Association between Cervical Microbiota and HPV: Could This Be the Key to Complete Cervical Cancer Eradication?
Abstract
Simple Summary
Abstract
1. Epidemiology of HPV
2. Screening and Histopathology of Cervical HPV Lesions
Principal Biomarkers for Cervical Cancer
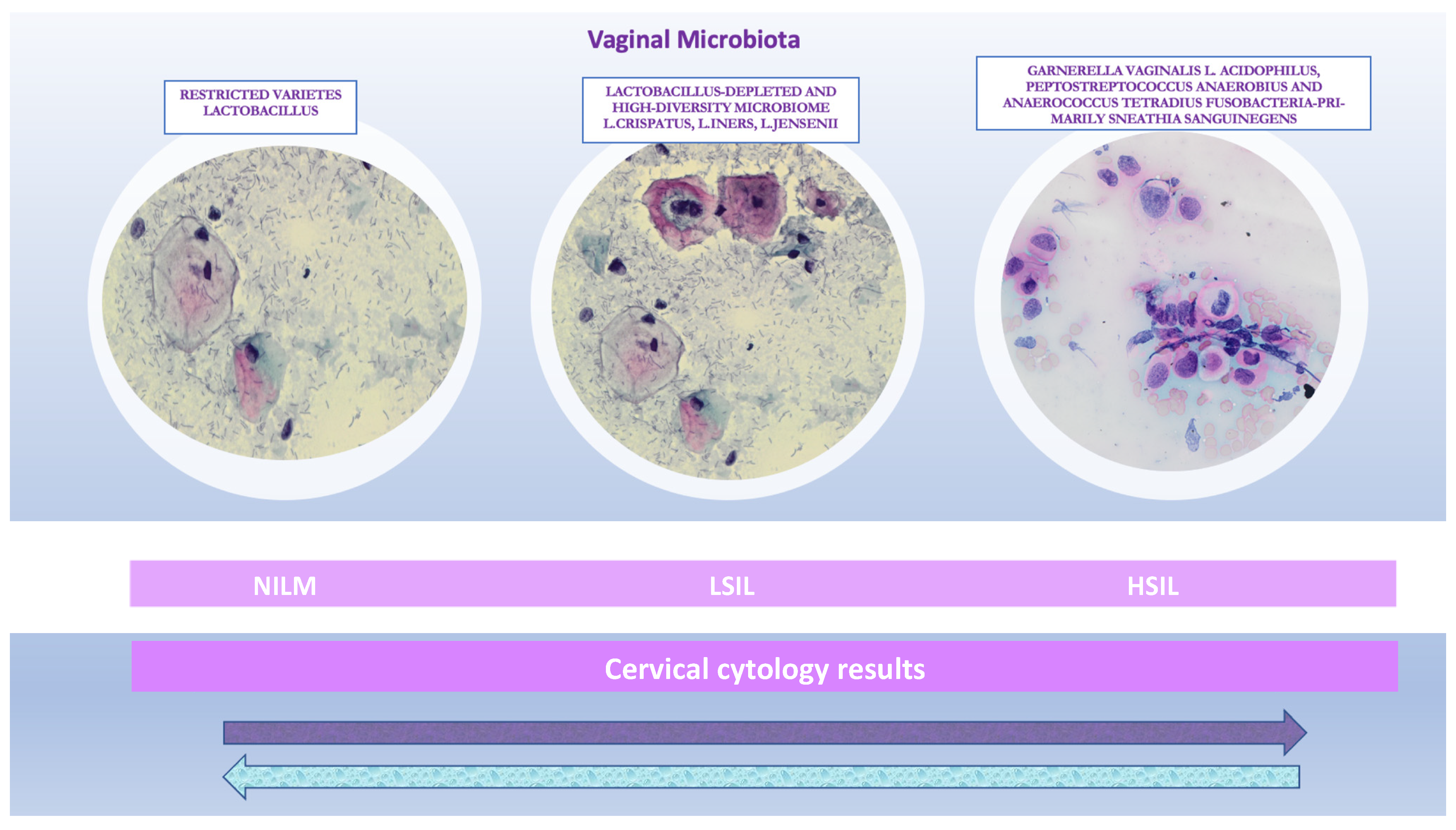
3. Diversity and Influencing Factors of Vaginal Microbiota
4. The Association of Cervical Microbiota with the Risk of CIN
5. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brianti, P.; De Flammineis, E.; Mercuri, S.R. Review of HPV-related diseases and cancers. New Microbiol. 2017, 40, 80–85. [Google Scholar] [PubMed]
- Soheili, M.; Keyvani, H.; Soheili, M.; Nasseri, S. Human papilloma virus: A review study of epidemiology, carcinogenesis, diagnostic methods, and treatment of all HPV-related cancers. Med. J. Islamic Repub. Iran 2021, 35, 65. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Koutsky, L.A. Natural history and epidemiological features of genital HPV infection. IARC Sci. Publ. 1992, 119, 25–52. [Google Scholar]
- McBride, A.A. Oncogenic human papillomaviruses. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160273. [Google Scholar] [CrossRef]
- Salambanga, C.; Zohoncon, T.M.; Traore, I.M.A.; Ouedraogo, R.A.; Djigma, W.F.; Ouedraog, C.; Simpore, J. High prevalence of high-risk human papillomavirus (HPV) infection among sexually active women in Ouagadougou. Med. Sante Trop. 2019, 29, 302–305. [Google Scholar] [CrossRef]
- Nielsen, A.; Iftner, T.; Norgaard, M.; Munk, C.; Junge, J.; Kjaer, S.K. The importance of low-risk HPV infection for the risk of abnormal cervical cytology/histology in more than 40,000 Danish women. Sex. Transm. Infect. 2012, 88, 627–632. [Google Scholar] [CrossRef]
- Morandell, D.; Rostek, U.; Bouvard, V.; Campo-Fernandez, B.; Fiedler, M.; Jansen-Durr, P.; Zwerschke, W. Human papillomavirus type 45 E7 is a transforming protein inducing retinoblastoma protein degradation and anchorage-independent cell cycle progression. Virology 2008, 379, 20–29. [Google Scholar] [CrossRef]
- de Sanjose, S.; Brotons, M.; Pavon, M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Bosch, F.X.; de Sanjose, S. Chapter 1: Human papillomavirus and cervical cancer--burden and assessment of causality. J. Natl. Cancer Inst. Monogr. 2003, 2003, 3–13. [Google Scholar] [CrossRef]
- Munoz, N.; Bosch, F.X.; de Sanjose, S.; Herrero, R.; Castellsague, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J. International Agency for Research on Cancer Multicenter Cervical Cancer Study, G. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef]
- Chen, H.C.; Schiffman, M.; Lin, C.Y.; Pan, M.H.; You, S.L.; Chuang, L.C.; Hsieh, C.Y.; Liaw, K.L.; Hsing, A.W.; Chen, C.J.; et al. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J. Natl. Cancer Inst. 2011, 103, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Mbuya, W.; Held, K.; McHaro, R.D.; Haule, A.; Mhizde, J.; Mnkai, J.; Mahenge, A.; Mwakatima, M.; Sembo, M.; Mwalongo, W.; et al. Depletion of Human Papilloma Virus E6- and E7-Oncoprotein-Specific T-Cell Responses in Women Living With HIV. Front. Immunol. 2021, 12, 742861. [Google Scholar] [CrossRef]
- Ostor, A.G. Natural history of cervical intraepithelial neoplasia: A critical review. Int. J. Gynecol. Pathol. 1993, 12, 186–192. [Google Scholar] [CrossRef]
- WHO. New Recommendations for Screening and Treatment to Prevent Cervical Cancer. 2021. Available online: https://www.who.int/news/item/06-07-2021-new-recommendations-for-screening-and-treatment-to-prevent-cervical-cancer (accessed on 6 July 2021).
