Simple Summary
Intercropping of different greening plant species is a regular management practice in landscape engineering. However, how this approach works on the remediation of heavy metal soil is not fully known. Here, the effect of intercropping two greening species, Euonymus japonicus and Photinia × fraseri on phytoremediation efficiency was studied in a Cd/Cu/Zn-contaminated field. This study provides (1) a feasible biotechnique for improving phytoremediation efficiency using greening plants and (2) a more practical work for multiple HM-polluted soil than research on single-polluted soil.
Abstract
Intercropping plants for phytoremediation is a promising strategy in heavy metal-polluted soils. In this study, two typical greening plant species, Euonymus japonicus (E. japonicus) and Photinia × fraseri (P. × fraseri), were intercropped in a Cd/Cu/Zn-contaminated field. The phytoremediation efficiency was investigated by measuring the plant biomass, metal concentration, and mycorrhizal colonisation, as well as the effects on soil properties, including soil pH; soil total N; and available N, P, K, Cd, Cu, and Zn. The results showed that, compared with the monoculture system, intercropping significantly lowered the available Cd, Cu, and Zn contents, significantly improved the total and available N contents in rhizosphere soils of both plant species, and increased the hyphae colonisation rate of P. × fraseri. In both plants, intercropping significantly improved the total plant biomass. Furthermore, the concentrations Zn and Cd in the root of E. japonicus and Cu concentration in the root of P. × fraseri were enhanced by 58.16%, 107.74%, and 20.57%, respectively. Intercropping resulted in plants accumulating higher amounts of Cd, Cu, and Zn. This was particularly evident in the total amount of Cd in E. japonicus, which was 2.2 times greater than that in the monoculture system. Therefore, this study provides a feasible technique for improving phytoremediation efficiency using greening plants.
1. Introduction
With the rapid expansion of nonferrous metal mining and smelting activities in China, agricultural fields across the country have been widely contaminated by heavy metals (HMs), which has resulted in a significant risk to the public health. Derived from parent rocks, HMs can be released during the leaching and heating of nonferrous metallic ores and irrigation with the surface water impacted by mining discharges. In addition, the atmospheric deposition of metal-containing dust also impacts agricultural fields located far away, which have sprouted across the regions rich in nonferrous metal resources of China, primarily in Hunan, Guangxi, Guangdong, and Jiangxi Provinces [1]. As reported by the Chinese National Soil Pollution Investigation Bulletin (2014) [2], approximately 19.4% of agricultural land is polluted by HMs, and multi-polluted soil comprises a very high proportion. Generally, multiple metal-combined stresses were more toxic to plants than a single metal stress [3,4,5]. Possible co-toxic mechanisms include the aggravation of oxidative stress [6,7], alteration of plant water [6], mineral nutrition [8], and hormonal status [9], resulting in greater growth reductions compared to a single metal stress [8,10]. However, compared to the research on single metal pollution, fewer studies have been conducted to investigate the phytoremediation techniques for multiple HM-polluted soil.
Intercropping is regarded as an effective technique for enhancing the phytoremediation efficiency [11,12]. For instance, intercropping Pteris vittata L. with Morus alba L. or Castanea mollissima Bl. with Camellia sinensis L. significantly enhanced the metal accumulation capacity of both plants by increasing the biomass and metal concentration compared to a monoculture system [13,14]. Intercropping may alter the mutual rhizosphere environments of both plants by accelerating the root exudation [15], improving the metal bioavailability [15], and enhancing the soil enzyme activity and microbial diversity [16,17], among others. Intercropping also strengthens the metal tolerance of plants by increasing the photosynthesis and oxidation resistance [16,17]. Many studies have introduced intercropping systems with hyperaccumulators to remediate contaminated soils. However, disposal of the polluted plant materials remains unfeasible, thereby limiting the use of this technique. Greening plants have been recommended as ideal low-cost candidates for phytoextraction owing to their economical use of follow-up materials [18,19,20]. The intercropping of different greening plant species is a regular management practice in landscape engineering. However, to the best of our knowledge, no studies investigating the effect of intercropping greening plants on metal removal in HM-combined polluted soil have been conducted to date. This pattern can be utilized in polluted agricultural lands, especially for the greening business, which has been occupied a large area of fertile land for marketing.
Arbuscular mycorrhizal fungi (AMF) are beneficial fungi belonging to the phylum Mucoromycota and subphylum Glomeromycotina, which can establish obligately symbiotic association by interacting with about 80% of terrestrial plants [21,22].AMF have beneficial effects on the host plants adapting to heavy metal stress [23]. They interfere with many plant metabolisms, mainly through metal immobilization, nutrient balance, and the activation of transporters involved in HM uptake and transport [24]. Although AM symbioses have been found in most terrestrial plant species, different AM symbioses showed a wide range of plasticity in metal assimilation, reaching from preventing metal transference on the one hand to the highlighting metal accumulation on the other hand. For example, the inoculation of AMF significantly restricted As uptake and retained more As in roots by upregulating MsPT4 and MsMT2 in Medicago sativa L. [25]. In contrast, the inoculation of AMF led to higher Cd accumulation in Solanum nigrum L. by regulating the metal transporters (Nramp5 and HMA3) in the intercropping system with rice and S. nigrum [26]. Furthermore, many studies have revealed that the intercropping system increased or decreased AMF diversity by regulating the rhizosphere environment and root distribution [27,28,29]. To date, the contribution of AMF to intercropping greening plants for phytoremediation is still unclear.
Euonymus japonicus Thunb. (E. japonicus) and Photinia × fraseri Dress. (P. × fraseri) are very popular ornamental plant for parks and gardens in China. E. japonicus has variegated or yellow leaves [30], while P. × fraseri has very striking foliage with new bright red leaves and dark green older ones [31]. Furthermore, both of them have bright branches and vast root systems, which can grow well in infertile and contaminated soil [19,32]. Due to the high economic and ecological value, they have been widely applied to landscapes in China. In this study, these two greening plant species were planted in monoculture and intercropping systems to explore the changes in the rhizosphere environment and heavy metal accumulation in Cd/Cu/Zn-polluted soils.
