, R.D.; Hellenthal, R.A.; Palma, R.L.; Johnson, K.P.; Clayton, D.H. The chewing lice: World Checklist and Biological Overview; Illinois Natural History Survey Special Publication: Champaign, IL, USA, 2003. [Google Scholar]
- Linardi, P.M.; Guimarães, L.R. Systematic review of genera and subgenera of Rhopalopsyllinae (Siphonaptera: Rhopalopsyllidae) by phenetic and cladistic methods. J. Med. Entomol. 1993, 30, 161–170. [Google Scholar] [CrossRef]
- Acosta, R.; Morrone, J.J. Clave ilustrada para la identificación de los taxones supraespecíficos de siphonaptera de México. Acta Zool. Mex. 2003, 89, 39–53. [Google Scholar] [CrossRef]
- Lareschi, M.; Sánchez, J.; Autino, A. A review of the fleas (Insecta: Siphonaptera) from Argentina. Zootaxa 2016, 4103, 239–258. [Google Scholar] [CrossRef]
- Krantz, G.W.; Walter, D.E. A manual of Acarology, 3rd ed.; Texas Tech University Press: Lubbock, TX, USA, 2009. [Google Scholar]
- Margolis, L.; Esch, G.W.; Holmes, J.C.; Kuris, A.M.; Schad, G.A. The use of ecological terms in parasitology (report of an ad hoc committee of the American Society of Parasitologists). J. Parasitol. 1982, 68, 131–133. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Hui, F.K.C.; Taskinen, S.; Pledger, S.; Foster, S.D.; Warton, D.I. Model-based approaches to unconstrained ordination. Methods Ecol. Evol. 2015, 6, 399–411. [Google Scholar] [CrossRef]
- Warton, D.I.; Blanchet, F.G.; O’Hara, R.O.O.; Taskinen, S.; Walker, S.C.; Hui, F.K.C. So many variables: Joint modeling in community ecology. Trends Ecol. Evol. 2015, 30, 766–779. [Google Scholar] [CrossRef]
- Niku, J.; Brooks, W.; Herliansyah, R.; Hui, F.K.C.; Taskinen, S.; Warton, D.I. gllvm: Generalized Linear Latent Variable Models. R Package Version 2017, 1, 38. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.r-project.org/. (accessed on 15 December 2021).
- Kristensen, K.; Nielsen, A.; Berg, C.W.; Skaug, H.; Bell, B.M. TMB: Automatic Differentiation and Laplace Approximation. J. Stat. Softw. 2016, 70, 1–21. [Google Scholar] [CrossRef]
- Niku, J.; Hui, F.K.C.; Taskinen, S.; Warton, D.I. Gllvm-Fast analysis of multivariate abundance data with Generalized Linear Latent Variable Models in R. 10. Methods Ecol. Evol. 2019, 10, 2173–2182. [Google Scholar] [CrossRef]
- Gaussen, H.; Bagnouls, F. Saison Seche et Indice Xerotermique; Université de Toulouse, Faculté des Sciences: Toulouse, France, 1953. [Google Scholar]
- Dunn, P.K.; Smyth, G.K. Randomized quantile residuals. J. Comput. Graph. Stat. 1996, 5, 236–244. [Google Scholar]
- Skoruppa, M.K.; Pearce, B.; Woodin, M.C.; Hickman, G.C. Ectoparasites of burrowing owls (Athene cunicularia hypugaea) wintering in southern Texas. Tex. J. Sci. 2006, 58, 73–79. [Google Scholar]
- Belthoff, J.R.; Bernhardt, S.A.; Ball, C.L.; Gregg, M.; Johnson, D.H.; Ketterling, R.; Price, E.; Tinker, J.K. Burrowing owls, Pulex irritans, and plague. Vector Borne Zoonotic Dis. 2015, 15, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Catanach, T.A.; Valim, M.P.; Weckstein, J.D.; Johnson, K.P. Cophylogenetic analysis of lice in the Colpocephalum complex (Phthiraptera: Amblycera). Zool. Scr. 2018, 47, 72–83. [Google Scholar] [CrossRef]
