Increasing Temperature Facilitates Polyp Spreading and Medusa Appearance of the Invasive Hydrozoan Craspedacusta sowerbii
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Effect of Temperature on Polyp Colonies
2.2. Thermal Tolerance and Suitable Habitats Models of Medusae Stage
3. Results
3.1. Effect of Temperature on the Polyp Colony Structure
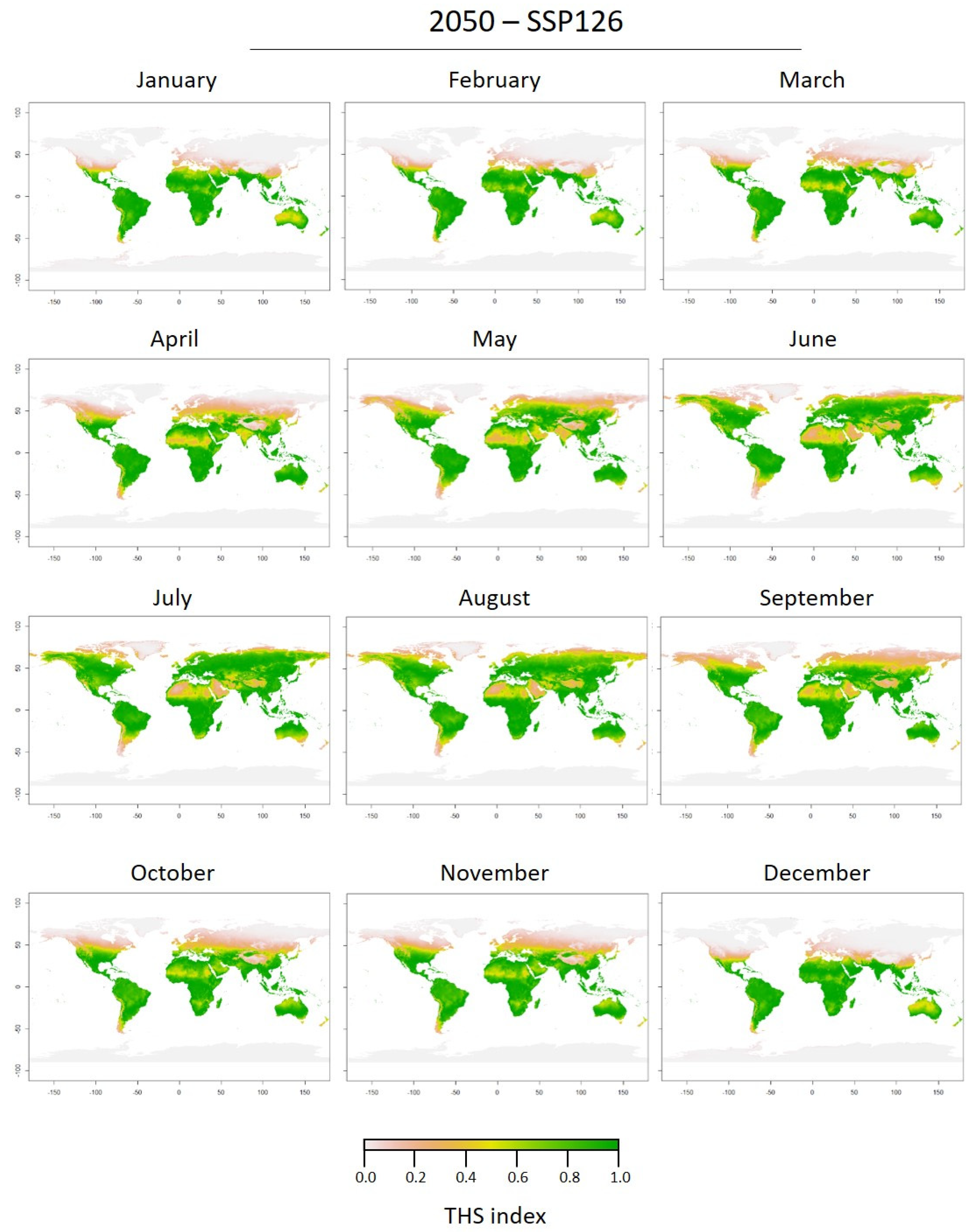
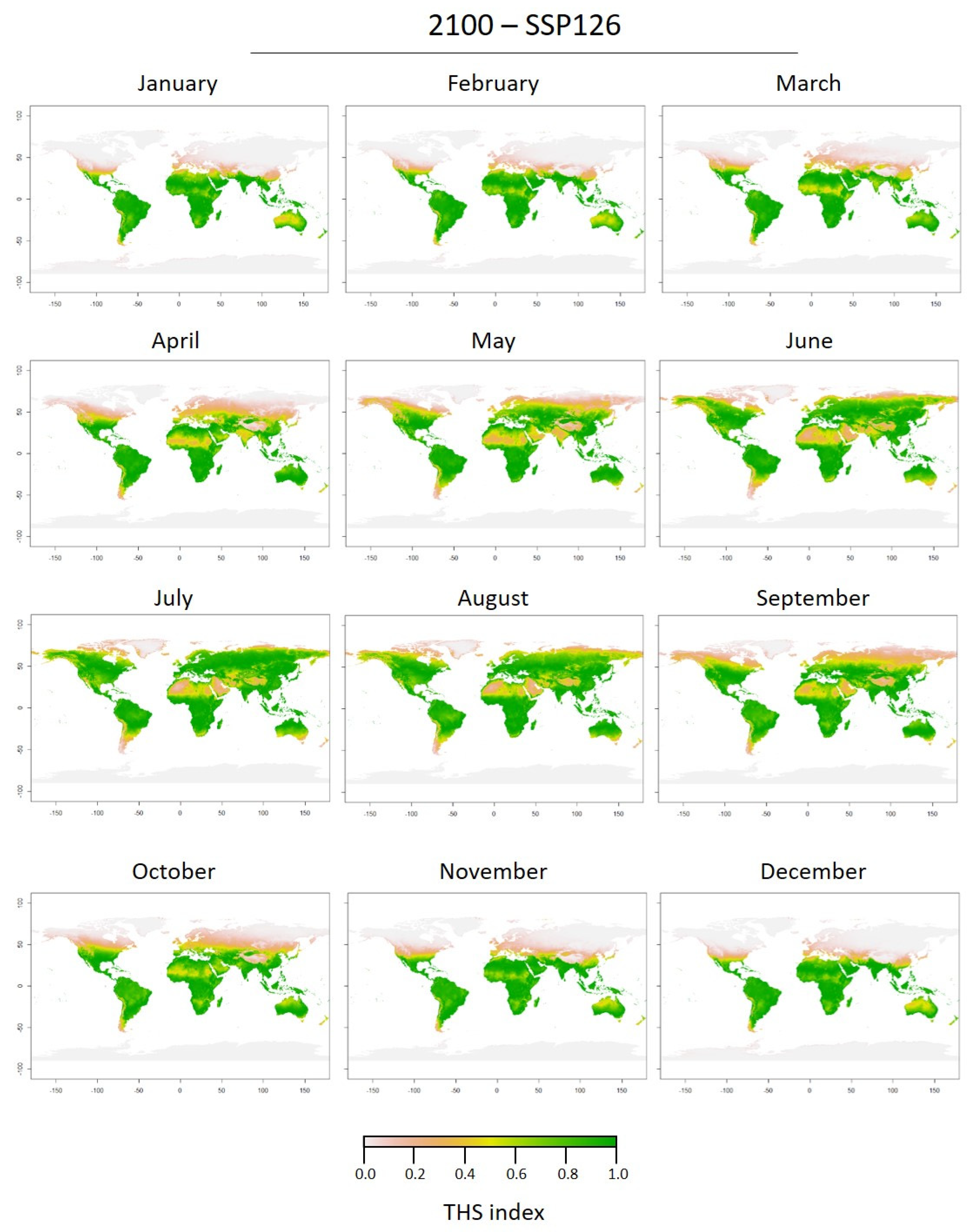
3.2. Thermal Tolerance of Medusae Stages and Thermal Habitat Suitability
4. Discussion
4.1. Effect of Temperature on Polyp Fitness
4.2. Effect of Temperature on the Medusa Stage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, C.-L.; Somero, G.N. The Impact of Ocean Warming on Marine Organisms. Chin. Sci. Bull. 2014, 59, 468–479. [Google Scholar] [CrossRef]
- Ramos, J.E.; Pecl, G.T.; Moltschaniwskyj, N.A.; Semmens, J.M.; Souza, C.A.; Strugnell, J.M. Population Genetic Signatures of a Climate Change Driven Marine Range Extension. Sci. Rep. 2018, 8, 9558. [Google Scholar] [CrossRef] [PubMed]
- Sarà, G.; Palmeri, V.; Rinaldi, A.; Montalto, V.; Helmuth, B. Predicting Biological Invasions in Marine Habitats through Eco-Physiological Mechanistic Models: A Case Study with the Bivalve B Rachidontes Pharaonis. Divers. Distrib. 2013, 19, 1235–1247. [Google Scholar] [CrossRef]
- Sarà, G.; Mangano, M.C.; Johnson, M.; Mazzola, A. Integrating Multiple Stressors in Aquaculture to Build the Blue Growth in a Changing Sea. Hydrobiologia 2018, 809, 5–17. [Google Scholar] [CrossRef]
- Bosch-Belmar, M.; Piraino, S.; Sarà, G. Predictive Metabolic Suitability Maps for the Thermophilic Invasive Hydroid Pennaria disticha Under Future Warming Mediterranean Sea Scenarios. Front. Mar. Sci. 2022, 9, 810555. [Google Scholar] [CrossRef]
- Cooley, S.R.; Kite-Powell, H.L.; Doney, S.C. Ocean Acidification’s Potential to Alter Global Marine Ecosystem Services. Oceanography 2009, 22, 172–181. [Google Scholar] [CrossRef]
- Barz, K.; Hirche, H.-J. Seasonal Development of Scyphomedusae and the Predatory Impact of Aurelia aurita on the Zooplankton Community in the Bornholm Basin (Central Baltic Sea). Mar. Biol. 2005, 147, 465–476. [Google Scholar] [CrossRef]
- Daskalov, G.M.; Grishin, A.N.; Rodionov, S.; Mihneva, V. Trophic Cascades Triggered by Overfishing Reveal Possible Mechanisms of Ecosystem Regime Shifts. Proc. Natl. Acad. Sci. USA 2007, 104, 10518–10523. [Google Scholar] [CrossRef]