We hypothesised that the soil characteristics and AMF would be affected by intercropping systems in a manner that improved the phytoremediation efficiency. The aims of this study were to evaluate the heavy metal remediation efficiency of the intercropping system and study the changes in the rhizosphere environment and AMF colonisation, as well as their subsequent roles in the phytoremediation of metal-contaminated soil.
2. Materials and Methods
2.1. Soil and Site Description
The field experiment was performed in paddy soils at a mining site in Zhejiang Province, China (Latitude 30.08219°, Longitude 119.046672°). The heavy metal concentrations and major chemical characteristics of the soil are presented in Table 1. E. japonicus and P. × fraseri seedlings were collected from the Zhejiang Senhe Seedling Company. The experimental design of the field plot experiment was completely randomised with four replicates and three different cultivation systems: (1) E. japonicus, (2) P. × fraseri, and (3) E. japonicus intercropped with P. × fraseri. The row space of E. japonicus and P. × fraseri was set as 0.5 × 0.4 m, and the planting plot was 8 m2 (60 plants in total of every plot; Figure S1). Healthy and uniform plants (1 year old) were planted in the designated field plots. Plants were watered and weeds were removed according to the routine management practices of the local farmers. After 340 d of growth, the plants were harvested, and the planted soil was sampled for further analysis.

Table 1.
The soil chemical characteristics and heavy metal concentrations in the soil.
2.2. Plant and soil Cd, Cu, and Zn Concentration
The plant roots were washed with deionised water; The rhizospheric soil (the cohesive soil from the plant roots) was brushed from the roots, mixed, air-dried, and sieved by 1 mm for further analysis. Leaves, stems, and roots were separated; dried at 70 °C to a constant weight; ground into a powder; passed through the sieves (2 mm); and stored at room temperature before analysis. The sample was subsequently digested in HNO3 at 160 °C for 12 h prior to analysis. The soil samples were digested with a mixture of water/HCl/HNO3 (1:1:4, v/v) in a microwave oven (MARS-240, USA CEM, North Carolina, USA) [18]. The concentrations of Cd, Cu, and Zn were measured using inductively coupled plasma mass spectrometry (ICP-MS; 7700, Agilent, Palo Alto, CA, USA).
2.3. Soil Extractable Cd, Cu, Zn; Dissolved Organic Carbon; and pH
Cd, Cu, and Zn were extracted using the Community Bureau of Reference (BCR) sequential extraction method [34]. The soil samples were treated with a 0.11-M acetic acid solution and then analysed using ICP-MS (ICP-MS, 7700, Agilent, Palo Alto, USA). The dissolved organic carbon (DOC) was extracted with distilled water at a solid-to-water ratio of 1:2.5 (w/v) and then shaken at 200 rpm for 2 h at 20 °C. The suspension was filtered through a 0.45-μm membrane filter and then measured using a carbon analyser (Multi N/C 3100, Analytikjena, Jena, Germany) [35]. The soil organic matter was determined using the dichromate wet oxidation method [36]. The soil pH was determined according to the ratio of soil to water 1:2.5 (w/v) for dissolution and subsequently examined using a pH meter (PHS-2F, Leici, Shanghai, China)
2.4. Detection of the Root Mycorrhization Rate
The plant roots were washed with water and separated randomly into segments (1 to 2 cm). They were then treated with 10% KOH for 1.2 h at 90 °C, washed with water and acidified with 5% (v/v) HCl solution for 8 min at 25 °C, and stained with 0.2% (w/v) trypan blue solution at 90 °C for 2 h [37]. The root segments were analysed for mycorrhizal colonisation using the magnified line intersection method (OLYMPUS DP74, Tokyo, Japan) [38].
2.5. Calculation of Bioconcentration Factor, Land Equivalent Ratio, and Metal Removal Equivalent Ratio
The plant bioconcentration factor (BCF) is commonly used to assess the potential of plants to remove heavy metals and was calculated using the following formulas [39]:
BCFR = (HM concentration of root)/(HM concentration in soil)
BCFS = (HM concentration of stem)/(HM concentration in soil)
BCFL = (HM concentration of leaf))/(HM concentration in soil)
The land equivalent ratio (LER) is commonly used to assess the intercropping advantage and is calculated as follows [40]:
where YAI and YAM indicate the yields of plant A in the intercropping and monoculture systems, respectively. YBI and YBM indicate the yields of plant B in the intercropping and monoculture systems, respectively. LER > 1 or <1 indicates the yield advantage or disadvantage of intercropping, while LER = 1 shows the same resource utilisation efficiency between intercropping and the monoculture.
LER = (YAI/YAM) + (YBI/YBM)
The metal removal equivalent ratio (MRER) is defined by the following formula [41]:
where XAI and XAM represent the heavy metal content of plant A in the intercropping and monoculture systems, respectively. XBI and XBM represent the heavy metal content of plant B in the intercropping and monoculture systems, respectively.
MRER= (XAI/XAM) + (XBI × XBM)
2.6. Statistical Analysis
The experimental data were calculated using Excel 2013 (shown as the mean ± standard error), followed by statistical analysis using SPSS 20.0. The means were subjected to a test of statistical significance using Duncan’s multiple range test at a 5% probability level [18].