- Fontham, E.T.; Wolf, A.M.; Church, T.R.; Etzioni, R.; Flowers, C.R.; Herzig, A.; Guerra, C.E.; Oeffinger, K.C.; Shih, Y.C.T.; Walter, L.C. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA A Cancer J. Clin. 2020, 70, 321–346. [Google Scholar] [CrossRef]
- Obermair, H.M.; Bennett, K.F.; Brotherton, J.M.; Smith, M.A.; McCaffery, K.J.; Dodd, R.H. Australian National Cervical Screening Program renewal: Attitudes and experiences of general practitioners, and obstetricians and gynaecologists. Aust. N. Z. J. Obstet. Gynaecol. 2021, 61, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.F.; Muller, T.R.; Olsen, A. Australian women’s cervical cancer screening attendance as a function of screening barriers and facilitators. Soc. Sci. Med. 2019, 220, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Chan, C.W.; Chow, K.M.; Yang, S.; Luo, Y.; Cheng, H.; Wang, H. Understanding the cervical screening behaviour of Chinese women: The role of health care system and health professions. Appl. Nurs. Res. 2018, 39, 58–64. [Google Scholar] [CrossRef]
- Schiffman, M.; Wentzensen, N. A Suggested Approach to Simplify and Improve Cervical Screening in the United States. J. Low. Genit. Tract Dis. 2016, 20, 1–7. [Google Scholar] [CrossRef][Green Version]
- von Karsa, L.; Arbyn, M.; De Vuyst, H.; Dillner, J.; Dillner, L.; Franceschi, S.; Patnick, J.; Ronco, G.; Segnan, N.; Suonio, E. European guidelines for quality assurance in cervical cancer screening. Summary of the supplements on HPV screening and vaccination. Papillomavirus Res. 2015, 1, 22–31. [Google Scholar] [CrossRef]
- ASCO. Secondary Prevention of Cervical Cancer: American Society of Clinical Oncology Resource-Stratified Clinical Practice Guideline. 2016. Available online: https://www.asco.org/sites/new-www.asco.org/files/content-files/practice-and-guidelines/documents/2016-rs-secondary-prev-cervical-slides2.pdf (accessed on 1 January 2016).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Nayar, R.; Wilbur, D.C. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015, 123, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Tsikouras, P.; Zervoudis, S.; Manav, B.; Tomara, E.; Iatrakis, G.; Romanidis, C.; Bothou, A.; Galazios, G. Cervical cancer: Screening, diagnosis and staging. J. BUON 2016, 21, 320–325. [Google Scholar] [PubMed]
- Ronco, G.; Giorgi-Rossi, P.; Carozzi, F.; Confortini, M.; Dalla Palma, P.; Del Mistro, A.; Ghiringhello, B.; Girlando, S.; Gillio-Tos, A.; De Marco, L.; et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: A randomised controlled trial. Lancet Oncol. 2010, 11, 249–257. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Monsonego, J. Global challenges of cervical cancer prevention. Eur. J. Gynaec. Oncol.-IssN 2000, 392, 2936. [Google Scholar]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Pirog, E.C. Cervical adenocarcinoma: Diagnosis of human papillomavirus–positive and human papillomavirus–negative tumors. Arch. Pathol. Lab. Med. 2017, 141, 1653–1667. [Google Scholar] [CrossRef]
- Holl, K.; Nowakowski, A.M.; Powell, N.; McCluggage, W.G.; Pirog, E.C.; Collas De Souza, S.; Tjalma, W.A.; Rosenlund, M.; Fiander, A.; Castro Sánchez, M. Human papillomavirus prevalence and type-distribution in cervical glandular neoplasias: Results from a E uropean multinational epidemiological study. Int. J. Cancer 2015, 137, 2858–2868. [Google Scholar] [CrossRef]