- Smith, A.S.; Alexander Jr, J.E. Potential Effects of the Freshwater Jellyfish Craspedacusta sowerbii on Zooplankton Community Abundance. J. Plankton Res. 2008, 30, 1323–1327. [Google Scholar] [CrossRef]
- Ko, G.W.; Dineshram, R.; Campanati, C.; Chan, V.B.; Havenhand, J.; Thiyagarajan, V. Interactive Effects of Ocean Acidification, Elevated Temperature, and Reduced Salinity on Early-Life Stages of the Pacific Oyster. Environ. Sci. Technol. 2014, 48, 10079–10088. [Google Scholar] [CrossRef]
- Folino-Rorem, N.C.; Reid, M.; Peard, T. Culturing the Freshwater Hydromedusa, Craspedacusta sowerbii under Controlled Laboratory Conditions. Invertebr. Reprod. Dev. 2016, 60, 17–27. [Google Scholar] [CrossRef]
- Mayor, D.J.; Sommer, U.; Cook, K.B.; Viant, M.R. The Metabolic Response of Marine Copepods to Environmental Warming and Ocean Acidification in the Absence of Food. Sci. Rep. 2015, 5, 13690. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, V.; Lobel, P.S. Size Affects Digestive Responses to Increasing Temperature in Fishes: Physiological Implications of Being Small under Climate Change. Mar. Ecol. 2016, 37, 813–820. [Google Scholar] [CrossRef]
- Pauly, D.; Cheung, W.W. Sound Physiological Knowledge and Principles in Modeling Shrinking of Fishes under Climate Change. Glob. Change Biol. 2018, 24, e15–e26. [Google Scholar] [CrossRef]
- Parent, G.H. La Découverte Lorraine de Craspedacusta Sowerbyi Lank. Dans Son Contexte Chorologique et Écologique Européen. Bull. Soc. D’Histoire Nat. Moselle 1982, 43, 317–337. [Google Scholar]
- Didžiulis, V. Invasive Alien Species. Fact Sheet–Craspedacusta sowerbyi. Online Database of the North European and Baltic Network on Invasive Alien Species; NOBANIS: Akureyri, Iceland, 2006. [Google Scholar]
- Lüskow, F.; López-González, P.J.; Pakhomov, E.A. Freshwater Jellyfish in Northern Temperate Lakes: Craspedacusta sowerbii in British Columbia, Canada. Aquat. Biol. 2021, 30, 69–84. [Google Scholar] [CrossRef]
- Marchessaux, G.; Lüskow, F.; Sarà, G.; Pakhomov, E.A. Predicting the Current and Future Global Distribution of the Invasive Freshwater Hydrozoan Craspedacusta sowerbii. Sci. Rep. 2021, 11, 23099. [Google Scholar] [CrossRef]
- Ozbek, M.; Sömek, H. Invasive Freshwater Jellyfish Craspedacusta sowerbii (Lankester, 1880) in Turkey: New Locality Record and Habitat Limnoecology, with an Overview of Distributional Data in the Middle East and Balkans. Acta Aquat. Turc. 2020, 16, 487–497. [Google Scholar]
