Acute and Chronic Effects of Fin Amputation on Behavior Performance of Adult Zebrafish in 3D Locomotion Test Assessed with Fractal Dimension and Entropy Analyses and Their Relationship to Fin Regeneration
Abstract
Simple Summary
Abstract
1. Introduction
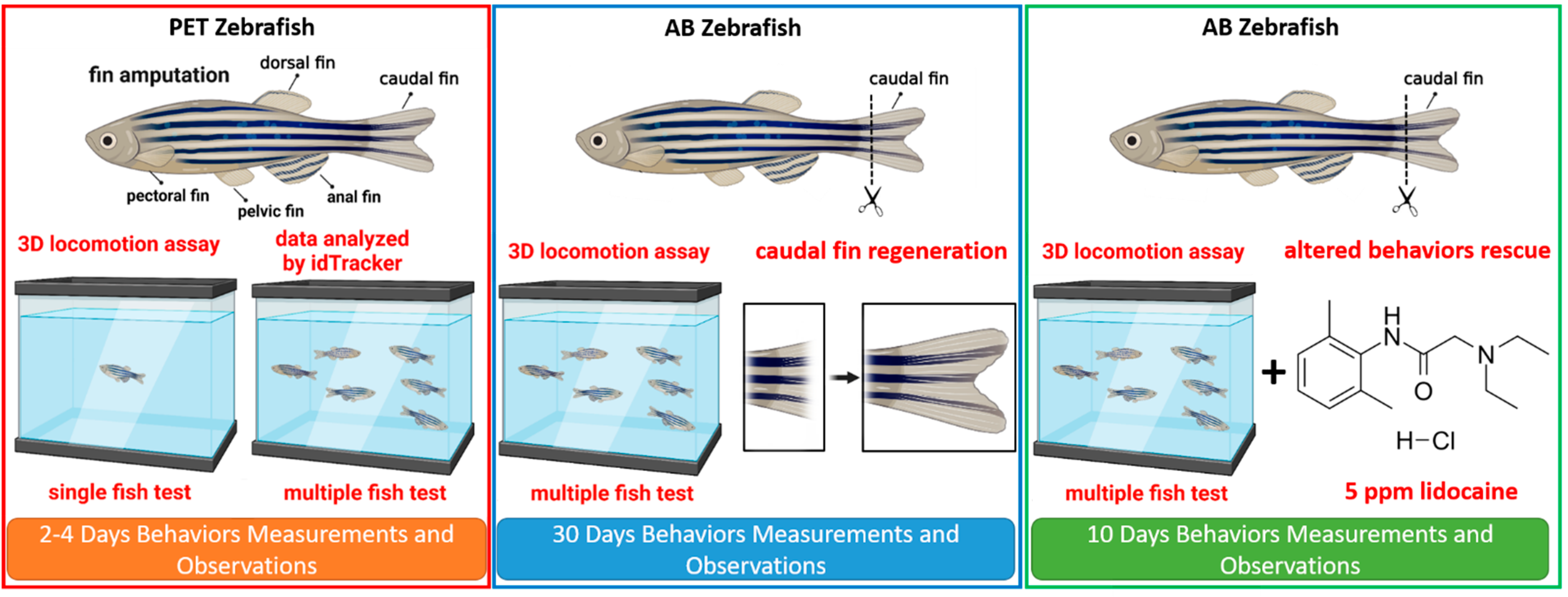
2. Materials and Methods
2.1. Zebrafish Maintenance
2.2. Fin amputation and Treatments
2.3. Three-Dimensional Locomotion Test and Locomotion Trajectories Analysis
2.4. The Mathematic Calculation for Fractal Dimension
2.5. The Mathematic Calculation for Entropy
2.6. Statistical Analyses
2.7. Principal Components Analysis (PCA) and Clustering Analysis
3. Results
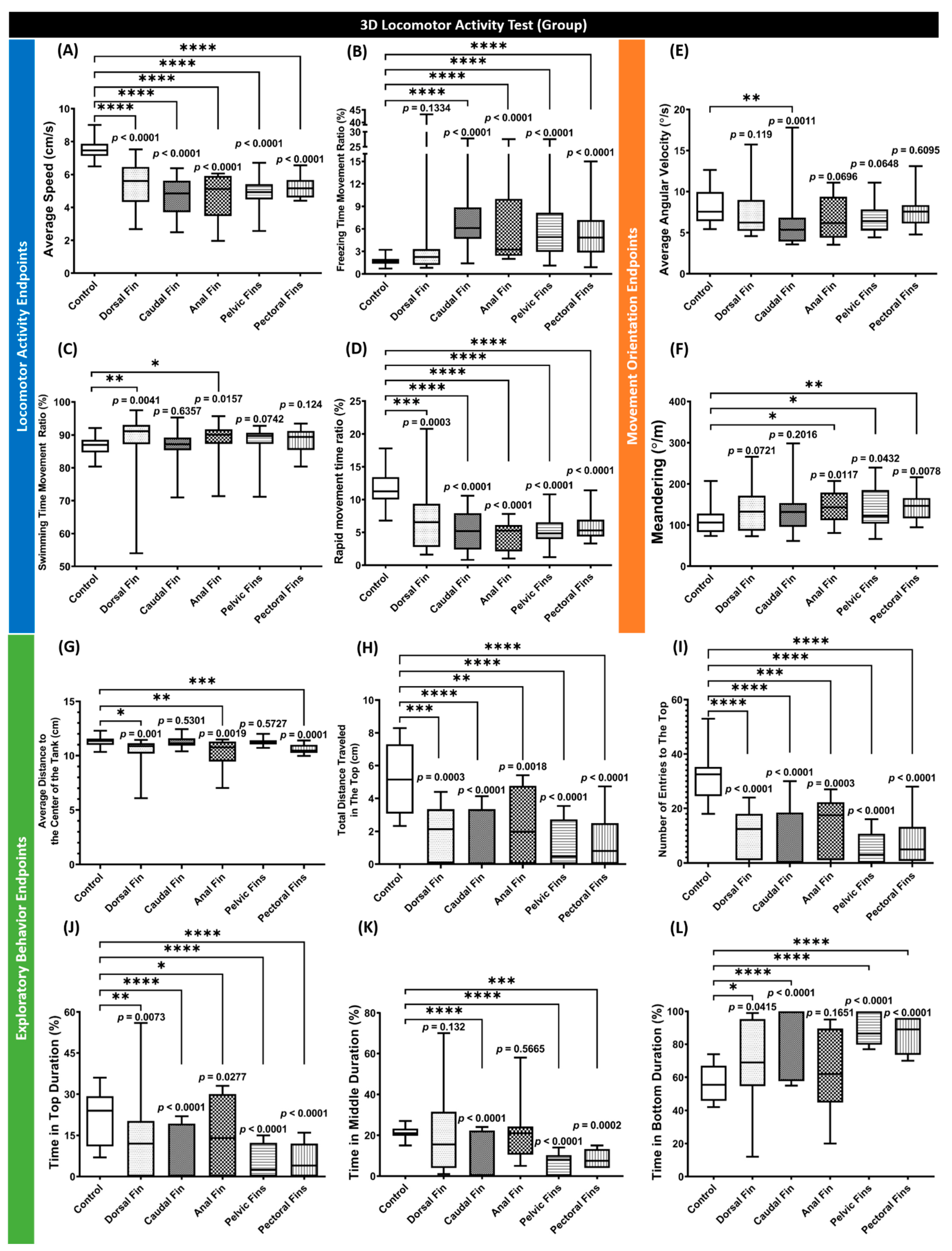
3.1. Behavior Performance of Grouped Fin-Amputated Fishes in 3D Locomotor Activity Test
3.2. Behavior Performance of Individual Fin-Amputated Fish in 3D Locomotor Activity Test
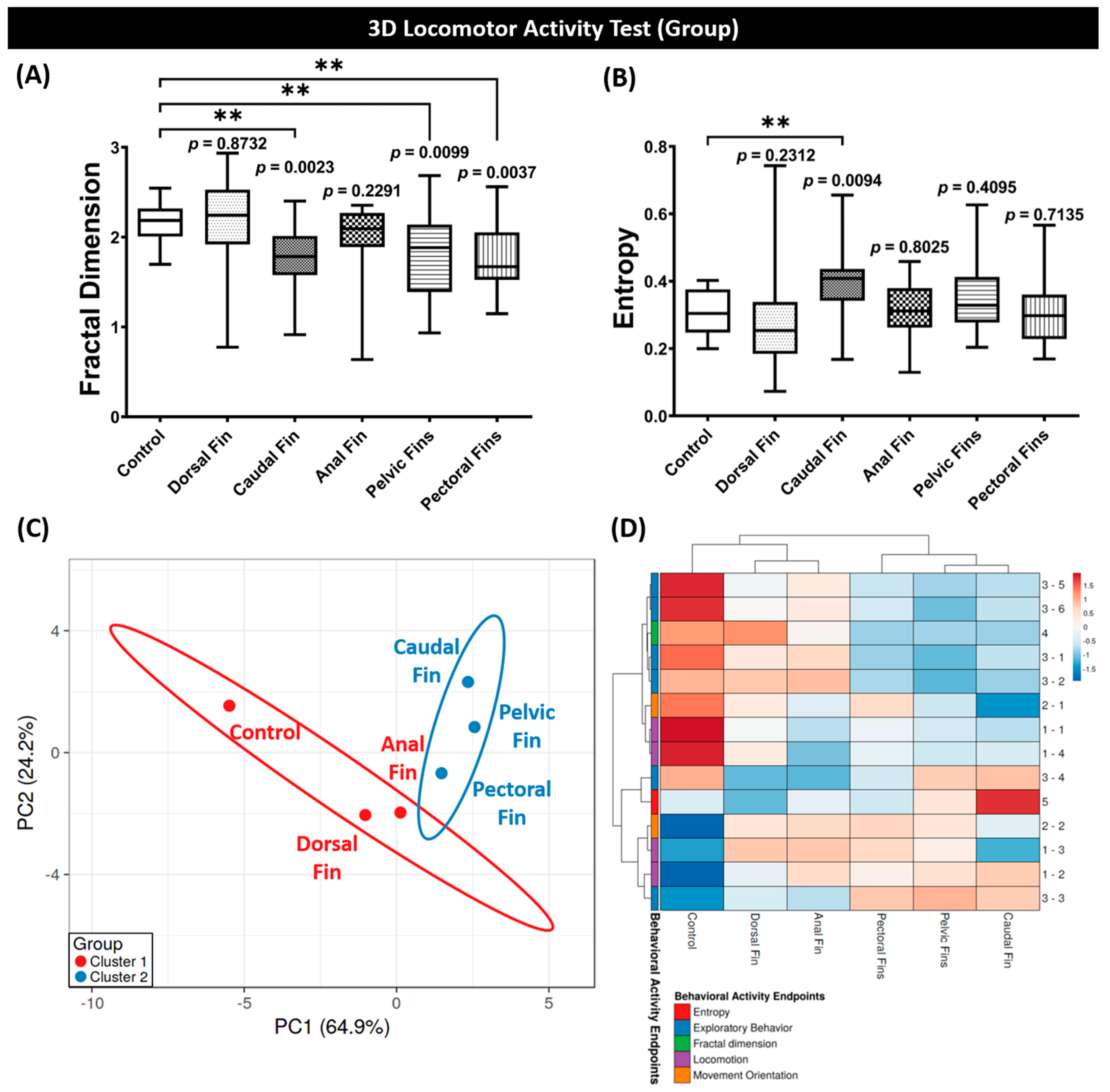
3.3. Fractal Dimension, Entropy, PCA, and Hierarchical Clustering Analyses of Grouped Zebrafish Behavior Performance after Fins Amputation
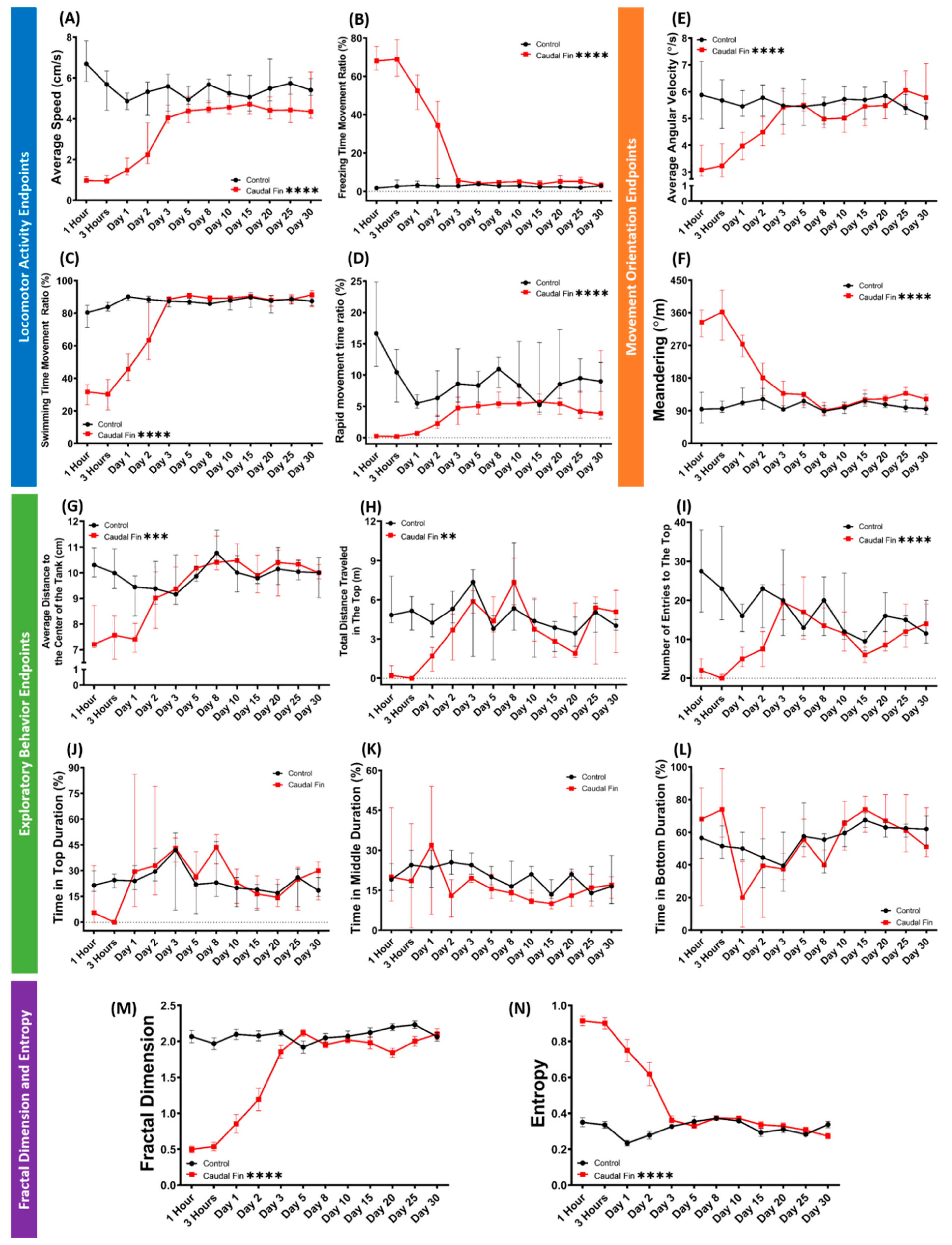
3.4. Recovery of Behavior Performance of Fish after Caudal Fin Amputation in a Grouped 3D Locomotor Activity Test
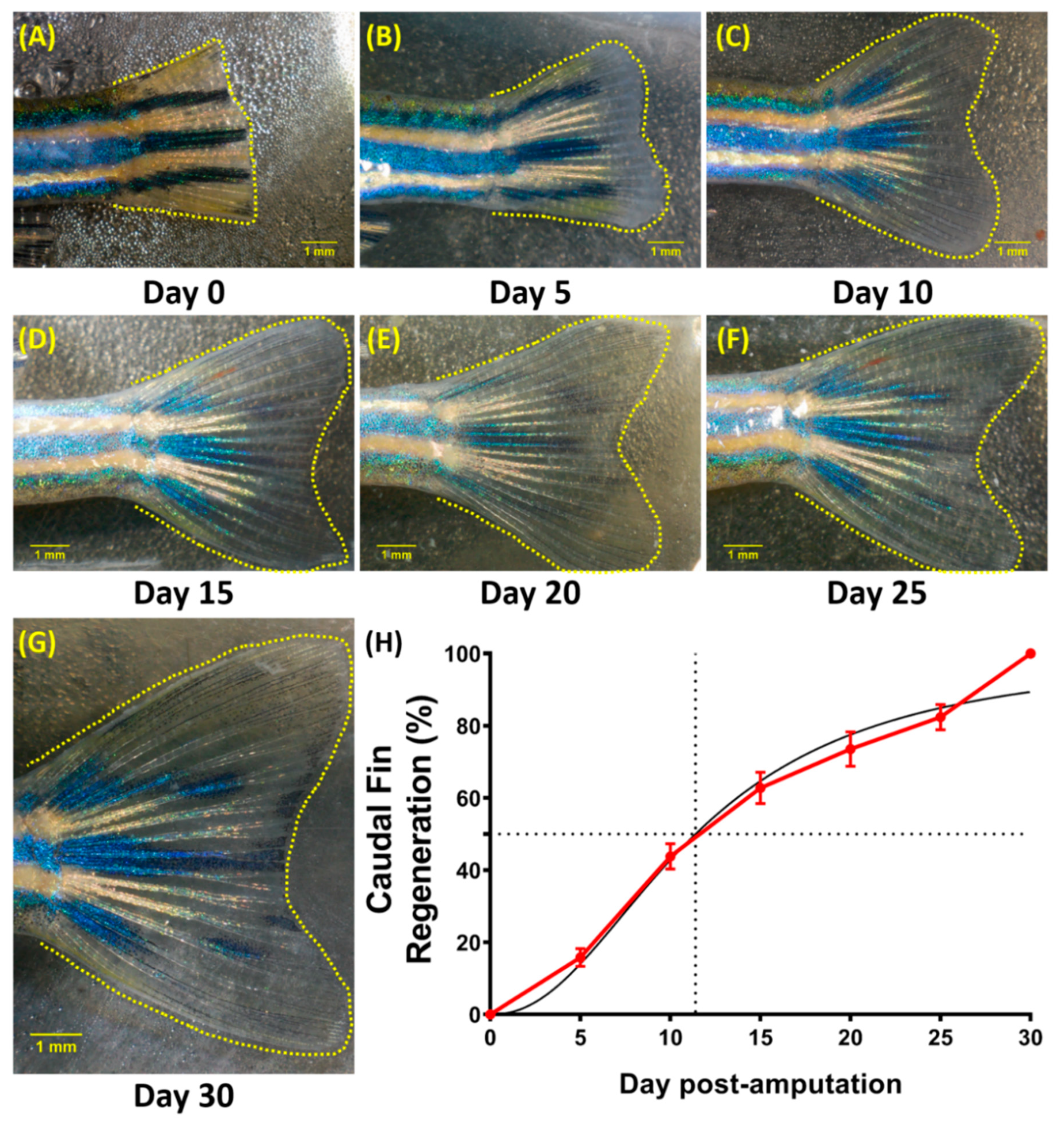
3.5. Regeneration of the Caudal Fin in Adult Zebrafish
3.6. Behavior Recovery after Caudal Fin Amputation by Lidocaine
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A

| Index | Behavior Endpoints (Units) | Definition | Belong to |
|---|---|---|---|
| 1-1 | Average speed (cm s−1) | Total distance traveled by fish divided by total time duration | Locomotor Activity Endpoints |
| 1-2 | Freezing time movement ratio (%) | Total percentage of time when fish’s speed is less than 1 cm s−1 | |
| 1-3 | Swimming time movement ratio (%) | Total percentage of time when fish’s speed is between 1 and 10 cm s−1 | |
| 1-4 | Rapid movement ratio (%) | Total percentage of time when fish’s speed is more than 10 cm s−1 | |
| 2-1 | Average angular velocity (°/s) | Average of magnitude and direction of zebrafish angular speed | Movement Orientation Endpoints |
| 2-2 | Meandering (°/m) | Movement without a fixed direction or path | |
| 3-1 | Time in top (%) | Total percentage of time when fish stayed in the top portion of the test tank | Exploratory Behavior Endpoints |
| 3-2 | Time in middle (%) | Total percentage of time when fish stayed in the middle portion of the test tank | |
| 3-3 | Time in bottom (%) | Total percentage of time when fish stayed in the bottom portion of the test tank | |
| 3-4 | Thigmotaxis (cm) | Average distance to the center of the tank | |
| 3-5 | Total distance traveled in top (cm) | Total distance traveled in the top portion of the novel tank | |
| 3-6 | Number of entries to the top | Total times fish enter the upper half of the tank | |
| 4 | Fractal Dimensions | Complexity of fractal patterns as a ratio of the change in detail to the change proportionally | Fractal Dimensions |