- Stolnicu, S.; Barsan, I.; Hoang, L.; Patel, P.; Terinte, C.; Pesci, A.; Aviel-Ronen, S.; Kiyokawa, T.; Alvarado-Cabrero, I.; Pike, M.C. International Endocervical Adenocarcinoma Criteria and Classification (IECC): A new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am. J. Surg. Pathol. 2018, 42, 214. [Google Scholar] [CrossRef]
- Tjalma, W. HPV negative cervical cancers and primary HPV screening. Facts Views Vis. ObGyn 2018, 10, 107. [Google Scholar]
- Szarewski, A.; Ambroisine, L.; Cadman, L.; Austin, J.; Ho, L.; Terry, G.; Liddle, S.; Dina, R.; McCarthy, J.; Buckley, H. Comparison of predictors for high-grade cervical intraepithelial neoplasia in women with abnormal smears. Cancer Epidemiol. Prev. Biomark. 2008, 17, 3033–3042. [Google Scholar] [CrossRef]
- Mayrand, M.-H.; Franco, E.L. Integrating novel primary-and secondary-prevention strategies: The next challenge for cervical cancer control. Future Oncol. 2010, 6, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; von Knebel Doeberitz, M. Biomarkers in cervical cancer screening. Dis. Markers 2007, 23, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, K.; Wentzensen, N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol. Prev. Biomark. 2008, 17, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Lawicki, S.; Bedkowska, E.; Gacuta-Szumarska, E.; Knapp, P.; Szmitkowski, M. The plasma levels and diagnostic utility of stem cell factor (SCF) and macrophage-colony stimulating factor (M-CSF) in cervical cancer patients. Pol. Merkur. Lek. 2008, 25, 38–42. [Google Scholar]
- Murphy, N.; Ring, M.; Heffron, C.C.; King, B.; Killalea, A.G.; Hughes, C.; Martin, C.M.; McGuinness, E.; Sheils, O.; O’Leary, J.J. p16INK4A, CDC6, and MCM5: Predictive biomarkers in cervical preinvasive neoplasia and cervical cancer. J. Clin. Pathol. 2005, 58, 525–534. [Google Scholar] [CrossRef]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schutte, U.M.; Zhong, X.; Koenig, S.S.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132ra152. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome 2016, 4, 58. [Google Scholar] [CrossRef]
- Kalof, A.N.; Cooper, K. p16INK4a immunoexpression: Surrogate marker of high-risk HPV and high-grade cervical intraepithelial neoplasia. Adv. Anat. Pathol. 2006, 13, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Trunk, M.J.; Dallenbach-Hellweg, G.; Ridder, R.; Petry, K.U.; Ikenberg, H.; Schneider, V.; von Knebel Doeberitz, M. Morphologic characteristics of p16INK4a-positive cells in cervical cytology samples. Acta Cytol. 2004, 48, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Bergeron, C.; Denton, K.J.; Ridder, R.; European, C.C.S.G. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: Results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol. 2011, 119, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, C.; Ikenberg, H.; Sideri, M.; Denton, K.; Bogers, J.; Schmidt, D.; Alameda, F.; Keller, T.; Rehm, S.; Ridder, R.; et al. Prospective evaluation of p16/Ki-67 dual-stained cytology for managing women with abnormal Papanicolaou cytology: PALMS study results. Cancer Cytopathol. 2015, 123, 373–381. [Google Scholar] [CrossRef]
- Kruse, A.J.; Baak, J.P.; de Bruin, P.C.; Jiwa, M.; Snijders, W.P.; Boodt, P.J.; Fons, G.; Houben, P.W.; The, H.S. Ki-67 immunoquantitation in cervical intraepithelial neoplasia (CIN): A sensitive marker for grading. J. Pathol. 2001, 193, 48–54. [Google Scholar] [CrossRef]
- FDA. Premarket Approval (PMA). 2020. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P190024 (accessed on 3 October 2020).