- Marchessaux, G.; Bejean, M. From Frustules to Medusae: A New Culture System for the Study of the Invasive Hydrozoan Craspedacusta sowerbii in the Laboratory. Invertebr. Biol. 2020, 139, e12308. [Google Scholar] [CrossRef]
- Payne, F. A Study of the Fresh-Water Medusa, Craspedacusta ryderi. J. Morphol. 1924, 38, 387–429. [Google Scholar] [CrossRef]
- Lundberg, S.; Svensson, J.-E.; Petrusek, A. Craspedacusta Invasions in Sweden. Int. Ver. Theor. Angew. Limnol. Verh. 2005, 29, 899–902. [Google Scholar] [CrossRef]
- Walter, K.; Graf, H.-F. On the Changing Nature of the Regional Connection between the North Atlantic Oscillation and Sea Surface Temperature. J. Geophys. Res. Atmos. 2002, 107, ACL-7. [Google Scholar] [CrossRef]
- Kearney, M.R.; Matzelle, A.; Helmuth, B. Biomechanics Meets the Ecological Niche: The Importance of Temporal Data Resolution. J. Exp. Biol. 2012, 215, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Freitas, V.; Cardoso, J.F.; Lika, K.; Peck, M.A.; Campos, J.; Kooijman, S.A.; Van der Veer, H.W. Temperature Tolerance and Energetics: A Dynamic Energy Budget-Based Comparison of North Atlantic Marine Species. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3553–3565. [Google Scholar] [CrossRef] [PubMed]
- Romanes, G.J. The New Freshwater Jelly Fish: Physiology of the Freshwater Medusa. Nature 1880, 22, 179–181. [Google Scholar] [CrossRef][Green Version]
- McClary, A. The Effect of Temperature on Growth and Reproduction in Craspedacusta sowerbii. Ecology 1959, 40, 158–162. [Google Scholar] [CrossRef]
- Dodds, R.B.; Hall, K.D. Environmental and Physiological Studies of the Freshwater Jellyfish Craspedacusta sowerbyi. Bios 1984, 55, 75–83. [Google Scholar]
- Zhang, Y.-W.; Xiao-Fu, P.A.N.; Xiao-Ai, W.; Jiang, W.-S.; Qian, L.I.U.; Jun-Xing, Y. Effects of Osmotic Pressure, Temperature and Stocking Density on Survival and Sexual Reproduction of Craspedacusta sowerbii. Zool. Res. 2016, 37, 90. [Google Scholar]
- Thomas, I.M. Craspedacusta sowerbii in South Australia, with Some Notes on Its Habits. T Roy Soc South Aust 1950, 74, 59–65. [Google Scholar]
- Kassambara, A.; Kassambara, M.A. Package ‘Ggpubr’. R Package Version 01 STHDA. 2020, Volume 6. Available online: http://www.sthda.com/english/ (accessed on 1 April 2022).