| 5 | Entropy | The uncertainty and degree of disorder | Entropy |
| Behavior Endpoints | Statistic Results | p Value | p Value Summary | F (DFn, DFd) |
|---|---|---|---|---|
| Average Speed | Row Factor | <0.0001 | **** | F (6.135, 207.5) = 18.38 |
| Column Factor | <0.0001 | **** | F (1, 34) = 238.9 | |
| Average Angular Velocity | Row Factor | <0.0001 | **** | F (7.294, 246.7) = 5.732 |
| Column Factor | <0.0001 | **** | F (1, 34) = 39.60 | |
| Meandering | Row Factor | <0.0001 | **** | F (5.240, 177.2) = 41.20 |
| Column Factor | <0.0001 | **** | F (1, 34) = 211.8 | |
| Freezing Movement Ratio | Row Factor | <0.0001 | **** | F (4.654, 157.4) = 83.65 |
| Column Factor | <0.0001 | **** | F (1, 34) = 312.8 | |
| Swimming Movement Ratio | Row Factor | <0.0001 | **** | F (5.351, 180.9) = 67.91 |
| Column Factor | <0.0001 | **** | F (1, 34) = 169.4 | |
| Rapid Movement Ratio | Row Factor | 0.0005 | *** | F (5.072, 171.5) = 4.691 |
| Column Factor | <0.0001 | **** | F (1, 34) = 130.5 | |
| Time in Top Percentage | Row Factor | <0.0001 | **** | F (3.798, 128.4) = 10.12 |
| Column Factor | 0.1063 | ns | F (1, 34) = 2.753 | |
| Time in Middle Percentage | Row Factor | <0.0001 | **** | F (4.633, 156.7) = 6.089 |
| Column Factor | 0.0694 | ns | F (1, 34) = 3.515 | |
| Time in Bottom Percentage | Row Factor | <0.0001 | **** | F (4.904, 165.9) = 12.47 |
| Column Factor | 0.829 | ns | F (1, 34) = 0.04737 | |
| Average Thigmotaxis | Row Factor | <0.0001 | **** | F (5.837, 197.4) = 24.46 |
| Column Factor | 0.0001 | *** | F (1, 34) = 18.70 | |
| Total Distance Traveled in the Top | Row Factor | <0.0001 | **** | F (6.054, 204.7) = 13.27 |
| Column Factor | 0.0057 | ** | F (1, 34) = 8.702 | |
| Total Entries to the Top | Row Factor | 0.0003 | *** | F (6.138, 207.6) = 4.375 |
| Column Factor | <0.0001 | **** | F (1, 34) = 57.43 | |
| Fractal Dimension | Row Factor | <0.0001 | **** | F (6.387, 216.0) = 37.20 |
| Column Factor | <0.0001 | **** | F (1, 34) = 223.5 | |
| Entropy | Row Factor | <0.0001 | **** | F (4.935, 166.9) = 44.15 |
| Column Factor | <0.0001 | **** | F (1, 34) = 151.8 |
| Behavior Endpoints | Statistic Results | p Value | p Value Summary | F (DFn, DFd) |
|---|---|---|---|---|
| Average Speed | Row Factor | <0.0001 | **** | F (5.373, 355.4) = 16.72 |
| Column Factor | <0.0001 | **** | F (3, 68) = 48.45 | |
| Control vs. Caudal Fin | <0.0001 | **** | - | |
| Control vs. Caudal Fin + Lidocaine | <0.0001 | **** | ||
| Control vs. 5 ppm Lidocaine | 0.1178 | ns | ||
| Average Angular Velocity | Row Factor | <0.0001 | **** | F (6.271, 475.7) = 4.849 |
| Column Factor | <0.0001 | **** | F (3, 531) = 27.90 | |
| Control vs. Caudal Fin | <0.0001 | **** | - | |
| Control vs. Caudal Fin + Lidocaine | <0.0001 | **** | ||
| Control vs. 5 ppm Lidocaine | 0.0151 | * | ||
| Meandering | Row Factor | <0.0001 | **** | F (4.546, 300.7) = 31.50 |