- Peeters, E.; Wentzensen, N.; Bergeron, C.; Arbyn, M. Meta-analysis of the accuracy of p16 or p16/Ki-67 immunocytochemistry versus HPV testing for the detection of CIN2+/CIN3+ in triage of women with minor abnormal cytology. Cancer Cytopathol. 2019, 127, 169–180. [Google Scholar] [CrossRef]
- Wright, T.C., Jr.; Behrens, C.M.; Ranger-Moore, J.; Rehm, S.; Sharma, A.; Stoler, M.H.; Ridder, R. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: Results from a sub-study nested into the ATHENA trial. Gynecol. Oncol. 2017, 144, 51–56. [Google Scholar] [CrossRef]
- Petry, K.U.; Schmidt, D.; Scherbring, S.; Luyten, A.; Reinecke-Luthge, A.; Bergeron, C.; Kommoss, F.; Loning, T.; Ordi, J.; Regauer, S.; et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 Dual-stained cytology. Gynecol. Oncol. 2011, 121, 505–509. [Google Scholar] [CrossRef]
- Wentzensen, N.; Schwartz, L.; Zuna, R.E.; Smith, K.; Mathews, C.; Gold, M.A.; Allen, R.A.; Zhang, R.; Dunn, S.T.; Walker, J.L.; et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin. Cancer Res. 2012, 18, 4154–4162. [Google Scholar] [CrossRef]
- Yu, L.L.; Chen, W.; Lei, X.Q.; Qin, Y.; Wu, Z.N.; Pan, Q.J.; Zhang, X.; Chang, B.F.; Zhang, S.K.; Guo, H.Q.; et al. Evaluation of p16/Ki-67 dual staining in detection of cervical precancer and cancers: A multicenter study in China. Oncotarget 2016, 7, 21181–21189. [Google Scholar] [CrossRef]
- Tay, T.K.Y.; Lim, K.L.; Hilmy, M.H.; Thike, A.A.; Goh, S.T.; Song, L.H.; Hwang, J.S.G.; Mantoo, S. Comparison of the sensitivity and specificity of p16/Ki-67 dual staining and HPV DNA testing of abnormal cervical cytology in the detection of histology proven cervical intraepithelial neoplasia grade 2 and above (CIN 2+). Malays J. Pathol. 2017, 39, 257–265. [Google Scholar] [PubMed]
- Possati-Resende, J.C.; Fregnani, J.H.; Kerr, L.M.; Mauad, E.C.; Longatto-Filho, A.; Scapulatempo-Neto, C. The Accuracy of p16/Ki-67 and HPV Test in the Detection of CIN2/3 in Women Diagnosed with ASC-US or LSIL. PLoS ONE 2015, 10, e0134445. [Google Scholar] [CrossRef] [PubMed]
- Hammes, L.S.; Tekmal, R.R.; Naud, P.; Edelweiss, M.I.; Kirma, N.; Valente, P.T.; Syrjanen, K.J.; Cunha-Filho, J.S. Up-regulation of VEGF, c-fms and COX-2 expression correlates with severity of cervical cancer precursor (CIN) lesions and invasive disease. Gynecol. Oncol. 2008, 110, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Kirma, N.; Hammes, L.S.; Liu, Y.G.; Nair, H.B.; Valente, P.T.; Kumar, S.; Flowers, L.C.; Tekmal, R.R. Elevated expression of the oncogene c-fms and its ligand, the macrophage colony-stimulating factor-1, in cervical cancer and the role of transforming growth factor-beta1 in inducing c-fms expression. Cancer Res. 2007, 67, 1918–1926. [Google Scholar] [CrossRef]
- Lawicki, S.; Bedkowska, G.E.; Gacuta-Szumarska, E.; Knapp, P.; Szmitkowski, M. Pretreatment plasma levels and diagnostic utility of hematopoietic cytokines in cervical cancer or cervical intraepithelial neoplasia patients. Folia Histochem. Cytobiol. 2012, 50, 213–219. [Google Scholar] [CrossRef]
- Zajkowska, M.; Zbucka-Kretowska, M.; Sidorkiewicz, I.; Lubowicka, E.; Gacuta, E.; Szmitkowski, M.; Chrostek, L.; Lawicki, S. Plasma levels and diagnostic utility of macrophage-colony stimulating factor, matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 as tumor markers in cervical cancer patients. Tumour. Biol. 2018, 40, 1010428318790363. [Google Scholar] [CrossRef]
- Lubowicka, E.; Zbucka-Kretowska, M.; Sidorkiewicz, I.; Zajkowska, M.; Gacuta, E.; Puchnarewicz, A.; Chrostek, L.; Szmitkowski, M.; Lawicki, S. Diagnostic Power of Cytokine M-CSF, Metalloproteinase 2 (MMP-2) and Tissue Inhibitor-2 (TIMP-2) in Cervical Cancer Patients Based on ROC Analysis. Pathol. Oncol. Res. 2020, 26, 791–800. [Google Scholar] [CrossRef]
- Sidorkiewicz, I.; Zbucka-Kretowska, M.; Zareba, K.; Lubowicka, E.; Zajkowska, M.; Szmitkowski, M.; Gacuta, E.; Lawicki, S. Plasma levels of M-CSF and VEGF in laboratory diagnostics and differentiation of selected histological types of cervical cancers. BMC Cancer 2019, 19, 398. [Google Scholar] [CrossRef]
- Atoe, K.; Ehimigbai, R.; Ayinbuomwan, E.; Onovughakpo-Sakpa, O. Assessing the utility of a cytokine in the diagnosis of cervical intraepithelial neoplasm among women in Benin city, Nigeria. Afr. J. Health Saf. Environ. 2020, 1, 101–112. [Google Scholar] [CrossRef]
- van de Wijgert, J.H.; Borgdorff, H.; Verhelst, R.; Crucitti, T.; Francis, S.; Verstraelen, H.; Jespers, V. The vaginal microbiota: What have we learned after a decade of molecular characterization? PLoS ONE 2014, 9, e105998. [Google Scholar] [CrossRef]
- Brotman, R.M. Vaginal microbiome and sexually transmitted infections: An epidemiologic perspective. J. Clin. Investig. 2011, 121, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.S.; Walker, J.; Fairley, C.K.; Chen, M.Y.; Tabrizi, S.N.; Donovan, B.; Kaldor, J.M.; McNamee, K.; Urban, E.; Walker, S.; et al. Prevalent and incident bacterial vaginosis are associated with sexual and contraceptive behaviours in young Australian women. PLoS ONE 2013, 8, e57688. [Google Scholar] [CrossRef]