- VanderWeele, T.J.; Mathur, M.B. Some Desirable Properties of the Bonferroni Correction: Is the Bonferroni Correction Really so Bad? Am. J. Epidemiol. 2019, 188, 617–618. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘Ggplot2’. Create Elegant Data Vis. Using Gramm. Graph. Version 2016, 2, 1–189. Available online: https://cran.microsoft.com/snapshot/2015-01-06/web/packages/ggplot2/ggplot2.pdf (accessed on 1 May 2022).
- Padfield, D.; O’Sullivan, H. RTPC: Functions for Fitting Thermal Performance Curves Curves (R package Version 1.0.0.) [Computer Software]. 2020. Available online: https://github.com/padpadpadpad/rTPC (accessed on 1 May 2022).
- Boatman, T.G.; Lawson, T.; Geider, R.J. A Key Marine Diazotroph in a Changing Ocean: The Interacting Effects of Temperature, CO2 and Light on the Growth of Trichodesmium erythraeum IMS101. PLoS ONE 2017, 12, e0168796. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L.; et al. The Beijing Climate Center Climate System Model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Gemitzi, A. Predicting Land Cover Changes Using a CA Markov Model under Different Shared Socioeconomic Pathways in Greece. GISci. Remote Sens. 2021, 58, 425–441. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) Experimental Design and Organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Jakovčev-Todorović, D.; DJikanović, V.; Skorić, S.; Cakić, P. Freshwater Jellyfish Craspedacusta sowerbyi Lankester, 1880 (Hydrozoa, Olindiidae): 50 Years’ Observations in Serbia. Arch. Biol. Sci. 2010, 62, 123–127. [Google Scholar] [CrossRef]