| Column Factor | <0.0001 | **** | F (3, 68) = 82.13 | |
| Control vs. Caudal Fin | <0.0001 | **** | - | |
| Control vs. Caudal Fin + Lidocaine | <0.0001 | **** | ||
| Control vs. 5 ppm Lidocaine | 0.0015 | ** | ||
| Freezing Movement Ratio | Row Factor | <0.0001 | **** | F (3.972, 262.7) = 53.38 |
| Column Factor | <0.0001 | **** | F (3, 68) = 146.8 | |
| Control vs. Caudal Fin | <0.0001 | **** | - | |
| Control vs. Caudal Fin + Lidocaine | <0.0001 | **** | ||
| Control vs. 5 ppm Lidocaine | 0.3648 | ns | ||
| Swimming Movement Ratio | Row Factor | <0.0001 | **** | F (4.531, 299.7) = 51.26 |
| Column Factor | <0.0001 | **** | F (3, 68) = 84.62 | |
| Control vs. Caudal Fin | <0.0001 | **** | - | |
| Control vs. Caudal Fin + Lidocaine | 0.9693 | ns | ||
| Control vs. 5 ppm Lidocaine | 0.9997 | ns | ||
| Rapid Movement Ratio | Row Factor | <0.0001 | **** | F (4.544, 300.5) = 9.073 |
| Column Factor | <0.0001 | **** | F (3, 68) = 19.06 | |
| Control vs. Caudal Fin | <0.0001 | **** | - | |
| Control vs. Caudal Fin + Lidocaine | <0.0001 | **** | ||
| Control vs. 5 ppm Lidocaine | 0.7126 | ns | ||
| Time in Top Percentage | Row Factor | 0.0001 | *** | F (4.407, 291.5) = 5.747 |
| Column Factor | <0.0001 | **** | F (3, 68) = 26.42 | |
| Control vs. Caudal Fin | 0.195 | ns | - | |
| Control vs. Caudal Fin + Lidocaine | <0.0001 | **** | ||
| Control vs. 5 ppm Lidocaine | <0.0001 | **** | ||
| Time in Middle Percentage | Row Factor | 0.0017 | ** | F (4.975, 329.1) = 3.968 |
| Column Factor | <0.0001 | **** | F (3, 68) = 17.10 | |
| Control vs. Caudal Fin | 0.2742 | ns | - | |
| Control vs. Caudal Fin + Lidocaine | <0.0001 | **** | ||
| Control vs. 5 ppm Lidocaine | 0.8823 | ns | ||
| Time in Bottom Percentage | Row Factor | <0.0001 | **** | F (5.262, 348.1) = 6.507 |
| Column Factor | <0.0001 | **** | F (3, 68) = 45.48 | |
| Control vs. Caudal Fin | 0.8192 | ns | - | |
| Control vs. Caudal Fin + Lidocaine | <0.0001 | **** | ||
| Control vs. 5 ppm Lidocaine | 0.0002 | *** | ||
| Average Thigmotaxis | Row Factor | <0.0001 | **** | F (5.086, 336.4) = 25.97 |
| Column Factor | <0.0001 | **** | F (3, 68) = 20.50 | |
| Control vs. Caudal Fin | <0.0001 | **** | - | |
| Control vs. Caudal Fin + Lidocaine | <0.0001 | **** | ||
| Control vs. 5 ppm Lidocaine | <0.0001 | **** | ||
| Total Distance Traveled in the Top | Row Factor | <0.0001 | **** | F (4.811, 318.2) = 14.97 |
| Column Factor | <0.0001 | **** | F (3, 68) = 16.62 | |
| Control vs. Caudal Fin | <0.0001 | **** | - | |
| Control vs. Caudal Fin + Lidocaine | 0.495 | ns | ||
| Control vs. 5 ppm Lidocaine | <0.0001 | **** | ||
| Total Entries to the Top | Row Factor | <0.0001 | **** | F (5.962, 394.4) = 9.835 |
| Column Factor | <0.0001 | **** | F (3, 68) = 34.53 | |
| Control vs. Caudal Fin | <0.0001 | **** | - | |
| Control vs. Caudal Fin + Lidocaine | 0.9996 | ns | ||
| Control vs. 5 ppm Lidocaine | <0.0001 | **** | ||