- Chico, R.M.; Mayaud, P.; Ariti, C.; Mabey, D.; Ronsmans, C.; Chandramohan, D. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-Saharan Africa: A systematic review. JAMA 2012, 307, 2079–2086. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Brooks, J.P.; Serrano, M.G.; Sheth, N.U.; Girerd, P.H.; Edwards, D.J.; Strauss, J.F.; The Vaginal Microbiome, C.; Jefferson, K.K.; Buck, G.A. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology (Reading) 2014, 160, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- White, B.A.; Creedon, D.J.; Nelson, K.E.; Wilson, B.A. The vaginal microbiome in health and disease. Trends Endocrinol. Metab. 2011, 22, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal microbiome: Rethinking health and disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef]
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus iners: Friend or Foe? Trends Microbiol. 2017, 25, 182–191. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Rampersaud, R.; Planet, P.J.; Randis, T.M.; Kulkarni, R.; Aguilar, J.L.; Lehrer, R.I.; Ratner, A.J. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J. Bacteriol. 2011, 193, 1034–1041. [Google Scholar] [CrossRef]
- Macklaim, J.M.; Gloor, G.B.; Anukam, K.C.; Cribby, S.; Reid, G. At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners AB-1. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4688–4695. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, E.; Dellino, M.; Cafforio, P.; Paradiso, A.V.; Cormio, G.; D’Oronzo, S. Breast cancer: An update on treatment-related infertility. J. Cancer Res. Clin. Oncol. 2020, 146, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Grassini, D.; Cascardi, E.; Sarotto, I.; Annaratone, L.; Sapino, A.; Berrino, E.; Marchiò, C. Unusual patterns of HER2 expression in breast cancer: Insights and perspectives. Pathobiology 2022, 89, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Annaratone, L.; Cascardi, E.; Vissio, E.; Sarotto, I.; Chmielik, E.; Sapino, A.; Berrino, E.; Marchio, C. The Multifaceted Nature of Tumor Microenvironment in Breast Carcinomas. Pathobiology 2020, 87, 125–142. [Google Scholar] [CrossRef]
- Mandar, R.; Punab, M.; Borovkova, N.; Lapp, E.; Kiiker, R.; Korrovits, P.; Metspalu, A.; Krjutskov, K.; Nolvak, H.; Preem, J.K.; et al. Complementary seminovaginal microbiome in couples. Res. Microbiol. 2015, 166, 440–447. [Google Scholar] [CrossRef]
- Hickey, R.J.; Zhou, X.; Settles, M.L.; Erb, J.; Malone, K.; Hansmann, M.A.; Shew, M.L.; Van Der Pol, B.; Fortenberry, J.D.; Forney, L.J. Vaginal microbiota of adolescent girls prior to the onset of menarche resemble those of reproductive-age women. mBio 2015, 6, e00097-15. [Google Scholar] [CrossRef]
- Eschenbach, D.A.; Thwin, S.S.; Patton, D.L.; Hooton, T.M.; Stapleton, A.E.; Agnew, K.; Winter, C.; Meier, A.; Stamm, W.E. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin. Infect. Dis. 2000, 30, 901–907. [Google Scholar] [CrossRef]
- MacIntyre, D.A.; Chandiramani, M.; Lee, Y.S.; Kindinger, L.; Smith, A.; Angelopoulos, N.; Lehne, B.; Arulkumaran, S.; Brown, R.; Teoh, T.G.; et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci. Rep. 2015, 5, 8988. [Google Scholar] [CrossRef]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Fadrosh, D.; Chang, K.; Silver, M.I.; Viscidi, R.P.; Burke, A.E.; Ravel, J.; Gravitt, P.E. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2014, 21, 450–458. [Google Scholar] [CrossRef]
- Boskey, E.R.; Cone, R.A.; Whaley, K.J.; Moench, T.R. Origins of vaginal acidity: High D/L lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 2001, 16, 1809–1813. [Google Scholar] [CrossRef]
- Spear, G.T.; French, A.L.; Gilbert, D.; Zariffard, M.R.; Mirmonsef, P.; Sullivan, T.H.; Spear, W.W.; Landay, A.; Micci, S.; Lee, B.H.; et al. Human alpha-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J. Infect. Dis. 2014, 210, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Fichorova, R.N.; Chen, P.L.; Morrison, C.S.; Doncel, G.F.; Mendonca, K.; Kwok, C.; Chipato, T.; Salata, R.; Mauck, C. The Contribution of Cervicovaginal Infections to the Immunomodulatory Effects of Hormonal Contraception. mBio 2015, 6, e00221-15. [Google Scholar] [CrossRef] [PubMed]
- Moreno, V.; Bosch, F.X.; Munoz, N.; Meijer, C.J.; Shah, K.V.; Walboomers, J.M.; Herrero, R.; Franceschi, S.; International Agency for Research on Cancer. Multicentric Cervical Cancer Study, G. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: The IARC multicentric case-control study. Lancet 2002, 359, 1085–1092. [Google Scholar] [CrossRef]
- Tamma, R.; Annese, T.; Ruggieri, S.; Brunetti, O.; Longo, V.; Cascardi, E.; Mastropasqua, M.G.; Maiorano, E.; Silvestris, N.; Ribatti, D. Inflammatory cells infiltrate and angiogenesis in locally advanced and metastatic cholangiocarcinoma. Eur. J. Clin. Investig. 2019, 49, e13087. [Google Scholar] [CrossRef] [PubMed]
