Black Ginseng Ameliorates Cellular Senescence via p53-p21/p16 Pathway in Aged Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparations
2.2. Animals and Experimental Design
2.3. Primary Mouse Embryonic Fibroblasts (MEFs), Primary Hepatocytes Isolation and Human Embryonic Kidney (HEK) 293 Cell Cultures
2.4. Cellular Senescence Induction by Ionizing Radiation (IR) and Replicative Senescence (RS)
2.5. Senescence-Associated β-Galactosidase (SA-β-gal) Staining
2.6. Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
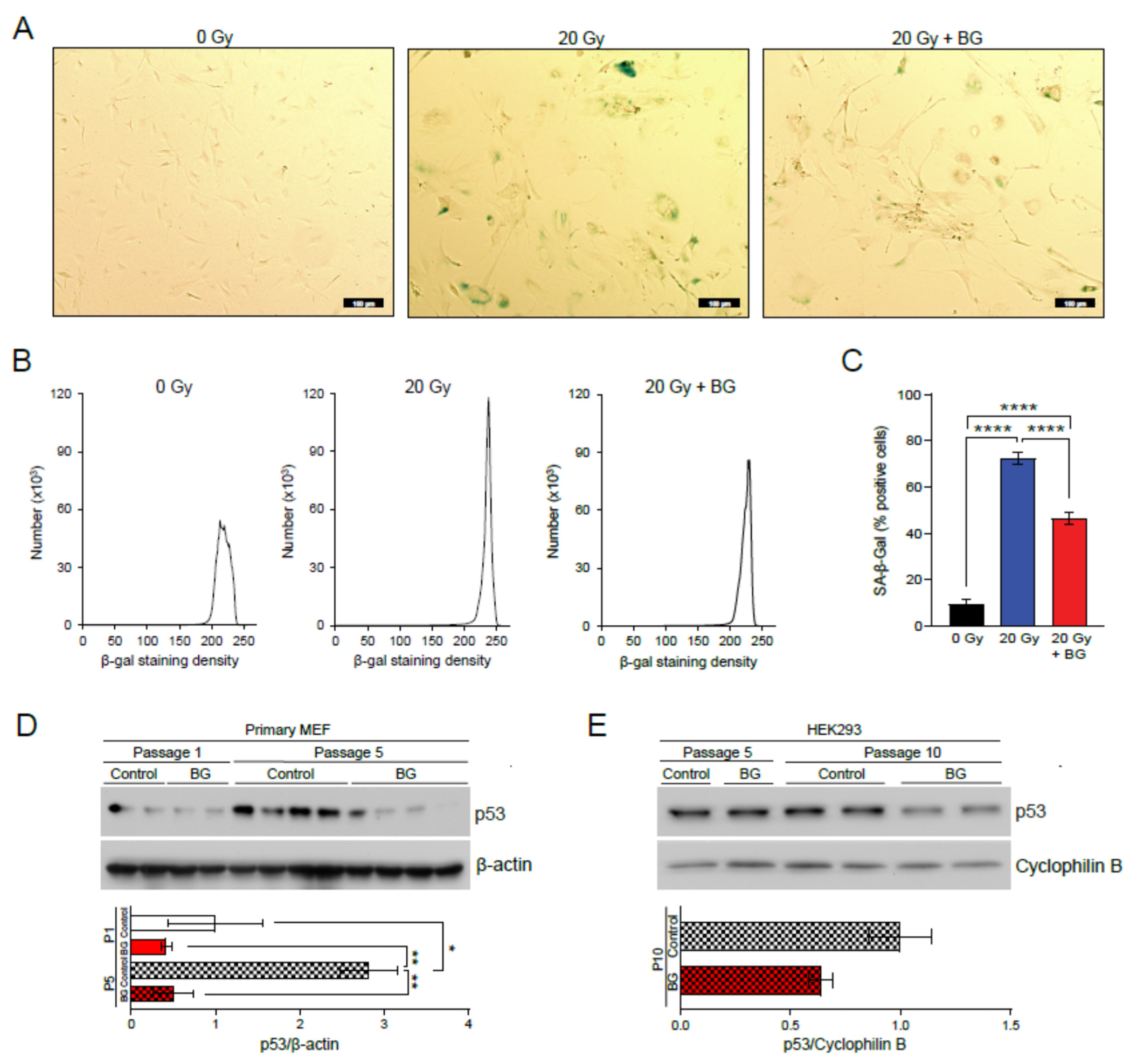
3.1. Black Ginseng Delays Cellular Senescence on MEFs
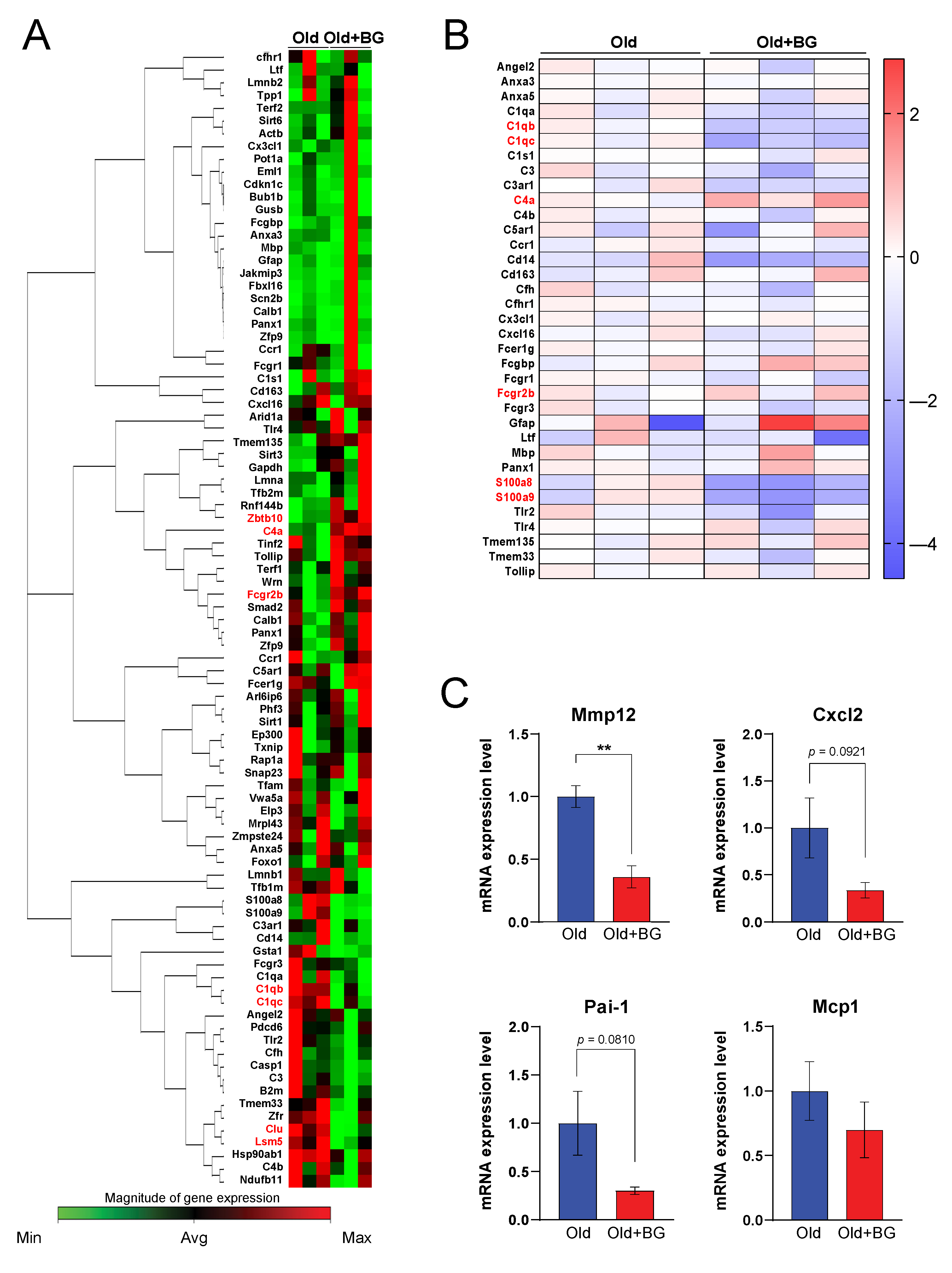
3.2. Black Ginseng Alters Hepatic Gene Expression Profiles Related to Aging-Associated Pathways in Aged Mice
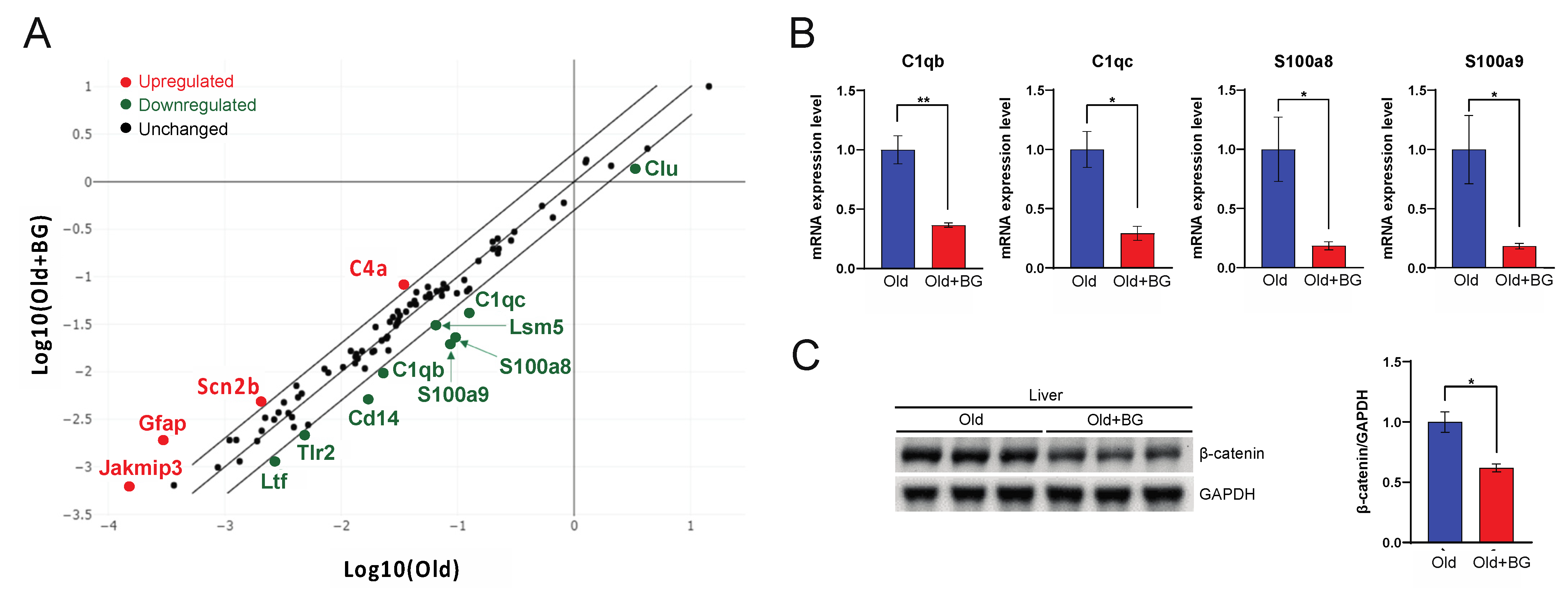
3.3. Black Ginseng Regulates the Complement System and a Component of Wnt Signaling in Aged Mouse Livers
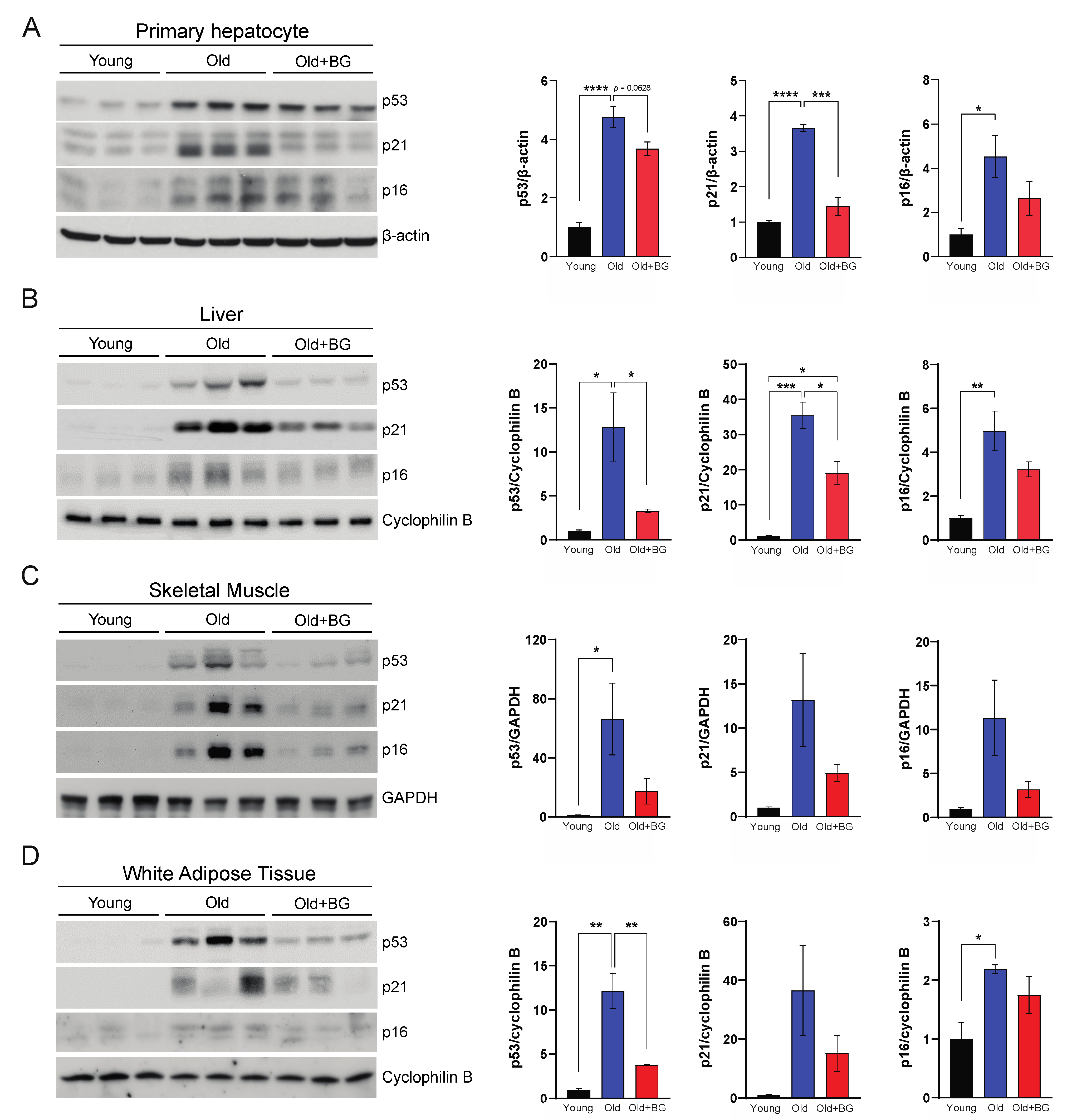
3.4. Black Ginseng Supplementation Ameliorates Canonical Cellular Senescence Pathways in Metabolically Active Organs in Aged Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.-G.; Yan, Y.Z.; Jin, X.