| Fractal Dimension | Row Factor | <0.0001 | **** | F (5.275, 317.3) = 21.00 |
| Column Factor | <0.0001 | **** | F (3, 62) = 77.90 | |
| Control vs. Caudal Fin | <0.0001 | **** | - | |
| Control vs. Caudal Fin + Lidocaine | 0.382 | ns | ||
| Control vs. 5 ppm Lidocaine | 0.0941 | ns | ||
| Entropy | Row Factor | <0.0001 | **** | F (4.455, 267.9) = 21.61 |
| Column Factor | <0.0001 | **** | F (3, 62) = 110.4 | |
| Control vs. Caudal Fin | <0.0001 | **** | - | |
| Control vs. Caudal Fin + Lidocaine | 0.0017 | ** | ||
| Control vs. 5 ppm Lidocaine | <0.0001 | **** |
References
- McClenahan, P.; Troup, M.; Scott, E.K. Fin-tail coordination during escape and predatory behavior in larval zebrafish. PLoS ONE 2012, 7, e32295. [Google Scholar] [CrossRef] [PubMed]
- Danos, N.; Lauder, G.V. The ontogeny of fin function during routine turns in zebrafish Danio rerio. J. Exp. Biol. 2007, 210, 3374–3386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Uemura, Y.; Kato, K.; Kawakami, K.; Kimura, Y.; Oda, Y.; Higashijima, S.-I. Neuronal circuits that control rhythmic pectoral fin movements in zebrafish. J. Neurosci. 2020, 40, 6678–6690. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.; Lawrence, C. The Laboratory Zebrafish; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Gong, Z.; Korzh, V. Fish Development and Genetics: The Zebrafish and Medaka Models; World Scientific Publishing Company: Singapore, 2004. [Google Scholar]
- Green, M.H.; Ho, R.K.; Hale, M.E. Movement and function of the pectoral fins of the larval zebrafish (Danio rerio) during slow swimming. J. Exp. Biol. 2011, 214, 3111–3123. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, D.; Hale, M. Development of zebrafish (Danio rerio) pectoral fin musculature. J. Morphol. 2005, 266, 241–255. [Google Scholar] [CrossRef]
- Thorsen, D.H.; Cassidy, J.J.; Hale, M.E. Swimming of larval zebrafish: Fin–axis coordination and implications for function and neural control. J. Exp. Biol. 2004, 207, 4175–4183. [Google Scholar] [CrossRef]
- Drucker, E.; Jensen, J. Pectoral fin locomotion in the striped surfperch. I. Kinematic effects of swimming speed and body size. J. Exp. Biol. 1996, 199, 2235–2242. [Google Scholar] [CrossRef]
- Webb, P.W. The biology of fish swimming. Mech. Physiol. Anim. Swim. 1994, 4562, 45–62. [Google Scholar]
- Fu, C.; Cao, Z.-D.; Fu, S.-J. The effects of caudal fin amputation on metabolic interaction between digestion and locomotion in juveniles of three cyprinid fish species with different metabolic modes. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2013, 164, 456–465. [Google Scholar] [CrossRef]
- Harris, J. The role of the fins in the equilibrium of the swimming fish: I. Wind-tunnel tests on a model of Mustelus canis (Mitchill). J. Exp. Biol. 1936, 13, 476–493. [Google Scholar] [CrossRef]
- Webb, P. Effects of partial caudal-fin amputation on the kinematics and metabolic rate of underyearling sockeye salmon (Oncorhynchus nerka) at steady swimming speeds. J. Exp. Biol. 1973, 59, 565–582. [Google Scholar] [CrossRef]