- Coulombe, H.N. Behavior and population ecology of the burrowing owl, Speotyto cunicularia, in the Imperial Valley of California. Condor 1971, 73, 162–176. [Google Scholar] [CrossRef]
- Baladrón, A.V.; Cavalli, M.; Bó, M.S.; Isacch, J.P. Nest dimensions, burrow-lining, and decoration behavior of burrowing owls in the Pampas. J. Raptor Res. 2021, 55, 255–266. [Google Scholar] [CrossRef]
- Clayton, D.H.; Lee, P.L.M.; Tompkins, D.M.; Brodie, E.D., III. Reciprocal natural selection on host-parasite phenotypes. Am. Nat. 1999, 154, 261–270. [Google Scholar] [CrossRef]
- Dik, B. Chewing-lice species (Phthiraptera) found on domestic and wild birds in Turkey. Turk. Parazitol. Derg. 2010, 34, 55–60. [Google Scholar]
- Whatton, J.F.; Eckerlin, R.P. Snowy Owl Brings New Record of Chewing Louse to Virginia. Banisteria 2016, 46, 29–30. [Google Scholar]
- Faraji, F.; Halliday, B. Five new species of mites (Acari: Laelapidae) associated with large Australian cockroaches (Blattodea: Blaberidae). Int. J. Acarol. 2009, 35, 245–264. [Google Scholar] [CrossRef]
- Martins-Hatano, F.; Raices, D.S.; Gazeta, G.S.; Serra-Freire, N.M.; Gettinger, D.; Bergallo, H.G. Community composition of laelapine mites (Acari: Laelapidae) associated with the nests and fur of Cerradomys subflavus (Wagner, 1842). J. Nat. Hist. 2011, 45, 1679–1688. [Google Scholar] [CrossRef]
- Bush, S.E.; Malenke, J.R. Host defence mediates interspecific competition in ectoparasites. J. Anim. Ecol. 2008, 77, 558–564. [Google Scholar] [CrossRef]
- Eswarappa, S.M.; Estrela, S.; Brown, S.P. Within-host dynamics of multi-species infections: Facilitation, competition and virulence. PLoS ONE 2012, 7, e38730. [Google Scholar] [CrossRef] [PubMed]
- Moyer, B.R.; Gardiner, D.W.; Clayton, D.H. Impact of feather molt on ectoparasites: Looks can be deceiving. Oecologia 2002, 131, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Doña, J.; Proctor, H.; Serrano, D.; Johnson, K.P.; Oddy-van Oploo, A.; Huguet-Tapia, J.C.; Ascunce, M.S.; Jovani, R. Feather mites play a role in cleaning host feathers: New insights from DNA metabarcoding and microscopy. Mol. Ecol. 2019, 28, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Liberman, V.; Khokhlova, I.S.; Degen, A.A.; Krasnov, B.R. Reproductive consequences of host age in a desert flea. Parasitology 2013, 140, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L. Behavior and ecology of burrowing owls in the Oakland municipal airport. Condor 1971, 73, 177–192. [Google Scholar] [CrossRef]
- Forsman, E.D.; Wight, H.M. Allopreening in owls: What are its functions? Auk 1979, 96, 525–531. [Google Scholar]
- Václav, R.; Calero-Torralbo, M.A.; Valera, F. Ectoparasite load is linked to ontogeny and cell-mediated immunity in an avian host system with pronounced hatching asynchrony. Biol. J. Linn. Soc. 2008, 94, 463–473. [Google Scholar] [CrossRef]
- Bize, P.; Roulin, A.; Tella, J.L.; Richner, H. Female-biased mortality in experimentally parasitized Alpine swift Apus melba nestlings. Funct. Ecol. 2005, 19, 405–413. [Google Scholar] [CrossRef]