3. Results
3.1. Plant Biomass
Plant biomass is an important factor for evaluating the efficiency of phytoremediation. The dry weights of the root, stem, leaf, and total plant were determined over the course of the experimental period (340 d) (Figure 1). P. × fraseri had a higher stem (by 146.25%) than E. japonicus. Intercropping significantly enhanced the stem and total biomass for E. japonicus and roots, leaves, and total biomass for P. × fraseri. For example, the stem, leaf, and total plant dry weights of E. japonicus increased by 72.71%, 93.85%, and 61.60%, respectively. The improvement in root and leaf dry weights was more pronounced in P. × fraseri, with 165.56% and 146.82% increases compared to the monoculture system, respectively. The total biomass of P. × fraseri in the intercropping system was 62.21% higher than that for P. × fraseri in the monoculture system.
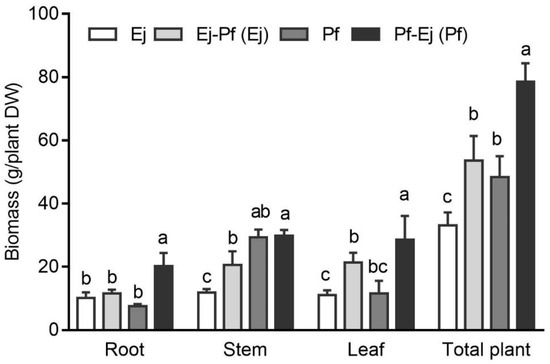
Figure 1.
Biomass of P. × fraseri and E. japonicus in the monoculture and intercropping systems. Ej and Pf refer to E. japonicus and P. × fraseri in the monoculture system, respectively; Ej-Pf and Pf-Ej (Pf) refer to E. japonicus and P. × fraseri in the intercropping system, respectively. All values are presented as the mean ± standard error (n = 4), and bars with different lowercase letters indicate significant (p < 0.05) differences between the treatments and control, according to Duncan’s test.
3.2. Concentration of Total Cd/Cu/Zn in Plants
As shown in Figure 2, E. japonicus accumulated significantly higher (p < 0.05) levels of Cu, Zn, and Cd in the roots but lower Cu, Zn, and Cd in the leaves than P. × fraseri in the monoculture system over the course of the experimental period. E. japonicus also showed less Cd concentration in stems when compared to P. × fraseri. Intercropping significantly increased the translocation of Cd (by 107.74%) and Zn (by 58.16%) in roots (p < 0.05) (Figure 2A,C) and reduced the translocation of Cd in stems (by 39.20%) and Cu in roots (by 30.42%) E. japonicus (p < 0.05) (Figure 2A,B), while intercropping decreased the translocation of Cu (by 46.64%) and Zn (by 20.57%) in roots but increased the translocation of Cu in leaves of P. × fraseri (p < 0.05) (Figure 2B,C).
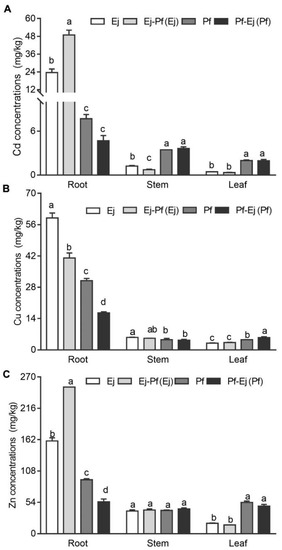
Figure 2.
The Cd, Cu, and Zn concentrations of P. × fraseri and E. japonicus in the monoculture and intercropping systems. Ej and Pf refer to E. japonicus and P. × fraseri in the monoculture system, respectively; Ej-Pf and Pf-Ej (Pf) refer to E. japonicus and P. × fraseri in the intercropping system, respectively. The concentrations of Cd (A), Cu (B), and Zn (C) in the root, stem, and leaf for P. × fraseri and E. japonicus, respectively. All values are presented as the mean ± standard error (n = 4), and bars with different lowercase letters indicate significant (p < 0.05) differences between the treatments and control, according to Duncan’s test.
3.3. Total Cd/Cu/Zn content in P. × fraseri and E. japonicus
In the monoculture system, the distribution of the total metal content was similar to that of the metal concentrations, as noted in that 86.38% Cu, 77.78% Zn, and 94.08% Cd were accumulated in the roots of E. japonicus, while 12.92% Cu, 20% Zn, and 12.7% Cd were accumulated in the leaves of P. × fraseri (Figure 3). Intercropping significantly altered the accumulation of these metals in both plant species. For example, the total Cu content in the roots and leaves of P. × fraseri increased by 53.99% and 232.47%, respectively, whereas that in the roots of E. japonicus significantly decreased by 31.37% (p < 0.05) (Figure 3B). The total Zn content of the leaves in P. × fraseri increased by 130.04% (Figure 3C). The total content of Cd in the stems and leaves of P. × fraseri increased by 80.28% and 122.98%, respectively; furthermore, a significant increase was found in the total Cd content in the roots (by 70.61%) and stems (by 244.19%) of E. japonicus in the intercropping system when compared with the monoculture (Figure 3A). The total contents of Zn and Cd in the E. japonicus whole plant and the total content of Cu in the P. × fraseri whole plant were significantly higher (p < 0.05) in the intercropping system and increased by 42.07%, 78.13%, and 49.45% (Figure 3), respectively.
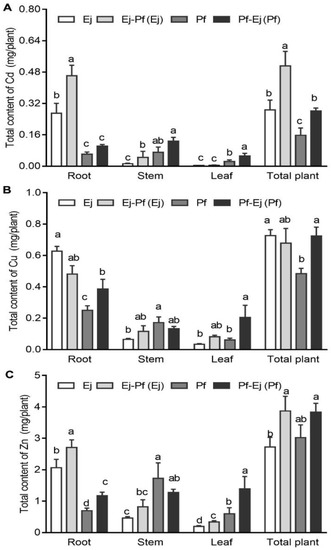
Figure 3.