- Webb, P. Effects of median-fin amputation on fast-start performance of rainbow trout (Salmo gairdneri). J. Exp. Biol. 1977, 68, 123–135. [Google Scholar] [CrossRef]
- Plaut, I. Effects of fin size on swimming performance, swimming behaviour and routine activity of zebrafish Danio rerio. J. Exp. Biol. 2000, 203, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Padilla, S.; Hunter, D.; Padnos, B.; Frady, S.; MacPhail, R. Assessing locomotor activity in larval zebrafish: Influence of extrinsic and intrinsic variables. Neurotoxicol. Teratol. 2011, 33, 624–630. [Google Scholar] [CrossRef]
- Cachat, J.; Stewart, A.; Utterback, E.; Hart, P.; Gaikwad, S.; Wong, K.; Kyzar, E.; Wu, N.; Kalueff, A.V. Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS ONE 2011, 6, e17597. [Google Scholar] [CrossRef]
- Green, J.; Collins, C.; Kyzar, E.J.; Pham, M.; Roth, A.; Gaikwad, S.; Cachat, J.; Stewart, A.M.; Landsman, S.; Grieco, F.; et al. Automated high-throughput neurophenotyping of zebrafish social behavior. J. Neurosci. Methods 2012, 210, 266–271. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef]
- Soleymani, A.; Cachat, J.; Robinson, K.; Dodge, S.; Kalueff, A.; Weibel, R. Integrating cross-scale analysis in the spatial and temporal domains for classification of behavioral movement. J. Spat. Inf. Sci. 2014, 2014, 1–25. [Google Scholar] [CrossRef]
- Stewart, A.M.; Grieco, F.; Tegelenbosch, R.A.; Kyzar, E.J.; Nguyen, M.; Kaluyeva, A.; Song, C.; Noldus, L.P.; Kalueff, A.V. A novel 3D method of locomotor analysis in adult zebrafish: Implications for automated detection of CNS drug-evoked phenotypes. J. Neurosci. Methods 2015, 255, 66–74. [Google Scholar] [CrossRef]
- Zhu, L.; Weng, W. Catadioptric stereo-vision system for the real-time monitoring of 3D behavior in aquatic animals. Physiol. Behav. 2007, 91, 106–119. [Google Scholar] [CrossRef]
- Cachat, J.M. Developing Zebrafish Models of Complex Phenotypes Relevant to Human Brain Disorders; Tulane University School of Science and Engineering: New Orleance, LA, USA, 2013; pp. 6–10. [Google Scholar]
- Bershadskii, A. An universal relation between fractal and Euclidean (topological) dimensions of random systems. Eur. Phys. J. B-Condens. Matter Complex Syst. 1998, 6, 381–382. [Google Scholar] [CrossRef]
- Power, W.L.; Tullis, T.E. Euclidean and fractal models for the description of rock surface roughness. J. Geophys. Res. Solid Earth 1991, 96, 415–424. [Google Scholar] [CrossRef]
- Nimkerdphol, K.; Nakagawa, M. Effect of sodium hypochlorite on zebrafish swimming behavior estimated by fractal dimension analysis. J. Biosci. Bioeng. 2008, 105, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Mandelbrot, B.B.; Mandelbrot, B.B. The Fractal Geometry of Nature; WH Freeman New York: New York, NY, USA, 1982; Volume 1. [Google Scholar]