- Tamma, R.; Limongelli, L.; Maiorano, E.; Pastore, D.; Cascardi, E.; Tempesta, A.; Carluccio, P.; Mastropasqua, M.G.; Capodiferro, S.; Covelli, C.; et al. Vascular density and inflammatory infiltrate in primary oral squamous cell carcinoma and after allogeneic hematopoietic stem cell transplantation. Ann. Hematol. 2019, 98, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Tamma, R.; Rutigliano, M.; Lucarelli, G.; Annese, T.; Ruggieri, S.; Cascardi, E.; Napoli, A.; Battaglia, M.; Ribatti, D. Microvascular density, macrophages, and mast cells in human clear cell renal carcinoma with and without bevacizumab treatment. Urol. Oncol. 2019, 37, 355.e11–355.e19. [Google Scholar] [CrossRef]
- Verginelli, F.; Pisacane, A.; Gambardella, G.; D’Ambrosio, A.; Candiello, E.; Ferrio, M.; Panero, M.; Casorzo, L.; Benvenuti, S.; Cascardi, E.; et al. Cancer of unknown primary stem-like cells model multi-organ metastasis and unveil liability to MEK inhibition. Nat. Commun. 2021, 12, 2498. [Google Scholar] [CrossRef]
- Vodstrcil, L.A.; Hocking, J.S.; Law, M.; Walker, S.; Tabrizi, S.N.; Fairley, C.K.; Bradshaw, C.S. Hormonal contraception is associated with a reduced risk of bacterial vaginosis: A systematic review and meta-analysis. PLoS ONE 2013, 8, e73055. [Google Scholar] [CrossRef]
- van de Wijgert, J.H.; Verwijs, M.C.; Turner, A.N.; Morrison, C.S. Hormonal contraception decreases bacterial vaginosis but oral contraception may increase candidiasis: Implications for HIV transmission. AIDS 2013, 27, 2141–2153. [Google Scholar] [CrossRef]
- Borgdorff, H.; Gautam, R.; Armstrong, S.D.; Xia, D.; Ndayisaba, G.F.; van Teijlingen, N.H.; Geijtenbeek, T.B.; Wastling, J.M.; van de Wijgert, J.H. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal. Immunol. 2016, 9, 621–633. [Google Scholar] [CrossRef]
- Achilles, S.L.; Hillier, S.L. The complexity of contraceptives: Understanding their impact on genital immune cells and vaginal microbiota. AIDS 2013, 27 (Suppl. S1), S5–S15. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M.; He, X.; Gajer, P.; Fadrosh, D.; Sharma, E.; Mongodin, E.F.; Ravel, J.; Glover, E.D.; Rath, J.M. Association between cigarette smoking and the vaginal microbiota: A pilot study. BMC Infect. Dis. 2014, 14, 471. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R.; Desmond, R.A.; Oh, M.K. Predictors of bacterial vaginosis in adolescent women who douche. Sex. Transm. Dis. 2004, 31, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M.; Ghanem, K.G.; Klebanoff, M.A.; Taha, T.E.; Scharfstein, D.O.; Zenilman, J.M. The effect of vaginal douching cessation on bacterial vaginosis: A pilot study. Am. J. Obstet. Gynecol. 2008, 198, 628.e1–628.e7. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.C.; Thai, T.N.; Tran, L.T.; Shete, S.S.; Ramondetta, L.M.; Basen-Engquist, K.M. Association Between Vaginal Douching and Genital Human Papillomavirus Infection Among Women in the United States. J. Infect. Dis. 2016, 214, 1370–1375. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Thomas, A.G.; Leybovich, E. Vaginal douching and adverse health effects: A meta-analysis. Am. J. Public Health 1997, 87, 1207–1211. [Google Scholar] [CrossRef]
- Foster, K.R.; Schluter, J.; Coyte, K.Z.; Rakoff-Nahoum, S. The evolution of the host microbiome as an ecosystem on a leash. Nature 2017, 548, 43–51. [Google Scholar] [CrossRef]
- Zeng, Q.; Wu, S.; Sukumaran, J.; Rodrigo, A. Models of microbiome evolution incorporating host and microbial selection. Microbiome 2017, 5, 127. [Google Scholar] [CrossRef]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Lederberg, J.; McCray, A.T. Ome SweetOmics—A genealogical treasury of words. Scientist 2001, 15, 8. [Google Scholar]
- Woese, C.R.; Fox, G.E. Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef] [PubMed]
- Curty, G.; de Carvalho, P.S.; Soares, M.A. The role of the cervicovaginal microbiome on the genesis and as a biomarker of premalignant cervical intraepithelial neoplasia and invasive cervical cancer. Int. J. Mol. Sci. 2019, 21, 222. [Google Scholar] [CrossRef] [PubMed]
- Audirac-Chalifour, A.; Torres-Poveda, K.; Bahena-Roman, M.; Tellez-Sosa, J.; Martinez-Barnetche, J.; Cortina-Ceballos, B.; Lopez-Estrada, G.; Delgado-Romero, K.; Burguete-Garcia, A.I.; Cantu, D.; et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLoS ONE 2016, 11, e0153274. [Google Scholar] [CrossRef]
- Conesa-Zamora, P. Immune responses against virus and tumor in cervical carcinogenesis: Treatment strategies for avoiding the HPV-induced immune escape. Gynecol. Oncol. 2013, 131, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Shulzhenko, N.; Lyng, H.; Sanson, G.F.; Morgun, A. Menage a trois: An evolutionary interplay between human papillomavirus, a tumor, and a woman. Trends Microbiol. 2014, 22, 345–353. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef]
- Gosmann, C.; Anahtar, M.N.; Handley, S.A.; Farcasanu, M.; Abu-Ali, G.; Bowman, B.A.; Padavattan, N.; Desai, C.; Droit, L.; Moodley, A.; et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity 2017, 46, 29–37. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Mitra, A.; Moscicki, A.B. Does the vaginal microbiota play a role in the development of cervical cancer? Transl. Res. 2017, 179, 168–182. [Google Scholar] [CrossRef]