The Cd, Cu, and Zn contents of P. × fraseri and E. japonicus in the monoculture and intercropping systems. Ej and Pf refer to E. japonicus and P. × fraseri in the monoculture system, respectively; Ej-Pf and Pf-Ej (Pf) refer to E. japonicus and P. × fraseri in the intercropping system, respectively. The total contents of Cd (A), Cu (B), and Zn (C) in the root, stem, leaf, and total plant, respectively. All values are presented as the mean ± standard error (n = 4), and bars with different lowercase letters indicate significant (p < 0.05) differences between the treatments and control, according to Duncan’s test.
3.4. BCF, MRER, and LER of P. × fraseri and E. japonicus in the Monoculture and Intercropping Systems
The BCF was calculated as the ratio of the metal concentration in the plant parts to that of the corresponding soil in which the plant was grown. Generally, the BCF of Cd was much higher than those of Zn and Cu (Table 2), indicating that Cd is easily absorbed by plants (Table 2). Intercropping significantly enhanced the BCF of Cd in the roots of E. japonicus and the stems and leaves of P. × fraseri (p < 0.05). A similar effect of intercropping was also noted in the BCF of Zn. Furthermore, the LER was 3.62, indicating that intercropping E. japonicus with P. × fraseri had a distinct yield advantage. Additionally, the MRERs of Cu, Zn, and Cd in the intercropping system were 2.48, 2.85, and 3.32, respectively, suggesting that the intercropping pattern has an advantage in removing heavy metals, particularly Cd.

Table 2.
The BCF, MRER, and LER of P. × fraseri and E. japonicus in different planting patterns.
3.5. Soil Chemical Characteristics and Heavy Metal Concentrations in Monoculture and Intercropping Systems
A comparison of the soil chemical characteristics, including pH; total N; SOM; and available N, P, K, Cu, Zn, and Cd, between the monoculture and intercropping systems is provided in Table 3. For both studied plants, the available Cu, Zn, and Cd of the soil in the intercropping system were significantly lower than in the monoculture system, except for Zn in the rhizosphere soil of P. × fraseri. Intercropping also enhanced the N level of the soil, as noted by the increase in the total and available N in E. japonicus (47.10% and 29.78%, respectively) and P. × fraseri (61.99% and 46.08%, respectively). In addition, the soil pH significantly decreased (p < 0.05) in the rhizosphere soil of intercropped E. japonicus compared with the monoculture. Furthermore, the available K significantly increased (p < 0.05) in the rhizosphere soil of E. japonicus and significantly decreased in the rhizosphere soil of P. × fraseri.

Table 3.
The soil chemical characteristics and heavy metal concentrations in the rhizosphere soil of P. × fraseri and E. japonicus in different planting patterns.
3.6. Mycorrhization Rate of P. × fraseri and E. japonicus in Monoculture and Intercropping Systems
To assess the influence of the intercropping system on the mycorrhization rate and plant performance, the degree of AMF colonisation was calculated (Figure 4). We observed that fungal hyphae and arbuscules were branched in the colonised roots in all the treatments (Figure 4A). In the monoculture system, P. × fraseri plants showed a significantly higher colonisation rate in the total, hyphae, and vesicles than E. japonicus plants by 44.51%, 96.95%, and 397.27% (p < 0.05), respectively (Figure 4B). However, substantially lower colonisation [H + A (%)] (64.88%) was observed in P. × fraseri plants compared to the monoculture system, and the proportion of root sectors containing hyphae (H (%)) was significantly higher (p < 0.05) in intercropped P. × fraseri plants than in monoculture plants by 21.2% (Figure 4B). In summary, the results suggest that mycorrhizal symbiosis was affected in intercropped plants.
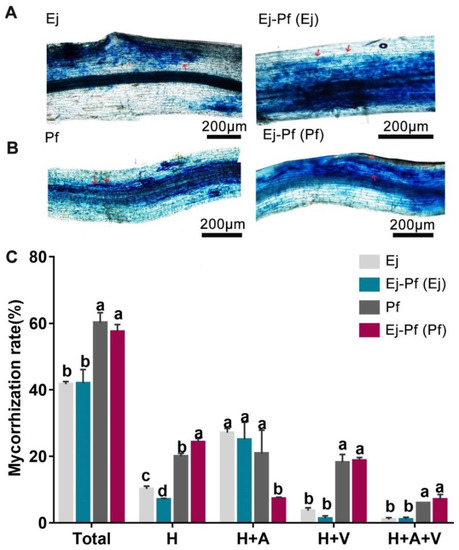
Figure 4.
The analysis of the mycorrhization rate of P. × fraseri and E. japonicus in the monoculture and intercropping systems. (A,B) Images of E. japonicus and P. × fraseri roots, bar = 200 µm. (C): Quantification of the mycorrhization rate level of P. × fraseri and E. japonicus plants. Ej and Pf refer to E. japonicus and P. × fraseri in the monoculture system, respectively; Ej-Pf and Pf-Ej (Pf) refer to E. japonicus and P. × fraseri in the intercropping system, respectively. A, H, and V represent “arbuscules”, “hyphae”, and “vesicles”, respectively; A + H (%) indicates the percentage of root with arbuscules and hyphae; A + V + H (%) represents the percentage of root with arbuscules, vesicles, and hyphae; H (%) indicates percentage of root with only hyphae; and V + H (%) indicates the percentage of root with vesicles and hyphae. Red arrows indicate arbuscules. All values are presented as the mean ± standard error (n = 4), and bars with different lowercase letters indicate significant (p < 0.05) differences between the treatments and control, according to Duncan’s test.