- Lewis, S.; Roberts, G.; Harris, M.P.; Prigmore, C.; Wanless, S. Fitness increases with partner and neighbour allopreening. Biol. Let. 2007, 3, 386–389. [Google Scholar] [CrossRef]
- Radford, A.N.; Plessis, M.A.D. Dual function of allopreening in the cooperatively breeding green woodhoopoe, Phoeniculus purpureus. Behav. Ecol. Sociobiol. 2006, 61, 221–230. [Google Scholar] [CrossRef]
- Kenny, E.; Birkhead, T.R.; Green, J.P. Allopreening in birds is associated with parental cooperation over offspring care and stable pair bonds across years. Behav. Ecol. 2017, 28, 1142–1148. [Google Scholar] [CrossRef]
- Gill, S.A. Strategic use of allopreening in family-living wrens. Behav. Ecol. Sociobiol. 2012, 66, 757–763. [Google Scholar]
- Teunissen, N.; Kingma, S.A.; Hall, M.L.; Aranzamendi, N.H.; Komdeur, J.; Peters, A. More than kin: Subordinates foster strong bonds with relatives and potential mates in a social bird. Behav. Ecol. 2018, 29, 1316–1324. [Google Scholar] [CrossRef]
- Møller, A.P.; Sorci, G.; Erritzøe, J. Sexual dimorphism in immune defense. Am. Nat. 1998, 152, 605–619. [Google Scholar] [CrossRef]
- Moreno, J.; Potti, J.; Yorio, P.; Borboroglu, P.G. Sex differences in cell-mediated immunity in the Magellanic penguin Spheniscus magellanicus. Ann. Zool. Fenn. 2001, 38, 111–116. [Google Scholar]
- Moreno-Rueda, G. Body-mass-dependent trade-off between immune response and uropygial gland size in house sparrows Passer domesticus. J. Avian Biol. 2015, 46, 40–45. [Google Scholar] [CrossRef]
- Valdebenito, J.O.; Halimubieke, N.; Lendvai, A.Z.; Figuerola, J.; Eichhorn, G.; Székely, T. Seasonal variation in sex-specific immunity in wild birds. Sci. Rep. 2021, 11, 1349. [Google Scholar] [CrossRef]
- Ronget, V.; Gaillard, J.M.; Coulson, T.; Garratt, M.; Gueyffier, F.; Lega, J.C.; Lemaître, J.F. Causes and consequences of variation in offspring body mass: Meta-analyses in birds and mammals. Biol. Rev. 2018, 93, 1–27. [Google Scholar] [CrossRef]
- Montalti, D.; Salibián, A. Uropygial gland size and avian habitat. Ornitol. Neotrop. 2000, 11, 297–306. [Google Scholar]
- Møller, A.P.; Rózsa, L. Parasite biodiversity and host defenses: Chewing lice and immune response of their avian hosts. Oecologia 2005, 142, 169–176. [Google Scholar] [CrossRef]
- Ancillotto, L.; Studer, V.; Howard, T.; Smith, V.S.; McAlister, E.; Beccaloni, J.; Manzia, F.; Renzopaoli, F.; Bosso, L.; Russo, D.; et al. Environmental drivers of parasite load and species richness in introduced parakeets in an urban landscape. Parasitol. Res. 2018, 117, 3591–3599. [Google Scholar] [CrossRef]
- Fernández-Gómez, L.; Palma, A.; Luna, Á.; Romero-Vidal, P.; Carrete, M. Diet Differences for Rural and Urban Burrowing Owls in Micromammals Composition. Bachelor’s Thesis, Environmental Sciences. Universidad Pablo de Olavide, Seville, Spain, 2019. [Google Scholar]
