Long-Term Exposure to Ozone and Fine Particulate Matter and Risk of Premature Coronary Artery Disease: Results from Genetics of Atherosclerotic Disease Mexican Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Air Pollution and Weather Data
2.3. Statistical Methods
3. Results
3.1. Characteristics of the Study Population
3.2. Ambient Ozone and PM2.5 Levels
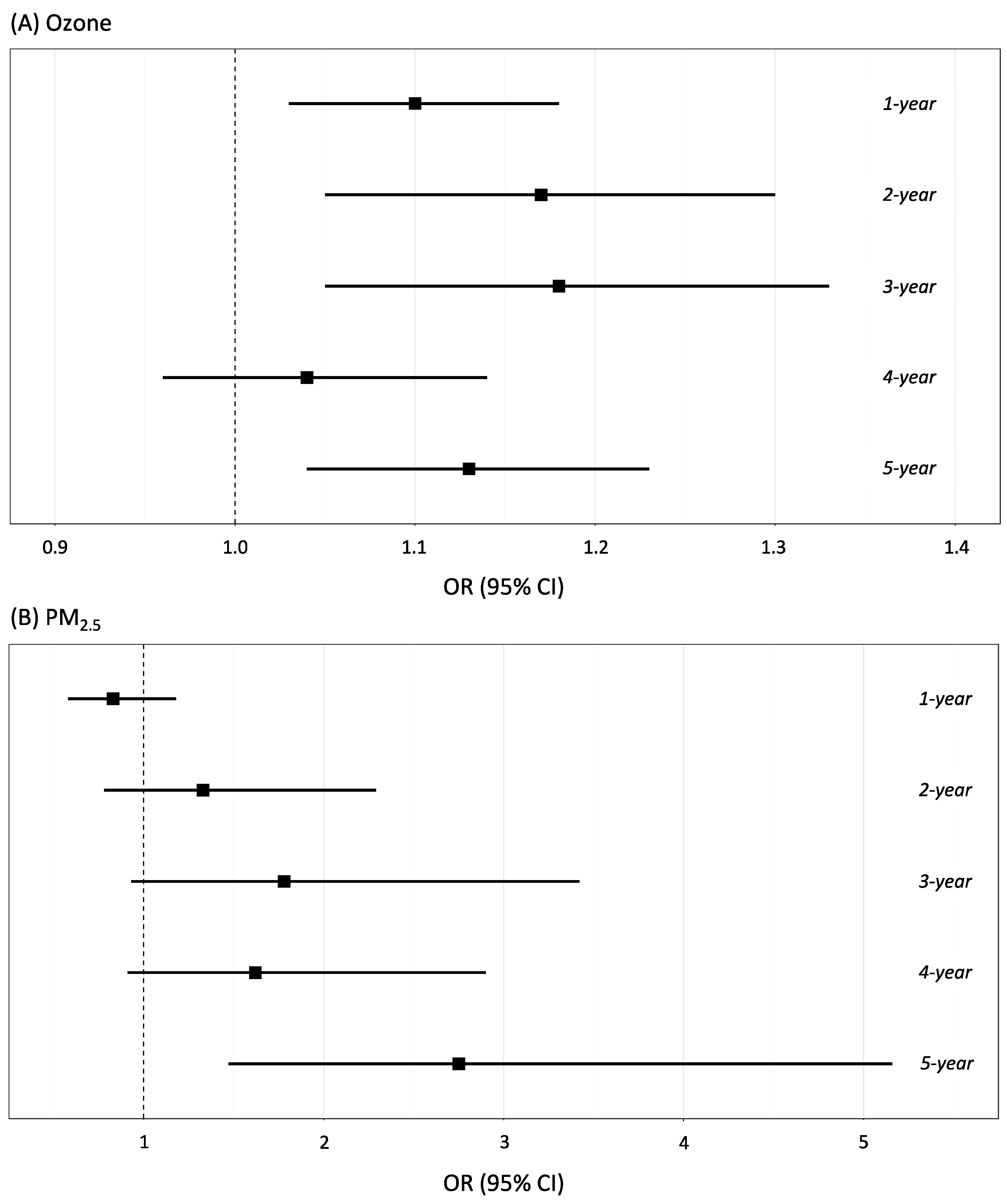
3.3. Association between Air Pollution Levels and pCAD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Velazquez Monroy, O.; Barinagarrementeria Aldatz, F.S.; Rubio Guerra, A.F.; Verdejo, J.; Mendez Bello, M.A.; Violante, R.; Pavia, A.; Alvarado-Ruiz, R.; Lara Esqueda, A. Morbidity and mortality by ischemic heart disease and stroke in Mexico. 2005. Arch. Cardiol. Mex. 2007, 77, 31–39. [Google Scholar] [PubMed]
- Yusuf, S.; Reddy, S.; Ounpuu, S.; Anand, S. Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001, 104, 2746–2753. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The world health report 2004—Changing History. Available online: https://apps.who.int/iris/bitstream/handle/10665/42891/924156265X.pdf?sequence=1&isAllowed=y (accessed on 1 April 2022).