4. Discussion
Phytoremediation with hyperaccumulators has been a promising technology for agricultural lands. However, the disposition of a hazardous biomass after mediation is still unfeasible. Thus, we perilously proposed a new technique of uptake and stabilizing the HMs from the contaminated soil by greening plants prior to their intravital transplantation for economical landscaping [18]. The pattern of high root retention (as also shown in the Figure 2) and low risk for defoliation (as in evergreen species) contribute to the feasibility of this technique for soil cleansing. More importantly, it is an eco-friendly technique, which would providing a feasible solution to the remediation of agricultural land polluted by HMs. In this study, we set up the intercropping system with different greening species, which aimed to further improve the efficiency of this technique.
4.1. Differences in P. × fraseri and E. japonicus Biomass in Monoculture and Intercropping Systems
Greening plants can be utilized for phytoremediation, as they have a marketable biomass compared with classical hyperaccumulators [18]. As observed in this study, the biomass of P. × fraseri and E. japonicus (48 and 33 g/plant, respectively) (Figure 1) are approximately 11–137 times larger than that of Sedum alfredii Hance. [40,42].
Intercropping significantly promoted the growth of both P. × fraseri and E. japonicus, as indicated by the high LER (Figure 1 and Table 2). Growth stimulation has also been observed in other systems, such as the intercropping of oilseed rape with Sedum alfredii [43], Sonchus asper (L.) Hill with Vicia faba Linn. [44], and Sedum alfredii with Zea mays Linn. [45]. This might because intercropping positively regulates the nutrient bioavailability through intertwined root exudation [15,46,47]. The root exudates could break the SOM–mineral connection, thus leading to the release of DOC in the soil [48]. As observed in this study, intercropping slightly decreased the SOM content (about 2 g/kg) and correspondingly increased the content of DOC in the Ej-Pf part (Table 1 and Table 3). It was also reported that the application of low-molecular-weight organic acids significantly reduced the SOM by 28.1% after 15 days [49]. Furthermore, the degradation of SOM by intercropping considerably affected the mycorrhization rate of both plant species (Figure 4), which, in turn, enhanced the total and available N of the rhizosphere soils (Table 3). A similar phenomenon was observed in the Manihot esculenta Crantz./Arachis hypogaea Linn. system [50,51].
The enhancement of growth was more pronounced in P. × fraseri compared to E. japonicus in the intercropping system. This might because P. × fraseri is a taller and broad shrub, which had a much higher biomass than E. japonicus (as seen in Figure S1). The fast growth of P. × fraseri could facilitate the allocation of more resources from the soil, including space, water, and nutrients. Similar results were also found in a previous study of intercropping red Solanum melongena L. (S. melongena) with green S. melongena and intercropping green S. melongena with black S. melongena, and rice (Oryza sativa Linn.) intercropping with Solanum nigrum L. decreased the growth of red eggplant, black S. melongena, and rice, respectively [26,52]. The growth discrepancy also changed the soil pH differently, as noted that P. × fraseri induced a strong decreasing effect on the soil pH, which even covered the increased effect of E. japonicus in the intercropping system (Table 3). Furthermore, AMF colonisation could influence the process of metal uptake by the plant. The total colonisation rate of P. × fraseri was higher than that of E. japonicus in both the monoculture and intercropping systems (Figure 4), indicating that intercropping affected the soil microbial community of the rhizosphere. It was also reported that Moso bamboo (Phyllostachys edulis J. Houz.) intercropping with Sedum plumbizincicola enhanced HM remediation through changing the abundances of the rhizospheric microbes [53]. The transcriptional regulation of AMF-induced genes involved in HM accumulation in plants and the nutritional status of soil requires further study.
4.2. Effect of Intercropping on Heavy Metal Accumulation and Phytoremediation Potential in P. × fraseri and E. japonicus
Interestingly, the enhanced DOC contents and decreased soil pH by intercropping failed to increase the bioavailability of heavy metals. In contrast, the bioavailable Cu and Cd were significantly decreased compared with the monoculture system (Table 3), which might because a large amount of them was assimilated by the plants. Intercropping significantly decreased the root/shoot ratio of E. japonicus while significantly enhancing the root/shoot ratio of P. × fraseri (as shown in Figure S2), It also disrupted the metal distribution among different organs, as observed in the increase in root Cd of E. japonicus and leaf Cu of P. × fraseri, while decreasing in the stem Cd of E. japonicus and the root Cu of P. × fraseri (Figure 2A,B), respectively. The change in the metal balance in the intercropping systems was also found in fava beans with Sedum alfredii [40], Galium aparine Linn. with Malachium Fr. ex Rchb. [54], and Moso bamboo with Sedum plumbizincicola [53].
Although the metal concentration in some organs was not altered or even lowered in the intercropping system, the total Cd/Cu contents in P. × fraseri and E. japonicus significantly increased. For example, the Cd concentration in the leaves of P. × fraseri did not change, whereas the total Cd accumulation in the leaves increased by 123%. The Cu concentration in the roots of P. × fraseri and Cd concentration in the stems of E. japonicus decreased by 46% and 41.49%, respectively, while the total Cu in roots and Cd content in the stems increased by 53.99% and 54.05%, respectively, in the intercropping system. Similar results have been reported in the Conyza canadensis/Cerastium glomeratum and Stellaria media/Malachium aquaticum systems [54,55]. These results indicate that phytoremediation efficiency depends not only on the metal concentration but also on the biomass of plants.
5. Conclusions
In this study, we demonstrated that intercropping with greening plants could promote heavy metal phytoextraction. In the intercropping system, E. japonicus showed a higher Cd phytoextraction efficiency than P. × fraseri, which was mainly ascribed to the high increase in the plant biomass. Additionally, the concentrations of the soil nutrients were greatly improved, especially the total and available N of the rhizosphere soils (Table 3), which supports the feasibility of this phytoremediation technique. Further studies are necessary to determine whether aspects such as optimal fertilisation and the application of microbial and mobilising agents may contribute to implementation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology11081133/s1, Figure S1: Picture and schematic diagram of field experiment; Figure S2: The analysis of root/shoot ratio of P. × fraseri and E. japonicus in monoculture and intercropping systems.
