Simple Summary
The production of bio-based materials, including organic acids, antibiotics, enzymes, ethanol, and hydrogen, is generally done by the cultivation of suspended cells rather than using immobilized cells. However, several studies suggest the application of productive biofilms as a reliable alternative for biocatalysis, with many advantages over suspended-growth systems. This review gives an overview of the breakthrough in the application of biofilm platforms for the sustainable production of valuable compounds, with particular insight into the latest advances in the production of recombinant proteins. Productive biofilms are shown to improve production rates and product yields, demonstrating great potential for industrial applications.
Abstract
In recent years, abundant research has been performed on biofilms for the production of compounds with biotechnological and industrial relevance. The use of biofilm platforms has been seen as a compelling approach to producing fine and bulk chemicals such as organic acids, alcohols, and solvents. However, the production of recombinant proteins using this system is still scarce. Biofilm reactors are known to have higher biomass density, operational stability, and potential for long-term operation than suspended cell reactors. In addition, there is an increasing demand to harness industrial and agricultural wastes and biorefinery residues to improve process sustainability and reduce production costs. The synthesis of recombinant proteins and other high-value compounds is mainly achieved using suspended cultures of bacteria, yeasts, and fungi. This review discusses the use of biofilm reactors for the production of recombinant proteins and other added-value compounds using bacteria and fungi.
1. Introduction
Biofilms are aggregates of microorganisms, such as bacteria, fungi, or algae, which are protected by a matrix of extracellular polymeric substances (EPS) that are usually attached to a solid surface that can be organic or inorganic [1,2]. Research in biofilms tends to focus on their detrimental effects on sectors such as health, food, and the maritime industry [3,4]. These effects range from persistent infections on medical devices [5], equipment clogging, heat transfer reduction and product degradation in the food industry [6], and the increment in frictional drag and consequent fuel consumption in marine vehicles [7]. The beneficial properties of biofilms include not only wastewater treatment [8], bioremediation, and removal of toxic pollutants [9,10], but also the production of added-value substances, such as organic acids, enzymes, alcohols, and recombinant proteins [11,12,13,14,15].
Recombinant proteins and other added-value compounds are being produced in biofilm reactors due to several advantages of this platform when compared to suspended cell systems. Biofilm reactors can (1) retain more biomass per unit volume, increasing production rates and yields, (2) resist stress conditions such as toxic compounds, (3) reduce the risk of washout (which eliminates the need for repeated inoculations during subsequent batch fermentation), and (4) reduce fermentation times and exhibit long-term activity [11,15,16,17]. However, some challenges need to be addressed, namely, (1) limitations of substrate and oxygen diffusion, which can increase population heterogeneity, (2) the complex maintenance of a pure culture in consecutive operations, (3) biofilm reactors are difficult to scale up [17], and (4) product secretion to the extracellular medium can be challenging, leading to difficulties in downstream processes [15].
Several studies propose the application of biofilms as robust, self-immobilized, and self-regenerating systems in the production of added-value chemicals and specific proteins [11,18,19]. Hence, this review intends to outline the advances in the production of recombinant proteins using biofilms, as well as to give an overview of the main added-value compounds produced using biofilms as a biocatalytic system.
2. Production of Added-Value Chemicals
In recent years, microbial biofilms have emerged as a new generation of biocatalysts due to their potential for the sustainable production of added-value chemicals [16,20,21], including organic acids, enzymes, polysaccharides, antimicrobial compounds, alcohols and solvents, and other products (Figure 1). This production typically resorts to a variety of biofilm reactors in which microorganisms attach to support materials [11,17,18,19]. The most common reactor types used to produce these substances are stirred-tank [22,23,24] and packed-bed reactors [25,26,27]. The packed-bed reactor is usually filled with densely packed solid supports, which provide high interfacial areas, whereas stirred-tank reactors integrate inserts and/or particles [19]. Additionally, membrane biofilm reactors with a porous gas-permeable membrane (e.g., silicone [28,29] and polysulphone [30]) are often used for these bioreactions [28,29]. Other configurations include fluidized-bed reactors [31,32], airlift reactors [33,34], bubble column reactors [35,36], rotating-disk reactors [37,38], or tubular biofilm reactors [39]. Thus, the choice of the reactor and feeding strategy (batch, fed-batch, and continuous mode) should be molded to the process conditions and nutritional requirements of the producing microorganisms.
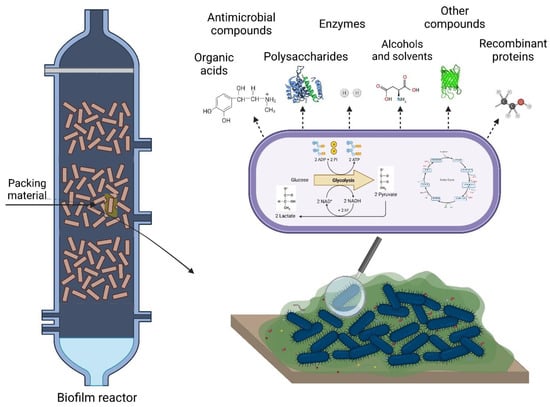
Figure 1.
Added-value compounds produced in biofilm reactors.
Several support materials have been employed for the immobilization of microorganisms in high biomass concentrations inside the reactors. The supports must be prone to adhesion of microorganisms, be widely available and inexpensive, resist high mechanical forces, and be non-toxic [16,18,19]. Synthetic materials employed as supports in biofilm reactors may include ceramics [26,40], silicone [41,42], polyethylene [43,44,45], polyurethane [46,47], clay bricks [27], polypropylene [48], and glass [39]. Natural polymers, such as alginate [49,50] and carrageenan [22], and some lignocellulosic materials, such as cotton [51,52], have also been used to immobilize microbial cells. Many agriculture-based waste materials have been used to create biofilm supports, such as corn stalks [53] or charcoal pellets produced from waste mushroom medium [54]. A good example is the loofah sponge, an inexpensive and environmentally friendly support matrix obtained from the ripped dried fruit of Luffa aegyptica [55,56], applied to produce lactic and gibberellic acids. Furthermore, many studies extensively implemented a specific class of plastic composite supports (PCS) for biomass immobilization due to the channeling of agricultural wastes to produce valuable compounds [24,38,57,58,59,60]. PCS are a blend of polypropylene, nutritious agricultural materials (e.g., oat hulls, soybean flour, and cornstarch), microbial nutrients (e.g., yeast extract, and bovine albumin), and mineral salts [60,61,62], usually produced in the form of chips [57], rings/disks [60], or tubes fixed to the agitator shaft of stirred-tank biofilm reactors [63]. Hence, this support simultaneously provides attachment areas for biofilm development and nutrients for the growth and synthesis of products.
Since the production of chemicals and fuels through biocatalytic processes in biorefineries is strongly impacted by raw material costs [64], it is driven by the utilization of renewable feedstocks, low in cost, abundant, and readily available, to sustainably produce commercially valuable products [65]. These raw materials do not compete with food crops and often comprise industrial wastes such as whey and milk permeates [25,55], molasses [46], olive mill wastewater [26], potato starch [56], and rice straw [66]. Potato waste and rice straw hydrolysates were used by Izmirlioglu and Demirci [67] and Todhanakasem et al. [68], respectively, as fermentation media to benefit from available and inexpensive waste materials to make ethanol production more sustainable. In another study, a complex medium containing the liquid fraction of deacetylated corn stover hydrolysate was used as a substrate for succinic acid production [65]. Although renewable feedstock may be cost-effective, their commercial feasibility requires a compromise between material costs and fermentation productivities and yields. Since the nutrients might be less accessible to microbial consumption, sometimes an additional step is needed to make their carbohydrate fraction available for microbial conversion [69]. During this pre-treatment, inhibitory compounds are produced, which can decrease production rates and yields, demanding an extra step for the removal of these substances and increasing the process costs [70]. Biofilms can tolerate such hazardous environments more easily than suspended cells, conferring a great advantage in this case.
2.1. Organic Acids
The production of a wide variety of organic acids in biofilm reactors is very popular due to their higher robustness to changing environmental conditions, in particular, a decrease in pH [19]. The organic acids produced in these systems include lactic, succinic, acetic, citric, fumaric, gibberellic, glycolic, propionic, and kojic acids (Table 1). Ho and colleagues reported a few studies on the production of lactic acid (widely used in chemical, pharmaceutical, and food industries [71]) in biofilm reactors, studying the characteristics of PCS and their effects on biofilm formation and lactic acid production [72], and the effects of different agricultural components on the properties of PCS [73]. These supports stimulated biofilm formation and improved the productivity of lactic acid in repeated-batch fermentations up to 4.3 g·L−1·h−1 at a starting glucose concentration of 100 g·L−1 [62]. The immobilized cells shortened the total fermentation time up to 61% and increased the lactic acid productivity of L. casei up to 70% relative to suspended cells. Following this, the fungi Rhizopus oryzae was used by Tay and Yang [51] to produce lactic acid in a rotating fibrous bed bioreactor. Glucose and cornstarch were the fermentation substrates tested. The highest lactic acid productivity of 2.5 g·L−1·h−1 was obtained from glucose in fed-batch fermentation with a yield of 90%, whereas a lactic acid yield close to 100% was achieved with cornstarch, despite the lower productivity of 1.65 g·L−1·h−1. Moreover, the immobilization with the cotton cloth restrained control and operation problems in the reactor observed with freely suspended fungal cells. More recently, Cuny et al. [39] used Lactobacillus delbrueckii to produce lactic acid in a horizontal tubular biofilm reactor. This biofilm system was operated in continuous mode for 3 weeks under different flow velocities and demonstrated good stability. The productivity increased with the flow velocity since, at low flow velocities, the higher retention times cause a strong pH drop generated by lactic acid accumulation, inhibiting the growth rate and production. The maximum productivity obtained was 10 g·L−1·h−1 with a product yield of 94%. The biofilm system demonstrated superior cell density and productivity of lactic acid over a batch culture by a factor of 19 and 6–8, respectively.
Urbance et al. [63,74] reported two works on the production of succinic acid by Actinobacillus succinogenes using PCS for biofilm formation. In their first study, they developed a medium supporting the growth and succinic acid production by A. succinogenes and screened customized PCS blends for cell immobilization and succinic acid production [74]. Then, the effectiveness of these supports was evaluated in repeated-batch and continuous fermentation with immobilized and suspended-cell systems [63]. For the continuous mode in the PCS bioreactor, as the dilution rate increased, succinic acid final concentrations and percentage yields decreased while productivity increased. A maximum of 8.8 g·L−1·h−1 was reached at a dilution rate of 1.2 h−1, whereas a maximum productivity of 7.0 g·L−1·h−1 was obtained at a dilution rate of 1.0 h−1 for suspended culture. In batch fermentation, A. succinogenes was able to tolerate high initial glucose concentrations. However, the overall production rate was higher at lower glucose concentrations (0.9 g·L−1·h−1), which suggests the need to continuously remove the succinic acid from the fermentation broth due to product inhibition.Another series of studies exploring the continuous production of succinic acid was performed by Bradfield and colleagues [42,65,75]. In their last study, Bradfield and Nicol [42] employed different types of biofilm supports (tightly bound wooden sticks, silicone-tubing segments, and loosely spaced wooden sticks) in three separate fermentations using a xylose feed stream. The results showed succinic acid yields on xylose of 0.55–0.68 g·g xylose−1, titers of 10.9–29.4 g·L−1, and productivities of 1.5–3.6 g·L−1·h−1 at different dilution rates. Although these levels were lower than the maximum achieved on glucose (4.4 g·L−1·h−1) in their previous work [75], the authors believe that succinic acid productions on xylose and glucose are comparable, suggesting that industrially relevant biomass feedstocks can be employed in the production of valuable compounds. Moreover, Ferone et al. [76] investigated the continuous anaerobic production of succinic acid by A. succinogenes for more than 5 months in a packed-bed biofilm reactor with Tygon rings as immobilization support. The bioreactor was fed with a synthetic medium simulating the composition of a lignocellulosic hydrolysate and carbon dioxide (CO2) for the succinic acid production pathway. The maximum succinic acid productivity (35 g·L−1·h−1) was obtained using glucose as a carbon source at a dilution rate of 1.9 h−1 and was the highest productivity reported so far using biofilms reactors. However, the optimum balance between succinic acid concentration, productivity, and sugar conversion was obtained at a dilution rate of 0.5 h−1 (43 g·L−1, 22 g·L−1·h−1, and 88% glucose conversion, respectively).
In addition to lactic acid, R. oryzae was employed by Cao et al. [77,78] in the production of fumaric acid from glucose in a rotating-disk biofilm reactor with polysulfone plastic disks mounted on a horizontal shaft. The authors created an integrated system of simultaneous/continuous production and recovery of fumaric acid by an adsorption column coupled to the reactor [78]. When R. oryzae produces fumaric acid, a decrease in the pH below a certain threshold may stop the fermentation. Therefore, adsorbent resins were used to remove the free acid and moderate the decrease in pH, thereby enhancing the fermentation rate and maintaining cell viability. As a result, this biofilm reactor reached a concentration of fumaric acid of 85 g·L−1, a yield of 91% (w/w), and maximum productivity of 4.25 g·L−1·h−1 within 20 h (compared to 72 h in the suspended-cell reactor). Conversely, in a stirred-tank fermentation, the productivity was 0.9 g·L−1·h−1, about 5 times lower than with biofilms. The same rotating-disk reactor was operated, supplementing the medium with CaCO3 to neutralize the pH, as an alternative to the adsorbent unit, and the fumaric acid productivity in the biofilm reactor was 3.78 g·L−1·h−1 within 24 h, about 5 times higher than with the stirred-tank fermenter, and the fermentation time was shortened by one-third [77]. The biofilm reactor was operated for 2 weeks without loss of biological activity.