- Vyshenska, D.; Lam, K.C.; Shulzhenko, N.; Morgun, A. Interplay between viruses and bacterial microbiota in cancer development. Semin. Immunol. 2017, 32, 14–24. [Google Scholar] [CrossRef]
- Curty, G.; Costa, R.L.; Siqueira, J.D.; Meyrelles, A.I.; Machado, E.S.; Soares, E.A.; Soares, M.A. Analysis of the cervical microbiome and potential biomarkers from postpartum HIV-positive women displaying cervical intraepithelial lesions. Sci. Rep. 2017, 7, 17364. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Weng, J.; Gao, Y.; Chen, X. Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: A cross-sectional study. BMC Infect. Dis. 2013, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjose, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers 2016, 2, 16086. [Google Scholar] [CrossRef]
- Laniewski, P.; Barnes, D.; Goulder, A.; Cui, H.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci. Rep. 2018, 8, 7593. [Google Scholar] [CrossRef]
- van de Wijgert, J.; Jespers, V. The global health impact of vaginal dysbiosis. Res. Microbiol. 2017, 168, 859–864. [Google Scholar] [CrossRef]
- Mitchell, C.; Marrazzo, J. Bacterial vaginosis and the cervicovaginal immune response. Am. J. Reprod. Immunol. 2014, 71, 555–563. [Google Scholar] [CrossRef]
- Navas-Molina, J.A.; Peralta-Sanchez, J.M.; Gonzalez, A.; McMurdie, P.J.; Vazquez-Baeza, Y.; Xu, Z.; Ursell, L.K.; Lauber, C.; Zhou, H.; Song, S.J.; et al. Advancing our understanding of the human microbiome using QIIME. Methods Enzymol. 2013, 531, 371–444. [Google Scholar] [CrossRef]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Lanier, B.R.; Moench, T.R.; Cone, R.A. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect. Dis. 2010, 10, 120. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 2015, 5, 16865. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.Y.; Kim, B.S.; Seo, S.S.; Kong, J.S.; Lee, J.K.; Park, S.Y.; Hong, K.M.; Kim, H.K.; Kim, M.K. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin. Microbiol. Infect. 2015, 21, 674.e1–674.e9. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, H.; Tsivtsivadze, E.; Verhelst, R.; Marzorati, M.; Jurriaans, S.; Ndayisaba, G.F.; Schuren, F.H.; van de Wijgert, J.H. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J. 2014, 8, 1781–1793. [Google Scholar] [CrossRef] [PubMed]
- Insinga, R.P.; Perez, G.; Wheeler, C.M.; Koutsky, L.A.; Garland, S.M.; Leodolter, S.; Joura, E.A.; Ferris, D.G.; Steben, M.; Hernandez-Avila, M.; et al. Incident cervical HPV infections in young women: Transition probabilities for CIN and infection clearance. Cancer Epidemiol. Biomark. Prev. 2011, 20, 287–296. [Google Scholar] [CrossRef]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Norenhag, J.; Du, J.; Olovsson, M.; Verstraelen, H.; Engstrand, L.; Brusselaers, N. The vaginal microbiota, human papillomavirus and cervical dysplasia: A systematic review and network meta-analysis. BJOG 2020, 127, 171–180. [Google Scholar] [CrossRef]
- Skapinyecz, J.; Smid, I.; Horvath, A.; Jeney, C.; Kardos, L.; Kovacs, P. Pelvic inflammatory disease is a risk factor for cervical cancer. Eur. J. Gynaecol. Oncol. 2003, 24, 401–404. [Google Scholar]
- Klatt, N.R.; Cheu, R.; Birse, K.; Zevin, A.S.; Perner, M.; Noel-Romas, L.; Grobler, A.; Westmacott, G.; Xie, I.Y.; Butler, J.; et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017, 356, 938–945. [Google Scholar] [CrossRef]
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Tracy, J.K.; Zenilman, J.M.; Ravel, J.; Gravitt, P.E. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J. Infect. Dis. 2014, 210, 1723–1733. [Google Scholar] [CrossRef]
- Kwasniewski, W.; Wolun-Cholewa, M.; Kotarski, J.; Warchol, W.; Kuzma, D.; Kwasniewska, A.; Gozdzicka-Jozefiak, A. Microbiota dysbiosis is associated with HPV-induced cervical carcinogenesis. Oncol. Lett. 2018, 16, 7035–7047. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Rani, A.; Metwally, A.; McGee, H.S.; Perkins, D.L. Analysis of the microbiome: Advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem. Biophys. Res. Commun. 2016, 469, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, Y.; Gao, W.; Pan, Y.; Gao, Y.; Shen, J.; Xiong, H. The direct and indirect association of cervical microbiota with the risk of cervical intraepithelial neoplasia. Cancer Med. 2018, 7, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Piyathilake, C.J.; Ollberding, N.J.; Kumar, R.; Macaluso, M.; Alvarez, R.D.; Morrow, C.D. Cervical Microbiota Associated with Higher Grade Cervical Intraepithelial Neoplasia in Women Infected with High-Risk Human Papillomaviruses. Cancer Prev. Res. 2016, 9, 357–366. [Google Scholar] [CrossRef]
- Tsay, J.J.; Wu, B.G.; Badri, M.H.; Clemente, J.C.; Shen, N.; Meyn, P.; Li, Y.; Yie, T.A.; Lhakhang, T.; Olsen, E.; et al. Airway Microbiota Is Associated with Upregulation of the PI3K Pathway in Lung Cancer. Am. J. Respir. Crit. Care Med. 2018, 198, 1188–1198. [Google Scholar] [CrossRef]
- Hyman, R.W.; Fukushima, M.; Jiang, H.; Fung, E.; Rand, L.; Johnson, B.; Vo, K.C.; Caughey, A.B.; Hilton, J.F.; Davis, R.W.; et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod. Sci. 2014, 21, 32–40. [Google Scholar] [CrossRef]