- Salud, S.A.D. Programa de Acción: Enfermedades Cardiovasculares e Hipertensión Arterial; Secretaría de Salud: Mexico City, Mexico, 2001. [Google Scholar]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Sikkel, M.B. Coronary artery disease and age: Beyond atherosclerosis. J. Physiol. 2013, 591, 5807–5808. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Qi, J.; Mao, H.; Wang, N.; Ye, X.; Zhou, L.; Tong, G.; Yang, J.; Pan, H.; Huang, J. Coronary plaque tissue characterization in patients with premature coronary artery disease. Int. J. Cardiovasc. Imaging 2020, 36, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Nasir, K.; Budoff, M.J.; Wong, N.D.; Scheuner, M.; Herrington, D.; Arnett, D.K.; Szklo, M.; Greenland, P.; Blumenthal, R.S. Family history of premature coronary heart disease and coronary artery calcification: Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2007, 116, 619–626. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Hayes, R.B.; Lim, C.; Zhang, Y.; Cromar, K.; Shao, Y.; Reynolds, H.R.; Silverman, D.T.; Jones, R.R.; Park, Y.; Jerrett, M.; et al. PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int. J. Epidemiology 2020, 49, 25–35. [Google Scholar] [CrossRef]
- Kaufman, J.D.; Adar, S.D.; Barr, R.G.; Budoff, M.; Burke, G.L.; Curl, C.L.; Daviglus, M.L.; Diez Roux, A.V.; Gassett, A.J.; Jacobs, D.R., Jr.; et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): A longitudinal cohort study. Lancet 2016, 388, 696–704. [Google Scholar] [CrossRef]
- Ugalde-Resano, R.; Riojas-Rodriguez, H.; Texcalac-Sangrador, J.L.; Cruz, J.C.; Hurtado-Diaz, M. Short term exposure to ambient air pollutants and cardiovascular emergency department visits in Mexico city. Environ. Res. 2022, 207, 112600. [Google Scholar] [CrossRef] [PubMed]
- Madrigano, J.; Kloog, I.; Goldberg, R.; Coull, B.A.; Mittleman, M.A.; Schwartz, J. Long-term exposure to PM2.5 and incidence of acute myocardial infarction. Environ. Health Perspect. 2013, 121, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.; Jorgensen, J.T.; Hoffmann, B.; Loft, S.; Brauner, E.V.; Prescott, E.; Ketzel, M.; Hertel, O.; Brandt, J.; Jensen, S.S.; et al. Long-Term Exposure to Air Pollution and Incidence of Myocardial Infarction: A Danish Nurse Cohort Study. Environ. Health Perspect. 2020, 128, 57003. [Google Scholar] [CrossRef]
- Yu, Y.; Yao, S.; Dong, H.; Ji, M.; Chen, Z.; Li, G.; Yao, X.; Wang, S.L.; Zhang, Z. Short-term effects of ambient air pollutants and myocardial infarction in Changzhou, China. Environ. Sci. Pollut. Res. 2018, 25, 22285–22293. [Google Scholar] [CrossRef]
- Hystad, P.; Larkin, A.; Rangarajan, S.; AlHabib, K.F.; Avezum, A.; Calik, K.B.T.; Chifamba, J.; Dans, A.; Diaz, R.; du Plessis, J.L.; et al. Associations of outdoor fine particulate air pollution and cardiovascular disease in 157 436 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet Planet. Health 2020, 4, e235–e245. [Google Scholar] [CrossRef]