A rotating-disk biofilm reactor was similarly used by Wang et al. [79] to produce citric acid by Aspergillus niger immobilized in polyurethane foam disks. The volumetric productivity obtained with the immobilized cell culture was 0.9 g·L−1·h−1 (weight yield of 72%), about 3 times higher than a stirred-tank fermenter with suspended cell culture (0.33 g·L−1·h−1; weight yield of 60%). Additionally, the immobilized biofilm was active for eight repeated-batch cycles without losing bioactivity. More recently, Yu et al. [80] developed a new carrier material termed PAF201 (polymeric porous foam made of polyurethane and carbon black) for A. niger immobilization with improved citric acid yield and productivity levels. PAF201 demonstrated improved cell immobilization and glucose consumption compared with other materials. Moreover, this carrier reduced the fermentation period (72 h) compared to planktonic cells (96 h). In a repeated fed-batch fermentation, the production of citric acid using cassava medium and immobilized A. niger showed maximum citric acid yields, concentrations, and productivity of 90%, 163 g·L−1, and 2.26 g·L−1·h−1, respectively, which were kept constant in all batches, demonstrating long-term stability. On the other side, the citric acid productivity of the suspended cell system was almost half of immobilized fermentation (1.41 g·L−1·h−1).
As for acetic acid, Horiuchi et al. [54] operated a packed-bed reactor with Acetobacter pasteurianus immobilized in charcoal pellets. The acetic acid productivity reached a maximum of 6.5 and 3.9 g·L−1·h−1 with a supply of O2-enriched air (40%) and normal aeration, respectively, indicating that the process was limited by oxygen transfer. The charcoal pellets were obtained at low cost from agricultural wastes and presented a porosity and specific surface area appropriate for bacterial adhesion; also promoting good operational stability since the system was continuously operated for 180 days. On the other side, Talabardon et al. [52] investigated the production of acetic acid from lactose and milk permeate, a by-product of the ultrafiltration of milk, using an anaerobic thermophilic co-culture of Clostridium thermolacticum and Moorella thermoautotrophica. In this fermentation process, C. thermolacticum converts lactose into lactic acid, which is thereby converted into acetic acid by M. thermoautotrophica. The fermentation kinetics were compared between a suspended cell reactor and an immobilized-cell fibrous-bed reactor in fed-batch fermentations at 58 °C. The acetic acid final concentration (22.0–22.5 g·L−1) and productivity (0.18–0.54 g·L−1·h−1) achieved in a fibrous-bed bioreactor using either lactose or milk permeate were significantly higher compared to those from the suspended cell fermentation (final concentration, 15 g·L−1; productivity, 0.06–0.08 g·L−1·h−1). The higher productivity of the fibrous-bed bioreactor was attributed to the higher cell density (20 g·L−1), approximately 10 times higher than in the planktonic culture (2 g·L−1). Additionally, the higher acetic acid yields and concentrations in the bioreactor were attributed to the mitigation of ethanol production as a by-product, and to the ability of the immobilized cells to adapt and tolerate higher product concentrations, respectively.
Gibberellic acid was produced from a milk permeate by the fungi Fusarium moniliforme immobilized in loofah sponge disks [55]. The effect of incubation temperature, initial pH, number of disks, and its reusability for gibberellic acid production was evaluated. The best gibberellic acid productivity of 15.6 mg·L−1·h−1 was reached at pH 5 after 6 days of incubation. Additionally, the F. moniliforme cells immobilized on the loofah sponge were reused in repeated batches and showed high production stability.
Liu et al. [59] used a PCS-immobilized bioreactor to produce kojic acid (an acid with strong metal chelating capacity widely used in cosmetic and food industries) by Aspergillus oryzae in repeated-batch fermentations. The use of a nitrogen-deficient (Ndef) medium created differences in mycelium morphology between the free suspension and the PCS-immobilized cultures. Mycelia in the Ndef medium had a feather-like structure, while in suspension, mycelia were more compact. These morphology changes were assumed to increase the surface area for absorbing more nutrients, which resulted in increased kojic acid production. In addition, RNA expression (kojA and kojT) under nitrogen starvation was 2.5 times higher than the control with full nitrogen, indicating that nitrogen deficiency influenced kojic acid production at the transcriptional level. This PCS immobilized fermentation system decreased the time needed to reach higher productions and productivities, where 83.47 g·L−1 of kojic acid was produced with a productivity of 3.09 g·L−1·day−1, which is higher than free-suspension in batch fermentation.

Table 1.
Different classes of organic acids produced in biofilm reactors.
Table 1.
Different classes of organic acids produced in biofilm reactors.
| Product | Producers | Substrate | Immobilization Material | Reactor Type | Process Time (h) | Maximum Productivity (g·L−1·h−1) | Productivity Increment c | Ref. |
|---|---|---|---|---|---|---|---|---|
| Lactic acid | Lactobacillus casei subsp. rhamnosus | Glucose as CS and YE as NS | PCS | Packed-bed reactor (B) b | 1584 | 4.3 | 1.5 | [62,72,73] |
| Stirred-tank reactor (C) | n.d. | 9.88 | n.a. | [24] | ||||
| Lactobacillus delbrueckii | Glucose as CS and YE as NS | Glass | Tubular biofilm reactor (C) | 504 | 10 | 6–8 | [39] | |
| MRS medium with molasses as CS | Polyurethane foam | Packed-bed biofilm reactor and stirred-tank reactor (C) | 1000 | 5 | 4 | [46] | ||
| Rhizopus oryzae | Glucose and cornstarch as CS | Cotton cloth | Rotating fibrous bed bioreactor (FB) | 200 | 2.5 | n.a. | [51] | |
| Potato starch | Loofah sponge | Airlift reactor (B) | 48 | 5 g·L−1 | 1.7 | [56] | ||
| Succinic acid | Actinobacillus succinogenes | Xylose as CS and YE as NS | Wooden sticks and silicone-tubing segments | n.d. (C) | 1500 | 3.6 | n.a. | [42] |
| Glucose as CS and YE as NS | PCS | Stirred-tank reactor (B, C) b | n.d. | 8.8 | 1.25 | [63,74] | ||
| Glucose and CO2 as CS, and YE as NS | Poraver beads | Packed-bed reactor (C) | 80 | 10.8 | n.a. | [81] | ||
| Tygon rings | 3600 | 35 | n.a. | [76] | ||||
| Fumaric acid | Rhizopus oryzae | Glucose as CS | Polysulfone plastic disks | Rotary biofilm contactor (FB) b | 20 a | 4.25 | 5 | [77,78] |
| Citric acid | Aspergillus niger | Sucrose as CS | Polyurethane foam | Rotary biofilm contactor (FB) b | 120 a | 0.90 | 3 | [79] |
| Sucrose and sugar cane juice as CS | Cellulose microfibrils | Recycle reactor (C, FB) | 624 | 2.08 | 1.8 | [82] | ||
| Glucose as CS dissolved in wheat bran extract and cassava-based medium | Polyurethane and carbon black foam | Flasks (FB) b | 72 a | 2.26 | 2 | [80] | ||
| Acetic acid | Acetobacter pasteurianus | Glucose as CS and ethanol as BS | Charcoal pellets | Packed-bed reactor (C) | 4320 | 6.45 | n.a. | [54] |
| Clostridium thermolacticum and Moorella thermoautotrophica | Lactose and milk permeate as CS and trypticase and YE as NS | Cotton towel overlaid with a stainless-steel wire cloth | Fibrous-bed bioreactor (B, FB) b | 336 a | 0.54 | 6 | [52] | |
| Propionic acid | Propionibacterium acidipropionici | Sorghum bagasse hemicellulosic hydrolysate | Sorghum bagasse | Glass column (B) b | 146 | 1.17 | 6 | [83] |
| Glycolic acid | Pseudomonas diminuta | Ethylene glycol as the BS | Stainless steel structured packing | Aerated trickle-bed biofilm reactor (C) | 1536 | 1.6 | 5 | [84] |
| Gibberellic acid | Fusarium moniliforme | Milk permeate | Loofah sponge | Shaking flask (B) b | 144 | 1.6 × 10−2 | 1.4 | [55] |
| Gluconic acid | Aspergillus niger | Deproteinized whey | Polyurethane foam | Erlenmeyer flasks (B) | 72 | 92 g·L−1 | 1.33 | [85] |
| Fatty acids (acetate, propionate, and butyrate) | Methanogens and acid-producing bacteria | Methane as BS | Hollow fiber membranes | Membrane biofilm reactor (B) b | 12 a | 0.42 | n.a. | [86] |
| Kojic acid | Aspergillus oryzae | Glucose as CS | PCS | Shaking flasks (B) b | 648 | 0.13 | >1 | [59] |
a batch duration; b repeated-batch or fed-batch mode; c Productivity increment corresponds to the productivity ratio between biofilms and suspended cell processes. When productivity increment is not reported, it was calculated as the ratio between the maximum productivity obtained with biofilms and the maximum productivity obtained with planktonic cultures. Abbreviations: B, batch culture; C, continuous feeding; FB, fed-batch culture; CS, carbon source; YE, yeast extract; NS, nitrogen source; BS, biotransformation substrate; PCS, plastic composite supports; MRS, De Man, Rogosa, and Sharpe broth; n.a., not applicable; n.d., not described.
2.2. Enzymes
The production of enzymes by the application of biofilm reactors has been scarcely investigated (Table 2). The production of cellulase, a lignocellulosic material with applicability in biofuel production and textile, paper, and pulp industries [87], using biofilm reactors was firstly reported by Webb et al. [88] using Trichoderma viride immobilized on stainless steel particles in a spouted-bed fermenter. They obtained a volumetric productivity of 31.5 U·L−1·h−1, which was more than three times higher compared with suspended cells. Since then, a few studies were performed on cellulase production in biofilms. Hui et al. [89] examined the stability of the Aspergillus terreus suspended cells and immobilized onto woven nylon pads with respect to cellulase production under repeated-batch fermentations. They found that the immobilization extended enzyme production for longer periods (about 120 days vs. 40 days) with a nearly 4.5-fold increase in productivity (with a cumulative enzyme activity of 453 U compared to 114 U) when compared to suspended cells.
Other ligninolytic enzymes, lignin (LiP) and manganese (MnG) peroxidases, were produced by the white-rot fungus Phanerochaete chrysosporium. Solomon and Petersen [30] described the production of these ligninolytic enzymes in a polysulfone membrane gradostat bioreactor. The study of the effect of operating parameters on enzyme production revealed higher activities at higher temperatures and lower glucose and ammonium concentrations. The maximum LiP and MnP were 35 and 96 U·L−1, respectively. The same biofilm system was used by Govender et al. [90] for the continuous production of MnP. In an initial screening, the authors optimized the effect of nutrient additives (Mn2+, Tween 80, and soybean-derived phospholipids) and oxygenation on MnP production and biofilm morphology and physiology. Oxygenation tangential to the biofilm has shown higher peroxidase activity (112 U·L−1) compared with oxygenation via a side arm (39 U·L−1) and bubbling O2 into the media (66 U·L−1). Additionally, the nutrient additives enhanced MnP activity both individually and when combined, resulting in a 58% increase in peroxidase activity compared to the conventional medium and a productivity of 1.3 U·L−1·h−1 under optimal conditions. In addition, Khiyami et al. [23] investigated the production of LiP and MnP in a biofilm stirred tank reactor holding PCS tubes. The addition of veratryl alcohol, a production activator, and aeration effectively improved the yield. The highest LiP and MnP activities were 50 and 63 U·L−1, respectively.
Yang et al. [91] described the application of Rhizopus arrhizus immobilized in polyurethane for lipase production. Lipase production was optimized regarding culture conditions where temperatures under 27 °C, a neutral pH, increasing levels of aeration, and the use of soybean flour and oils as nitrogen and carbon sources, respectively, enhanced lipase production and activity. The lipase productivity of immobilized cells during the repeated-batch fermentation in 250 mL flasks (17.6 U·mL−1·h−1) was about three times higher than a 5 L fermentor (6.1 U·mL−1·h−1), and the fermentation time was also shortened (nine and six consecutive batches in 140 h, respectively). This demonstrates the difficulty in reproducing the lab-scale results in large-scale biofilm reactors.

Table 2.
Different classes of enzymes produced in biofilm reactors.
Table 2.
Different classes of enzymes produced in biofilm reactors.
| Product | Producers | Substrate | Immobilization Material | Reactor Type | Process Time (h) | Maximum Productivity (U·L−1) | Productivity Increment c | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cellulase | Trichoderma viride | Glucose as CS | Stainless steel spheres | Spouted-bed reactor (C) | 336 | 31.5 U·L−1·h−1 | 3 | [88] |
| Aspergillus niger | Ground rice straw | Celite and polyurethane foams | Bubble column fermenter and shaking flasks (B) | 168 | 1400 | 2 | [66] | |
| Aspergillus terreus | Cellulose as CS | Woven nylon pads | n.d. (B) b | 2880 | 2400 | 4.5 | [89] | |
| Lignin peroxidase and Manganese peroxidase | Phanerochaete chrysosporium | Glucose as CS | Polysulfone capillary membrane | Membrane gradostat bioreactor (C) | 336 | LiP = 35 MnP = 96 | n.a. | [30] |
| PCS | Stirred-tank reactor (B) b | 144 a | LiP = 50 MnP = 63 | n.a. | [23] | |||
| Polystyrene foam | Shaking flasks (B) | 192 | MnP = 421 | 1.2 | [92] | |||
| Phospholipid-rich medium | Polysulfone capillary membrane | Membrane gradostat bioreactor (C) | 552 | 1.3 U·L−1·h−1 | n.a. | [90] | ||
| Lipase | Rhizopus arrhizus | Peanut oil as CS and soybean flour as NS | Polyurethane | Shaking flasks (B) b | 140 | 1.76 × 104 U·L−1·h−1 | n.a. | [91] |
a batch duration; b repeated-batch or fed-batch mode; c Productivity increment corresponds to the productivity ratio between biofilms and suspended cell processes. When productivity increment is not reported, it was calculated as the ratio between the maximum productivity obtained with biofilms and the maximum productivity obtained with planktonic cultures. Abbreviations: B, batch culture; C, continuous feeding; SC, semi-continuous feeding; CS, carbon source; PCS, plastic composite supports; LiP, Lignin peroxidase; MnP, Manganese peroxidase; U, activity unit; n.a., not applicable; n.d., not described.