- Buonomo, B.; Massarotti, C.; Dellino, M.; Anserini, P.; Ferrari, A.; Campanella, M.; Magnotti, M.; De Stefano, C.; Peccatori, F.A.; Lambertini, M. Reproductive issues in carriers of germline pathogenic variants in the BRCA1/2 genes: An expert meeting. BMC Med. 2021, 19, 205. [Google Scholar] [CrossRef]
- Dellino, M.; Silvestris, E.; Loizzi, V.; Paradiso, A.; Loiacono, R.; Minoia, C.; Daniele, A.; Cormio, G. Germinal ovarian tumors in reproductive age women: Fertility-sparing and outcome. Medicine 2020, 99, e22146. [Google Scholar] [CrossRef]
- Afiuni-Zadeh, S.; Boylan, K.L.M.; Jagtap, P.D.; Griffin, T.J.; Rudney, J.D.; Peterson, M.L.; Skubitz, A.P.N. Evaluating the potential of residual Pap test fluid as a resource for the metaproteomic analysis of the cervical-vaginal microbiome. Sci. Rep. 2018, 8, 10868. [Google Scholar] [CrossRef]
- Silvestris, E.; Cormio, G.; Skrypets, T.; Dellino, M.; Paradiso, A.V.; Guarini, A.; Minoia, C. Novel aspects on gonadotoxicity and fertility preservation in lymphoproliferative neoplasms. Crit. Rev. Oncol. Hematol. 2020, 151, 102981. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Morton, A.N.; Hocking, J.; Garland, S.M.; Morris, M.B.; Moss, L.M.; Horvath, L.B.; Kuzevska, I.; Fairley, C.K. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 2006, 193, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, E.; D’Oronzo, S.; Cafforio, P.; Kardhashi, A.; Dellino, M.; Cormio, G. In Vitro Generation of Oocytes from Ovarian Stem Cells (OSCs): In Search of Major Evidence. Int. J. Mol. Sci. 2019, 20, 6225. [Google Scholar] [CrossRef] [PubMed]
- Menard, J.P. Antibacterial treatment of bacterial vaginosis: Current and emerging therapies. Int. J. Womens Health 2011, 3, 295–305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dellino, M.; Minoia, C.; Paradiso, A.V.; De Palo, R.; Silvestris, E. Fertility Preservation in Cancer Patients During the Coronavirus (COVID-19) Pandemic. Front. Oncol. 2020, 10, 1009. [Google Scholar] [CrossRef] [PubMed]
- Bertuccini, L.; Russo, R.; Iosi, F.; Superti, F. Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. Int. J. Immunopathol. Pharmacol. 2017, 30, 163–167. [Google Scholar] [CrossRef]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef]
- Domig, K.J.; Kiss, H.; Petricevic, L.; Viernstein, H.; Unger, F.; Kneifel, W. Strategies for the evaluation and selection of potential vaginal probiotics from human sources: An exemplary study. Benef. Microbes 2014, 5, 263–272. [Google Scholar] [CrossRef]
- Laue, C.; Papazova, E.; Liesegang, A.; Pannenbeckers, A.; Arendarski, P.; Linnerth, B.; Domig, K.J.; Kneifel, W.; Petricevic, L.; Schrezenmeir, J. Effect of a yoghurt drink containing Lactobacillus strains on bacterial vaginosis in women—A double-blind, randomised, controlled clinical pilot trial. Benef. Microbes 2018, 9, 35–50. [Google Scholar] [CrossRef]
- Palma, E.; Recine, N.; Domenici, L.; Giorgini, M.; Pierangeli, A.; Panici, P.B. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: A promising solution against HPV-infection. BMC Infect. Dis. 2018, 18, 13. [Google Scholar] [CrossRef]
- Dellino, M.; Carriero, C.; Silvestris, E.; Capursi, T.; Paradiso, A.; Cormio, G. Primary Vaginal Carcinoma Arising on Cystocele Mimicking Vulvar Cancer. J. Obstet. Gynaecol. Can. 2020, 42, 1543–1545. [Google Scholar] [CrossRef]
- Giraldo, P.C.; Sanches, J.M.; Sparvolli, L.G.; Amaral, R.; Migliorini, I.; Gil, C.D.; Taddei, C.R.; Witkin, S.S.; Discacciati, M.G. Relationship between Papillomavirus vaccine, vaginal microbiome, and local cytokine response: An exploratory research. Braz. J. Microbiol. 2021, 52, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascardi, E.; Cazzato, G.; Daniele, A.; Silvestris, E.; Cormio, G.; Di Vagno, G.; Malvasi, A.; Loizzi, V.; Scacco, S.; Pinto, V.; et al. Association between Cervical Microbiota and HPV: Could This Be the Key to Complete Cervical Cancer Eradication? Biology 2022, 11, 1114. https://doi.org/10.3390/biology11081114
Cascardi E, Cazzato G, Daniele A, Silvestris E, Cormio G, Di Vagno G, Malvasi A, Loizzi V, Scacco S, Pinto V, et al. Association between Cervical Microbiota and HPV: Could This Be the Key to Complete Cervical Cancer Eradication? Biology. 2022; 11(8):1114. https://doi.org/10.3390/biology11081114
Chicago/Turabian StyleCascardi, Eliano, Gerardo Cazzato, Antonella Daniele, Erica Silvestris, Gennaro Cormio, Giovanni Di Vagno, Antonio Malvasi, Vera Loizzi, Salvatore Scacco, Vincenzo Pinto, and et al. 2022. "Association between Cervical Microbiota and HPV: Could This Be the Key to Complete Cervical Cancer Eradication?" Biology 11, no. 8: 1114. https://doi.org/10.3390/biology11081114
APA StyleCascardi, E., Cazzato, G., Daniele, A., Silvestris, E., Cormio, G., Di Vagno, G., Malvasi, A., Loizzi, V., Scacco, S., Pinto, V., Cicinelli, E., Maiorano, E., Ingravallo, G., Resta, L., Minoia, C., & Dellino, M. (2022). Association between Cervical Microbiota and HPV: Could This Be the Key to Complete Cervical Cancer Eradication? Biology, 11(8), 1114. https://doi.org/10.3390/biology11081114