- Mappes, T.; Mappes, J.; Kotiaho, J. Ectoparasites, nest site choice, and breeding success in the pied flycatcher. Oecologia 1994, 98, 147–149. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Khokhlova, I.S.; Fielden, L.J.; Burdelova, N.V. Development rates of two Xenopsilla flea species in relation to air temperature and humidity. Med. Vet. Entomol. 2001, 15, 249–258. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Khokhlova, I.S.; Fielden, L.J.; Burdelova, N.V. Effect of air temperature and humidity on the survival of pre-imaginal stages of two flea species (Siphonaptera: Pulicidae). J. Med. Entomol. 2001, 38, 629–637. [Google Scholar] [CrossRef]
- Heeb, P.; Kölliker, M.; Richner, H. Bird-ectoparasite interactions, nest humidity, and ectoparasite community structure. Ecology 2000, 81, 958–968. [Google Scholar]
- Dearborn, D.C.; Kark, S. Motivations for conserving urban biodiversity. Conserv. Biol. 2010, 24, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Alfaleh, F.A.; Alyousif, M.S.; Al-Quraishy, S.; Al-Shawa, Y.R. Eimeria biarmicus sp.n. (Apicomplexa: Eimeriidae) infecting falcons from the genus Falco in Saudi Arabia. Parasitol. Res. 2012, 110, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- de Andery, D.A.; Ferreira Junior, F.; Araújo, A.V.; da Vilela, D.R.; Marques, M.; Marin, S.Y.; Horta, R.S.; Ortiz, M.C.; Resende, J.S.; Martins, N.S. Health assessment of raptors in triage in Belo Horizonte, MG, Brazil. Rev. Bras. Cien. Avic. 2013, 15, 247–256. [Google Scholar] [CrossRef]
- Atyeo, W.T.; Braasch, N.L.; Norman, L. The feather mite genus Proctophyllodes (Sarcoptiformes: Proctophyllodidae). Bull. Univ. Nebr. State Mus. 1996, 39, 1–354. [Google Scholar]
- Brown, J.H. Sylvatic plague: The recovery of fleas from the burrowing owl and its burrow in a plague area in Alberta. Entomol. News 1994, 55, 15–18. [Google Scholar]
- Buscher, H.N. Echinoparyphium speotyto sp. n. (Trematoda: Echinostomatidae) from the burrowing owl in Oklahoma, with a discussion of the genus Echinoparyphium. J. Parasitol. 1978, 64, 52–58. [Google Scholar] [CrossRef]
- Daciuk, J.; Cicchino, A.C.; Mauri, R.; Capri, J.J. Notas faunísticas y bioecológicas de Península Valdes y Patagonia. XXIV. Artrópodos ectoparásitos de mamíferos y aves colectados en la Península Valdes y alrededores (Provincia de Chubut, Argentina). Physis C 1981, 39, 41–48. [Google Scholar]
- da Silva, A.S.; Zanette, R.A.; Lara, V.M.; Gressler, L.T.; Carregaro, A.B.; Santurio, J.M.; Monteiro, S.G. Gastrointestinal parasites of owls (Strigiformes) kept in captivity in the Southern region of Brazil. Parasitol. Res. 2009, 104, 485–487. [Google Scholar] [CrossRef]
- de Almeida Pedroso, L.G.; Hernandes, F.A. New records of feather mites (Acariformes: Astigmata) from non- passerine birds (Aves) in Brazil. Check List. Biodiv. Data J. 2016, 12, 2000. [Google Scholar]
- Drago, F.B.; Lunaschi, L.I.; Cabrera, N.E.; Barbieri, L. Helminth parasites of four species of strigiform birds from Central and Northeastern Argentina. Rev. Arg. Parasitol. 2015, 4, 15–23. [Google Scholar]
- Dusek, R.J.; Iko, W.M.; Hofmeister, E.K. Occurrence of West Nile virus infection in raptors at the Salton Sea, California. J. Wildl. Dis. 2010, 46, 889–895. [Google Scholar] [CrossRef]