- Acker, T.S.; Muscat, A.M. The Ecology of Craspedacusta sowerbii Lankester, a Freshwater Hydrozoan. Am. Midl. Nat. 1976, 95, 323–336. [Google Scholar] [CrossRef]
- Lytle, C.F. A Note on Distribution Patterns in Craspedacusta. Trans. Am. Microsc. Soc. 1960, 79, 461–469. [Google Scholar] [CrossRef]
- DeVries, D.R. The Freshwater Jellyfish Craspedacusta sowerbyi: A Summary of Its Life History, Ecology, and Distribution. J. Freshw. Ecol. 1992, 7, 7–16. [Google Scholar] [CrossRef]
- Gomes-Pereira, J.N.; Dionísio, G. Craspedacusta sowerbii Lankester, 1880 in Southern Portugal. BioInvasions Rec. 2013, 2, 133–136. [Google Scholar] [CrossRef]
- Dejdar, E. Die Süsswassermeduse Craspedacusta sowerbii Lankester in Monographischer Darstellung. Z. Für Morphol. Ökol. Tiere 1934, 28, 595–691. [Google Scholar] [CrossRef]
- Fuhrmann, O. Sur Craspedacusta sowerbyi Lank. et Un Nouveau Coelentéré d’eau Douce, Calpasoma Dactyloptera, Ng, n. Sp.(Note Préliminaire). Rev. Suisse Zool. 1939, 46, 363–368. [Google Scholar]
- Buchert, A. Craspedacusta sowerbyi Lank., Eine Süsswassermeduse Und Ihre Beiden Polypen-Typen in Der Ungarischen Fauna. Acta Zool. Acad. Sci. Hung 1960, 6, 29–55. [Google Scholar]
- Matthews, D.C. A Comparative Study of Craspedacusta sowerbyi and Calpasoma dactyloptera Life Cycles. BioStor 1966, 20, 246–259. [Google Scholar]
- Persch, H. Untersuchungen Über Microhydra Germanica Roch. Z. Wiss Zool. 1933, 144, 163–210. [Google Scholar]