- Miranda, J.J.; Herrera, V.M.; Chirinos, J.A.; Gomez, L.F.; Perel, P.; Pichardo, R.; Gonzalez, A.; Sanchez, J.R.; Ferreccio, C.; Aguilera, X.; et al. Major cardiovascular risk factors in Latin America: A comparison with the United States. The Latin American Consortium of Studies in Obesity (LASO). PLoS ONE 2013, 8, e54056. [Google Scholar] [CrossRef]
- Rivera-Andrade, A.; Luna, M.A. Trends and heterogeneity of cardiovascular disease and risk factors across Latin American and Caribbean countries. Prog. Cardiovasc. Dis. 2014, 57, 276–285. [Google Scholar] [CrossRef]
- EEA. Air Pollution Due to Ozone: Health Impacts and Effects of Climate Change; E.E.A.: Copenhagen, Denmark, 2015.
- U.S.E.E.P. Integrates Science Assessment (ISA) for Ozone and Related Photochemical Oxidants. EPA/600/R-10/076F.; U.S.E.E.P.: Washington, DC, USA, 2013.
- Wang, M.; Sampson, P.D.; Sheppard, L.E.; Stein, J.H.; Vedal, S.; Kaufman, J.D. Long-Term Exposure to Ambient Ozone and Progression of Subclinical Arterial Disease: The Multi-Ethnic Study of Atherosclerosis and Air Pollution. Environ. Health Perspect. 2019, 127, 57001. [Google Scholar] [CrossRef]
- Mirowsky, J.E.; Carraway, M.S.; Dhingra, R.; Tong, H.; Neas, L.; Diaz-Sanchez, D.; Cascio, W.; Case, M.; Crooks, J.; Hauser, E.R.; et al. Ozone exposure is associated with acute changes in inflammation, fibrinolysis, and endothelial cell function in coronary artery disease patients. Environ. Health 2017, 16, 126. [Google Scholar] [CrossRef]
- (INECC), I.N.d.E.y.C.C. Valoración Económica de Los Beneficios A La Salud de La Población Que Se Alcanzarían Por La Reducción De Las PM2.5 En tres Zonas Metropolitanas Mexicanas. INECC, 2014. Available online: https://www.gob.mx/cms/uploads/attachment/file/195224/2014_CGCSA_Beneficos_econ_micos_al_reducir_PM2.5.pdf (accessed on 1 January 2022).
- Trejo-Gonzalez, A.G.; Riojas-Rodriguez, H.; Texcalac-Sangrador, J.L.; Guerrero-Lopez, C.M.; Cervantes-Martinez, K.; Hurtado-Diaz, M.; de la Sierra-de la Vega, L.A.; Zuniga-Bello, P.E. Quantifying health impacts and economic costs of PM2.5 exposure in Mexican cities of the National Urban System. Int. J. Public Health 2019, 64, 561–572. [Google Scholar] [CrossRef]
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.; Saldiva, P.H.N.; Lavigne, E.; Matus, P.; et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Angeles-Martinez, J.; Posadas-Sanchez, R.; Bravo-Flores, E.; Gonzalez-Salazar, M.D.C.; Vargas-Alarcon, G. Common Variants in IL-20 Gene are Associated with Subclinical Atherosclerosis, Cardiovascular Risk Factors and IL-20 Levels in the Cohort of the Genetics of Atherosclerotic Disease (GEA) Mexican Study. Biomolecules 2020, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Posadas-Sanchez, R.; Lopez-Uribe, A.R.; Posadas-Romero, C.; Perez-Hernandez, N.; Rodriguez-Perez, J.M.; Ocampo-Arcos, W.A.; Fragoso, J.M.; Cardoso-Saldana, G.; Vargas-Alarcon, G. Association of the I148M/PNPLA3 (rs738409) polymorphism with premature coronary artery disease, fatty liver, and insulin resistance in type 2 diabetic patients and healthy controls. The GEA study. Immunobiology 2017, 222, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Mautner, G.C.; Mautner, S.L.; Froehlich, J.; Feuerstein, I.M.; Proschan, M.A.; Roberts, W.C.; Doppman, J.L. Coronary artery calcification: Assessment with electron beam CT and histomorphometric correlation. Radiology 1994, 192, 619–623. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- DeLong, D.M.; DeLong, E.R.; Wood, P.D.; Lippel, K.; Rifkind, B.M. A comparison of methods for the estimation of plasma low- and very low-density lipoprotein cholesterol. The Lipid Research Clinics Prevalence Study. JAMA 1986, 256, 2372–2377. [Google Scholar] [CrossRef]
- Vargas-Alarcón, G.; González-Salazar, M.d.C.; Hernández-Díaz Couder, A.; Sánchez-Muñoz, F.; Ramírez-Bello, J.; Rodríguez-Pérez, J.M.; Posadas-Sánchez, R. Association of the rs17574 DPP4 Polymorphism with Premature Coronary Artery Disease in Diabetic Patients: Results from the Cohort of the GEA Mexican Study. Diagnostics 2022, 12, 1716. [Google Scholar] [CrossRef]
- Baecke, J.A.; Burema, J.; Frijters, J.E. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am. J. Clin. Nutr. 1982, 36, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Rojo, M.M.; Rothenberg, S.J.; Texcalac-Sangrador, J.L.; Just, A.C.; Kloog, I.; Rojas-Saunero, L.P.; Gutierrez-Avila, I.; Bautista-Arredondo, L.F.; Tamayo-Ortiz, M.; Romero, M.; et al. Children’s acute respiratory symptoms associated with PM2.5 estimates in two sequential representative surveys from the Mexico City Metropolitan Area. Environ. Res. 2020, 180, 108868. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.D. Local Models for Spatial Analysis, 2nd ed.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2019; p. 352. [Google Scholar]
- Kaufman, J.D.; Adar, S.D.; Allen, R.W.; Barr, R.G.; Budoff, M.J.; Burke, G.L.; Casillas, A.M.; Cohen, M.A.; Curl, C.L.; Daviglus, M.L.; et al. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Am. J. Epidemiol. 2012, 176, 825–837. [Google Scholar] [CrossRef]
- McGuinn, L.A.; Ward-Caviness, C.K.; Neas, L.M.; Schneider, A.; Diaz-Sanchez, D.; Cascio, W.E.; Kraus, W.E.; Hauser, E.; Dowdy, E.; Haynes, C.; et al. Association between satellite-based estimates of long-term PM2.5 exposure and coronary artery disease. Environ. Res. 2016, 145, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Heiss, G.; Rose, K.M.; Whitsel, E.A.; Lurmann, F.; London, S.J. Prospective analysis of traffic exposure as a risk factor for incident coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Environ. Health Perspect. 2008, 116, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-term exposure to air pollution and incidence of cardiovascular events in women. N. Engl. J. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef]
- Puett, R.C.; Hart, J.E.; Yanosky, J.D.; Paciorek, C.; Schwartz, J.; Suh, H.; Speizer, F.E.; Laden, F. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ. Health Perspect. 2009, 117, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Global Air Quality Guidelines; WHO: Geneva, Switzerland, 2021.