- Eom, H.-J.; Liu, Y.; Kwak, G.-S.; Heo, M.; Song, K.S.; Chung, Y.D.; Chon, T.-S.; Choi, J. Inhalation toxicity of indoor air pollutants in Drosophila melanogaster using integrated transcriptomics and computational behavior analyses. Sci. Rep. 2017, 7, 46473. [Google Scholar] [CrossRef]
- Ji, C.; Lee, S.; Choi, K.; Kwak, I.; Lee, S.; Cha, E.; Lee, S.; Chon, T. Monitoring of movement behaviors of chironomid larvae after exposure to diazinon using fractal dimension and self-organizing map. Int. J. Des. Nat. Ecodynamics 2007, 2, 1. [Google Scholar] [CrossRef]
- Deakin, A.G.; Spencer, J.W.; Cossins, A.R.; Young, I.S.; Sneddon, L.U. Welfare challenges influence the complexity of movement: Fractal analysis of behaviour in zebrafish. Fishes 2019, 4, 8. [Google Scholar] [CrossRef]
- Thomson, J.S.; Deakin, A.G.; Cossins, A.R.; Spencer, J.W.; Young, I.S.; Sneddon, L.U. Acute and chronic stress prevents responses to pain in zebrafish: Evidence for stress-induced analgesia. J. Exp. Biol. 2020, 223, jeb224527. [Google Scholar] [CrossRef]
- Liu, Y.; Chon, T.-S.; Baek, H.; Do, Y.; Choi, J.H.; Chung, Y.D. Permutation entropy applied to movement behaviors of Drosophila melanogaster. Mod. Phys. Lett. B 2011, 25, 1133–1142. [Google Scholar] [CrossRef]
- Eguiraun, H.; López-de-Ipiña, K.; Martinez, I. Application of entropy and fractal dimension analyses to the pattern recognition of contaminated fish responses in aquaculture. Entropy 2014, 16, 6133–6151. [Google Scholar] [CrossRef]
- Bandt, C.; Pompe, B. Permutation entropy: A natural complexity measure for time series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef]
- Eguiraun, H.; Casquero, O.; Sørensen, A.J.; Martinez, I. Reducing the Number of Individuals to Monitor Shoaling Fish Systems–Application of the Shannon Entropy to Construct a Biological Warning System Model. Front. Physiol. 2018, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 2008, 83, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. J. Vis. Exp. 2012, 69, e4196. [Google Scholar] [CrossRef]
- Kim, J.; Shin, W. How to do random allocation (randomization). Clin. Orthop. Surg. 2014, 6, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, J.; Saszik, S.; DeLorenzo, A.S.; Hardesty, H.R. Establishing and maintaining a low-cost zebrafish breeding and behavioral research facility. Behav. Res. Methods Instrum. Comput. 1999, 31, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Barba-Escobedo, P.A.; Gould, G.G. Visual social preferences of lone zebrafish in a novel environment: Strain and anxiolytic effects. Genes Brain Behav. 2012, 11, 366–373. [Google Scholar] [CrossRef]
- Kurta, A.; Palestis, B.G. Effects of ethanol on the shoaling behavior of zebrafish (Danio rerio). Dose-Response 2010, 8, 527–532. [Google Scholar] [CrossRef]
- Sullivan, L. Power and Sample Size Determination. Available online: https://sphweb.bumc.bu.edu/otlt/mph-modules/bs/bs704_power/bs704_power_print.html (accessed on 28 August 2020).