2.3. Polysaccharides
Compared to the other substances, the production of polysaccharides using biofilm reactors has barely been studied (Table 3). Bacterial cellulose was successfully produced by Cheng et al. [93,94] using Acetobacter xylinum immobilized in a PCS biofilm reactor. The high biomass density accumulated on the PCS resulted in a bacterial cellulose production of 7.05 g·L−1, about 2.5-fold higher than with the suspended-growth reactors (2.82 g·L−1) [94]. Moreover, improved mechanical properties (elastic deformation, strain at break, and mechanical strength) and thermal stability were observed for the PCS-grown bacterial cellulose. Higher production values were obtained more recently by Rahman et al. [95] which, similarly to Meleigy and Khalaf [55], used a natural loofah sponge as a scaffold for cell immobilization, in this case, using Gluconacetobacter kombuchae for the production of bacterial cellulose for 15 days. Bacterial cellulose production was compared between immobilized and non-immobilized cells, where immobilization on loofah supports resulted in approximately two times more product than in the absence of support. Moreover, several cultivation parameters were analyzed and optimized, including the initial pH, static or shaking conditions, inoculum size, nitrogen source, carbon/nitrogen ratio, and supplements that facilitate cellulose production (ethanol and acetic acid). A maximum cellulose production of 24 g·L−1 was obtained under shaking conditions, at an initial pH of 5.5, using yeast extract as a nitrogen source and a C/N ratio of 40 supplemented with ethanol.
Likewise, pullulan production was extensively investigated by Cheng et al. using Aureobasidium pullulans immobilized in PCS tubes connected to a stirred-tank reactor [58,96,97,98]. First, they tested numerous types of PCS with different compositions and assessed the effects of various pH profiles on pullulan production and biofilm formation [58]. A pullulan concentration of 32.9 g·L−1 with a purity of 96% was achieved in the biofilm reactor, which was 1.8 times higher than in a cell suspension, although the production rate was lower (0.44 g·L−1·h−1 vs. 0.68 g·L−1·h−1, respectively). Subsequently, they optimized the concentrations of sucrose and nitrogen sources (ammonium sulfate and yeast extract) in the medium for pullulan production using a Response Surface Methodology [96]. Medium optimization improved pullulan production up to 60.7 g·L−1 in 7 days, which was 2.4-fold higher than suspensions. Lastly, the effects of different concentrations of ammonium sulfate and sucrose and dilution rates were evaluated for continuous pullulan production [98]. The maximum pullulan production rate was improved compared with their previous studies (1.33 g·L−1·h−1 at a dilution rate of 0.16 h−1).
Additionally, Mesquita et al. [99] studied the production of xanthan gum with Xanthomonas campestris immobilized in polyurethane, and evaluated the storage stability and capacity for recycling the immobilized cells. The volumetric xanthan productivity with immobilized cells (0.62 g·L−1·h−1) was higher than in suspended-growth culture (0.12 g·L−1·h−1), indicating that immobilization improved the production of xanthan gum. Additionally, the immobilized cells demonstrated the capacity to be reused up to six times without losing significant activity. In a more recent study, Nejadmansouri et al. [100] compared the production of xanthan gum on different types of supports, demonstrating the improvement in xanthan production compared with the control without supports.

Table 3.
Different classes of polysaccharides produced in biofilm reactors.
Table 3.
Different classes of polysaccharides produced in biofilm reactors.
| Product | Producers | Substrate | Immobilization Material | Reactor Type | Process Time (h) | Maximum Productivity (g·L−1·h−1) | Productivity Increment b | Ref. |
|---|---|---|---|---|---|---|---|---|
| Bacterial cellulose | Acetobacter xylinum | Corn steep liquor with fructose as CS | PCS | Stirred-tank reactor (B) | 120 | 5.9 × 10−2 | 2.5 | [93,94] |
| Gluconacetobacter kombuchae | Sucrose as CS and YE as NS | Loofah sponge | Shaking flasks (B) | 360 | 6.7 × 10−2 | 2 | [95] | |
| Gluconacetobacter xylinum | Corn steep liquor with fructose | PCS | Rotating-disk bioreactor (B) a | 120 | 1.0 × 10−2 | n.a. | [38] | |
| Pullulan | Aureobasidium pullulans | Sucrose as CS, ammonium sulfate and YE as NS | PCS | Stirred-tank reactor (B, C, FB) | 168 | 1.33 | 3 | [58,96,97,98] |
| Xanthan gum | Xanthomonas campestris | YM medium with sucrose as CS | Polyurethane | Shaking flask (B) | 96 | 0.62 | 3.6 | [99] |
| YPD broth | Polyethylene | n.d. (B) | 72 | 8 g·L−1 | 2.5 | [100] |
a repeated-batch or fed-batch mode; b Productivity increment corresponds to the productivity ratio between biofilms and suspended cell processes. When productivity increment is not reported, it was calculated as the ratio between the maximum productivity obtained with biofilms and the maximum productivity obtained with planktonic cultures. Abbreviations: B, batch culture; C, continuous feeding; FB, fed-batch culture; CS, carbon source; YE, yeast extract; NS, nitrogen source; PCS, plastic composite supports; n.a., not applicable; n.d., not described.
2.4. Antimicrobial Compounds
Antibiotic production is usually performed using suspended-cell cultures [49]. However, cell immobilization proved to be efficacious and enhanced the productivity of antibiotics (including neomycin and cephalosporin) and other antimicrobial compounds such as bacteriocins and other proteins with bactericidal activity (Table 4) [101]. Pediococcus acidilactici immobilized on κ-carrageenan/locust bean gum gel beads was explored by Naghmouch et al. [102] for pediocin production in MRS broth and supplemented whey permeate medium. Pediocin is a bacteriocin with inhibitory action against some food-borne pathogenic and spoilage microorganisms involved in foodborne outbreaks [103]. An increased pediocin volumetric productivity was obtained in a repeated-cycle batch with immobilized cells (5461 AU·mL−1·h−1) compared with free cells (342 AU·mL−1·h−1). Moreover, the maximum activity of pediocin was reached after 0.75- and 2-h incubation cycles in MRS broth and whey permeate medium (2048 AU·mL−1·h−1), respectively, indicating the feasibility of using a low-cost medium such as whey permeate for high pediocin production.
Liu et al. [104] used a similar reactor type and immobilization supports for nisin production (a biopreservative for the food industry [105]) by Lactococcus lactis. Laboratory media (5.2 × 107 AU·L−1·h−1) and whey permeate (1.0 × 107 AU·L−1·h−1) originated similar productivities, which introduced whey permeate as an economical alternative for sustainable production of bacteriocins. Furthermore, the bioreactor was continuously operated for 6 months without clogging or contaminations, indicating long-term stability. Pongtharangkul and Demirci [106,107,108,109] also performed a set of studies on nisin production using a biofilm reactor with PCS tubes immobilizing L. lactis. The high biomass density attained with biofilm reactors was reflected in a shorter lag time of nisin production in comparison to the suspended-cell reactor, and sucrose (1100 IU·mL−1) increased nisin production substantially by 1.9-fold as related to glucose (579 IU·mL−1); however, high concentrations of sucrose stimulated lactic acid production, negatively affecting nisin production, as well as high magnesium concentrations [106]. Additionally, the levels of nisin production were greatly affected by the pH, andproduction in the biofilm reactor (3553 IU·mL−1) was about 1.8 times higher than in the suspended-cell system (2018 IU·mL−1) [107]. In a fed-batch fermentation, nisin production was enhanced for both suspended-cell (4188 IU·mL−1) and biofilm (4314 IU·mL−1) reactors, achieving 1.8- and 2.3-fold higher nisin titers than their respective batch fermentation due to the mitigation of substrate limitation and product inhibition [108]. Lastly, the implementation of an online recovery unit of silicic acid (adsorbent) coupled with a micro-filter module successfully recovered nisin from the fermentation broth and significantly improved nisin production (7445 IU·mL−1), approximately 4-fold when compared with the batch fermentation without the online recovery (1897 IU·mL−1) [109].
Srivastava and Kundu [34] produced Cephalosporin-C using Cephalosporium acremonium immobilized on an inert porous Siran carrier in an airlift reactor. Cephalosporin-C productivity was significantly improved in biofilm reactors (7.1 × 10−3 g·L−1·h−1) compared to suspended cell cultures (4.3 × 10−3 g·L−1·h−1). By using a similar reactor, Srinivasulu et al. [49] immobilized Streptomyces marinensis in alginate beads to produce neomycin, and also compared the effect of dilution rate and the use of planktonic cells on volumetric productivity. The maximum neomycin productivity with immobilized cells was 7.5 × 10−3 g·L−1·h−1 at a dilution rate of 0.065 h−1, about 2.5 times higher than with suspended cells.
More recently, Ercan and Demirci [110,111,112,113] performed stepped studies on the production of human lysozyme using the fungi Kluyveromyces lactis on PCS-grid biofilm reactors. Lysozyme is a lytic enzyme targeting bacterial cell walls with application in medicine, cosmetics, and food industries. Firstly, the growth conditions of K. lactis and the fermentation medium were optimized to maximize lysozyme production and biofilm formation on PCS [110,111]. The optimum conditions for lysozyme and biomass productions were different, so a shift in pH and aeration was done after biofilm formation to increase lysozyme secretion, achieving a lysozyme production of 173 U·mL−1. Later, the authors conducted fed-batch and continuous fermentations under the optimum operation conditions determined above [112]. Regarding the fed-batch fermentation, an initial feeding of glucose and continuous addition of lactose showed the highest lysozyme concentration and productivity (187 U·mL−1 and 5.9 U·mL−1·h−1, respectively) compared to their previous study in batch conditions (173 U·mL−1 and 4 U·mL−1·h−1). Continuous fermentation also supported significantly higher productivity (7.5 U·mL−1·h−1) over batch and fed-batch fermentations in a biofilm reactor and suspended cell reactor (4 U·mL−1·h−1). Finally, fermentation in a biofilm reactor was coupled to an online recovery system using silicic acid as an adsorbent to enhance lysozyme production and recovery [113]. The adsorption and desorption conditions of the recovery system were optimized, accomplishing 96% lysozyme adsorption and 98% desorption. The simultaneous fermentation and online lysozyme recovery improved the production to 280 U·mL−1, which was 63% higher than without the online recovery system (173 U·mL−1), demonstrating, just as Pongtharangkul and Demirci [109] did, that the use of recovery systems to recuperate bioactive compounds during fermentation has great potential to enhance the effectiveness of these processes.

Table 4.
Different classes of antimicrobial compounds produced in biofilm reactors.
Table 4.
Different classes of antimicrobial compounds produced in biofilm reactors.
| Product | Producers | Substrate | Immobilization Material | Reactor Type | Process Time (h) | Maximum Productivity | Productivity Increment c | Ref. |
|---|---|---|---|---|---|---|---|---|
| Nisin | Lactococcus lactis subsp. lactis | Whey permeate | k-carrageenan/locust bean gum gel beads | Stirred-tank reactor (B) b | 1 a | 5.7 × 106 AU·L−1·h−1 | 6.7 | [22] |
| Lactose and whey permeate as CS | Spiral wound fibrous matrix | Packed-bed reactor (C) | 4320 | 5.2 × 107 AU·L−1·h−1 | n.a. | [104] | ||
| Sucrose as CS | PCS | Stirred-tank reactor (B, FB) b | 12 | 7.6 × 106 IU·L−1·h−1 | 1.8 | [106,107,108,109] | ||
| Pediocin | Pediococcus acidilactici | MRS medium | Spiral wound fibrous matrix | Packed-bed biofilm reactor (C) | 2160 | 4.2 × 105 AU·L−1·h−1 | n.a. | [114] |
| MRS medium and supplemented whey permeate medium | k-carrageenan/locust bean gum gel beads | Stirred-tank reactor (B) b | 0.75 a | 5.5 × 106 AU·L−1·h−1 | 16 | [102] | ||
| Cephalosporin-C | Cephalosporium acremonium | Sucrose as CS | Siran beads | Airlift reactor (FB) | 180 | 7.1 × 10−3 g·L−1·h−1 | 1.65 | [34] |
| Neomycin | Streptomyces marinensis | Maltose as CS | Alginate beads | Airlift reactor (C) | 16 | 7.5 × 10−3 g·L−1·h−1 | 2.5 | [49] |
| Erlenmeyer flasks | 96 | 6.7 × 10−2 g·L−1·h−1 | 1.3 | [115] | ||||
| Lysozyme | Kluyveromyces lactis | Lactose as CS | PCS | Stirred-tank reactor (B, C, FB) | 74 | 2.8 × 105 U·L−1 | 1.8 | [110,111,112,113] |
a batch duration; b repeated-batch or fed-batch mode; c Productivity increment corresponds to the productivity ratio between biofilms and suspended cell processes. When productivity increment is not reported, it was calculated as the ratio between the maximum productivity obtained with biofilms and the maximum productivity obtained with planktonic cultures. Abbreviations: B, batch culture; C, continuous feeding; FB, fed-batch culture; CS, carbon source; NS, nitrogen source; PCS, plastic composite support; MRS, De Man, Rogosa, and Sharpe broth; U, activity unit; AU, Anson unit; IU, international unit; n.a., not applicable.
2.5. Alcohols and Solvents
The production of alcohols and solvents, such as ethanol, butanol, and acetone, is a classic example of the use of biofilm reactors in the biotechnological scope (Table 5). Ethanol production was largely studied in different geometries of biofilm reactors. Kunduru and Pometto [116] investigated the continuous production of ethanol in a packed-bed reactor with PCS chips carrying Zymomonas mobilis or Saccharomyces cerevisiae in a long-term fermentation for 60 days. A maximum volumetric ethanol productivity of 536 and 76 g·L−1·h−1 were obtained for Z. mobilis and S. cerevisiae at dilution rates of 15 and 3 h−1, respectively, and these values were 100- and 15-fold higher than those obtained in suspension cultures. Later, lower productivity values of 2.31 g·L−1·h−1 were obtained by Izmirlioglu and Demirci [67] in a biofilm reactor with PCS-grid tubes immobilizing S. cerevisiae. The optimal growth parameters for S. cerevisiae in this biofilm reactor were found to be 34 °C, pH 4.2, and 100 rpm, reaching an ethanol concentration of 37 g·L−1 and a theoretical yield of 92%. The high porosity of PCS increased the surface area and established a very dense biofilm. In a different study, Shen et al. [117] surpassed the mass transfer limitations commonly observed in ethanol production by syngas fermentation through the use of a horizontal rotating packed bed (h-RPB) reactor. Biofilms of Clostridium carboxidivorans were immobilized on high-density polyethylene carriers and contacted the liquid and headspace alternately by the continuous reactor rotation. The gas transfer was more prominent in the headspace phase of the h-RPB reactor, which contributed significantly to cell growth and ethanol production. The reactor was continuously operated for 190 days at various rotational speeds, headspace pressures, and dilution rates. The maximum ethanol titer and productivity were 7.0 g·L−1 and 6.7 g·L−1·day−1, respectively, achieved at a dilution rate of 0.96 day−1, which was about 3.3-fold higher than those obtained in continuous stirred-tank reactors. The combination of a simple mechanical design, inexpensive parts for assembly, low power, and high ethanol demands make this reactor system efficient for syngas fermentation.