- Tapia, V.; Steenland, K.; Vu, B.; Liu, Y.; Vasquez, V.; Gonzales, G.F. PM2.5 exposure on daily cardio-respiratory mortality in Lima, Peru, from 2010 to 2016. Environ. Health 2020, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, D.S.; Martins, L.C.; Arbex, M.A.; Pamplona, Y.A.P. Atmospheric Pollution and Hospitalization for Cardiovascular and Respiratory Diseases in the City of Manaus from 2008 to 2012. Sci. World J. 2020, 2020, 8458359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodriguez-Camargo, L.A.; Sierra-Parada, R.J.; Blanco-Becerra, L.C. Spatial analysis of PM2.5 concentrations in Bogota according to the World Health Organization air quality guidelines for cardiopulmonary diseases, 2014–2015. Biomedica 2020, 40, 137–152. [Google Scholar] [CrossRef]
- Vedal, S.; Campen, M.J.; McDonald, J.D.; Larson, T.V.; Sampson, P.D.; Sheppard, L.; Simpson, C.D.; Szpiro, A.A. National Particle Component Toxicity (NPACT) initiative report on cardiovascular effects. Res. Rep. Health Eff. Inst. 2013, 5–8. [Google Scholar]
- Osornio-Vargas, A.R.; Bonner, J.C.; Alfaro-Moreno, E.; Martinez, L.; Garcia-Cuellar, C.; Ponce-de-Leon Rosales, S.; Miranda, J.; Rosas, I. Proinflammatory and cytotoxic effects of Mexico City air pollution particulate matter in vitro are dependent on particle size and composition. Environ. Health Perspect. 2003, 111, 1289–1293. [Google Scholar] [CrossRef]
- Moreno, T.; Querol, X.; Pey, J.; Minguillon, M.C.; Perez, N.; Alastuey, A.; Bernabe, R.M.; Blanco, S.; Cardenas, B.; Eichinger, W.; et al. Spatial and temporal variations in inhalable CuZnPb aerosols within the Mexico City pollution plume. J. Environ. Monit. 2008, 10, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Leon, N.; Serrano-Lomelin, J.; Sanchez, B.N.; Quintana-Belmares, R.; Vega, E.; Vazquez-Lopez, I.; Rojas-Bracho, L.; Lopez-Villegas, M.T.; Vadillo-Ortega, F.; De Vizcaya-Ruiz, A.; et al. TNFalpha and IL-6 Responses to Particulate Matter in Vitro: Variation According to PM Size, Season, and Polycyclic Aromatic Hydrocarbon and Soil Content. Environ. Health Perspect. 2016, 124, 406–412. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Edgerton, S.A.; Vega, E. Chemical composition of PM2.5 and PM10 in Mexico City during winter 1997. Sci. Total Environ. 2002, 287, 177–201. [Google Scholar] [CrossRef]
- Sancini, G.; Farina, F.; Battaglia, C.; Cifola, I.; Mangano, E.; Mantecca, P.; Camatini, M.; Palestini, P. Health risk assessment for air pollutants: Alterations in lung and cardiac gene expression in mice exposed to Milano winter fine particulate matter (PM2.5). PLoS ONE 2014, 9, e109685. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, J.; Ning, R.; Du, Z.; Liu, J.; Batibawa, J.W.; Duan, J.; Sun, Z. The critical role of endothelial function in fine particulate matter-induced atherosclerosis. Part. Fibre Toxicol. 2020, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Han, I.K.; Shao, M.; Hu, M.; Zhang, O.J.; Tang, X. PM2.5 constituents and oxidative DNA damage in humans. Environ. Sci. Technol. 2009, 43, 4757–4762. [Google Scholar] [CrossRef] [PubMed]