- Fontanelli-Vaz, F.; Teixeira, V.N. New records of three hippoboscid species on newly captured birds from nature in Paraná, Brazil. Rev. Bras. Parasitol. Vet. 2016, 25, 501–503. [Google Scholar]
- Franson, J.C. Protozoal hepatitis in a western burrowing owl (Athene cunicularia hypugaea). Southw. Nat. 2017, 62, 75–77. [Google Scholar] [CrossRef]
- González-Acuña, D.; Rodrigo Muñoz, C.; Cicchino, A.; Figueroa, R.A. Lice of Chilean owls: A first description. J. Raptor Res. 2006, 40, 301–302. [Google Scholar] [CrossRef]
- Goulart, T.M. Ácaros Asociados à “Avoante” Zenaida Auriculata (Des Murs, 1847) na Regiao de Campinas-SP, Brasil. Tesis de Maestría; Universidade Estadual de Campinas: Campinas, Brazil, 2011. [Google Scholar]
- Graciolli, G.; Barros de Carvalho, C.J. Hippoboscidae (Diptera, Hippoboscoidea) no Estado do Paraná, Brasil: Chaves de identificação, hospedeiros e distribuição geográfica. Rev. Bras. Zool. 2003, 20, 667–674. [Google Scholar] [CrossRef]
- Hernández-Orts, J.S.; Pinacho-Pinacho, C.D.; García-Varela, M.; Kostadinova, A. Maritrema corai n. sp. (Digenea: Microphallidae) from the white ibis Eudocimus albus (Linnaeus) (Aves: Threskiornithidae) in Mexico. Parasitol. Res. 2015, 115, 547–559. [Google Scholar] [CrossRef]
- Jellison, W.L. The burrowing owl as a host of the argasid tick, Ornithodoros parkeri. Public Health Rep. 1940, 55, 206–208. [Google Scholar] [CrossRef]
- Kinsella, J.M.; Foster, G.W.; Forrester, D.J. Parasitic helminths of five species of owls from Florida, U.S.A. Comp. Parasitol. 2001, 68, 130–134. [Google Scholar]
- Kurey, W.J. Ectoparasitic Acarina (Analgoidea) from Non-Passeriform Birds of North America. Master’s Thesis, Youngstown State University, Youngstown, OH, USA, 1976. [Google Scholar]
- Liébana, M.S.; Santillán, M.Á.; Cicchino, A.C.; Sarasola, J.H.; Martínez, P.; Cabezas, S.; Bó, M.S. Ectoparasites in free-ranging American kestrels in Argentina: Implications for the transmission of viral diseases. J. Raptor Res. 2011, 45, 335–341. [Google Scholar] [CrossRef]
- Llyuh, M.P.; Goncharov, A.I. On the fleas IDS. Caucasian. Ornithol. Gaz. 2005, 17, 5–8. (In Bielorussian) [Google Scholar]
- Loomis, R.B. The chigger mites of Kansas (Acarina, Trombiculidae). Univ. Kansas Sci. Bull. 1956, 37, 1195–1442. [Google Scholar]
- Marietto-Gonçalves, G.A.; Martins, T.F.; Andreatti Filho, R.L. Chewing lice (Insecta, Phthiraptera) parasitizing birds in Botucatu, SP, Brazil. Rev. Bras. C. Vet. 2012, 19, 206–212. [Google Scholar] [CrossRef]
- Mascarenhas, C.S.; Bernardon, F.F.; Gastal, S.; Müller, G. Checklist of the parasitic nasal mites of birds in Brazil. Syst. App. Acarol. 2018, 23, 1672–1692. [Google Scholar] [CrossRef]
- Mueller, N.S.; Mueller, H.C.; Berger, D.D. Host records and phenology of louse-flies on Wisconsin birds. Trans. Wis. Acad. Sci. Arts Lett. 1969, 57, 189–207. [Google Scholar]
- Need, J.T.; Dale, W.E.; Keirans, J.E.; Dasch, G.A. Annotated list of ticks (Acari: Ixodidae, Argasidae) reported in Peru: Distribution, hosts, and bibliography. J. Med. Entomol. 1991, 28, 590–597. [Google Scholar] [CrossRef]