Gross et al. [29] used two recombinant Pseudomonas sp. strains (Pseudomonas sp. strain VLB120 pBT10 and P. putida PpS81 pBT10) in a silicone membrane biofilm reactor to continuously produce 1-octanol from octane. The volumetric productivities of both biofilms were 0.74 and 1.3 g·L−1·day−1 for about 30 and 7 days, respectively, similarly to the suspended cell reactor (1.0 g·L−1·day−1). Bioreactor aeration enhanced octanol synthesis by P. putida and decreased synthesis by Pseudomonas sp. strain VLB120, possibly due to the metabolization of octanol by the host’s alcohol dehydrogenases.
More recently, Hoschek et al. [118] used a dual-species biofilm of cyanobacterium Synechocystis sp. and Pseudomonas taiwanensis, both carrying the recombinant cyclohexane monooxygenase responsible for the oxyfunctionalization of cyclohexane to cyclohexanol. Their complementary properties regarding O2 metabolism resulted in higher cell densities compared to single-species biofilms since P. taiwanensis consumed the O2 fed to the capillary reactor, avoiding the inhibition of the Synechocystis sp. growth. This cooperation enabled the continuous cyclohexane conversion in cyclohexanol for a month with a productivity of 0.2 g·L−1·h−1.
The production of solvents (acetone, butanol, and ethanol—ABE) by solventogenic Clostridia (e.g., Clostridium acetobutylicum and Clostridium beijerinckii) fermentation has been attempted using microbial biofilms in order to make ABE production environmentally favorable by the use of renewable resources such as corn derivates, whey permeates, or different molasses [119,120]. Lee et al. [121] investigated the production of butanol by suspended or polyvinyl alcohol-immobilized cultures of C. beijerinckii in batch and continuous fermentations. The ratio of acetone/butanol was affected by the addition of acetate and butyrate, which enhanced the production of solvents, presumably due to a shift in the metabolic pathway toward solvent production. The addition of butyrate significantly increased butanol production in both immobilized and freely suspended cells. During continuous mode, the butanol productivity and yield were 0.40 g·L−1·h−1 and 0.44 g-butanol·g-glucose−1, respectively, about 2 times higher than those obtained with suspended cells (0.22 g·L−1·h−1 and 0.24 g-butanol·g-glucose−1). Moreover, supplementation with butyrate shifted the acetone/butanol ratio to 1:3 and prevented strain degeneration for 150 days, even in the presence of high butanol concentrations. In turn, Napoli et al. [122] used C. acetobutylicum immobilized in Tygon rings loaded in a packed-bed reactor for continuous butanol production for 750 h under several operational conditions (dilution rates, pH, and substrate concentrations). A complex media supplemented with lactose and yeast extract was employed to reproduce the nutritional characteristics of cheese whey wastewater. The maximum butanol productivity was 4.4 g·L−1·h−1 at a dilution rate of 1.0 h−1. Ethanol and acetone were also produced at lower concentrations alongside butanol (butanol selectivity of 88%). In addition, the existence of pH gradients towards the bottom layers of the biofilms was demonstrated, requiring a pH in the bulk higher than the optimal pH for suspended cell processes.

Table 5.
Different classes of alcohols and solvents produced in biofilm reactors.
Table 5.
Different classes of alcohols and solvents produced in biofilm reactors.
| Product | Producers | Substrate | Immobilization Material | Reactor Type | Process Time (h) | Maximum Productivity (g·L−1·h−1) | Productivity Increment c | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ethanol | Zymononas mobilis | Glucose as CS and YE as NS | PCS | Packed-bed reactor (C) | 1440 | 536 | 100 | [116] |
| Rice straw hydrolysate | Plastic and corn silk composites carriers | Packed-bed reactor (B, C) b | 120 | YP/S = 0.47 g·g−1 | n.a. | [68] | ||
| Saccharomyces cerevisiae | Starch | Loofah sponge | Packed-bed reactor (B) b | 168 a | 0.25 | 1 | [123] | |
| Potato waste hydrolysate | PCS | Stirred-tank reactor (B) b | 48 | 2.31 | n.a. | [67] | ||
| Clostridium carboxidivorans | Fructose as CS and syngas as BS | AnoxKaldnes K1 carriers | Horizontal rotating packed-bed reactor (C) | 4560 | 0.28 | n.a. | [117] | |
| 1-Octanol | Recombinant Pseudomonas putida | Octane as BS | Silicone membrane | Biofilm membrane reactor (C) | 720 | 5.0 × 10−2 | 1.3 | [29] |
| Cyclohexanol | Synechocystis sp. and Pseudomonas taiwanensis | Cyclohexane as BS | Glass | Capillary reactor (C) | 720 | 0.2 | n.a. | [118] |
| 1,3-propanediol | Klebsiella pneumoniae | Glycerol as CS | Porous hydrophobic polyurethane | Fixed-bed reactor (FB) b | 1460 | 1.7 | 1.1 | [47] |
| ABE solvents (acetone, butanol, and ethanol) | Clostridia beijerinckii | Glucose as CS and YE as NS | Corn stalk pieces | Biofilm reactor (C) | 480 | 5.06 | 23 | [53] |
| Clostridium acetobutylicum | Lactose as CS and yeast extract as NS | Tygon rings | Packed-bed biofilm reactor (C) | 750 | 4.4 | n.a. | [122] |
a batch duration; b repeated-batch or fed-batch mode; c Productivity increment corresponds to the productivity ratio between biofilms and suspended cell processes. When productivity increment is not reported, it was calculated as the ratio between the maximum productivity obtained with biofilms and the maximum productivity obtained with planktonic cultures. Abbreviations: B, batch culture; C, continuous feeding; FB, fed-batch culture; CS, carbon source; YE, yeast extract; NS, nitrogen source; BS, biotransformation substrate; PCS, plastic composite support; YP/S, ethanol yield; n.a., not applicable.
2.6. Other Compounds
Apart from organic acids, enzymes, polysaccharides, alcohols, and antimicrobial substances, other chemicals such as hydrogen, (S)-styrene oxide, benzaldehyde, and dihydroxyacetone have been produced using biofilm reactors (Table 6). In similar studies, Manssouri et al. [44] and Inoue et al. [43] produced hydrogen from sucrose-based synthetic wastewater in stirred anaerobic sequencing batch biofilm reactors by an anaerobic sludge immobilized on low-density polyethylene pellets. Using different feeding strategies, maximum molar hydrogen productivities of 39.9 mol·m−3·day−1 (batch) and 81.2 mol·m−3·day−1 (fed-batch) were obtained, respectively. More recently, Kongjan et al. [124] compared the application of a granule up-flow anaerobic sludge blanket reactor and an up-flow anaerobic packed-bed reactor with plastic biofilm supports for the continuous production of hydrogen by using a microbial consortium composed of moderate thermophilic cultures. The H2 production rate and yield at the optimal cultivation conditions were higher for the granules reactor (0.63 L-H2·L−1·h−1 and 0.25 L-H2·g-xylose−1, respectively) compared with the biofilm reactor (0.55 L-H2·L−1·h−1 and 0.22 L-H2·g-xylose−1, respectively), with acetate and butyrate as the main metabolite products. However, the maximum H2 production rate of 0.81 L-H2·L−1·h−1 was achieved by the biofilm reactor, though the H2 yield was lower (0.16 L-H2·g-xylose−1). Lower production rate values were obtained by Renaudie et al. [125] using a continuous hollow fiber liquid/gas membrane bioreactor originally seeded with sludge from a wastewater treatment plant containing C. beijerinckii, Clostridium pasteurianum, and Enterobacter sp. A maximum hydrogen productivity of 0.26 L-H2·L−1·h−1 was achieved, with acetate and butyrate being the main metabolite products from the glucose feed.
Furthermore, some studies reported continuous (S)-styrene oxide production through the epoxidation of styrene using the engineered Pseudomonas sp. strain VLB120DC as a biocatalyst attached in biofilm membrane reactors. Gross et al. [126] reached a maximum (S)-styrene oxide volumetric productivity of 70 g·L−1·day−1 using a tubular membrane reactor with a silicone membrane. This process was conducted for more than 50 days with no substrate or product mass transfer limitations, although high biomass concentrations introduced diffusional limitations of oxygen. On the other side, Halan et al. [40] employed a membrane biofilm reactor equipped with a microporous central ceramic unit for aeration and cell attachment, and obtained a maximum (S)-styrene oxide productivity of 28 g·L−1·day−1.
Additionally, the production of dihydroxyacetone (DHA) by Gluconobacter oxydans immobilized on silicone-coated Ralu-rings was investigated by Hekmat et al. [41] using a packed-bed bubble column reactor. Although the DHA yield from glycerol fermentation with and without cell immobilization was similar (0.87 and 0.85 kg·kg−1, respectively), DHA productivity was improved from 3.7 kg·m−3·h−1 using suspended biomass to 5.9 kg·m−3·h−1 with immobilized cells. The silicone matrix was demonstrated to be biocompatible, durable, mechanically stable, and have high oxygen permeability.
More recently, Roukas [48] attempted the production of carotene by the fungus Blakeslea trispora in a modified rotary biofilm reactor (MRBR) with polypropylene disks mounted on a polypropylene shaft. The MRBR enhanced the carotene production six times at optimal conditions compared with the conventional stirred-tank reactor (57.5 and 9.4 mg·L−1·day−1).

Table 6.
Different classes of other added-value compounds produced in biofilm reactors.
Table 6.
Different classes of other added-value compounds produced in biofilm reactors.
| Product | Producers | Substrate | Immobilization Material | Reactor Type | Process Time (h) | Maximum Productivity (g·L−1·h−1) | Productivity Increment c | Ref. |
|---|---|---|---|---|---|---|---|---|
| Hydrogen | Anaerobic sludge | Sucrose-based synthetic wastewater | Low-density polyethylene | Stirred anaerobic sequencing batch biofilm reactor (FB, B) b | 2 a | 3.4 × 10−3 mol-H2·L−1·h−1 | n.a. | [43,44] |
| High-density polyethylene | Packed-bed reactor (C) | 336–504 | 0.12 L-H2·L−1·h−1 | n.a. | [45] | |||
| Species of Thermoanaerobacterium | Xylose as CS | Plastic carriers | Up-flow anaerobic packed-bed reactor (C) | 1368 | 0.81 L-H2·L−1·h−1 | n.a. | [124] | |
| Activated sludge | Glucose as CS | Hollow-fiber membrane module of polytetrafluoroethylene | Liquid/gas membrane bioreactor (C) | 92 | 0.26 L-H2·L−1·h−1 | n.a. | [125] | |
| Polyhydroxyalkanoates | Bacillus sp. | Mineral salt media with date syrup | PCS | Stirred-tank reactor (B) b | 30 a | 0.195 | 1.4 | [127] |
| Mixed microbial cultures | Acetic acid and fermented greenhouse residues | Biofilm carriers | Reactor tank | 5400 | 35 mg·g substrate−1·h−1 | n.a. | [128] | |
| (S)-Styrene oxide | Pseudomonas sp. strain VLB120ΔC | Glucose as CS and styrene as BS | Silicone membrane | Tubular membrane reactor (C) | 1200 | 2.92 | n.a. | [126] |
| Styrene as BS | Microporous ceramic aeration unit | Biofilm membrane reactor (C) | 720 | 1.17 | n.a. | [40] | ||
| Dihydroxyacetone | Gluconobacter oxydans | Glycerol as CS and YE as NS | Silicone-coated Ralu rings | Packed-bed bubble column reactor (FB) b | 432 | 5.9 | 1.6 | [41,129] |
| Poly(3-hydroxybutyrate) | Alcaligenes eutrophus | Glucose as CS | Anion exchange resin | Packed-bed reactor (C) | 74 | 0.04 | n.a. | [130] |
| Carotene | Blakeslea trispora | Glucose and corn steep liquor as CS | Polypropylene disks | Rotary biofilm reactor (C) | 144 | 2.4 × 10−3 | 6 | [48] |
a batch duration; b repeated-batch or fed-batch mode; c Productivity increment corresponds to the productivity ratio between biofilms and suspended cell processes. When productivity increment is not reported, it was calculated as the ratio between the maximum productivity obtained with biofilms and the maximum productivity obtained with planktonic cultures. Abbreviations: B, batch culture; C, continuous feeding; FB, fed-batch culture; SC, semi-continuous feeding; CS, carbon source; YE, yeast extract; NS, nitrogen source; BS, biotransformation substrate; PCS, plastic composite support; n.a., not applicable.
3. Recombinant Proteins
Recombinant proteins (RPs) are a type of proteins obtained by the isolation and engineering of the gene sequence that encodes the target protein, followed by its introduction into a selected expression vector (Figure 2) [131,132].
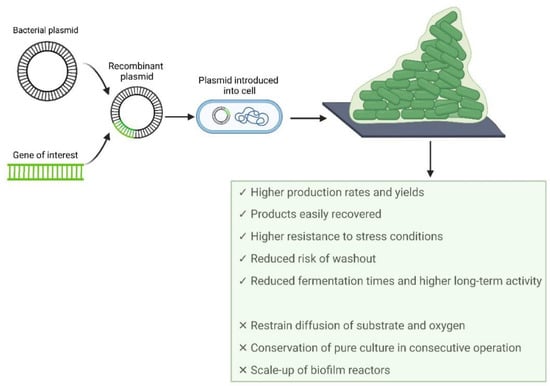
Figure 2.
Production of recombinant proteins in biofilms: advantages and limitations.