- Cigankova, H.; Mikuska, P.; Hegrova, J.; Krajcovic, J. Comparison of oxidative potential of PM1 and PM2.5 urban aerosol and bioaccessibility of associated elements in three simulated lung fluids. Sci. Total Environ. 2021, 800, 149502. [Google Scholar] [CrossRef]
- Bauersachs, R.; Zeymer, U.; Briere, J.B.; Marre, C.; Bowrin, K.; Huelsebeck, M. Burden of Coronary Artery Disease and Peripheral Artery Disease: A Literature Review. Cardiovasc. Ther. 2019, 2019, 8295054. [Google Scholar] [CrossRef]
- Tibaut, M.; Petrovic, D. Oxidative Stress Genes, Antioxidants and Coronary Artery Disease in Type 2 Diabetes Mellitus. Hematol. Agents Med. Chem. 2016, 14, 23–38. [Google Scholar] [CrossRef]
- Janik, R.; Kubov, M.; Schieber, B. The ground-level ozone concentration in beech (Fagus sylvatica L.) forests in the West Carpathian Mountains. Environ. Monit. Assess. 2020, 192, 233. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jacob, D.J.; Liao, H.; Qiu, Y.; Shen, L.; Zhai, S.; Bates, K.H.; Sulprizio, M.P.; Song, S.; Lu, X.; et al. Ozone pollution in the North China Plain spreading into the late-winter haze season. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Lange, S.S. Comparing apples to oranges: Interpreting ozone concentrations from observational studies in the context of the United States ozone regulatory standard. Sci. Total Environ. 2018, 644, 1547–1556. [Google Scholar] [CrossRef]
- Peralta, O.; Ortinez-Alvarez, A.; Torres-Jardon, R.; Suarez-Lastra, M.; Castro, T.; Ruiz-Suarez, L.G. Ozone over Mexico City during the COVID-19 pandemic. Sci. Total Environ. 2021, 761, 143183. [Google Scholar] [CrossRef]
- Ballester, F.; Rodriguez, P.; Iniguez, C.; Saez, M.; Daponte, A.; Galan, I.; Taracido, M.; Arribas, F.; Bellido, J.; Cirarda, F.B.; et al. Air pollution and cardiovascular admissions association in Spain: Results within the EMECAS project. J. Epidemiol. Community Health 2006, 60, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.C.; Chuang, K.J.; Chien, L.C.; Chen, W.J.; Chang, W.T. Urban air pollution and emergency admissions for cerebrovascular diseases in Taipei, Taiwan. Eur. Heart J. 2006, 27, 1238–1244. [Google Scholar] [CrossRef]
- Barnett, A.G.; Williams, G.M.; Schwartz, J.; Best, T.L.; Neller, A.H.; Petroeschevsky, A.L.; Simpson, R.W. The effects of air pollution on hospitalizations for cardiovascular disease in elderly people in Australian and New Zealand cities. Environ. Health Perspect. 2006, 114, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, P.E.; Klein, M.; Peel, J.L.; Sarnat, S.E.; Sarnat, J.A. Multipollutant modeling issues in a study of ambient air quality and emergency department visits in Atlanta. J. Expo. Sci. Environ. Epidemiol. 2007, 17 (Suppl. S2), S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Lee, K.K.; McAllister, D.A.; Hunter, A.; Nair, H.; Whiteley, W.; Langrish, J.P.; Newby, D.E.; Mills, N.L. Short term exposure to air pollution and stroke: Systematic review and meta-analysis. BMJ 2015, 350, h1295. [Google Scholar] [CrossRef] [PubMed]
- Mustafic, H.; Jabre, P.; Caussin, C.; Murad, M.H.; Escolano, S.; Tafflet, M.; Perier, M.C.; Marijon, E.; Vernerey, D.; Empana, J.P.; et al. Main air pollutants and myocardial infarction: A systematic review and meta-analysis. JAMA 2012, 307, 713–721. [Google Scholar] [CrossRef]