- Reisinger, E. Zur Entwicklungsgeschichte Und Entwicklungsmechanik von Craspedacusta (Hydrozoa, Limnotrachylina). Z. Morphol. Ökol. Tiere 1957, 45, 656–698. [Google Scholar] [CrossRef]
- Hashimoto, H. Feeding Behavior and Reproduction of the Hydroid of the Fresh-Water Medusa, Craspedacusta sowerbyi Lankester. Bull. Fac. Edu. Shizuoka Univ. Nat. Sci. 1973, 23, 45–55. [Google Scholar]
- Prusina, I.; Sarà, G.; De Pirro, M.; Dong, Y.-W.; Han, G.-D.; Glamuzina, B.; Williams, G.A. Variations in Physiological Responses to Thermal Stress in Congeneric Limpets in the Mediterranean Sea. J. Exp. Mar. Biol. Ecol. 2014, 456, 34–40. [Google Scholar] [CrossRef]
- Boco, S.R.; Pitt, K.A.; Melvin, S.D. Extreme, but Not Moderate Climate Scenarios, Impart Sublethal Effects on Polyps of the Irukandji Jellyfish, Carukia Barnesi. Sci. Total Environ. 2019, 685, 471–479. [Google Scholar] [CrossRef]
- Gambill, M.; Møller, L.F.; Peck, M.A. Effects of Temperature on the Feeding and Growth of the Larvae of the Invasive Ctenophore Mnemiopsis leidyi. J. Plankton Res. 2015, 37, 1001–1005. [Google Scholar] [CrossRef]
- Angilletta, M.J., Jr.; Angilletta, M.J. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009; p. 289. [Google Scholar]
- Matzelle, A.J.; Sarà, G.; Montalto, V.; Zippay, M.; Trussell, G.C.; Helmuth, B. A Bioenergetics Framework for Integrating the Effects of Multiple Stressors: Opening a ‘Black Box’in Climate Change Research. Am. Malacol. Bull. 2015, 33, 150–160. [Google Scholar] [CrossRef]
- Boero, F. The Ecology of Marine Hydroids and Effects of Environmental Factors: A Review. Mar. Ecol. 1984, 5, 93–118. [Google Scholar] [CrossRef]