The production of recombinant proteins requires the selection of an expression system, which should consider transcriptional and translational issues [131,132], followed by the selection of a suitable host between bacteria, yeast, filamentous fungi, mammalian, plant, and insect cells [133,134]. Despite the variety of host cells available, the selection tends to be narrowed to a few options as the host selection should take into consideration the intrinsic protein properties, level of production, cell growth, scalability potential, regulatory issues, and production cost when moving towards the industrial scale [12,135]. However, due to the considerable differences in the physicochemical properties of proteins [136], it might be difficult to predict if a target protein will be obtained in a high amount and in an active form (for example, inclusion body formation or protein inactivity may impair the yield of the target protein) [132], often requiring the development of new strategies for optimizing the production of a recombinant protein. RPs have been used in different fields of everyday life like biotechnological, food, and medical industries (Table 7).

Table 7.
Recombinant proteins produced by biotechnological processes.
The production of recombinant proteins has been mainly performed in suspended cell cultures. However, some studies have revealed that biofilm reactors can be a more attractive platform for their production [146,147,148]. The insertion of a gene into a multicopy plasmid imposes an added metabolic burden on the host cell due to the metabolites and energy required for the replication of plasmid DNA and the synthesis of recombinant proteins [131,149]. In planktonic cells, these events often lead to a decrease in cellular growth and biomass yields and, consequently, a decrease in the production level of the target protein [149]. On the other hand, since cells in biofilms grow more slowly than their planktonic counterparts [150], fewer resources are channeled towards replication, reducing the metabolic burden associated with plasmid maintenance [148]. Additionally, an increase in biofilm formation was evidenced due to the presence of expression vectors in bacterial cells [146]. Since stress conditions can induce biofilm formation [151], the metabolic burden related to recombinant gene expression may stimulate biofilm formation [146] and increase the production of the target protein relative to planktonic cells [146,148,152].
The production of recombinant proteins using biofilm platforms has been scarcely studied in recent decades. It predominantly resorts to bacterial cells, such as Escherichia coli [148,153,154] or Bacillus subtilis [155], fungal cells, such as A. niger [140] or A. oryzae [141], and proteins such as β-galactosidase [156,157,158] and enhanced green fluorescent protein (eGFP) [146,147,152] (Table 8).
The production of recombinant proteins in biofilms was evaluated using different platforms: microplates [144,155], parallel-plate flow cell (PPFC) systems [156,157,158], and a modified Robbins device [146,153]. Microplates are regularly used for screening assays as they are easy to handle, high-throughput platforms, and can be used in static or controlled shaking conditions [159,160]. PPFC systems enable in situ and real-time visualization of cell adhesion and biofilm formation, and require a lower medium volume when compared to modified Robbins devices; however, PPFCs have lower throughput when compared to microplates and modified Robbins devices [161]. The Robbins device was first developed to monitor biofilm formation in water systems, and since then, several modifications have been introduced, wherein some used a custom-made and semi-circular flow cell with a set of characterized hydrodynamic features [160].
Recombinant protein synthesis in biofilms was first described in bacterial biofilms in 1992 by Huang et al. [156]. They tested the production of β-galactosidase in the E. coli DH5α strain using a PPFC system. The production of β-galactosidase was only quantifiable when isopropyl β-D-1-thiogalactopyranoside (IPTG) induction was performed, with the maximum production being obtained 24 h after induction with yields of 0.08, 0.1, and 0.12 pg·cell−1 for IPTG concentrations of 0.17, 0.34, and 0.51 mM, respectively [156]. Huang et al. [157] continued to study the production of β-galactosidase with another plasmid using the same host and cultivation and induction conditions. This study revealed that β-galactosidase production reached its peak for 0.17 and 0.34 mM IPTG with 0.027 and 0.036 pg·cell−1, respectively, after 36 h of induction, and 0.050 pg·cell−1 for 0.51 mM IPTG after 48 h of induction. Moreover, β-galactosidase mRNA synthesis rates increased 4-fold under 0.17 mM IPTG, and almost 12-fold under 0.34 and 0.51 mM IPTG after 36 h of induction. Nevertheless, the production of β-galactosidase did not follow the same ratio of mRNA synthesis rate, suggesting that mRNA was less stable at higher expression levels [157].

Table 8.
Synopsis of the published work on the production of recombinant proteins in bacterial biofilms.
Table 8.
Synopsis of the published work on the production of recombinant proteins in bacterial biofilms.
| Recombinant Protein | Host | Cultivation Conditions | Production Levels | Productivity Increment c | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reactor | Surface Material | Culture Medium | Temp.(°C) | Hydrodynamics | Time (Days) | Induction | |||||
| β-galactosidase | Escherichia coli DH5α (pMJR1750) | PPFC | Glass | M9 minimal | 37 | Laminar flow (Re = 20) | 4–5 | IPTG (0.17–0.51 mM) | 0.08–0.12 pg·cell−1 | 0.25 | [156] |
| Escherichia coli DH5α (pTKW106) | 0.027–0.050 pg·cell−1 | n.a. | [157] | ||||||||
| eGFP | Escherichia coli ATCC 33456 | PPFC | Glass | LB | 37 | Laminar flow (Re = 32) | 6 | - | 0.01–0.16 g·L−1 | n.d. | [148] |
| Escherichia coli JM109(DE3) | Flow cell | PVC | Nutrient medium a | 30 | Turbulent flow (Re = 4600) | 12 | - | 5.8 fg·cell−1 | 30 | [146] | |
| DM and LB | 12 | - | 5.7–12 fg·cell−1 | 10 | [154] | ||||||
| LB | 11 | IPTG (2 mM) | 17 fg·cell−1 | n.a. | [147] | ||||||
| LB and M9ZB | 10 | - | 1.51–15.96 fg·cell−1 | 4 | [162] | ||||||
| TB | Transient flow (Re = 2300) and Turbulent flow (Re = 4600) | 7 | - | 8.8–21.5 fg·cell−1 | 4 | [163] | |||||
| D-Amino acid oxidase | Escherichia coli TOP10 | Static and shaken reactors | - | HSG4 | 30 | Static conditions | 7 | IPTG (0.1 mM) | 1.2 U·g−1 | n.a. | [164] |
| Cellulose nanofibers | 170 rpm | 2.1 U·g−1 | n.a. | ||||||||
| Iturin A | Bacillus subtilis | 24-well plates | - | LB | 28 | Static conditions | 6 | - | 0.6 g·L−1 | n.a. | [155] |
| mCherry, EgTrp and EgA31 (part of fusion proteins) | Bacillus subtilis | Well plates with a 22 mm2 surface area and agar plates | - | MSgg | 30 | Static conditions | 3 | - | n.d. | n.d. | [144] |
| GFP (as part of the GLA-GFP fusion protein) | Aspergillus niger | SFB and RFB reactor | Cotton cloth attached to a stainless-steel cylinder | Modified Vogel’s medium | 25 | Static conditions 100, 400, and 600 rpm | 33–34 | - | 0.1 g·L−1 0.8 g·L−1 | n.a. | [140] |
| GFP (as part of the GLA-GFP fusion protein) | Aspergillus oryzae | BfR fungal reactor | Stainless steel packing | Complex medium b | 30 | n.d. | 3 | - | n.a. | n.d. | [141] |
a Nutrient medium composed of 0.55 g·L−1 glucose, 0.25 g·L−1 peptone, 0.125 g·L−1 yeast extract, and phosphate buffer (0.188 g L−1 KH2PO4 and 0.26 g L−1 Na2HPO4), pH 7.0; b Complex medium composed of 5 g·L−1 soluble starch, 5 g·L−1 casein peptone, and 5 g·L−1 yeast extract; c Productivity increment corresponds to the productivity ratio between biofilms and suspended cell processes. When productivity increment is not reported, it was calculated as the ratio between the maximum productivity obtained with biofilms and the maximum productivity obtained with planktonic cultures; Abbreviations: Temp., temperature; PPFC, parallel-plate flow cell; LB, Lysogeny broth; DM, Diluted medium; Re, Reynolds number; IPTG, isopropyl β-D-1-thiogalactopyranoside; PVC, polyvinyl chloride; SFB, static fibrous bed; RFB, rotating fibrous bed; BfR, biofilm reactor: n.a., not applicable; n.d., not described. Units: pg·cell−1, picogram of protein per cell; fg·cell−1, femtogram of protein per cell; U, activity unit.
In 2007, O’Connell et al. [148] investigated the production of eGFP in a biofilm system. An E. coli strain harboring a pEGFP plasmid was investigated in a PPFC reactor and the authors studied the impact of ampicillin concentration on cell fluorescence, revealing that low antibiotic concentrations (between 33 and 100 ppm) lead to 60% of strongly eGFP-producing cells [148]. Further, the results revealed that the biofilm environment enhanced plasmid maintenance and heterologous protein production when compared to planktonic cells, in contrast to what was previously described by Huang et al. [156,157].
Since 2016, Gomes and collaborators have been studying the eGFP production in E. coli JM109(DE3) strain, both in planktonic and biofilm cells [12,146,147,152,153,154,162,163]. All studies were performed in a modified Robbins device with controlled temperature (30 °C) and hydrodynamic conditions (turbulent flow, Reynolds number of 4600, and shear stress of 0.3 Pa) throughout the assays, except for Soares et al. [163] on which turbulent and transient flow were compared. Initially, Gomes et al. [146] compared eGFP-specific production in biofilms versus planktonic cells. Experiments revealed that specific eGFP production in biofilms was about 30-fold higher than in the planktonic state, even without optimization of cultivation conditions (5.8 and 0.18 fg·cell−1 in biofilm and planktonic state, respectively). Afterward, Gomes et al. [154] compared eGFP production by using two different culture media (Lysogeny Broth (LB) and Diluted Medium (DM)) combined with different antibiotic concentrations (20 and 30 µg·mL−1 kanamycin). LB medium (composed of 10 g·L−1 tryptone and 5 g·L−1 yeast extract) has a substantial amount of carbon and nitrogen and is a medium regularly used for the expression of recombinant proteins [165]; the DM medium (composed of 0.55 g·L−1 glucose, 0.25 g·L−1 peptone, and 0.125 g·L−1 yeast extract) was described as a suitable medium for biofilm development [166]. The eGFP expression was higher in LB supplemented with 20 µg·mL−1 kanamycin with a specific production of 12 fg·cell−1, in opposition to 5.7 and 6.2 fg·cell−1 obtained with DM containing 20 and 30 µg·mL−1 kanamycin, respectively. Gomes et al. [154] concluded that eGFP production was higher in the LB medium and that the antibiotic concentration had no effect on the expression of eGFP. Subsequently, Gomes et al. [153] determined a set of techniques that could be performed to monitor and quantify fluorescent recombinant protein expression in biofilm cells. This study used LB medium and revealed that the biofilm population became increasingly heterogeneous during the assay, which corroborates O’Connell’s results [148]. Concerning the special distribution, eGFP-expressing cells were mostly located in the external layers of the biofilm [153]. Gomes et al. [147] also investigated the eGFP protein expression in non-induced and induced biofilms, resorting to chemical induction with IPTG at a final concentration of 2 mM. The experiment revealed that eGFP levels remained constant in the induced biofilm culture over the operation time, with a specific concentration of around 17 fg·cell−1, whereas in the non-induced biofilms, the eGFP production decreased by about 31%. Subsequently, Soares et al. [162] investigated the influence of nutrient conditions on recombinant protein production in biofilms comparing LB and M9ZB media. M9ZB favored biofilm development, but it had an inhibitory effect on eGFP expression, possibly due to the presence of glucose in medium composition. On the other hand, LB medium favored the number of eGFP-expressing cells and eGFP yield, probably due to the higher nitrogen content compared to M9ZB. Recently, Soares et al. [163] investigated the influence of hydrodynamics on biofilm formation and eGFP expression using Terrific Broth (TB) medium. They compared a transient flow regime ( = 2300) with a turbulent flow regime ( = 4600), revealing that higher biofilm eGFP production was obtained at the higher flow rate with a maximum eGFP production of 21.5 fg·cell−1 (2.5-fold more than under transient flow conditions).
Although GFP protein production has been mainly studied in bacterial biofilms, some research has been performed with fungal biofilms using GFP as a fusion protein. In 2005, Talabardon et al. [140] studied a recombinant A. niger strain containing a gene that encodes the glucoamylase-GFP (GLA-GFP) fusion protein to study the glucoamylase and GFP protein production in both suspension and immobilized biofilm cells. This study compared a static fibrous bed (SFB) and a rotating fibrous bed (RFB) under different hydrodynamics conditions and found that the RFB biofilm was able to produce 0.8 g·L−1 of glucoamylase and GFP, about six times more than in an SFB reactor and ten times more than in suspended cells.
Zune et al. [141] attempted the production of the GLA-GFP fusion protein in A. oryzae using a biofilm reactor with a stainless steel packing, whereas one bioreactor was fully immersed in the liquid medium and the other had a periodic immersion of the biofilm. The results revealed that the GFP fluorescence was similar in suspended cell cultures and biofilm reactors under high shear stress conditions. The production of the fusion protein in the two different configurations of the biofilm reactor was evaluated, revealing that both achieved similar yield values.
D-Amino acid oxidase (DAAO) is a native protein from Rhodosporidium toruloides and its production was performed in E. coli TOP10 [164]. The experiment compared static and shaken cultivation after IPTG induction, showing that DAAO protein production was nearly 2-fold higher in shaken conditions compared with static conditions.
Although most recombinant protein production in biofilms is performed in Gram-negative bacteria such as E. coli, some studies used Gram-positive bacteria such as B. subtilis to produce iturin A [155] and the fusion protein TasA [144]. Rahman et al. [155] used the B. subtilis 168 strain for the production of iturin A in biofilms at different temperatures, with the best production (0.6 g·L−1) being obtained at 28 °C. Vogt et al. [144] also used B. subtilis biofilms and engineered a fusion protein of TasA with the red fluorescent protein mCherry, showing that the fusion TasA-mCherry was homogeneously and abundantly distributed within the biofilm. In the same work, the production of TasA with Echinococus granulosus antigenic peptides (paramyosin and tropomyosin) was performed, indicating that antigens could be expressed in the biofilm state and were located in the biofilm matrix [144].
4. Overall Advantages and Limitations of Productive Biofilms
Biofilm reactors exhibit good operational stability with the possibility of long operation periods, increased tolerance to toxic substrates and products, robustness of the immobilized cells towards fluctuating process conditions, and high cell densities, increasing the volumetric productivity rates of several products even on dilute feed streams. On the other hand, productive biofilms may face limited oxygen and substrate diffusion and may be prone to contamination in consecutive operations. Despite these limitations, biofilm reactors have a high potential to be used in biotechnology/biotransformation processes.