- Shah, A.S.; Langrish, J.P.; Nair, H.; McAllister, D.A.; Hunter, A.L.; Donaldson, K.; Newby, D.E.; Mills, N.L. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet 2013, 382, 1039–1048. [Google Scholar] [CrossRef]
- Romieu, I.; Gouveia, N.; Cifuentes, L.A.; de Leon, A.P.; Junger, W.; Vera, J.; Strappa, V.; Hurtado-Diaz, M.; Miranda-Soberanis, V.; Rojas-Bracho, L.; et al. Multicity study of air pollution and mortality in Latin America (the ESCALA study). Res. Rep. Health Eff. Inst. 2012, 171, 5–86. [Google Scholar]
- Bravo, M.A.; Son, J.; de Freitas, C.U.; Gouveia, N.; Bell, M.L. Air pollution and mortality in Sao Paulo, Brazil: Effects of multiple pollutants and analysis of susceptible populations. J. Expo. Sci Environ. Epidemiol. 2016, 26, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Franck, U.; Leitte, A.M.; Suppan, P. Multiple exposures to airborne pollutants and hospital admissions due to diseases of the circulatory system in Santiago de Chile. Sci. Total Environ. 2014, 468–469, 746–756. [Google Scholar] [CrossRef]
- Day, D.B.; Xiang, J.; Mo, J.; Li, F.; Chung, M.; Gong, J.; Weschler, C.J.; Ohman-Strickland, P.A.; Sundell, J.; Weng, W.; et al. Association of Ozone Exposure With Cardiorespiratory Pathophysiologic Mechanisms in Healthy Adults. JAMA Intern. Med. 2017, 177, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Kahle, J.J.; Neas, L.M.; Devlin, R.B.; Case, M.W.; Schmitt, M.T.; Madden, M.C.; Diaz-Sanchez, D. Interaction effects of temperature and ozone on lung function and markers of systemic inflammation, coagulation, and fibrinolysis: A crossover study of healthy young volunteers. Environ. Health Perspect. 2015, 123, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Dias, D.; Tchepel, O. Spatial and Temporal Dynamics in Air Pollution Exposure Assessment. Int. J. Environ. Res. Public Health 2018, 15, 558. [Google Scholar] [CrossRef]
- Lash, T.L.; VanderWeele, T.J.; Haneuse, S.; Rothman, K.J. Modern Epidemiology; Wolters Kluwer: Philadelphia, PA, USA, 2021. [Google Scholar]
- Zamarron-Licona, E.; Rodriguez-Perez, J.M.; Posadas-Sanchez, R.; Vargas-Alarcon, G.; Banos-Gonzalez, M.A.; Borgonio-Cuadra, V.M.; Perez-Hernandez, N. Variants of PCSK9 Gene Are Associated with Subclinical Atherosclerosis and Cardiometabolic Parameters in Mexicans. The GEA Project. Diagnostics 2021, 11, 774. [Google Scholar] [CrossRef]
- Fewell, Z.; Davey Smith, G.; Sterne, J.A. The impact of residual and unmeasured confounding in epidemiologic studies: A simulation study. Am. J. Epidemiol. 2007, 166, 646–655. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Control | pCAD | p-Value |
|---|---|---|---|
| Overall | 869 (53.8%) | 746 (46.2%) | |
| Participant sex | |||
| Male | 326 (37.5%) | 603 (80.8%) | |
| Female | 543 (62.5%) | 143 (19.2%) | <0.0001 * |
| Age (years) | 51.9 ± 9.0 | 53.9 ± 7.7 | <0.0001 & |
| BMI (kg/m2) | 28.5 ± 4.5 | 29.0 ± 4.5 | 0.005 & |
| BMI classification (kg/m2) | |||
| Normal (18.5–24.9) | 193 (22.2%) | 127 (17.0%) | |
| Overweight (25–29.9) | 398 (45.8%) | 341 (45.7%) | |
| Obesity (>30.0) | 278 (32.0%) | 278 (37.3%) | 0.01 * |
| Education | |||
| <Elementary school | 257 (29.6%) | 398 (53.4%) | |
| Junior high school | 320 (36.8%) | 186 (24.9%) | |