Author Contributions
Conceptualisation, J.L., G.Q. and B.G.; methodology, B.G.; data curation, G.Q.; writing—original draft preparation, J.L.; writing—review, C.L. and B.G.; visualisation, J.L., C.L., H.L. and Q.F.; supervision, Q.F. and B.G.; funding acquisition, J.L. and B.G.; revising and editing, Y.L.; and writing—reviewing and editing, X.C. and Q.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Science and Technology of Zhejiang Province, China (2015C03020 and 2019C02008), Natural Science Foundation of China (No. 41001184 and 42007120), Geological Exploration Fund of Zhejiang Province (2020006), and State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AMF | Arbuscular mycorrhiza fungi |
| BCR | Community Bureau of Reference |
| BCF | Calculation of bioconcentration factor |
| DOC | Dissolved organic carbon |
| Ej/E. japonicus | Euonymus japonicus |
| HMs | Heavy metals |
| LER | Land equivalent ratio |
| MRER | Metal removal equivalent ratio |
| Pf/P. × fraseri | Photinia × fraseri Dress |
| SOM | Soil organic matter |
References
- Hu, Y.; Cheng, H.; Tao, S. The challenges and solutions for cadmium-contaminated rice in China: A critical review. Environ. Int. 2016, 92, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environmental Protection. A National Survey of Soil Pollution Bulletin; Ministry of Environmental Protection: Beijing, China, 2014. Available online: https://huanbao.bjx.com.cn/news/20151209/689681.shtml (accessed on 25 July 2022).
- Sasmaz, M.; Öbek, E.; Sasmaz, A. Bioaccumulation of cadmium and thallium in Pb-Zn tailing waste water by Lemna minor and Lemna gibba. Appl. Geochem. 2019, 100, 287–292. [Google Scholar] [CrossRef]
- Chen, W.; Peng, L.; Hu, K.; Zhang, Z.; Peng, C.; Teng, C.; Zhou, K. Spectroscopic response of soil organic matter in mining area to Pb/Cd heavy metal interaction: A mirror of coherent structural variation. J. Hazard. Mater. 2020, 393, 122425. [Google Scholar] [CrossRef]
- Bamagoos, A.A.; Alharby, H.F.; Abbas, G. Differential Uptake and Translocation of Cadmium and Lead by Quinoa: A Multivariate Comparison of Physiological and Oxidative Stress Responses. Toxics 2022, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Pandey, P.; Rajpoot, R.; Rani, A.; Dubey, R. Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 2014, 251, 1047–1065. [Google Scholar] [CrossRef]
- Kaya, C.; Akram, N.A.; Sürücü, A.; Ashraf, M. Alleviating effect of nitric oxide on oxidative stress and antioxidant defence system in pepper (Capsicum annuum L.) plants exposed to cadmium and lead toxicity applied separately or in combination. Sci. Hortic. 2019, 255, 52–60. [Google Scholar] [CrossRef]
- He, H.; Wang, X.; Wu, M.; Guo, L.; Fan, C.; Peng, Q. Cadmium and lead affect the status of mineral nutrients in alfalfa grown on a calcareous soil. Soil Sci. Plant Nutr. 2020, 66, 506–514. [Google Scholar] [CrossRef]
- Zhou, M.; Han, R.; Ghnaya, T.; Lutts, S. Salinity influences the interactive effects of cadmium and zinc on ethylene and polyamine synthesis in the halophyte plant species Kosteletzkya pentacarpos. Chemosphere 2018, 209, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Ronzan, M.; Piacentini, D.; Fattorini, L.; Della Rovere, F.; Eiche, E.; Riemann, M.; Altamura, M.; Falasca, G. Cadmium and arsenic affect root development in Oryza sativa L. negatively interacting with auxin. Environ. Exp. Bot. 2018, 151, 64–75. [Google Scholar] [CrossRef]
- De Conti, L.; Ceretta, C.A.; Melo, G.W.; Tiecher, T.L.; Silva, L.O.; Garlet, L.P.; Mimmo, T.; Cesco, S.; Brunetto, G. Intercropping of young grapevines with native grasses for phytoremediation of Cu-contaminated soils. Chemosphere 2019, 216, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Niu, H.; Wang, Z.; Song, J.; Long, A.; Cao, M.; Luo, J. Cadmium subcellular distribution and chemical form in Festuca arundinacea in different intercropping systems during phytoremediation. Chemosphere 2021, 276, 130137. [Google Scholar] [CrossRef]
- Ma, Y.-h.; Fu, S.-l.; Zhang, X.-p.; Zhao, K.; Chen, H.Y. Intercropping improves soil nutrient availability, soil enzyme activity and tea quantity and quality. Appl. Soil Ecol. 2017, 119, 171–178. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Feng, W.; Xin, L.; Xu, Z. Phytoextraction potential of Pteris vittata L. co-planted with woody species for As, Cd, Pb and Zn in contaminated soil. Sci. Total Environ. 2019, 650, 594–603. [Google Scholar] [CrossRef]
- Li, Z.-R.; Wang, J.-X.; An, L.-Z.; Tan, J.-B.; Zhan, F.-D.; Wu, J.; Zu, Y.-Q. Effect of root exudates of intercropping Vicia faba and Arabis alpina on accumulation and sub-cellular distribution of lead and cadmium. Int. J. Phytoremediation 2019, 21, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Liu, C.; Li, J.; Luo, Y.; Yang, Q.; Zhang, W.; Yang, P.; Feng, B. Responses of rhizosphere soil properties, enzyme activities and microbial diversity to intercropping patterns on the Loess Plateau of China. Soil Tillage Res. 2019, 195, 104355. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C. Dynamic response of enzymatic activity and microbial community structure in metal (loid)-contaminated soil with tree-herb intercropping. Geoderma 2019, 345, 5–16. [Google Scholar] [CrossRef]