Biofilm processes have been a recurring choice to produce bulk chemicals with a low ecological footprint, employing agro-wastes and biorefinery residues for their bio-conversion into valuable chemical and pharmaceutic compounds to meet economic process sustainability. In this sense, productive biofilms could have a huge potential for application in diverse areas, such as in the production of chemicals, biofuels, food additives, and bioactive compounds. Regarding the production of chemicals, to the best of our knowledge, this has only been reported at a bench and pilot scale, while the production of recombinant proteins in biofilm platforms is in its initial stage.
5. Future Directions
Due to the advantages of biofilm platforms over suspended cell cultures, the biotechnology industry should consider the implementation of large-scale biofilm reactors. The main advances are likely to come from the continuous development and optimization of support materials, bioreactor configurations and operating conditions, the creation of in situ biofilm monitoring strategies, and the development of suitable biofilm reactor scale-up criteria and product recovery systems. Complementary strategies such as genetic engineering of the producing microorganisms can also increase biofilm formation and even specific production rates. However, it is necessary to consider that the behavior of biofilm cultures can be hard to predict, and the lack of biofilm reproducibility can be an obstacle to its industrial application. Consequently, a study on parameters for scale-up should be performed, such as culture conditions, mass and heat transfer constraints, kinetics, and production modeling.
Author Contributions
Conceptualization, F.J.M.M., F.M.C. and L.C.G.; writing—F.M.C., A.A. and M.M.F.; original draft preparation, F.M.C. and A.A.; writing—review and editing, F.M.C., A.A, L.C.G. and F.J.M.M.; supervision, F.J.M.M. and L.C.G.; funding acquisition, F.J.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by: LA/P/0045/2020 (ALiCE), UIDB/00511/2020, and UIDP/00511/2020 (LEPABE), which were funded by national funds through FCT/MCTES (PIDDAC); project PTDC/BII-BIO/29589/2017-POCI-01-0145-FEDER-029589 funded by FEDER funds through COMPETE2020-Programa Operacional Competitividade e Internacionalização (POCI) and by national funds (PIDDAC) through FCT/MCTES. A.A. acknowledges the receipt of a Ph.D. grant from the Portuguese Foundation of Science and Technology (FCT) (2020.07427). L.C.G. thanks FCT for the financial support of her work through the Scientific Employment Stimulus-Individual Call-[CEECIND/01700/2017].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Corte, L.; Casagrande Pierantoni, D.; Tascini, C.; Roscini, L.; Cardinali, G. Biofilm Specific Activity: A Measure to Quantify Microbial Biofilm. Microorganisms 2019, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry-A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.G. The role of biofilms in reprocessing medical devices. Am. J. Infect. Control 2013, 41, S77–S80. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Chandra, N.; Kumar, S. The Role of Biofilms in Medical Devices and Implants. In Biofilms in Human Diseases: Treatment and Control; Kumar, S., Chandra, N., Singh, L., Hashmi, M.Z., Varma, A., Eds.; Springer International Publishing: Cham, Switzenland, 2019; pp. 151–165. [Google Scholar]
- Moreira, J.M.R.; Fulgêncio, R.; Alves, P.; Machado, I.; Bialuch, I.; Melo, L.F.; Simões, M.; Mergulhão, F.J. Evaluation of SICAN performance for biofouling mitigation in the food industry. Food Control 2016, 62, 201–207. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R. Marine Biofilms: A Successful Microbial Strategy with Economic Implications. Front. Mar. Sci. 2018, 5, 126. [Google Scholar] [CrossRef]
- Li, L.; He, Z.; Liang, T.; Sheng, T.; Zhang, F.; Wu, D.; Ma, F. Colonization of biofilm in wastewater treatment: A review. Environ. Pollut. 2022, 293, 118514. [Google Scholar] [CrossRef]
- Edwards, S.J.; Kjellerup, B.V. Applications of biofilms in bioremediation and biotransformation of persistent organic pollutants, pharmaceuticals/personal care products, and heavy metals. Appl. Microbiol. Biotechnol. 2013, 97, 9909–9921. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, J.; Yuan, X.; Jiang, X.; Wang, Y.; Zhong, C.; Xu, D.; Gu, T.; Wang, F. Bacterial biofilms as platforms engineered for diverse applications. Biotechnol. Adv. 2022, 57, 107932. [Google Scholar] [CrossRef]
- Cheng, K.C.; Demirci, A.; Catchmark, J.M. Advances in biofilm reactors for production of value-added products. Appl. Microbiol. Biotechnol. 2010, 87, 445–456. [Google Scholar] [CrossRef]
- Soares, A.; Azevedo, A.; Gomes, L.C.; Mergulhao, F.J. Recombinant protein expression in biofilms. AIMS Microbiol. 2019, 5, 232–250. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, Y.; Zhang, X.; Gao, H.; Mou, L.; Wu, M.; Zhang, W.; Xin, F.; Jiang, M. Biofilm application in the microbial biochemicals production process. Biotechnol. Adv. 2021, 48, 107724. [Google Scholar] [CrossRef] [PubMed]
- Germec, M.; Demirci, A.; Turhan, I. Biofilm reactors for value-added products production: An in-depth review. Biocatal. Agric. Biotechnol. 2020, 27, 101662. [Google Scholar] [CrossRef]
- Ercan, D.; Demirci, A. Current and future trends for biofilm reactors for fermentation processes. Crit. Rev. Biotechnol. 2015, 35, 1–14. [Google Scholar] [CrossRef]
- Todhanakasem, T. Developing microbial biofilm as a robust biocatalyst and its challenges. Biocatal. Biotransformation 2017, 35, 86–95. [Google Scholar] [CrossRef]
- Demirci, A.; Pongtharangkul, T.; Pometto, A.L. Applications of biofilm reactors for production of value-added products by microbial fermentation. In Biofilms in the Food Environment, 2nd ed.; Blaschek, H.P., Wang, H.H., Agle, M.E., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 167–190. [Google Scholar]
- Mahdinia, E.; Demirci, A. Biofilms in Fermentation for the Production of Value-Added Products. In Microbial Biofilms; CRC Press: Boca Raton, FL, USA, 2020; pp. 73–108. [Google Scholar]
- Muffler, K.; Lakatos, M.; Schlegel, C.; Strieth, D.; Kuhne, S.; Ulber, R. Application of biofilm bioreactors in white biotechnology. Adv. Biochem. Eng. Biotechnol. 2014, 146, 123–161. [Google Scholar] [CrossRef]
- Rosche, B.; Li, X.Z.; Hauer, B.; Schmid, A.; Buehler, K. Microbial biofilms: A concept for industrial catalysis? Trends Biotechnol. 2009, 27, 636–643. [Google Scholar] [CrossRef]
- Halan, B.; Buehler, K.; Schmid, A. Biofilms as living catalysts in continuous chemical syntheses. Trends Biotechnol. 2012, 30, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Fliss, I.; Lacroix, C. High nisin-Z production during repeated-cycle batch cultures in supplemented whey permeate using immobilized Lactococcus lactis UL719. Int. Dairy J. 2001, 11, 953–960. [Google Scholar] [CrossRef]
- Khiyami, M.A.; Pometto, A.L., 3rd; Kennedy, W.J. Ligninolytic enzyme production by Phanerochaete chrysosporium in plastic composite support biofilm stirred tank bioreactors. J. Agric. Food Chem. 2006, 54, 1693–1698. [Google Scholar] [CrossRef]
- Cotton, J.C.; Pometto, A.L., 3rd; Gvozdenovic-Jeremic, J. Continuous lactic acid fermentation using a plastic composite support biofilm reactor. Appl. Microbiol. Biotechnol. 2001, 57, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Roukas, T.; Kotzekidou, P. Continuous production of lactic acid from deproteinized whey by coimmobilized lactobacillus casei and lactococcus lactis cells in a packed-bed reactor. Food Biotechnol. 1996, 10, 231–242. [Google Scholar] [CrossRef]
- Monti, M.; Scoma, A.; Martinez, G.; Bertin, L.; Fava, F. Uncoupled hydrogen and volatile fatty acids generation in a two-step biotechnological anaerobic process fed with actual site wastewater. New Biotechnol. 2015, 32, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Lienhardt, J.; Schripsema, J.; Qureshi, N.; Blaschek, H.P. Butanol production by Clostridium beijerinckii BA101 in an immobilized cell biofilm reactor: Increase in sugar utilization. Appl. Biochem. Biotechnol. 2002, 98–100, 591–598. [Google Scholar] [CrossRef]
- Venkatadri, R.; Irvine, R.L. Cultivation of Phanerochaete chrysosporium and production of lignin peroxidase in novel biofilm reactor systems: Hollow fiber reactor and silicone membrane reactor. Water Res. 1993, 27, 591–596. [Google Scholar] [CrossRef]
- Gross, R.; Buehler, K.; Schmid, A. Engineered catalytic biofilms for continuous large scale production of n-octanol and (S)-styrene oxide. Biotechnol. Bioeng. 2013, 110, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.S.; Petersen, F.W. Membrane bioreactor production of lignin and manganese peroxidase. Membr. Technol. 2002, 2002, 6–8. [Google Scholar] [CrossRef]
- Dumsday, G.J.; Zhou, B.; Buhmann, S.; Stanley, G.A.; Pamment, N.B. Continuous Ethanol Production by Escherichia Coli KO11 in Continuous Stirred Tank and Fluidized Bed Fermenters. Australas. Biotechnol. 1997, 7, 300–303. [Google Scholar]
- Barros, A.R.; Cavalcante de Amorim, E.L.; Reis, C.M.; Shida, G.M.; Silva, E.L. Biohydrogen production in anaerobic fluidized bed reactors: Effect of support material and hydraulic retention time. Int. J. Hydrogen Energy 2010, 35, 3379–3388. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.L.; Bai, S. Modeling of continuous L(+)-lactic acid production with immobilized R. oryzae in an airlift bioreactor. Biochem. Eng. J. 1999, 3, 87–90. [Google Scholar] [CrossRef]
- Srivastava, P.; Kundu, S. Studies on cephalosporin-C production in an air lift reactor using different growth modes of Cephalosporium acremonium. Process Biochem. 1999, 34, 329–333. [Google Scholar] [CrossRef]
- Vassilev, N.B.; Vassileva, M.C.; Spassova, D.I. Production of gluconic acid by Aspergillus niger immobilized on polyurethane foam. Appl. Microbiol. Biotechnol. 1993, 39, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lee, C.W.; Chang, H.N. Citric acid production by Aspergillus niger immobilized on polyurethane foam. Appl. Microbiol. Biotechnol. 1989, 30, 141–143. [Google Scholar] [CrossRef]
- Amin, G.; Doelle, H.W. Production of high ethanol concentrations from glucose using a vertical rotating immobilized cell reactor of the bacterium zymomonas mobilis. Acta Biotechnol. 1990, 10, 35–40. [Google Scholar] [CrossRef]
- Lin, S.-P.; Hsieh, S.-C.; Chen, K.-I.; Demirci, A.; Cheng, K.-C. Semi-continuous bacterial cellulose production in a rotating disk bioreactor and its materials properties analysis. Cellulose 2014, 21, 835–844. [Google Scholar] [CrossRef]
- Cuny, L.; Pfaff, D.; Luther, J.; Ranzinger, F.; Ödman, P.; Gescher, J.; Guthausen, G.; Horn, H.; Hille-Reichel, A. Evaluation of productive biofilms for continuous lactic acid production. Biotechnol. Bioeng. 2019, 116, 2687–2697. [Google Scholar] [CrossRef]
- Halan, B.; Schmid, A.; Buehler, K. Maximizing the productivity of catalytic biofilms on solid supports in membrane aerated reactors. Biotechnol. Bioeng. 2010, 106, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, D.; Bauer, R.; Neff, V. Optimization of the microbial synthesis of dihydroxyacetone in a semi-continuous repeated-fed-batch process by in situ immobilization of Gluconobacter oxydans. Process Biochem. 2007, 42, 71–76. [Google Scholar] [CrossRef]
- Bradfield, M.F.; Nicol, W. Continuous succinic acid production from xylose by Actinobacillus succinogenes. Bioprocess Biosyst. Eng. 2016, 39, 233–244. [Google Scholar] [CrossRef]
- Inoue, R.K.; Lima, D.M.; Rodrigues, J.A.; Ratusznei, S.M.; Zaiat, M. Effect of organic loading rate and fill time on the biohydrogen production in a mechanically stirred AnSBBR treating synthetic sucrose-based wastewater. Appl. Biochem. Biotechnol. 2014, 174, 2326–2349. [Google Scholar] [CrossRef]
- Manssouri, M.; Rodrigues, J.A.; Ratusznei, S.M.; Zaiat, M. Effects of organic loading, influent concentration, and feed time on biohydrogen production in a mechanically stirred AnSBBR treating sucrose-based wastewater. Appl. Biochem. Biotechnol. 2013, 171, 1832–1854. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, W.; Ferrasse, J.-H.; Giudici-Orticoni, M.-T.; Soric, A. Effect of hydraulic retention time on a continuous biohydrogen production in a packed bed biofilm reactor with recirculation flow of the liquid phase. Int. J. Hydrogen Energy 2018, 43, 18883–18895. [Google Scholar] [CrossRef]
- Rangaswamy, V.; Ramakrishna, S.V. Lactic acid production by Lactobacillus delbrueckii in a dual reactor system using packed bed biofilm reactor. Lett. Appl. Microbiol. 2008, 46, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.A.; Moon, C.; Kang, C.H.; Kong, S.W.; Sang, B.I.; Um, Y. Microbial fed-batch production of 1,3-propanediol using raw glycerol with suspended and immobilized Klebsiella pneumoniae. Appl. Biochem. Biotechnol. 2010, 161, 491–501. [Google Scholar] [CrossRef]
- Roukas, T. Modified rotary biofilm reactor: A new tool for enhanced carotene productivity by Blakeslea trispora. J. Clean. Prod. 2018, 174, 1114–1121. [Google Scholar] [CrossRef]
