Mercury-Induced Oxidative Stress Response in Benthic Foraminifera: An In Vivo Experiment on Amphistegina lessonii
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Individual Collection and Experimental Setup
2.2. Electrophoresis and Western Blotting
2.3. Antioxidant Activity Assays
2.3.1. Total Superoxide Dismutase Assay
2.3.2. Reduced Glutathione Assay
2.3.3. Glutathione-Related Enzymes Assays
2.4. Statistical Analysis
3. Results
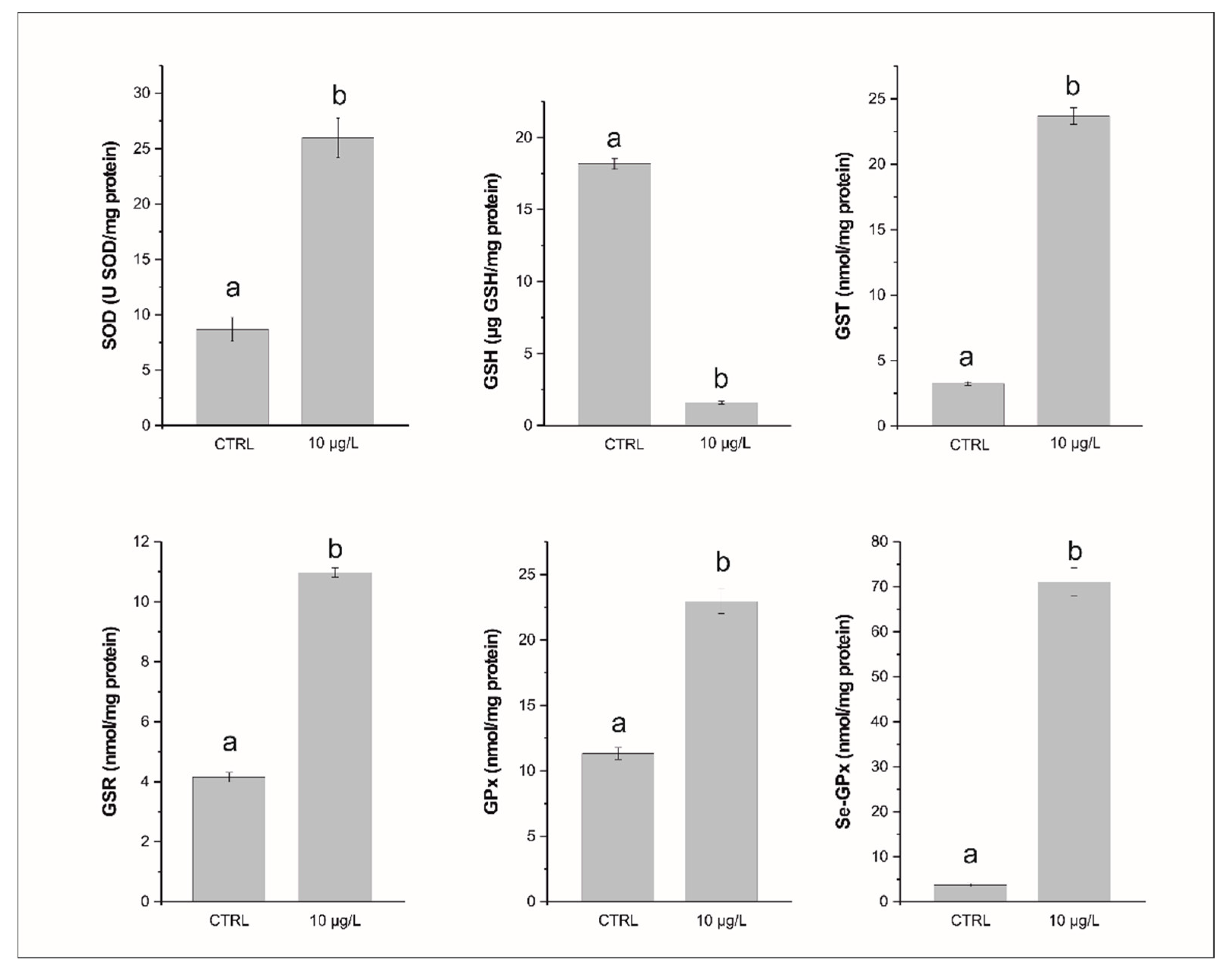
3.1. Glutathione and Enzymatic Biomarkers
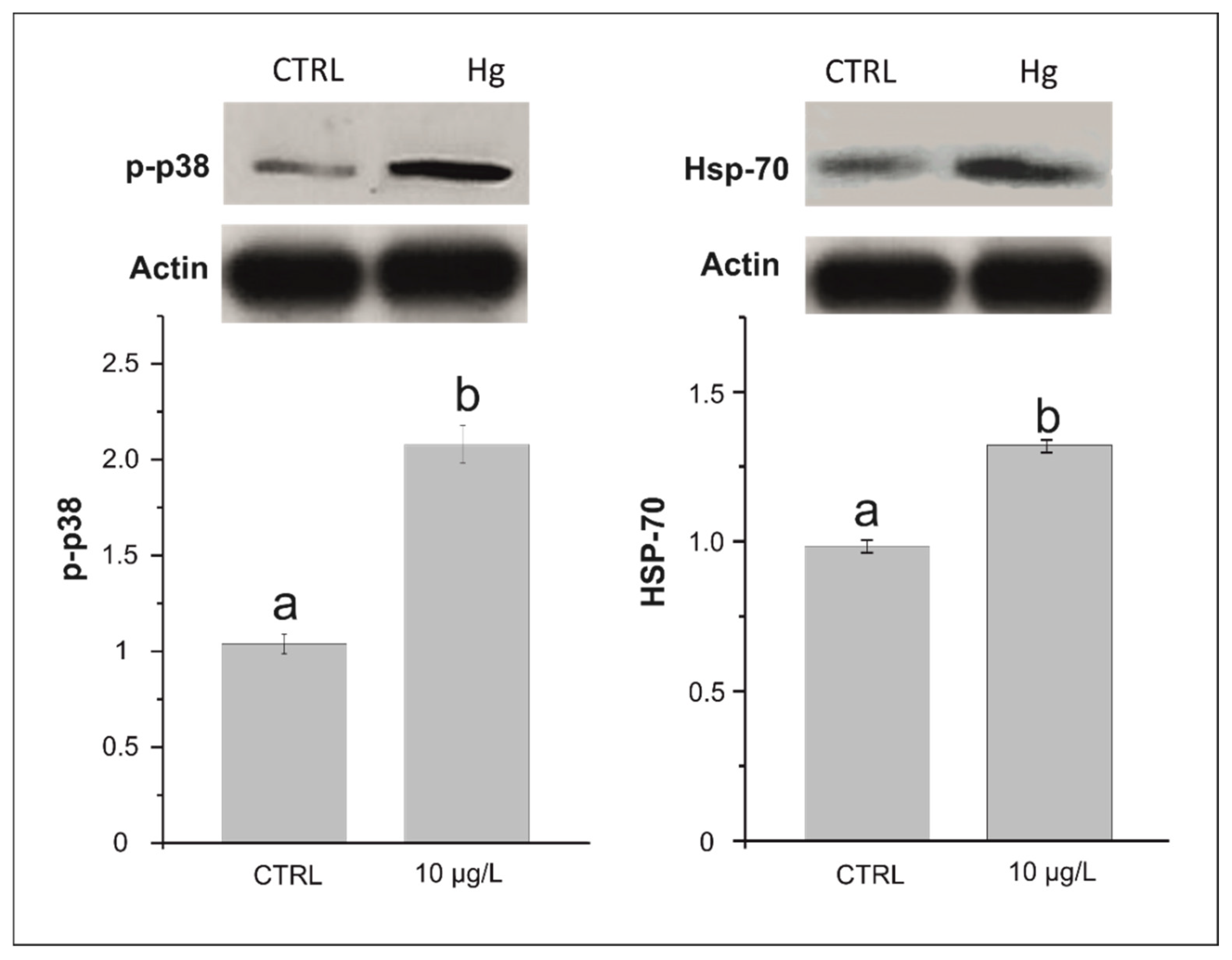
3.2. Effects on p38 MAPK Phosphorylation and HSP 70
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stankovic, S.M.; Kalaba, P.; Stankovic, A.R. Biota as toxic metal indicators. Environ. Chem. Lett. 2014, 12, 63–84. [Google Scholar] [CrossRef]
- Stankovic, S.; Stankovic, A.R. Bioindicators of toxic metals. In Green Materials for Energy, Products and Depollution, Environmental Chemistry for a Sustainable World; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 151–228. [Google Scholar] [CrossRef]
- Gu, B.; Bian, Y.; Miller, C.L.; Dong, W.; Jiang, X.; Liang, L. Mercury reduction and complexation by natural organic matter in anoxic environments. Proc. Natl. Acad. Sci. USA 2011, 108, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tsui, M.T.-K.; Lee, E.; Fowler, J.; Jia, Z. Uptake, efflux, and toxicity of inorganic and methyl mercury in the endothelial cells (EA.hy926). Sci. Rep. 2020, 10, 9023. [Google Scholar] [CrossRef] [PubMed]
- Markert, B.A.; Breure, A.M.; Zechmeister, H.G. Definitions, strategies and principles for bioindication/biomonitoring of the environment. In Trace Metals and other Contaminants in the Environment; Markert, B.A., Breure, A.M., Zechmeister, H.G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 3–39. [Google Scholar] [CrossRef]
- Volkova, P.; Geras’kin, S. ‘Omic’ technologies as a helpful tool in radioecological research. J. Environ. Radioact. 2018, 189, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Hagger, J.A.; Jones, M.B.; Leonard, D.R.P.; Owen, R.; Galloway, T.S. Biomarkers and integrated environmental risk assessment: Are there more questions than answers? Integr. Environ. Assess. Manag. 