-J.; Kim, Y.K.; Uddin, M.R.; Kim, Y.B.; Bae, H.; Kim, Y.C.; Lee, S.W.; Park, S.U. Ginsenoside content in the leaves and roots of Panax ginseng at different ages. Life Sci. J. 2012, 9, 670–683. [Google Scholar]
- Lee, M.R.; Yun, B.S.; In, O.H.; Sung, C.K. Comparative study of Korean white, red, and black ginseng extract on cholinesterase inhibitory activity and cholinergic function. J. Ginseng Res. 2011, 35, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metwaly, A.M.; Lianlian, Z.; Luqi, H.; Deqiang, D. Black ginseng and its saponins: Preparation, phytochemistry and pharmacological effects. Molecules 2019, 24, 1856. [Google Scholar] [CrossRef] [Green Version]
- Choudhry, Q.N.; Kim, J.H.; Cho, H.T.; Heo, W.; Lee, J.-J.; Lee, J.H.; Kim, Y.J. Ameliorative effect of black ginseng extract against oxidative stress-induced cellular damages in mouse hepatocytes. J. Ginseng Res. 2019, 43, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, Y.-J.; Jeon, J.-N.; Wang, C.; Min, J.-W.; Noh, H.-Y.; Yang, D.-C. Effect of white, red and black ginseng on physicochemical properties and ginsenosides. Plant Foods Hum. Nutr. 2015, 70, 141–145. [Google Scholar] [CrossRef]