- Guo, B.; Liang, Y.; Fu, Q.; Ding, N.; Liu, C.; Lin, Y.; Li, H.; Li, N. Cadmium stabilization with nursery stocks through transplantation: A new approach to phytoremediation. J. Hazard. Mater. 2012, 199, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Bai, Y.; Hong, X.; Sun, L.; Liu, Y. Particulate matter and heavy metal deposition on the leaves of Euonymus japonicus during the East Asian monsoon in Beijing, China. PLoS ONE 2017, 12, e0179840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattei, P.; Gnesini, A.; Gonnelli, C.; Marraccini, C.; Masciandaro, G.; Macci, C.; Doni, S.; Iannelli, R.; Lucchetti, S.; Nicese, F.P. Phytoremediated marine sediments as suitable peat-free growing media for production of red robin photinia (Photinia x fraseri). Chemosphere 2018, 201, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [Green Version]
- Errisson, I.; Hognason, J.; Vidarsdottir, H.; Gottskalksson, G.; Gunnarsson, G.; Sverrisson, J.; Gudbjartsson, T.; Dhalaria, R.; Kumar, D.; Kumar, H. Arbuscular Mycorrhizal Fungi as Potential Agents in Ameliorating Heavy Metal Stress in Plants. Agronomy 2020, 10, 815. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Y.; Chen, S.; Polle, A.; Rennenberg, H.; Luo, Z.B. Physiological and molecular mechanisms of heavy metal accumulation in nonmycorrhizal versus mycorrhizal plants. Plant Cell Environ. 2019, 42, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, Y.; Jiang, X.; Chen, B.; Zhang, X. Arbuscular mycorrhizal fungi alleviate arsenic toxicity to Medicago sativa by influencing arsenic speciation and partitioning. Ecotoxicol. Environ. Saf. 2018, 157, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qin, J.; Li, J.; Lai, Z.; Li, H. Upland rice intercropping with Solanum nigrum inoculated with arbuscular mycorrhizal fungi reduces grain Cd while promoting phytoremediation of Cd-contaminated soil. J. Hazard. Mater. 2021, 406, 124325. [Google Scholar] [CrossRef]
- Lian, T.; Mu, Y.; Ma, Q.; Cheng, Y.; Gao, R.; Cai, Z.; Jiang, B.; Nian, H. Use of sugarcane–soybean intercropping in acid soil impacts the structure of the soil fungal community. Sci. Rep. 2018, 8, 14488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, X.; Wang, Y.; Li, Z.; Larousse, M.; Pere, A.; da Rocha, M.; Zhan, F.; He, Y.; Pu, L.; Panabières, F. Root-associated microbiota drive phytoremediation strategies to lead of Sonchus Asper (L.) Hill as revealed by intercropping-induced modifications of the rhizosphere microbiome. Environ. Sci. Pollut. Res. 2022, 29, 23026–23040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Mu, Y.; Li, X.; Li, S.; Sang, P.; Wang, X.; Wu, H.; Xu, N. Response of the arbuscular mycorrhizal fungi diversity and community in maize and soybean rhizosphere soil and roots to intercropping systems with different nitrogen application rates. Sci. Total Environ. 2020, 740, 139810. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Riaguas, A.; Fernández-de Córdova, M.; Llorent-Martínez, E.J. Phenolic profile and antioxidant activity of Euonymus japonicus Thunb. Nat. Prod. Res. 2020, 36, 1–5. [Google Scholar] [CrossRef]
- Larraburu, E.E.; Apóstolo, N.M.; Llorente, B.E. Anatomy and morphology of photinia (Photinia × fraseri Dress) in vitro plants inoculated with rhizobacteria. Trees 2010, 24, 635–642. [Google Scholar] [CrossRef]
- Lin, X.; Shu, D.; Zhang, J.; Chen, J.; Zhou, Y.; Chen, C. Dynamics of particle retention and physiology in Euonymus japonicus Thunb. var. aurea-marginatus Hort. with severe exhaust exposure under continuous drought. Environ. Pollut. 2021, 285, 117194. [Google Scholar] [CrossRef]
- Guo, B.; Hong, C.; Tong, W.; Xu, M.; Huang, C.; Yin, H.; Lin, Y.; Fu, Q. Health risk assessment of heavy metal pollution in a soil-rice system: A case study in the Jin-Qu Basin of China. Sci. Rep. 2020, 10, 11490. [Google Scholar] [CrossRef]
- Kartal, Ş.; Aydın, Z.; Tokalıoğlu, Ş. Fractionation of metals in street sediment samples by using the BCR sequential extraction procedure and multivariate statistical elucidation of the data. J. Hazard. Mater. 2006, 132, 80–89. [Google Scholar] [CrossRef]
- Qu, X.; Fu, H.; Mao, J.; Ran, Y.; Zhang, D.; Zhu, D. Chemical and structural properties of dissolved black carbon released from biochars. Carbon 2016, 96, 759–767. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Wiley and Sons: Hoboken, NJ, USA, 1996; Volume 5, pp. 961–1010. [Google Scholar]