- Srinivasulu, B.; Prakasham, R.S.; Jetty, A.; Srinivas, S.; Ellaiah, P.; Ramakrishna, S.V. Neomycin production with free and immobilized cells of Streptomyces marinensis in an airlift reactor. Process Biochem. 2002, 38, 593–598. [Google Scholar] [CrossRef]
- Chaganti, S.; Reddy Shetty, P.; Rao, A.; Yadav, J. Production of L-(+)-lactic acid by Lactobacillus delbrueckii immobilized in functionalized alginate matrices. World J. Microbiol. Biotechnol. 2008, 24, 1411–1415. [Google Scholar] [CrossRef]
- Tay, A.; Yang, S.T. Production of L(+)-lactic acid from glucose and starch by immobilized cells of Rhizopus oryzae in a rotating fibrous bed bioreactor. Biotechnol. Bioeng. 2002, 80, 1–12. [Google Scholar] [CrossRef]
- Talabardon, M.; Schwitzguébel, J.P.; Péringer, P.; Yang, S.T. Acetic acid production from lactose by an anaerobic thermophilic coculture immobilized in a fibrous-bed bioreactor. Biotechnol. Prog. 2000, 16, 1008–1017. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Yang, F.; Zhang, C. Continuous acetone-butanol-ethanol production by corn stalk immobilized cells. J. Ind. Microbiol. Biotechnol. 2009, 36, 1117–1121. [Google Scholar] [CrossRef]
- Horiuchi, J.; Tabata, K.; Kanno, T.; Kobayashi, M. Continuous acetic acid production by a packed bed bioreactor employing charcoal pellets derived from waste mushroom medium. J. Biosci. Bioeng. 2000, 89, 126–130. [Google Scholar] [CrossRef]
- Meleigy, S.A.; Khalaf, M.A. Biosynthesis of gibberellic acid from milk permeate in repeated batch operation by a mutant Fusarium moniliforme cells immobilized on loofa sponge. Bioresour. Technol. 2009, 100, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Shahri, S.Z.; Vahabzadeh, F.; Mogharei, A. Lactic acid production by loofah-immobilized Rhizopus oryzae through one-step fermentation process using starch substrate. Bioprocess Biosyst. Eng. 2020, 43, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Kunduru, M.R.; Pometto, A.L. Evaluation of plastic composite-supports for enhanced ethanol production in biofilm reactors. J. Ind. Microbiol. 1996, 16, 241–248. [Google Scholar] [CrossRef]
- Cheng, K.C.; Demirci, A.; Catchmark, J.M. Effects of plastic composite support and pH profiles on pullulan production in a biofilm reactor. Appl. Microbiol. Biotechnol. 2010, 86, 853–861. [Google Scholar] [CrossRef]
- Liu, J.M.; Yu, T.C.; Lin, S.P.; Hsu, R.J.; Hsu, K.D.; Cheng, K.C. Evaluation of kojic acid production in a repeated-batch PCS biofilm reactor. J. Biotechnol. 2016, 218, 41–48. [Google Scholar] [CrossRef]
- Velázquez, A.C.; Pometto, A.L., 3rd; Ho, K.L.; Demirci, A. Evaluation of plastic-composite supports in repeated fed-batch biofilm lactic acid fermentation by Lactobacillus casei. Appl. Microbiol. Biotechnol. 2001, 55, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Demirci, A.; Pometto, A.L. Repeated-batch fermentation in biofilm reactors with plastic-composite supports for lactic acid production. Appl. Microbiol. Biotechnol. 1995, 43, 585–589. [Google Scholar] [CrossRef]
- Ho, K.L.; Pometto, A.L., 3rd; Hinz, P.N. Optimization of L-(+)-lactic acid production by ring and disc plastic composite supports through repeated-batch biofilm fermentation. Appl. Environ. Microbiol. 1997, 63, 2533–2542. [Google Scholar] [CrossRef]
- Urbance, S.E.; Pometto, A.L., 3rd; Dispirito, A.A.; Denli, Y. Evaluation of succinic acid continuous and repeat-batch biofilm fermentation by Actinobacillus succinogenes using plastic composite support bioreactors. Appl. Microbiol. Biotechnol. 2004, 65, 664–670. [Google Scholar] [CrossRef]
- Van Dien, S. From the first drop to the first truckload: Commercialization of microbial processes for renewable chemicals. Curr. Opin. Biotechnol. 2013, 24, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Bradfield, M.F.; Mohagheghi, A.; Salvachúa, D.; Smith, H.; Black, B.A.; Dowe, N.; Beckham, G.T.; Nicol, W. Continuous succinic acid production by Actinobacillus succinogenes on xylose-enriched hydrolysate. Biotechnol. Biofuels 2015, 8, 181. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, S.W.; Lee, J.S. Production of cellulase and xylanase in a bubble column using immobilized Aspergillus niger KKS. Appl. Biochem. Biotechnol. 1995, 53, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Izmirlioglu, G.; Demirci, A. Ethanol production in biofilm reactors from potato waste hydrolysate and optimization of growth parameters for Saccharomyces cerevisiae. Fuel 2016, 181, 643–651. [Google Scholar] [CrossRef]
- Todhanakasem, T.; Salangsing, O.-l.; Koomphongse, P.; Kaewket, S.; Kanokratana, P.; Champreda, V. Zymomonas mobilis Biofilm Reactor for Ethanol Production Using Rice Straw Hydrolysate Under Continuous and Repeated Batch Processes. Front. Microbiol. 2019, 10, 1777. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Leonov, P.S.; Flores-Alsina, X.; Gernaey, K.V.; Sternberg, C. Microbial biofilms in biorefinery—Towards a sustainable production of low-value bulk chemicals and fuels. Biotechnol. Adv. 2021, 50, 107766. [Google Scholar] [CrossRef]
- Rodrigues, C.; Vandenberghe, L.P.S.; Woiciechowski, A.L.; de Oliveira, J.; Letti, L.A.J.; Soccol, C.R. 24—Production and Application of Lactic Acid. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Negi, S., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 543–556. [Google Scholar]
- Ho, K.G.; Pometto, A.I.; Hinz, P.N.; Demirci, A. Nutrient leaching and end product accumulation in plastic composite supports for L-(+)-lactic Acid biofilm fermentation. Appl. Environ. Microbiol. 1997, 63, 2524–2532. [Google Scholar] [CrossRef]
- Ho, K.L.; Pometto, A.L., III; Hinz, P.N.; Dickson, J.S.; Demirci, A. Ingredient selection for plastic composite supports for L-(+)-lactic acid biofilm fermentation by Lactobacillus casei subsp. rhamnosus. Appl. Environ. Microbiol. 1997, 63, 2516–2523. [Google Scholar] [CrossRef]
- Urbance, S.E.; Pometto, A.L.; DiSpirito, A.A.; Demirci, A. Medium Evaluation and Plastic Composite Support Ingredient Selection for Biofilm Formation and Succinic Acid Production by Actinobacillus succinogenes. Food Biotechnol. 2003, 17, 53–65. [Google Scholar] [CrossRef]
- Bradfield, M.F.A.; Nicol, W. Continuous succinic acid production by Actinobacillus succinogenes in a biofilm reactor: Steady-state metabolic flux variation. Biochem. Eng. J. 2014, 85, 1–7. [Google Scholar] [CrossRef][Green Version]
- Ferone, M.; Raganati, F.; Ercole, A.; Olivieri, G.; Salatino, P.; Marzocchella, A. Continuous succinic acid fermentation by Actinobacillus succinogenes in a packed-bed biofilm reactor. Biotechnol. Biofuels 2018, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Du, J.; Chen, C.; Gong, C.S.; Tsao, G.T. Production of fumaric acid by immobilized rhizopus using rotary biofilm contactor. Appl. Biochem. Biotechnol. 1997, 63–65, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Du, J.; Gong, C.S.; Tsao, G.T. Simultaneous Production and Recovery of Fumaric Acid from Immobilized Rhizopus oryzae with a Rotary Biofilm Contactor and an Adsorption Column. Appl. Environ. Microbiol. 1996, 62, 2926–2931. [Google Scholar] [CrossRef] [PubMed]
- Jianlong, W. Production of citric acid by immobilized Aspergillus niger using a rotating biological contactor (RBC). Bioresour. Technol. 2000, 75, 245–247. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Sun, W.; Xi, X.; Zhao, N.; Huang, Z.; Ying, Z.; Liu, L.; Liu, D.; Niu, H.; et al. Continuous citric acid production in repeated-fed batch fermentation by Aspergillus niger immobilized on a new porous foam. J. Biotechnol. 2018, 276–277, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, K.; Bradfield, M.F.; Nicol, W. Succinic acid-producing biofilms of Actinobacillus succinogenes: Reproducibility, stability and productivity. Appl. Microbiol. Biotechnol. 2014, 98, 7379–7386. [Google Scholar] [CrossRef][Green Version]
- Sankpal, N.V.; Joshi, A.P.; Kulkarni, B.D. Citric acid production by Aspergillus niger immobilized on cellulose microfibrils: Influence of morphology and fermenter conditions on productivity. Process Biochem. 2001, 36, 1129–1139. [Google Scholar] [CrossRef]
- Castro, P.G.M.; Maeda, R.N.; Rocha, V.A.L.; Fernandes, R.P.; Pereira Jr, N. Improving propionic acid production from a hemicellulosic hydrolysate of sorghum bagasse by means of cell immobilization and sequential batch operation. Biotechnol. Appl. Biochem. 2021, 68, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Hauer, B.; Rosche, B. Catalytic biofilms on structured packing for the production of glycolic acid. J. Microbiol. Biotechnol. 2013, 23, 195–204. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Chatterjee, S.; Chatterjee, B.P.; Banerjee, P.C.; Guha, A.K. Production of gluconic acid from whey by free and immobilized Aspergillus niger. Int. Dairy J. 2005, 15, 299–303. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, L.; Hu, S.; Yuan, Z.; Guo, J. High-Rate Production of Short-Chain Fatty Acids from Methane in a Mixed-Culture Membrane Biofilm Reactor. Environ. Sci. Technol. Lett. 2018, 5, 662–667. [Google Scholar] [CrossRef]
- Jayasekara, S.; Ratnayake, R. Microbial Cellulases: An Overview and Applications. In Cellulose; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Webb, C.; Fukuda, H.; Atkinson, B. The production of cellulase in a spouted bed fermentor using cells immobilized in biomass support particles. Biotechnol. Bioeng. 1986, 28, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Amirul, A.A.; Yahya, A.; Azizan, M. Cellulase production by free and immobilized Aspergillus terreus. World J. Microbiol. Biotechnol. 2010, 26, 79–84. [Google Scholar] [CrossRef]
- Govender, S.; Pillay, V.L.; Odhav, B. Nutrient manipulation as a basis for enzyme production in a gradostat bioreactor. Enzym. Microb. Technol. 2010, 46, 603–609. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Cui, F.; Tan, T. Production of lipase by repeated batch fermentation with immobilized Rhizopus arrhizus. Process Biochem. 2005, 40, 2095–2103. [Google Scholar] [CrossRef]
- Urek, R.O.; Pazarlioğlu, N.K. A novel carrier for Phanerochaete chrysosporium immobilization. Artif. Cells Blood Substit. Biotechnol. 2004, 32, 563–574. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Catchmark, J.M.; Demirci, A. Effect of different additives on bacterial cellulose production by Acetobacter xylinum and analysis of material property. Cellulose 2009, 16, 1033–1045. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Catchmark, J.M.; Demirci, A. Enhanced production of bacterial cellulose by using a biofilm reactor and its material property analysis. J. Biol. Eng. 2009, 3, 12. [Google Scholar] [CrossRef]
- Rahman, S.S.A.; Vaishnavi, T.; Vidyasri, G.S.; Sathya, K.; Priyanka, P.; Venkatachalam, P.; Karuppiah, S. Production of bacterial cellulose using Gluconacetobacter kombuchae immobilized on Luffa aegyptiaca support. Sci. Rep. 2021, 11, 2912. [Google Scholar] [CrossRef]
- Cheng, K.C.; Demirci, A.; Catchmark, J.M. Enhanced pullulan production in a biofilm reactor by using response surface methodology. J. Ind. Microbiol. Biotechnol. 2010, 37, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-C.; Demirci, A.; Catchmark, J.M.; Puri, V.M. Effects of initial ammonium ion concentration on pullulan production by Aureobasidium pullulans and its modeling. J. Food Eng. 2011, 103, 115–122. [Google Scholar] [CrossRef]
- Cheng, K.C.; Demirci, A.; Catchmark, J.M. Continuous pullulan fermentation in a biofilm reactor. Appl. Microbiol. Biotechnol. 2011, 90, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, R.A.; Hassemer, G.; Marchiori, V.; Kiedis, J.; Valduga, E.; Junges, A.; Malvessi, E.; Cansian, R.L.; Zeni, J. Synthesis of Xanthan Gum from Xanthomonas campestris Immobilized in Polyurethane. Ind. Biotechnol. 2018, 14, 276–281. [Google Scholar] [CrossRef]
- Nejadmansouri, M.; Shad, E.; Razmjooei, M.; Safdarianghomsheh, R.; Delvigne, F.; Khalesi, M. Production of xanthan gum using immobilized Xanthomonas campestris cells: Effects of support type. Biochem. Eng. J. 2020, 157, 107554. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Naghmouchi, K.; Fliss, I.; Drider, D.; Lacroix, C. Pediocin PA-1 production during repeated-cycle batch culture of immobilized Pediococcus acidilactici UL5 cells. J. Biosci. Bioeng. 2008, 105, 513–517. [Google Scholar] [CrossRef]
- Klaenhammer, T.R. Bacteriocins of lactic acid bacteria. Biochimie 1988, 70, 337–349. [Google Scholar] [CrossRef]
- Liu, X.; Chung, Y.-K.; Yang, S.-T.; Yousef, A.E. Continuous nisin production in laboratory media and whey permeate by immobilized Lactococcus lactis. Process Biochem. 2005, 40, 13–24. [Google Scholar] [CrossRef]
- Benmechernene, Z.; Fernandez-No, I.; Kihal, M.; Böhme, K.; Calo-Mata, P.; Barros-Velazquez, J. Recent patents on bacteriocins: Food and biomedical applications. Recent Pat. DNA Gene Seq. 2013, 7, 66–73. [Google Scholar] [CrossRef]