- Kang, K.S.; Kim, H.Y.; Pyo, J.S.; Yokozawa, T. Increase in the Free Radical Scavenging Activity of Ginseng by Heat-Processing. Biol. Pharm. Bull. 2006, 29, 750–754. [Google Scholar] [CrossRef] [Green Version]
- An, M.-Y.; Lee, S.R.; Hwang, H.-J.; Yoon, J.-G.; Lee, H.-J.; Cho, J.A. Antioxidant and Anti-Inflammatory Effects of Korean Black Ginseng Extract through ER Stress Pathway. Antioxidants 2021, 10, 62. [Google Scholar] [CrossRef]
- Lee, S.-R.; Kim, M.-R.; Yon, J.-M.; Baek, I.-J.; Park, C.G.; Lee, B.J.; Yun, Y.W.; Nam, S.-Y. Black ginseng inhibits ethanol-induced teratogenesis in cultured mouse embryos through its effects on antioxidant activity. Toxicol. Vitro 2009, 23, 47–52. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Childs, B.G.; Durik, M.; Baker, D.J.; Van Deursen, J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Paez-Ribes, M.; González-Gualda, E.; Doherty, G.; Muñoz-Espín, D. Targeting senescent cells in translational medicine. EMBO Mol. Med. 2019, 11, e10234. [Google Scholar] [CrossRef] [PubMed]