- Salkind, N.J. Encyclopedia of Research Design; Sage: Thousand Oaks, CA, USA, 2010. [Google Scholar]
- Thomson, J.S.; Al-Temeemy, A.A.; Isted, H.; Spencer, J.W.; Sneddon, L.U. Assessment of behaviour in groups of zebrafish (Danio rerio) using an intelligent software monitoring tool, the chromatic fish analyser. J. Neurosci. Methods 2019, 328, 108433. [Google Scholar] [CrossRef]
- Deakin, A.G.; Buckley, J.; AlZu’bi, H.S.; Cossins, A.R.; Spencer, J.W.; Al’Nuaimy, W.; Young, I.S.; Thomson, J.S.; Sneddon, L.U. Automated monitoring of behaviour in zebrafish after invasive procedures. Sci. Rep. 2019, 9, 9042. [Google Scholar] [CrossRef]
- Pérez-Escudero, A.; Vicente-Page, J.; Hinz, R.C.; Arganda, S.; De Polavieja, G.G. idTracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 2014, 11, 743–748. [Google Scholar] [CrossRef]
- Audira, G.; Sampurna, B.; Juniardi, S.; Liang, S.-T.; Lai, Y.-H.; Hsiao, C.-D. A simple setup to perform 3D locomotion tracking in zebrafish by using a single camera. Inventions 2018, 3, 11. [Google Scholar] [CrossRef]
- De Lombaert, M.; Rick, E.L.; Krugner-Higby, L.A.; Wolman, M.A. Behavioral characteristics of adult zebrafish (Danio rerio) after MS222 anesthesia for fin excision. J. Am. Assoc. Lab. Anim. Sci. 2017, 56, 377–381. [Google Scholar] [PubMed]
- Schroeder, P.G.; Sneddon, L.U. Exploring the efficacy of immersion analgesics in zebrafish using an integrative approach. Appl. Anim. Behav. Sci. 2017, 187, 93–102. [Google Scholar] [CrossRef]
- Nordgreen, J.; Tahamtani, F.M.; Janczak, A.M.; Horsberg, T.E. Behavioural effects of the commonly used fish anaesthetic tricaine methanesulfonate (MS-222) on zebrafish (Danio rerio) and its relevance for the acetic acid pain test. PLoS ONE 2014, 9, e92116. [Google Scholar] [CrossRef] [PubMed]
- Geraudie, J.; Monnot, M.J.; Brulfert, A.; Ferretti, P. Caudal fin regeneration in wild type and long-fin mutant zebrafish is affected by retinoic acid. Int. J. Dev. Biol. 2002, 39, 373–381. [Google Scholar]
- Adams, D.C.; Anthony, C.D. Using randomization techniques to analyse behavioural data. Anim. Behav. 1996, 51, 733–738. [Google Scholar] [CrossRef]
- Privitera, G.J. Student Study Guide With IBM® SPSS® Workbook for Essential Statistics for the Behavioral Sciences; SAGE Publications: Thousand Oaks, CA, USA, 2015. [Google Scholar]
- Salkind, N.J. Statistics for People Who (Think They) Hate Statistics: Excel 2007 Edition; SAGE: Thousand Oaks, CA, USA, 2009. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Hussain, A.; Audira, G.; Malhotra, N.; Uapipatanakul, B.; Chen, J.-R.; Lai, Y.-H.; Huang, J.-C.; Chen, K.H.-C.; Lai, H.-T.; Hsiao, C.-D. Multiple Screening of Pesticides Toxicity in Zebrafish and Daphnia Based on Locomotor Activity Alterations. Biomolecules 2020, 10, 1224. [Google Scholar] [CrossRef]
- Audira, G.; Siregar, P.; Chen, J.-R.; Lai, Y.-H.; Huang, J.-C.; Hsiao, C.-D. Systematical exploration of the common solvent toxicity at whole organism level by behavioral phenomics in adult zebrafish. Environ. Pollut. 2020, 266, 115239. [Google Scholar] [CrossRef]
- Audira, G.; Lai, Y.H.; Huang, J.C.; Chen, K.H.C.; Hsiao, C.D. Phenomics Approach to Investigate Behavioral Toxicity of Environmental or Occupational Toxicants in Adult Zebrafish (Danio rerio). Curr. Protoc. 2021, 1, e223. [Google Scholar] [CrossRef]
- Bui Thi, N.H.; Nguyen Thi, N.A.; Audira, G.; Siregar, P.; Liang, S.-T.; Huang, J.-C.; Hsiao, C.-D. Chronic Exposure to Low Concentration Lead Chloride-Induced Anxiety and Loss of Aggression and Memory in Zebrafish. Int. J. Mol. Sci. 2020, 21, 1844. [Google Scholar] [CrossRef] [PubMed]
- de Paiva Magalhaes, D.; Buss, D.F.; Da Cunha, R.A.; Linde-Arias, A.R.; Baptista, D.F. Analysis of individual versus group behavior of zebrafish: A model using pH sublethal effects. Bull. Environ. Contam. Toxicol. 2012, 88, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.L.; Amaral, L.A.; Hausdorff, J.M.; Ivanov, P.C.; Peng, C.-K.; Stanley, H.E. Fractal dynamics in physiology: Alterations with disease and aging. Proc. Natl. Acad. Sci. USA 2002, 99, 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, K.; Haskell, M.; Glasbey, C.; Jones, R.; Lawrence, A. Fractal analysis of animal behaviour as an indicator of animal welfare. Anim. Welf. 2004, 13, 99–103. [Google Scholar]
- Butail, S.; Ladu, F.; Spinello, D.; Porfiri, M. Information flow in animal-robot interactions. Entropy 2014, 16, 1315–1330. [Google Scholar] [CrossRef]
- Ladu, F.; Mwaffo, V.; Li, J.; Macrì, S.; Porfiri, M. Acute caffeine administration affects zebrafish response to a robotic stimulus. Behav. Brain Res. 2015, 289, 48–54. [Google Scholar] [CrossRef]
- Pfefferli, C.; Jaźwińska, A. The art of fin regeneration in zebrafish. Regeneration 2015, 2, 72–83. [Google Scholar] [CrossRef]
- White, L.J.; Thomson, J.S.; Pounder, K.C.; Coleman, R.C.; Sneddon, L.U. The impact of social context on behaviour and the recovery from welfare challenges in zebrafish, Danio rerio. Anim. Behav. 2017, 132, 189–199. [Google Scholar] [CrossRef]
- Reilly, S.C.; Quinn, J.P.; Cossins, A.R.; Sneddon, L.U. Behavioural analysis of a nociceptive event in fish: Comparisons between three species demonstrate specific responses. Appl. Anim. Behav. Sci. 2008, 114, 248–259. [Google Scholar] [CrossRef]
- Maximino, C. Modulation of nociceptive-like behavior in zebrafish (Danio rerio) by environmental stressors. Psychol. Neurosci. 2011, 4, 149–155. [Google Scholar] [CrossRef]
- Audira, G.; Siregar, P.; Strungaru, S.-A.; Huang, J.-C.; Hsiao, C.-D. Which Zebrafish Strains Are More Suitable to Perform Behavioral Studies? A Comprehensive Comparison by Phenomic Approach. Biology 2020, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-c.; Lawson, N.D.; Weinstein, B.M.; Johnson, S.L. reg6 is required for branching morphogenesis during blood vessel regeneration in zebrafish caudal fins. Dev. Biol. 2003, 264, 263–274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Molony, V.; Kent, J.; Robertson, I. Behavioural responses of lambs of three ages in the first three hours after three methods of castration and tail docking. Res. Vet. Sci. 1993, 55, 236–245. [Google Scholar] [CrossRef]
- Lawrence, C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture 2007, 269, 1–20. [Google Scholar] [CrossRef]