- Pence, D.B.; Bergan, J.F. Hypopi (Acari: Hypoderatidae) from Owls (Aves: Strigiformes: Strigidae). J. Med. Entomol. 1996, 33, 828–834. [Google Scholar] [CrossRef]
- Philips, J.R. A review and checklist of the parasitic mites (Acarina) of the Falconiformes and Strigiformes. J. Raptor Res. 2000, 34, 210–231. [Google Scholar]
- Redig, P.T.; Cooper, J.E.; Remple, D.; Hunter, D.B. Raptor Biomedicine; Minneapolis, University of Minnesota Press: Minneapolis, MN, USA, 1993. [Google Scholar]
- Riding, C.S. Effects of Old Nest Material on Occupancy and Reuse of Artificial Burrows, and Breeding Dispersal by Burrowing Owls (Athene cunicularia) in Southwestern Idaho. Ph.D. Thesis, Boise State University, Boise, ID, USA, 2010. [Google Scholar]
- Schwan, T.G. Nosopsyllus fasciatus parasiting house mice on Southeast Farallon Island, California (Siphonaptera: Ceratophyllidae). Pan-Pac. Entomol. 1984, 60, 345–349. [Google Scholar]
- Seery, B.D.; Biggins, D.E.; Montenieri, J.A.; Enscore, R.E.; Tandia, D.T.; Gage, K.L. Treatment of black-tailed prairie dog burrows with deltamethrin to control fleas (Insecta: Siphonaptera) and plague. J. Med. Entomol. 2003, 40, 718–722. [Google Scholar] [CrossRef]
- Silva, T.M.; Sakai-Okamoto, A.; Firmino da Silva, L.A.; Domeneghetti-Smaniotto, B.; Da Silva, R.J.; Andreatti-Filho, R.L. New record of Pelecitus sp. (Nematoda, Onchocercidae) as a parasite of Athene cunicularia (Strigiformes, Strigidae) in southeastern Brazil. Rev. Bras. Parasitol. Vet. 2014, 23, 274–275. [Google Scholar]
- Skoracki, M.; Unsoeld, M.; Marciniak, N.; Sikora, B. Diversity of quill mites of the family Syringophilidae (Acari: Prostigmata) parasitizing owls (Aves: Strigiformes) with remarks on the host-parasite relationships. J. Med. Entomol. 2016, 53, 815–826. [Google Scholar] [CrossRef]
- Smith, B.W. Nest-Site Selection, Ectoparasites, and Mitigation Techniques: Studies of Burrowing Owls and Artificial Burrow Systems in Southwestern Idaho. Master’S Thesis, Boise State University, Boise, ID, USA, 1999. [Google Scholar]
- Thompson, G.B. XXX.-A list of the type-hosts of the Mallophaga and the lice described from them. Ann. Mag. Nat. Hist. 1950, 3, 365–382. [Google Scholar] [CrossRef]
- Vitaliano, S.N.; Soares, H.S.; Pena, H.F.J.; Dubey, J.P.; Gennari, S.M. Serologic evidence of Toxoplasma gondii infection in wild birds and mammals from southeast Brazil. J. Zoo Wildl. Med. 2014, 45, 197–199. [Google Scholar] [CrossRef]


| Burrowing Owls | Mites | Lice | Fleas | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Prevalance | Mean ± SD | Range | Prevalence | Mean ± SD | Range | Prevalence | Mean ± SD | Range | |
| Global | 869 | 1.75% | 2.60 ± 4.31 | 1–18 | 8.76% | 3.01 ± 3.12 | 1–17 | 3.50% | 1.70 ± 1.02 | 1–5 |
| Males | 237 | 0.70% | 4.00 ± 6.87 | 1–18 | 4.21% | 3.69 ± 4.01 | 1–17 | 1.52% | 1.77 ± 1.24 | 1–5 |
| Females | 301 | 1.05% | 1.67 ± 0.71 | 1–3 | 4.56% | 2.38 ± 1.82 | 1–8 | 1.99% | 1.65 ± 0.86 | 1–3 |
| SU | 331 | 1.61% | 1.00 ± 0.00 | 1–1 | 4.94% | 2.60 ± 2.32 | 1–9 | 2.30% | 1.95 ± 1.13 | 1–5 |
| Chicks | 380 | 1.52% | 2.69 ± 4.63 | 1–8 | 5.02% | 3.37 ± 3.59 | 1–17 | 2.22% | 1.95 ± 1.13 | 1–5 |