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, R.; Jat, P. Mechanisms of cellular senescence: Cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 2021, 9, 485. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Cellular senescence: A translational perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Palmer, A.K.; Xu, M.; Zhu, Y.; Pirtskhalava, T.; Weivoda, M.M.; Hachfeld, C.M.; Prata, L.G.; van Dijk, T.H.; Verkade, E.; Casaclang-Verzosa, G. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 2019, 18, e12950. [Google Scholar] [CrossRef]
- Roos, C.M.; Zhang, B.; Palmer, A.K.; Ogrodnik, M.B.; Pirtskhalava, T.; Thalji, N.M.; Hagler, M.; Jurk, D.; Smith, L.A.; Casaclang-Verzosa, G. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 2016, 15, 973–977. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Sen, J.M.; Gorospe, M. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef]
- Lee, S.-J.; Chandrasekran, P.; Mazucanti, C.H.; O’Connell, J.F.; Egan, J.M.; Kim, Y. Dietary curcumin restores insulin homeostasis in diet-induced obese aged mice. Aging 2022, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qin, L.; Feng, R.; Hu, G.; Sun, H.; He, Y.; Zhang, R. Emerging senolytic agents derived from natural products. Mech. Ageing Dev. 2019, 181, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Saba, E.; Irfan, M.; Kim, M.; Yi-Le Chan, J.; Jeon, B.S.; Choi, S.K.; Rhee, M.H. The anti-inflammatory and anti-nociceptive effects of Korean black ginseng. Phytomedicine 2019, 54, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-H.; Kim, K.-J.; Chei, S.; Seo, Y.-J.; Lee, K.; Lee, B.-Y. Korean red ginseng and Korean black ginseng extracts, JP5 and BG1, prevent hepatic oxidative stress and inflammation induced by environmental heat stress. J. Ginseng Res. 2020, 44, 267–273. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Goldblatt, P.J.; Hinton, D.E.; Lipsky, M.M.; Chacko, J.; Trump, B.F. Mouse liver cell culture. Vrito 1981, 17, 913–925. [Google Scholar]
- Park, J.Y.; Lee, D.-S.; Kim, C.-E.; Shin, M.-S.; Seo, C.-S.; Shin, H.-K.; Hwang, G.S.; An, J.M.; Kim, S.-N.; Kang, K.S. Effects of fermented black ginseng on wound healing mediated by angiogenesis through the mitogen-activated protein kinase pathway in human umbilical vein endothelial cells. J. Ginseng Res. 2018, 42, 524–531. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, D.-Y.; Kim, Y.; Oklahoma State University, Stillwater, OK, USA. 2022; Unpublished work.