2006, 2, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Hook, S.E.; Gallagher, E.P.; Batley, G.E. The role of biomarkers in the assessment of aquatic ecosystem health: Biomarkers in the assessment of aquatic ecosystem health. Integr. Environ. Assess. Manag. 2014, 10, 327–341. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Varela, Z.; Franco, D.; Fernández, J.A.; Aboal, J.R. Can proteomics contribute to biomonitoring of aquatic pollution? A critical review. Environ. Poll. 2020, 267, 115473. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, D.R. Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Rev. Méd. Vét. 2003, 154, 427–430. [Google Scholar]
- Ganesan, K.; Sukalingam, K.; Xu, B. Solanum trilobatum L. Ameliorate thioacetamide-induced oxidative stress and hepatic damage in albino rats. Antioxidants 2017, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.E.; Madgwick, D.K.; Takemoto, D.J. Protein kinase C as a stress sensor. Cell. Signal. 2007, 19, 1820–1829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gopalakrishna, R.; Jaken, S. Protein kinase C signaling and oxidative stress. Free Radical Biol. Med. 2000, 28, 1349–1361. [Google Scholar] [CrossRef]
- Kim, B.M.; Rhee, J.S.; Jeong, C.B.; Seo, J.S.; Park, G.S.; Lee, Y.M.; Lee, J.S. Heavy metals induce oxidative stress and trigger oxidative stress-mediated heat shock protein (HSP) modulation in the intertidal copepod Tigriopus japonicus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 166, 65–74. [Google Scholar] [CrossRef]
- Trapp, J.; Armengaud, J.; Salvador, A.; Chaumot, A.; Geffard, O. Next-generation proteomics: Toward customized biomarkers for environmental biomonitoring. Environ. Sci. Technol. 2014, 48, 13560–13572. [Google Scholar] [CrossRef] [PubMed]
- Frontalini, F.; Coccioni, R. Benthic foraminifera as bioindicators of pollution: A review of Italian research over the last three decades. Rev. Micropaleontol. 2011, 54, 115–127. [Google Scholar] [CrossRef]
- Frontalini, F.; Curzi, D.; Giordano, F.M.; Bernhard, J.M.; Falcieri, E.; Coccioni, R. Effects of lead pollution on Ammonia parkinsoniana (foraminifera): Ultrastructural and microanalytical approaches. Eur. J. Histochem. 2015, 59, 2460. [Google Scholar] [CrossRef] [PubMed]