- Caputo, L.; Huovinen, P.; Sommaruga, R.; Gómez, I. Water Transparency Affects the Survival of the Medusa Stage of the Invasive Freshwater Jellyfish Craspedacusta sowerbii. Hydrobiologia 2018, 817, 179–191. [Google Scholar] [CrossRef]
- Fuentes, R.; Cárdenas, L.; Abarzua, A.; Caputo, L. Southward Invasion of Craspedacusta sowerbii across Mesotrophic Lakes in Chile: Geographical Distribution and Genetic Diversity of the Medusa Phase. Freshw. Sci. 2019, 38, 193–202. [Google Scholar] [CrossRef]
- Caputo, L.; Fuentes, R.; Woelfl, S.; Castañeda, L.E.; Cárdenas, L. Phenotypic Plasticity of Clonal Populations of the Freshwater Jellyfish Craspedacusta sowerbii (Lankester, 1880) in Southern Hemisphere Lakes (Chile) and the Potential Role of the Zooplankton Diet. Austral Ecol. 2021, 46, 1192–1197. [Google Scholar] [CrossRef]
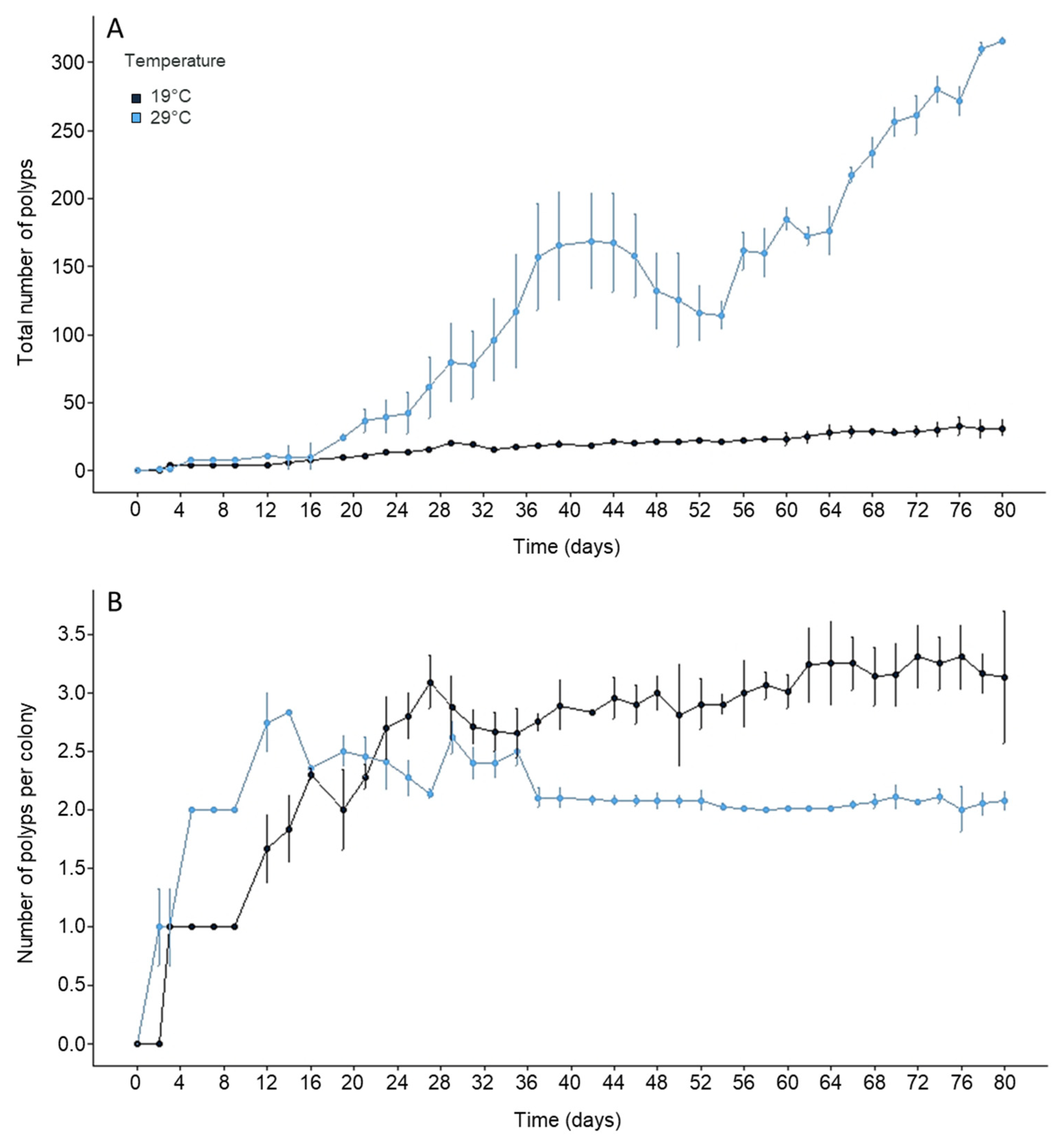
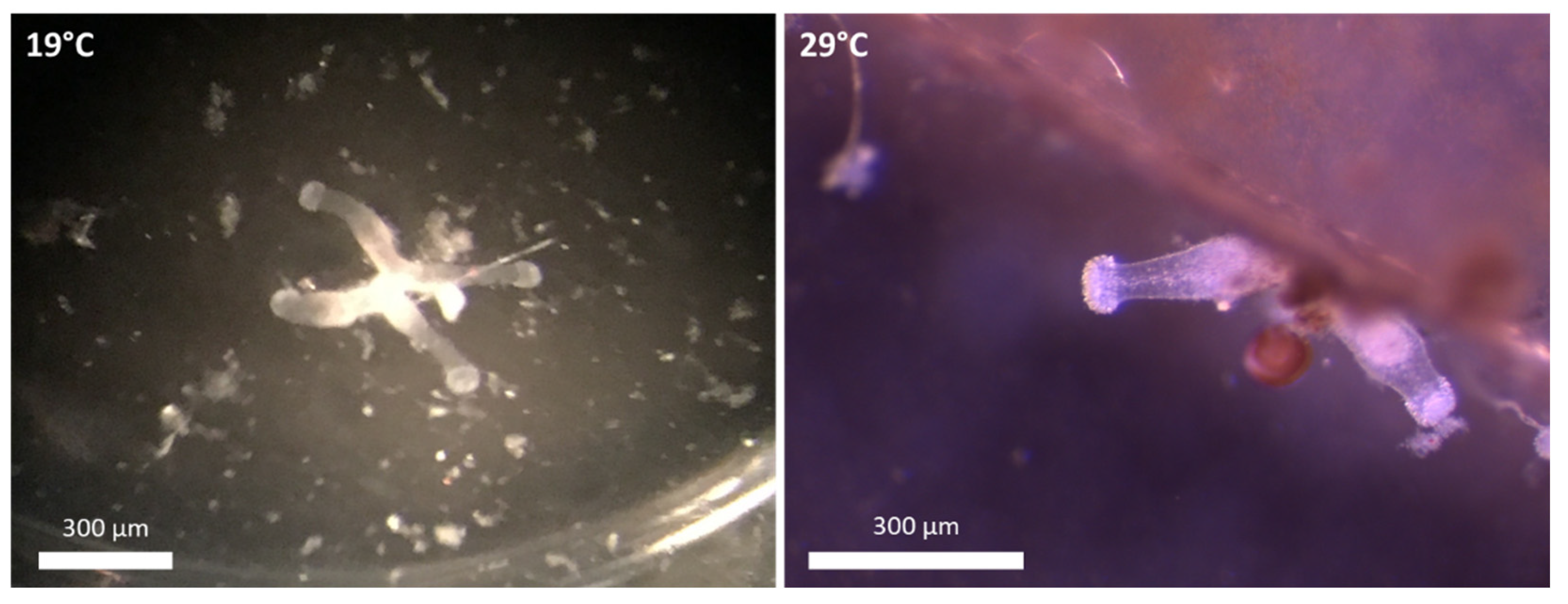
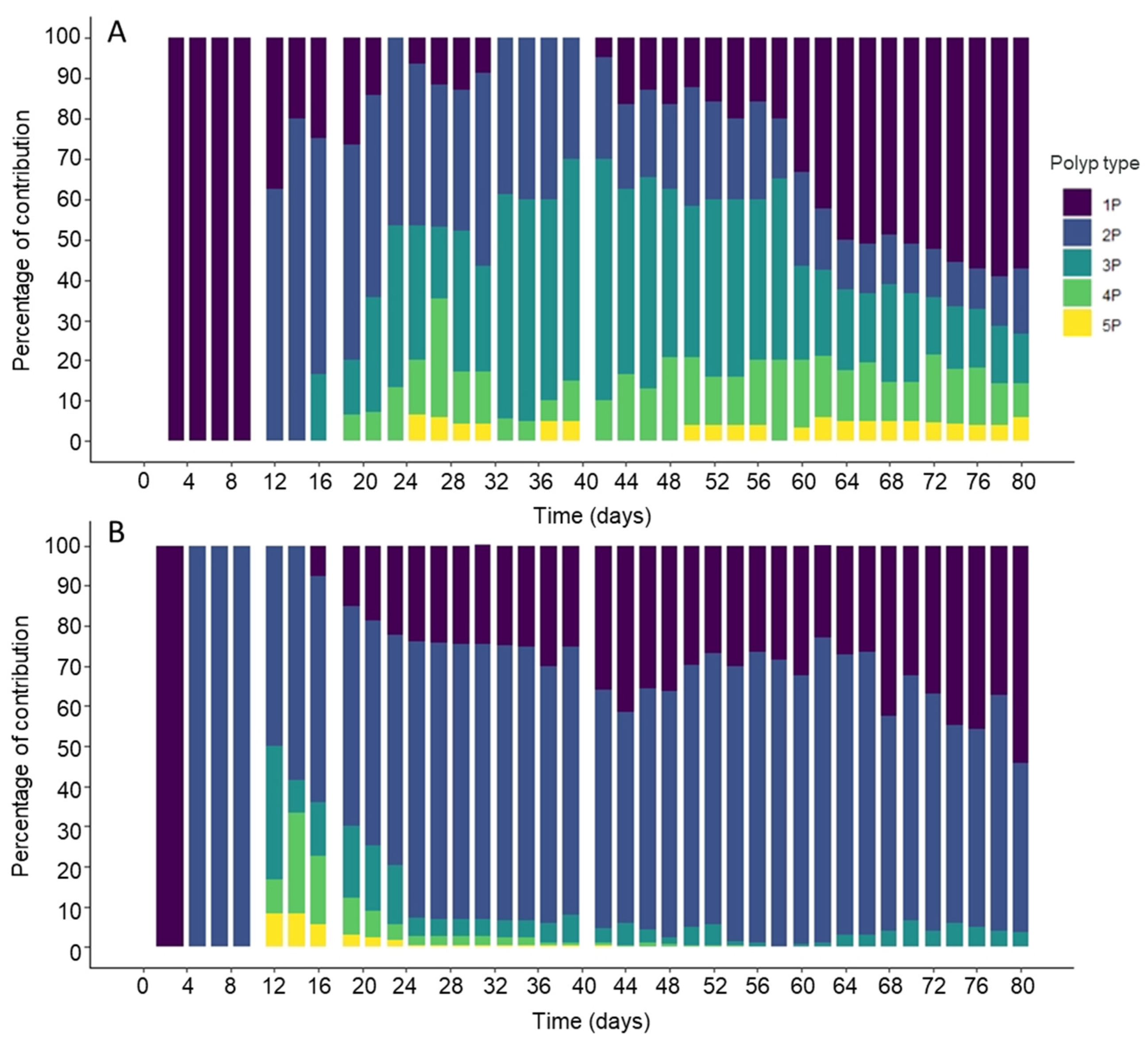

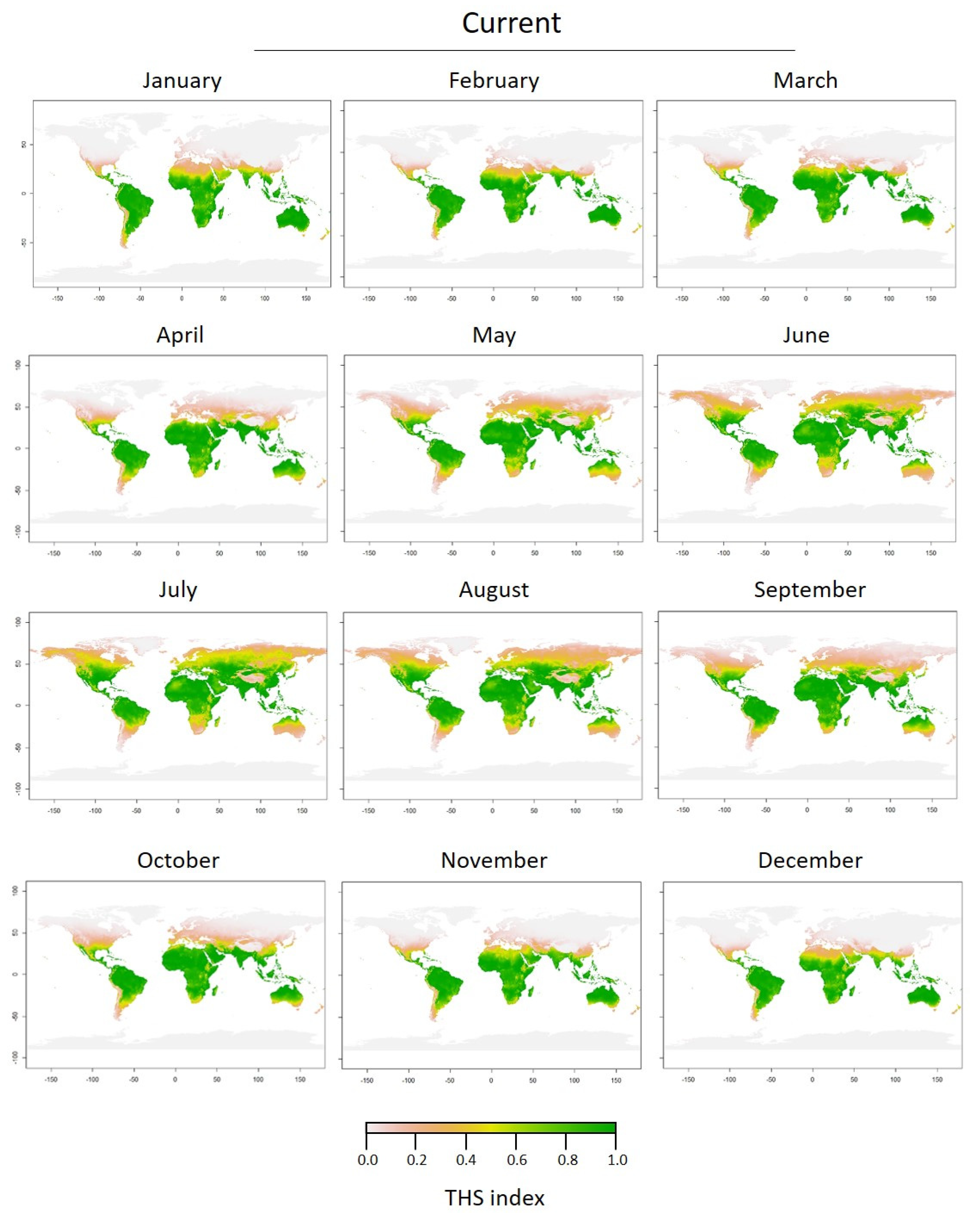
| Colony Types | 19 °C | 29 °C | F Value | p Value |
|---|---|---|---|---|
| 1 polyp | 33 ± 30 | 30 ± 21 | 0.32 | 0.430 |
| 2 polyps | 30 ± 17 | 65 ± 13 | 89.22 | <0.001 * |
| 3 polyps | 32 ± 15 | 6 ± 6 | 93.57 | <0.001 * |
| 4 polyps | 13 ± 5 | 3 ± 5 | 73.91 | <0.001 * |
| 5 polyps | 4 ± 2 | 1 ± 2 | 25.06 | <0.001 * |
| Colony Types | 19 °C | 29 °C | F Value | p Value |
|---|---|---|---|---|
| 1 polyp | 3 ± 1 | 2 ± 0 | 3.02 | 0.158 |
| 2 polyps | 12 ± 2 | 5 ± 2 | 29.40 | 0.006 * |
| 3 polyps | 16 ± 5 | 12 ± 5 | 0.96 | 0.383 |