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Schonthaler, H.B.; Guinea-Viniegra, J.; Wculek, S.K.; Ruppen, I.; Ximénez-Embún, P.; Guío-Carrión, A.; Navarro, R.; Hogg, N.; Ashman, K.; Wagner, E.F. S100A8-S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity 2013, 39, 1171–1181. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Fergusson, M.M.; Castilho, R.M.; Liu, J.; Cao, L.; Chen, J.; Malide, D.; Rovira, I.I.; Schimel, D.; Kuo, C.J. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 2007, 317, 803–806. [Google Scholar] [CrossRef] [Green Version]
- Naito, A.T.; Sumida, T.; Nomura, S.; Liu, M.-L.; Higo, T.; Nakagawa, A.; Okada, K.; Sakai, T.; Hashimoto, A.; Hara, Y. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell 2012, 149, 1298–1313. [Google Scholar] [CrossRef] [Green Version]
- Nusse, R.; Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiong, Y.; Chen, W.; Wu, L.J.E.; Medicine, T. Wnt/β-catenin signaling may induce senescence of chondrocytes in osteoarthritis. Exp. Ther. Med. 2020, 20, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Hu, Q.; Hu, Y.; Hafner, K.; Costa, R.; van den Berg, A.; Königshoff, M. Chronic WNT/β-catenin signaling induces cellular senescence in lung epithelial cells. Cell. Signal. 2020, 70, 109588. [Google Scholar] [CrossRef] [PubMed]
- Damalas, A.; Kahan, S.; Shtutman, M.; Ben-Ze’ev, A.; Oren, M. Deregulated β-catenin induces a p53-and ARF-dependent growth arrest and cooperates with Ras in transformation. EMBO J. 2001, 20, 4912–4922. [Google Scholar] [CrossRef] [Green Version]
- Prange, W.; Breuhahn, K.; Fischer, F.; Zilkens, C.; Pietsch, T.; Petmecky, K.; Eilers, R.; Dienes, H.P.; Schirmacher, P.J.T. Beta-catenin accumulation in the progression of human hepatocarcinogenesis correlates with loss of E-cadherin and accumulation of p53, but not with expression of conventional WNT-1 target genes. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2003, 201, 250–259. [Google Scholar] [CrossRef]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef] [Green Version]
- Schafer, M.J.; Miller, J.D.; LeBrasseur, N.K. Cellular senescence: Implications for metabolic disease. Mol. Cell. Endocrinol. 2017, 455, 93–102. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, K.K.; Jiang, X.; Xu, A.; Cheng, K.K. The role of adipose tissue senescence in obesity-and ageing-related metabolic disorders. Clin. Sci. 2020, 134, 315–330. [Google Scholar] [CrossRef]
- Minamino, T.; Orimo, M.; Shimizu, I.; Kunieda, T.; Yokoyama, M.; Ito, T.; Nojima, A.; Nabetani, A.; Oike, Y.; Matsubara, H. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat. Med. 2009, 15, 1082–1087. [Google Scholar] [CrossRef]
- Filipová, A.; Diaz-Garcia, D.; Bezrouk, A.; Čížková, D.; Havelek, R.; Vávrová, J.; Dayanithi, G.; Řezacová, M. Ionizing radiation increases primary cilia incidence and induces multiciliation in C2C12 myoblasts. Cell Biol. Int. 2015, 39, 943–953. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Mooi, W.J.; Peeper, D.S. The essence of senescence. Genes Dev. 2010, 24, 2463–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.Y.; Souroullas, G.P.; Diekman, B.O.; Krishnamurthy, J.; Hall, B.M.; Sorrentino, J.A.; Parker, J.S.; Sessions, G.A.; Gudkov, A.V.; & Sharpless, N.E. Cells exhibiting strong p16INK4a promoter activation in vivo display features of senescence. Proc. Natl. Acad. Sci. USA 2019, 116, 2603–2611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Porath, I.; Weinberg, R.A. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 2005, 37, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Hara, E.; Smith, R.; Parry, D.; Tahara, H.; Stone, S.; Peters, G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol. Cell. Biol. 1996, 16, 859–867. [Google Scholar] [CrossRef] [Green Version]