- Losada Ros, M.T.; Al-Enezi, E.; Cesarini, E.; Canonico, B.; Bucci, C.; Alves Martins, M.V.; Papa, S.; Frontalini, F. Assessing the cadmium effects on the benthic foraminifer ammonia cf. parkinsoniana: An acute toxicity test. Water 2020, 12, 1018. [Google Scholar] [CrossRef]
- Saraswat, R.; Kurtarkar, S.R.; Mazumder, A.; Nigam, R. Foraminifers as indicators of marine pollution: A culture experiment with Rosalina leei. Mar. Poll. Bull. 2004, 48, 91–96. [Google Scholar] [CrossRef]
- Marques, J.A.; Abrantes, D.P.; Marangoni, L.F.; Bianchini, A. Ecotoxicological responses of a reef calcifier exposed to copper, acidification and warming: A multiple biomarker approach. Environ. Poll. 2020, 257, 113572. [Google Scholar] [CrossRef]
- Marques, J.A.; de Barros Marangoni, L.F.; Bianchini, A. Combined effects of sea water acidification and copper exposure on the symbiont-bearing foraminifer Amphistegina gibbosa. Coral Reefs 2017, 36, 489–501. [Google Scholar] [CrossRef]
- Prazeres, M.; Martins, S.E.; Bianchini, A. Assessment of water quality in coastal waters of Fernando de Noronha, Brazil: Biomarker analyses in Amphistegina lessonii. J. Foraminifer. Res. 2012, 42, 56–65. [Google Scholar] [CrossRef]
- Prazeres, M.d.F.; Martins, S.E.; Bianchini, A. Biomarkers response to zinc exposure in the symbiont-bearing foraminifer Amphistegina lessonii (Amphisteginidae, Foraminifera). J. Exp. Mar. Biol. Ecol. 2011, 407, 116–121. [Google Scholar] [CrossRef]
- Betti, M.; Ciacci, C.; Abramovich, S.; Frontalini, F. Protein extractions from Amphistegina lessonii: Protocol development and optimization. Life 2021, 11, 418. [Google Scholar] [CrossRef]
- Heinz, P.; Marten, R.A.; Linshy, V.N.; Haap, T.; Geslin, E.; Köhler, H.R. 70 kD stress protein (Hsp70) analysis in living shallow-water benthic foraminifera. Mar. Biol. Res. 2012, 8, 677–681. [Google Scholar] [CrossRef][Green Version]
- Langer, M.R.; Mouanga, G.H. Invasion of amphisteginid foraminifera in the Adriatic Sea. Biol. Invas. 2016, 18, 1335–1349. [Google Scholar] [CrossRef]
- Weinmann, A.E.; Rödder, D.; Lötters, S.; Langer, M.R. Traveling through time: The past, present and future biogeographic range of the invasive foraminifera Amphistegina spp. in the Mediterranean Sea. Mar. Micropaleontol. 2013, 105, 30–39. [Google Scholar] [CrossRef]