- Pongtharangkul, T.; Demirci, A. Evaluation of culture medium for nisin production in a repeated-batch biofilm reactor. Biotechnol. Prog. 2006, 22, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Pongtharangkul, T.; Demirci, A. Effects of pH profiles on nisin production in biofilm reactor. Appl. Microbiol. Biotechnol. 2006, 71, 804–811. [Google Scholar] [CrossRef]
- Pongtharangkul, T.; Demirci, A. Effects of fed-batch fermentation and pH profiles on nisin production in suspended-cell and biofilm reactors. Appl. Microbiol. Biotechnol. 2006, 73, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Pongtharangku, T.; Demirci, A. Online recovery of nisin during fermentation and its effect on nisin production in biofilm reactor. Appl. Microbiol. Biotechnol. 2007, 74, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Ercan, D.; Demirci, A. Production of human lysozyme in biofilm reactor and optimization of growth parameters of Kluyveromyces lactis K7. Appl. Microbiol. Biotechnol. 2013, 97, 6211–6221. [Google Scholar] [CrossRef] [PubMed]
- Ercan, D.; Demirci, A. Enhanced human lysozyme production in biofilm reactor by Kluyveromyces lactis K7. Biochem. Eng. J. 2014, 92, 2–8. [Google Scholar] [CrossRef]
- Ercan, D.; Demirci, A. Effects of fed-batch and continuous fermentations on human lysozyme production by Kluyveromyces lactis K7 in biofilm reactors. Bioprocess Biosyst. Eng. 2015, 38, 2461–2468. [Google Scholar] [CrossRef]
- Ercan, D.; Demirci, A. Enhanced human lysozyme production by Kluyveromyces lactis K7 in biofilm reactor coupled with online recovery system. Biochem. Eng. J. 2015, 98, 68–74. [Google Scholar] [CrossRef]
- Cho, H.Y.; Yousef, A.E.; Yang, S.T. Continuous production of pediocin by immobilized Pediococcus acidilactici PO2 in a packed-bed bioreactor. Appl. Microbiol. Biotechnol. 1996, 45, 589–594. [Google Scholar] [CrossRef]
- Srinivasulu, B.; Adinarayana, K.; Ellaiah, P. Investigations on neomycin production with immobilized cells of Streptomyces marinensis NUV-5 in calcium alginate matrix. AAPS PharmSciTech 2003, 4, E57. [Google Scholar] [CrossRef][Green Version]
- Kunduru, M.R.; Pometto, A.L., 3rd. Continuous ethanol production by Zymomonas mobilis and Saccharomyces cerevisiae in biofilm reactors. J. Ind. Microbiol. 1996, 16, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Brown, R.C.; Wen, Z. Syngas fermentation by Clostridium carboxidivorans P7 in a horizontal rotating packed bed biofilm reactor with enhanced ethanol production. Appl. Energy 2017, 187, 585–594. [Google Scholar] [CrossRef]
- Hoschek, A.; Heuschkel, I.; Schmid, A.; Bühler, B.; Karande, R.; Bühler, K. Mixed-species biofilms for high-cell-density application of Synechocystis sp. PCC 6803 in capillary reactors for continuous cyclohexane oxidation to cyclohexanol. Bioresour. Technol. 2019, 282, 171–178. [Google Scholar] [CrossRef]
- Qureshi, N.; Schripsema, J.; Lienhardt, J.; Blaschek, H.P. Continuous solvent production by Clostridium beijerinckii BA101 immobilized by adsorption onto brick. World J. Microbiol. Biotechnol. 2000, 16, 377–382. [Google Scholar] [CrossRef]
- Qureshi, N.; Karcher, P.; Cotta, M.; Blaschek, H.P. High-productivity continuous biofilm reactor for butanol production. Appl. Biochem. Biotechnol. 2004, 114, 713–721. [Google Scholar] [CrossRef]
- Lee, S.-M.; Cho, M.O.; Park, C.H.; Chung, Y.-C.; Kim, J.H.; Sang, B.-I.; Um, Y. Continuous Butanol Production Using Suspended and Immobilized Clostridium beijerinckii NCIMB 8052 with Supplementary Butyrate. Energy Fuels 2008, 22, 3459–3464. [Google Scholar] [CrossRef]
- Napoli, F.; Olivieri, G.; Russo, M.E.; Marzocchella, A.; Salatino, P. Butanol production by Clostridium acetobutylicum in a continuous packed bed reactor. J. Ind. Microbiol. Biotechnol. 2010, 37, 603–608. [Google Scholar] [CrossRef]
- Chen, J.P.; Wu, K.W.; Fukuda, H. Bioethanol production from uncooked raw starch by immobilized surface-engineered yeast cells. Appl. Biochem. Biotechnol. 2008, 145, 59–67. [Google Scholar] [CrossRef]
- Kongjan, P.; Inchan, S.; Chanthong, S.; Jariyaboon, R.; Reungsang, A.; O-Thong, S. Hydrogen production from xylose by moderate thermophilic mixed cultures using granules and biofilm up-flow anaerobic reactors. Int. J. Hydrogen Energy 2019, 44, 3317–3324. [Google Scholar] [CrossRef]
- Renaudie, M.; Dumas, C.; Vuilleumier, S.; Ernst, B. Biohydrogen production in a continuous liquid/gas hollow fiber membrane bioreactor: Efficient retention of hydrogen producing bacteria via granule and biofilm formation. Bioresour. Technol. 2021, 319, 124203. [Google Scholar] [CrossRef]
- Gross, R.; Lang, K.; Bühler, K.; Schmid, A. Characterization of a biofilm membrane reactor and its prospects for fine chemical synthesis. Biotechnol. Bioeng. 2010, 105, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Khiyami, M.; Alfadul, S.; Bahkali, A. Polyhydroxyalkanoates production via Bacillus PCS biofilm and date palm syrup. J. Med. Plant Res. 2011, 5, 3312–3320. [Google Scholar]
- Bengtsson, S.; Karlsson, A.; Alexandersson, T.; Quadri, L.; Hjort, M.; Johansson, P.; Morgan-Sagastume, F.; Anterrieu, S.; Arcos-Hernandez, M.; Karabegovic, L.; et al. A process for polyhydroxyalkanoate (PHA) production from municipal wastewater treatment with biological carbon and nitrogen removal demonstrated at pilot-scale. New Biotechnol. 2017, 35, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Hekmat, D.; Bauer, R.; Fricke, J. Optimization of the microbial synthesis of dihydroxyacetone from glycerol with Gluconobacter oxydans. Bioprocess Biosyst. Eng. 2003, 26, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Norrlöw, O.; Wawrzynczyk, J.; Dey, E.S. Poly(3-hydroxybutyrate) biosynthesis in the biofilm of Alcaligenes eutrophus, using glucose enzymatically released from pulp fiber sludge. Appl. Environ. Microbiol. 2004, 70, 6776–6782. [Google Scholar] [CrossRef] [PubMed]
- Overton, T.W. Recombinant protein production in bacterial hosts. Drug Discov. Today 2014, 19, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef]
- Chen, R. Bacterial expression systems for recombinant protein production: E. coli and beyond. Biotechnol. Adv. 2012, 30, 1102–1107. [Google Scholar] [CrossRef]
- Gomes, L.; Mergulhão, F. Production of Recombinant Proteins in Escherichia coli Biofilms: Challenges and Opportunities. In Advances in Medicine and Biology; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2019; p. 181. [Google Scholar]
- Burdette, L.A.; Leach, S.A.; Wong, H.T.; Tullman-Ercek, D. Developing Gram-negative bacteria for the secretion of heterologous proteins. Microb. Cell Factories 2018, 17, 196. [Google Scholar] [CrossRef]
- Peng, M.; Margetts, T.J.; Rayana, N.P.; Sugali, C.K.; Dai, J.; Mao, W. The application of lentiviral vectors for the establishment of TGFβ2-induced ocular hypertension in C57BL/6J mice. Exp. Eye Res. 2022, 221, 109137. [Google Scholar] [CrossRef] [PubMed]
- Al-Aridhi, T. GFP-coated microparticles to quantify and compare wild-type desmin with known desmin mutations in human heart disease via quantitative live-cell fluorescence Imaging; Universität Bielefeld: Bielefeld, Germany, 2022. [Google Scholar]
- Hoffman, R.M. Application of GFP imaging in cancer. Lab. Investig. 2015, 95, 432–452. [Google Scholar] [CrossRef] [PubMed]
- Talabardon, M.; Yang, S.T. Production of GFP and glucoamylase by recombinant Aspergillus niger: Effects of fermentation conditions on fungal morphology and protein secretion. Biotechnol. Prog. 2005, 21, 1389–1400. [Google Scholar] [CrossRef]
- Zune, Q.; Delepierre, A.; Gofflot, S.; Bauwens, J.; Twizere, J.C.; Punt, P.J.; Francis, F.; Toye, D.; Bawin, T.; Delvigne, F. A fungal biofilm reactor based on metal structured packing improves the quality of a Gla::GFP fusion protein produced by Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2015, 99, 6241–6254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saqib, S.; Akram, A.; Halim, S.A.; Tassaduq, R. Sources of β-galactosidase and its applications in food industry. 3 Biotech 2017, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Husain, Q. Beta galactosidases and their potential applications: A review. Crit. Rev. Biotechnol. 2010, 30, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Vogt, C.M.; Schraner, E.M.; Aguilar, C.; Eichwald, C. Heterologous expression of antigenic peptides in Bacillus subtilis biofilms. Microb. Cell Factories 2016, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.V. Chapter 19 Medical Biotechnology Techniques and Applications. In Omics Technologies and Bio-Engineering; Academic Press: Cambridge, MA, USA, 2018; pp. 449–469. [Google Scholar]
- Gomes, L.; Mergulhão, F. Heterologous protein production in Escherichia coli biofilms: A non-conventional form of high cell density cultivation. Process Biochem. 2017, 57, 1–8. [Google Scholar] [CrossRef]
- Gomes, L.; Monteiro, G.; Mergulhao, F. The Impact of IPTG Induction on Plasmid Stability and Heterologous Protein Expression by Escherichia coli Biofilms. Int. J. Mol. Sci. 2020, 21, 576. [Google Scholar] [CrossRef]
- O’Connell, H.A.; Niu, C.; Gilbert, E.S. Enhanced high copy number plasmid maintenance and heterologous protein production in an Escherichia coli biofilm. Biotechnol. Bioeng. 2007, 97, 439–446. [Google Scholar] [CrossRef]
- Hoffmann, F.; Rinas, U. Stress induced by recombinant protein production in Escherichia coli. Adv. Biochem. Eng. Biotechnol. 2004, 89, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Role of Biofilms in Antimicrobial Resistance. ASAIO J. 2000, 46, S47–S52. [Google Scholar] [CrossRef] [PubMed]
- Landini, P. Cross-talk mechanisms in biofilm formation and responses to environmental and physiological stress in Escherichia coli. Res. Microbiol. 2009, 160, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.; Gomes, L.C.; Mergulhão, F.J. Comparing the Recombinant Protein Production Potential of Planktonic and Biofilm Cells. Microorganisms 2018, 6, 48. [Google Scholar] [CrossRef]
- Gomes, L.C.; Carvalho, D.; Briandet, R.; Mergulhao, F.J. Temporal variation of recombinant protein expression inEscherichia coli biofilms analysed at single-cell level. Process Biochem. 2016, 51, 1155–1161. [Google Scholar] [CrossRef]
- Gomes, L.C.; Mergulhao, F.J. Effects of antibiotic concentration and nutrient medium composition on Escherichia coli biofilm formation and green fluorescent protein expression. FEMS Microbiol. Lett. 2017, 364, fnx042. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Ano, T.; Shoda, M. Biofilm fermentation of iturin A by a recombinant strain of Bacillus subtilis 168. J. Biotechnol. 2007, 127, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Peretti, S.W.; Bryers, J.D. Plasmid retention and gene expression in suspended and biofilm cultures of recombinant Escherichia coli DH5alpha(pMJR1750). Biotechnol. Bioeng. 1992, 41, 211–220. [Google Scholar] [CrossRef]
- Huang, C.T.; Peretti, S.W.; Bryers, J.D. Effects of inducer levels on a recombinant bacterial biofilm formation and gene expression. Biotechnol. Lett. 1994, 16, 903–908. [Google Scholar] [CrossRef]
- Huang, C.T.; Peretti, S.W.; Bryers, J.D. Effects of medium carbon-to-nitrogen ratio on biofilm formation and plasmid stability. Biotechnol. Bioeng. 1994, 44, 329–336. [Google Scholar] [CrossRef]
- Gomes, L.C.; Moreira, J.M.; Teodósio, J.S.; Araújo, J.D.; Miranda, J.M.; Simões, M.; Melo, L.F.; Mergulhão, F.J. 96-well microtiter plates for biofouling simulation in biomedical settings. Biofouling 2014, 30, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.C.; Mergulhão, F.J.M. A Selection of Platforms to Evaluate Surface Adhesion and Biofilm Formation in Controlled Hydrodynamic Conditions. Microorganisms 2021, 9, 1993. [Google Scholar] [CrossRef]
- Alves, P.; Gomes, L.C.; Vorobii, M.; Rodriguez-Emmenegger, C.; Mergulhão, F.J. The potential advantages of using a poly(HPMA) brush in urinary catheters: Effects on biofilm cells and architecture. Colloids Surf. B Biointerfaces 2020, 191, 110976. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.; Gomes, L.; Monteiro, G.; Mergulhao, F. The Influence of Nutrient Medium Composition on Escherichia coli Biofilm Development and Heterologous Protein Expression. Appl. Sci. 2021, 11, 8667. [Google Scholar] [CrossRef]
- Soares, A.; Gomes, L.C.; Monteiro, G.A.; Mergulhão, F.J. Hydrodynamic Effects on Biofilm Development and Recombinant Protein Expression. Microorganisms 2022, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Chien, L.J.; Lee, C.K. Self-immobilized recombinant Acetobacter xylinum for biotransformation. Biochem. Eng. J. 2008, 43, 78–84. [Google Scholar] [CrossRef]
- Donovan, R.S.; Robinson, C.W.; Glick, B.R. Review: Optimizing inducer and culture conditions for expression of foreign proteins under the control of the lac promoter. J. Ind. Microbiol. 1996, 16, 145–154. [Google Scholar] [CrossRef]
- Teodosio, J.S.; Simoes, M.; Melo, L.F.; Mergulhao, F.J. Flow cell hydrodynamics and their effects on E. coli biofilm formation under different nutrient conditions and turbulent flow. Biofouling 2011, 27, 1–11. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).