- Huda, N.; Liu, G.; Hong, H.; Yan, S.; Khambu, B.; Yin, X.-M. Hepatic senescence, the good and the bad. World J. Gastroenterol. 2019, 25, 5069. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Gustafson, B.; Kirkland, J.L.; Smith, U. Cellular senescence: At the nexus between ageing and diabetes. Diabetologia 2019, 62, 1835–1841. [Google Scholar] [CrossRef] [Green Version]
- Mankhong, S.; Kim, S.; Moon, S.; Kwak, H.-B.; Park, D.-H.; Kang, J.-H. Experimental models of sarcopenia: Bridging molecular mechanism and therapeutic strategy. Cells 2020, 9, 1385. [Google Scholar] [CrossRef]
- Stein, G.H.; Drullinger, L.F.; Soulard, A.; Dulić, V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol. Cell Biol. 1999, 19, 2109–2117. [Google Scholar] [CrossRef] [Green Version]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L.; van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.M.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Emanuele, E.; Lucia, A.; Gálvez, B.G. ‘Adipaging’: Ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J. Physiol. 2016, 594, 3187–3207. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, M.; Nori, N.; Brunelli, A.; Zoico, E. How does adipose tissue contribute to inflammageing? Exp. Gerontol. 2021, 143, 111162. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunkelberger, J.R.; Song, W.-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef] [Green Version]
- Nayak, A.; Pednekar, L.; Reid, K.B.; Kishore, U. Complement and non-complement activating functions of C1q: A prototypical innate immune molecule. Innate Immun. 2012, 18, 350–363. [Google Scholar] [CrossRef]
- Nayak, A.; Ferluga, J.; Tsolaki, A.G.; Kishore, U. The non-classical functions of the classical complement pathway recognition subcomponent C1q. Immunol. Lett. 2010, 131, 139–150. [Google Scholar] [CrossRef]
- Kouser, L.; Madhukaran, S.P.; Shastri, A.; Saraon, A.; Ferluga, J.; Al-Mozaini, M.; Kishore, U. Emerging and novel functions of complement protein C1q. Front. Immunol. 2015, 6, 317. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.-J.; Park, J.-Y.; Choi, S.; Lee, J.-B.; Jung, H.; Kim, T.-D.; Yoon, S.R.; Choi, I.; Shim, S.; Park, Y.-J.J.B.; et al. Ginsenoside Rg3 regulates S-nitrosylation of the NLRP3 inflammasome via suppression of iNOS. Biochem. Biophys. Res. Commun. 2015, 463, 1184–1189. [Google Scholar] [CrossRef]
- Jung, J.-S.; Kim, D.-H.; Kim, H.-S. Ginsenoside Rh1 suppresses inducible nitric oxide synthase gene expression in IFN-γ-stimulated microglia via modulation of JAK/STAT and ERK signaling pathways. Biochem. Biophys. Res. Commun. 2010, 397, 323–328. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, J.-E.; Song, G.-Y.; Bae, J.-S. Rg × 365, a Rare Protopanaxatriol-Type Ginsenoside Fraction from Black Ginseng, Suppresses Inflammatory Gene iNOS via the Inhibition of p-STAT-1 and NF-κ B. Am. J. Chin. Med. 2020, 48, 1091–1102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-J.; Lee, D.-Y.; O’Connell, J.F.; Egan, J.M.; Kim, Y. Black Ginseng Ameliorates Cellular Senescence via p53-p21/p16 Pathway in Aged Mice. Biology 2022, 11, 1108. https://doi.org/10.3390/biology11081108
Lee S-J, Lee D-Y, O’Connell JF, Egan JM, Kim Y. Black Ginseng Ameliorates Cellular Senescence via p53-p21/p16 Pathway in Aged Mice. Biology. 2022; 11(8):1108. https://doi.org/10.3390/biology11081108
Chicago/Turabian StyleLee, Su-Jeong, Da-Yeon Lee, Jennifer F. O’Connell, Josephine M. Egan, and Yoo Kim. 2022. "Black Ginseng Ameliorates Cellular Senescence via p53-p21/p16 Pathway in Aged Mice" Biology 11, no. 8: 1108. https://doi.org/10.3390/biology11081108
APA StyleLee, S.-J., Lee, D.-Y., O’Connell, J. F., Egan, J. M., & Kim, Y. (2022). Black Ginseng Ameliorates Cellular Senescence via p53-p21/p16 Pathway in Aged Mice. Biology, 11(8), 1108. https://doi.org/10.3390/biology11081108