| 4 polyps | 19 ± 2 | 12 ± 4 | 7.35 | 0.050 * |
| 5 polyps | 25 ± 2 | 12 ± 2 | 63.37 | 0.001 * |
| Temperature (°C) | Mean Time (Days) to First Polyp Formation | Mean Number of Polyps Per Colony | Reference |
|---|---|---|---|
| 12 | - | 5.9 | [27] |
| 19 | 3.1 ± 0.6 | 3.1 ± 0.2 | This study |
| 20 | - | 2 - 4 | [27] |
| 24 | 5.1 ± 0.5 | 2.2 ± 0.1 | [11] |
| 25 | - | 1.8 to 3.8 | [27] |
| 26 | - | 2 to 6 | [11] |
| 28 | - | 3.8 | [27] |
| 29 | 2.1 ± 0.1 | 2.2 ± 0.2 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchessaux, G.; Lüskow, F.; Bejean, M.; Pakhomov, E.A. Increasing Temperature Facilitates Polyp Spreading and Medusa Appearance of the Invasive Hydrozoan Craspedacusta sowerbii. Biology 2022, 11, 1100. https://doi.org/10.3390/biology11081100
Marchessaux G, Lüskow F, Bejean M, Pakhomov EA. Increasing Temperature Facilitates Polyp Spreading and Medusa Appearance of the Invasive Hydrozoan Craspedacusta sowerbii. Biology. 2022; 11(8):1100. https://doi.org/10.3390/biology11081100
Chicago/Turabian StyleMarchessaux, Guillaume, Florian Lüskow, Mickaël Bejean, and Evgeny A. Pakhomov. 2022. "Increasing Temperature Facilitates Polyp Spreading and Medusa Appearance of the Invasive Hydrozoan Craspedacusta sowerbii" Biology 11, no. 8: 1100. https://doi.org/10.3390/biology11081100
APA StyleMarchessaux, G., Lüskow, F., Bejean, M., & Pakhomov, E. A. (2022). Increasing Temperature Facilitates Polyp Spreading and Medusa Appearance of the Invasive Hydrozoan Craspedacusta sowerbii. Biology, 11(8), 1100. https://doi.org/10.3390/biology11081100