| >Senior high school | 292 (33.6%) | 162 (21.7%) | <0.0001 * |
| Cigarette Smoking | |||
| Never smoker | 390 (44.9%) | 157 (21.1%) | |
| Former smoker | 281 (32.3%) | 477 (63.9%) | |
| Current smoker | 198 (22.8%) | 112 (15.0%) | <0.0001 * |
| Diabetes Mellitus | |||
| No | 775 (89.2%) | 485 (65.0%) | |
| Yes | 94 (10.8%) | 261 (35.0%) | <0.0001 * |
| HDL-C (mg/dL) | 46.8 ± 13.8 | 39.6 ± 10.9 | <0.0001 & |
| LDL-C (mg/dL) | 116.0 ± 31.5 | 98.3 ± 38.0 | <0.0001 & |
| Systolic blood pressure (mmHg) | 113.6 ± 15.9 | 118 ± 18.5 | <0.0001 & |
| Diastolic blood pressure (mmHg) | 70.2 ± 8.7 | 71.79 ± 9.9 | 0.007 & |
| Antihypertensive medication | |||
| No | 710 (81.7) | 247 (33.1) | |
| Yes | 159 (18.3) | 499 (66.9) | <0.0001 * |
| Physical activity | 7.8 ± 1.2 | 7.6 ± 1.3 | 0.0004 & |
| Total (N = 1615) | Controls (N = 869) | pCAD (N = 746) | p-Value | |
|---|---|---|---|---|
| Ozone (ppb) | ||||
| 1-year | 75.8 (68.5–81.2) | 75.9 (68.7–81.2) | 75.7 (68.5–80.8) | 0.22 |
| 2-year | 75.6 (71.4–82.6) | 75.5 (71.4–82.6) | 75.7 (71.5–81.8) | 0.38 |
| 3-year | 76.5 (71.8–83.1) | 76.3 (71.8–83.1) | 76.6 (71.8–81.9) | 0.05 |
| 4-year | 77.5 (73.1–84.9) | 77.3 (73.1–84.9) | 77.6 (73.2–83.6) | 0.14 |
| 5-year | 78.6 (73.7–84.9) | 78.4 (73.7–84.9) | 78.9 (73.7–84.7) | 0.02 |
| PM2.5 (μg/m3) | ||||
| 1-year | 24.6 (17.7–31.7) | 24.7 (17.8–29.9) | 24.5 (17.7–31.7) | 0.05 |
| 2-year | 23.9 (20.8–29.6) | 23.9 (21.1–29.6) | 23.9 (20.8–29.2) | 0.76 |
| 3-year | 23.6 (21.5–29.5) | 23.5 (21.6–29.3) | 23.7 (21.5–29.5) | <0.0001 |
| 4-year | 24.2 (21.9–29.8) | 24.0 (21.9–29.7) | 24.3 (21.9–29.8) | <0.0001 |
| 5-year | 24.7 (22.3–29.7) | 24.5 (22.3–29.7) | 25.0 (22.3–29.7) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posadas-Sánchez, R.; Vargas-Alarcón, G.; Cardenas, A.; Texcalac-Sangrador, J.L.; Osorio-Yáñez, C.; Sanchez-Guerra, M. Long-Term Exposure to Ozone and Fine Particulate Matter and Risk of Premature Coronary Artery Disease: Results from Genetics of Atherosclerotic Disease Mexican Study. Biology 2022, 11, 1122. https://doi.org/10.3390/biology11081122
Posadas-Sánchez R, Vargas-Alarcón G, Cardenas A, Texcalac-Sangrador JL, Osorio-Yáñez C, Sanchez-Guerra M. Long-Term Exposure to Ozone and Fine Particulate Matter and Risk of Premature Coronary Artery Disease: Results from Genetics of Atherosclerotic Disease Mexican Study. Biology. 2022; 11(8):1122. https://doi.org/10.3390/biology11081122
Chicago/Turabian StylePosadas-Sánchez, Rosalinda, Gilberto Vargas-Alarcón, Andres Cardenas, José Luis Texcalac-Sangrador, Citlalli Osorio-Yáñez, and Marco Sanchez-Guerra. 2022. "Long-Term Exposure to Ozone and Fine Particulate Matter and Risk of Premature Coronary Artery Disease: Results from Genetics of Atherosclerotic Disease Mexican Study" Biology 11, no. 8: 1122. https://doi.org/10.3390/biology11081122
APA StylePosadas-Sánchez, R., Vargas-Alarcón, G., Cardenas, A., Texcalac-Sangrador, J. L., Osorio-Yáñez, C., & Sanchez-Guerra, M. (2022). Long-Term Exposure to Ozone and Fine Particulate Matter and Risk of Premature Coronary Artery Disease: Results from Genetics of Atherosclerotic Disease Mexican Study. Biology, 11(8), 1122. https://doi.org/10.3390/biology11081122