| Adults | 489 | 0.23% | 2.00 ± 1.41 | 1–3 | 3.74% | 2.53 ± 2.32 | 1–9 | 1.29% | 1.27 ± 0.65 | 1–3 |
| Rural | 387 | 0.35% | 1.67 ± 1.15 | 1–3 | 4.09% | 3.43 ± 3.97 | 1–17 | 2.69% | 1.65 ± 1.07 | 1–5 |
| Urban | 482 | 1.40% | 2.83 ± 4.80 | 1–18 | 4.67% | 2.65 ± 2.11 | 1–8 | 0.82% | 1.86 ± 0.90 | 1–3 |
| 2016–2017 | 413 | 3.39% | 2.71 ± 4.44 | 1–18 | 9.20% | 3.26 ± 3.87 | 1–17 | 3.87% | 1.81 ± 1.16 | 1–5 |
| 2017–2018 | 456 | 2.19% | 1.00 ± 0.00 | 1–1 | 7.89% | 2.58 ± 1.87 | 1–8 | 2.41% | 1.72 ± 0.90 | 1–3 |
| Parasite | Component | Estimate | Std. Err. | z Value | p-Value |
|---|---|---|---|---|---|
| Lice | Age | −0.9389 | 0.0879 | −10.682 | <2 × 10−16 *** |
| Sex | −0.4041 | 0.0745 | −5.426 | 5.76 × 10−8 *** | |
| Habitat | 0.3051 | 0.1598 | 1.909 | 0.0562 * | |
| Nind/nest | −0.3988 | 0.0022 | −179.647 | <2 × 10−16 *** | |
| Aridity | 0.1138 | 0.1752 | 0.650 | 0.51577 | |
| Body mass | 0.0004 | 0.0007 | 0.603 | 0.54620 | |
| Age:Sex | 0.6932 | 0.1091 | 6.356 | 2.07 × 10−10 *** | |
| Fleas | Age | −1.1969 | 0.0683 | −17.514 | <2 × 10−16 *** |
| Sex | −0.0727 | 0.0125 | −5.818 | 5.94 × 10−9 *** | |
| Habitat | 3.4717 | 0.0805 | 43.115 | <2 × 10−16 *** | |
| Nind/nest | 0.3418 | 0.0469 | 7.287 | 3.17 × 10−13 *** | |
| Aridity | 0.9466 | 0.1409 | 6.718 | 1.84 × 10−11 *** | |
| Body mass | −0.0088 | 0.0008 | −11.187 | <2 × 10−16 *** | |
| Age:Sex | −0.2483 | 0.0045 | −54.809 | <2 × 10−16 *** | |
| Mites | Age | −2.6764 | 0.1419 | −18.862 | <2 × 10−16 *** |
| Sex | 1.8086 | 0.1631 | 11.090 | <2 × 10−16 *** | |
| Habitat | −1.2081 | 0.0805 | −15.003 | <2 × 10−16 *** | |
| Nind/nest | 0.1976 | 0.0761 | 2.593 | 0.00951 ** | |
| Aridity | −0.8150 | 0.0948 | −8.600 | <2 × 10−16 *** | |
| Body mass | −0.0103 | 0.0010 | −9.963 | <2 × 10−16 *** | |
| Age:Sex | −0.6797 | 0.0830 | −8.190 | 2.62 × 10−16 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáez-Ventura, Á.; López-Montoya, A.J.; Luna, Á.; Romero-Vidal, P.; Palma, A.; Tella, J.L.; Carrete, M.; Liébanas, G.M.; Pérez, J.M. Drivers of the Ectoparasite Community and Co-Infection Patterns in Rural and Urban Burrowing Owls. Biology 2022, 11, 1141. https://doi.org/10.3390/biology11081141
Sáez-Ventura Á, López-Montoya AJ, Luna Á, Romero-Vidal P, Palma A, Tella JL, Carrete M, Liébanas GM, Pérez JM. Drivers of the Ectoparasite Community and Co-Infection Patterns in Rural and Urban Burrowing Owls. Biology. 2022; 11(8):1141. https://doi.org/10.3390/biology11081141
Chicago/Turabian StyleSáez-Ventura, Ángeles, Antonio J. López-Montoya, Álvaro Luna, Pedro Romero-Vidal, Antonio Palma, José L. Tella, Martina Carrete, Gracia M. Liébanas, and Jesús M. Pérez. 2022. "Drivers of the Ectoparasite Community and Co-Infection Patterns in Rural and Urban Burrowing Owls" Biology 11, no. 8: 1141. https://doi.org/10.3390/biology11081141
APA StyleSáez-Ventura, Á., López-Montoya, A. J., Luna, Á., Romero-Vidal, P., Palma, A., Tella, J. L., Carrete, M., Liébanas, G. M., & Pérez, J. M. (2022). Drivers of the Ectoparasite Community and Co-Infection Patterns in Rural and Urban Burrowing Owls. Biology, 11(8), 1141. https://doi.org/10.3390/biology11081141