- Paull, G.C.; Filby, A.L.; Giddins, H.G.; Coe, T.S.; Hamilton, P.B.; Tyler, C.R. Dominance hierarchies in zebrafish (Danio rerio) and their relationship with reproductive success. Zebrafish 2010, 7, 109–117. [Google Scholar] [CrossRef]
- Pavlidis, M.; Digka, N.; Theodoridi, A.; Campo, A.; Barsakis, K.; Skouradakis, G.; Samaras, A.; Tsalafouta, A. Husbandry of zebrafish, Danio rerio, and the cortisol stress response. Zebrafish 2013, 10, 524–531. [Google Scholar] [CrossRef]
- Speedie, N.; Gerlai, R. Alarm substance induced behavioral responses in zebrafish (Danio rerio). Behav. Brain Res. 2008, 188, 168–177. [Google Scholar] [CrossRef]
- Waldman, B. Quantitative and developmental analyses of the alarm reaction in the zebra danio, Brachydanio rerio. Copeia 1982, 1982, 1–9. [Google Scholar] [CrossRef]
- Barreto, R.E.; Volpato, G.L. Caution for using ventilatory frequency as an indicator of stress in fish. Behav. Processes 2004, 66, 43–51. [Google Scholar] [CrossRef]
- Barcellos, L.J.G.; Ritter, F.; Kreutz, L.C.; Quevedo, R.M.; da Silva, L.B.; Bedin, A.C.; Finco, J.; Cericato, L. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture 2007, 272, 774–778. [Google Scholar] [CrossRef]
- Faustino, A.I.; Tacão-Monteiro, A.; Oliveira, R.F. Mechanisms of social buffering of fear in zebrafish. Sci. Rep. 2017, 7, 44329. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.F.; Faustino, A.I. Social information use in threat perception: Social buffering, contagion and facilitation of alarm responses. Commun. Integr. Biol. 2017, 10, 44329. [Google Scholar] [CrossRef]
- Mezzomo, N.J.; Fontana, B.D.; Müller, T.E.; Duarte, T.; Quadros, V.A.; Canzian, J.; Pompermaier, A.; Soares, S.M.; Koakoski, G.; Loro, V.L. Taurine modulates the stress response in zebrafish. Horm. Behav. 2019, 109, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, H.N.; Anandampillai, R. Systemic toxic effects of local anaesthetics. Anaesth. Intensive Care Med. 2019, 20, 215–218. [Google Scholar] [CrossRef]
- Sneddon, L.U. Clinical anesthesia and analgesia in fish. J. Exot. Pet Med. 2012, 21, 32–43. [Google Scholar] [CrossRef]
- Sloman, K.A.; Bouyoucos, I.A.; Brooks, E.J.; Sneddon, L.U. Ethical considerations in fish research. J. Fish Biol. 2019, 94, 556–577. [Google Scholar] [CrossRef]
- Park, I.-S.; Park, S.J.; Gil, H.W.; Nam, Y.K.; Kim, D.S. Anesthetic effects of clove oil and lidocaine-HCl on marine medaka (Oryzias dancena). Lab Anim. 2011, 40, 45–51. [Google Scholar] [CrossRef]
- Collymore, C.; Banks, E.K.; Turner, P.V. Lidocaine hydrochloride compared with MS222 for the euthanasia of zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 816–820. [Google Scholar]
- de Abreu, M.S.; Giacomini, A.C.; dos Santos, B.E.; Genario, R.; Marchiori, N.I.; da Rosa, L.G.; Kalueff, A.V. Effects of lidocaine on adult zebrafish behavior and brain acetylcholinesterase following peripheral and systemic administration. Neurosci. Lett. 2019, 692, 181–186. [Google Scholar] [CrossRef]
- Kudyakova, T.; Sarycheva, N.Y.; Kamenskii, A. Orientation and exploratory behavior and anxiety of CBA mice with anosmia induced by N-trimethylindole (skatole). Bull. Exp. Biol. Med. 2007, 143, 1–4. [Google Scholar] [CrossRef]
- Foehn, E.R.M. Adult and pediatric anesthesia/sedation for gastrointestinal procedures outside of the operating room. Curr. Opin. Anaesthesiol. 2015, 28, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.D.; Soanes, K.H. A larval zebrafish model of bipolar disorder as a screening platform for neuro-therapeutics. Behav. Brain Res. 2012, 233, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Quist, T.S.; Stevenson, T.J.; Dahlem, T.J.; Bonkowsky, J.L. Rapid and efficient zebrafish genotyping using PCR with high-resolution melt analysis. JoVE J. Vis. Exp. 2014, 84, e51138. [Google Scholar] [CrossRef]
- UCSF Institutional Animal Care and Use Program. Fin Clipping of Zebrafish. Available online: https://iacuc.ucsf.edu/sites/g/files/tkssra751/f/wysiwyg/STD%20PROCEDURE%20-%20Aquatic%20-%20Fin%20Clipping%20of%20Zebrafish.pdf (accessed on 6 June 2022).
- Azevedo, A.S.; Grotek, B.; Jacinto, A.; Weidinger, G.; Saúde, L. The regenerative capacity of the zebrafish caudal fin is not affected by repeated amputations. PLoS ONE 2011, 6, e22820. [Google Scholar] [CrossRef] [PubMed]
- Assessment European Commission Expert Working Group on Severity. Expert Working Group on Severity Classification of Scientific Procedures Performed on Animals. Available online: https://ec.europa.eu/environment/chemicals/lab_animals/pdf/report_ewg.pdf (accessed on 3 June 2022).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Audira, G.; Suryanto, M.E.; Chen, K.H.-C.; Vasquez, R.D.; Roldan, M.J.M.; Yang, C.-C.; Hsiao, C.-D.; Huang, J.-C. Acute and Chronic Effects of Fin Amputation on Behavior Performance of Adult Zebrafish in 3D Locomotion Test Assessed with Fractal Dimension and Entropy Analyses and Their Relationship to Fin Regeneration. Biology 2022, 11, 969. https://doi.org/10.3390/biology11070969
Audira G, Suryanto ME, Chen KH-C, Vasquez RD, Roldan MJM, Yang C-C, Hsiao C-D, Huang J-C. Acute and Chronic Effects of Fin Amputation on Behavior Performance of Adult Zebrafish in 3D Locomotion Test Assessed with Fractal Dimension and Entropy Analyses and Their Relationship to Fin Regeneration. Biology. 2022; 11(7):969. https://doi.org/10.3390/biology11070969
Chicago/Turabian StyleAudira, Gilbert, Michael Edbert Suryanto, Kelvin H.-C. Chen, Ross D. Vasquez, Marri Jmelou M. Roldan, Chun-Chuen Yang, Chung-Der Hsiao, and Jong-Chin Huang. 2022. "Acute and Chronic Effects of Fin Amputation on Behavior Performance of Adult Zebrafish in 3D Locomotion Test Assessed with Fractal Dimension and Entropy Analyses and Their Relationship to Fin Regeneration" Biology 11, no. 7: 969. https://doi.org/10.3390/biology11070969
APA StyleAudira, G., Suryanto, M. E., Chen, K. H.-C., Vasquez, R. D., Roldan, M. J. M., Yang, C.-C., Hsiao, C.-D., & Huang, J.-C. (2022). Acute and Chronic Effects of Fin Amputation on Behavior Performance of Adult Zebrafish in 3D Locomotion Test Assessed with Fractal Dimension and Entropy Analyses and Their Relationship to Fin Regeneration. Biology, 11(7), 969. https://doi.org/10.3390/biology11070969