- Liu, J.; Liu, J.; Chen, A.; Ji, M.; Chen, J.; Yang, X.; Gu, M.; Qu, H.; Xu, G. Analysis of tomato plasma membrane H+-ATPase gene family suggests a mycorrhiza-mediated regulatory mechanism conserved in diverse plant species. Mycorrhiza 2016, 26, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Klinnawee, L.; Zhou, Y.; Saridis, G.; Vijayakumar, V.; Brands, M.; Dörmann, P.; Gigolashvili, T.; Turck, F.; Bucher, M. AP2 transcription factor CBX1 with a specific function in symbiotic exchange of nutrients in mycorrhizal Lotus japonicus. Proc. Natl. Acad. Sci. USA 2018, 115, E9239–E9246. [Google Scholar] [CrossRef] [Green Version]
- Jamali, M.K.; Kazi, T.G.; Arain, M.B.; Afridi, H.I.; Jalbani, N.; Kandhro, G.A.; Shah, A.Q.; Baig, J.A. Heavy metal accumulation in different varieties of wheat (Triticum aestivum L.) grown in soil amended with domestic sewage sludge. J. Hazard. Mater. 2009, 164, 1386–1391. [Google Scholar] [CrossRef]
- Tang, L.; Hamid, Y.; Zehra, A.; Sahito, Z.A.; He, Z.; Beri, W.T.; Khan, M.B.; Yang, X. Fava bean intercropping with Sedum alfredii inoculated with endophytes enhances phytoremediation of cadmium and lead co-contaminated field. Environ. Pollut. 2020, 265, 114861. [Google Scholar] [CrossRef]
- Zeng, L.; Lin, X.; Zhou, F.; Qin, J.; Li, H. Biochar and crushed straw additions affect cadmium absorption in cassava-peanut intercropping system. Ecotoxicol. Environ. Saf. 2019, 167, 520–530. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Q.; Meng, Q.; Liu, Y.; Pan, F.; Luo, S.; Wu, Y.; Ma, L.; Yang, X. Chromosome doubling of Sedum alfredii Hance: A novel approach for improving phytoremediation efficiency. J. Environ. Sci. 2019, 86, 87–96. [Google Scholar] [CrossRef]
- Cao, X.; Wang, X.; Lu, M.; Hamid, Y.; Lin, Q.; Liu, X.; Li, T.; Liu, G.; He, Z.; Yang, X. The Cd phytoextraction potential of hyperaccumulator Sedum alfredii-oilseed rape intercropping system under different soil types and comprehensive benefits evaluation under field conditions. Environ. Pollut. 2021, 285, 117504. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Qin, L.; Zhan, F.; Wu, J.; Li, Y.; Chen, J.; Wang, J.; Hu, W. Effects of Intercropping of Sonchus asper and Vicia faba on plant cadmium accumulation and root responses. Pedosphere 2020, 30, 457–465. [Google Scholar] [CrossRef]
- Hei, L.; Wu, Q.-T.; Long, X.-X.; Hu, Y.-M. Effect of co-planting of Sedum alfredii and Zea mays on Zn-contaminated sewage sludge. Huan Jing Ke Xue Huanjing Kexue 2007, 28, 852–858. [Google Scholar] [PubMed]
- Yang, M.; Zhang, Y.; Qi, L.; Mei, X.; Liao, J.; Ding, X.; Deng, W.; Fan, L.; He, X.; Vivanco, J.M. Plant-plant-microbe mechanisms involved in soil-borne disease suppression on a maize and pepper intercropping system. PLoS ONE 2014, 9, e115052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qing, L.; Li-Na, S.; Xiao-Min, H. Metabonomics study on root exudates of cadmium hyperaccumulator Sedum alfredii. Chin. J. Anal. Chem. 2015, 43, 7–12. [Google Scholar] [CrossRef]
- Ling, W.; Sun, R.; Gao, X.; Xu, R.; Li, H. Low-molecular-weight organic acids enhance desorption of polycyclic aromatic hydrocarbons from soil. Eur. J. Soil Sci. 2015, 66, 339–347. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Khan, M.A.; Luo, W.; Xiang, Z.; Xu, W.; Zhong, B.; Ma, J.; Ye, Z.; Zhu, Y. Effect of plant extracts and citric acid on phytoremediation of metal-contaminated soil. Ecotoxicol. Environ. Saf. 2021, 211, 111902. [Google Scholar] [CrossRef]
- Tang, X.; Luo, S.; Huang, Z.; Wu, H.; Wang, J.; Shi, G.; He, L.; Xiong, F.; Jiang, J.; Liu, J. Changes in the physicochemical properties and microbial communities of rhizospheric soil after cassava/peanut intercropping. bioRxiv 2019, 570937. [Google Scholar] [CrossRef]
- Tang, X.; Zhong, R.; Jiang, J.; He, L.; Huang, Z.; Shi, G.; Wu, H.; Liu, J.; Xiong, F.; Han, Z. Cassava/peanut intercropping improves soil quality via rhizospheric microbes increased available nitrogen contents. BMC Biotechnol. 2020, 20, 13. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.; Lu, R.; Li, H.; Lin, L.; Li, L.; Xiang, J.; Chen, L.; Tang, Y. Effects of mutual intercropping on cadmium accumulation in seedlings of three varieties of eggplant. Int. J. Environ. Anal. Chem. 2021, 101, 1761–1772. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Li, C.; Zhang, X.; Gu, L.; Huang, Z.; Gai, X.; Huang, Z. Intercropping improves heavy metal phytoremediation efficiency through changing properties of rhizosphere soil in bamboo plantation. J. Hazard. Mater. 2021, 416, 125898. [Google Scholar] [CrossRef]
- Lu, Q.; Li, J.; Chen, F.; Liao, M.A.; Lin, L.; Tang, Y.; Liang, D.; Xia, H.; Lai, Y.; Wang, X. Effects of mutual intercropping on the cadmium accumulation in accumulator plants Stellaria media, Malachium aquaticum, and Galium aparine. Environ. Monit. Assess. 2017, 189, 622. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Liang, D.; Chen, F.; Liao, M.A.; Lin, L.; Tang, Y.; Lv, X.; Li, H.; Wang, Z.; Wang, X. Effects of mutual intercropping on cadmium accumulation by the accumulator plants Conyza canadensis, Cardamine hirsuta, and Cerastium glomeratum. Int. J. Phytoremediation 2018, 20, 855–861. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).