- El Kateb, A.; Stalder, C.; Stainbank, S.; Fentimen, R.; Spezzaferri, S. The genus Amphistegina (benthic foraminifera): Distribution along the southern Tunisian coast. BioInvasions Rec. 2018, 7, 391–398. [Google Scholar] [CrossRef]
- Weinmann, A.E.; Rödder, D.; Lötters, S.; Langer, M.R. Heading for new shores: Projecting marine distribution ranges of selected larger foraminifera. PLoS ONE 2013, 8, e62. [Google Scholar] [CrossRef] [PubMed]
- Stulpinaite, R.; Hyams-Kaphzan, O.; Langer, M.R. Alien and cryptogenic foraminifera in the Mediterranean Sea: A revision of taxa as part of the EU 2020 Marine Strategy Framework Directive. Mediterr. Mar. Sci. 2020, 21, 719–758. [Google Scholar] [CrossRef]
- Hallock, P.; Williams, D.E.; Fisher, E.M.; Toler, S.K. Bleaching in foraminifera with algal symbionts: Implications for reef monitoring and risk asessment. Anuário Inst. Geociênc. 2006, 29, 108–128. [Google Scholar] [CrossRef]
- Hallock, P.; Lidz, B.H.; Cockey-Burkhard, E.M.; Donnelly, K.B. Foraminifera as bioindicators in coral reef assessment and monitoring: The FORAM Index. Environ. Monit. Assess. 2003, 81, 221–238. [Google Scholar] [CrossRef]
- Prazeres, M.; Martínez-Colón, M.; Hallock, P. Foraminifera as bioindicators of water quality: The FORAM Index revisited. Environ. Poll. 2020, 257, 113612. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eliahu, N.; Herut, B.; Rahav, E.; Abramovich, S. Shell growth of large benthic foraminifera under heavy metals pollution: Implications for geochemical monitoring of coastal environments. Int. J. Environ. Res. Public Health 2020, 17, 3741. [Google Scholar] [CrossRef] [PubMed]
- Frontalini, F.; Nardelli, M.P.; Curzi, D.; Martín-González, A.; Sabbatini, A.; Negri, A.; Losada, M.T.; Gobbi, P.; Coccioni, R.; Bernhard, J.M. Benthic foraminiferal ultrastructural alteration induced by heavy metals. Mar. Micropaleontol. 2018, 138, 83–89. [Google Scholar] [CrossRef]
- Bresler, V.; Yanko, V. Acute toxicity of heavy metals for benthic epiphytic foraminifera Pararotalia spinigera (le calvez) and influence of seaweed-derived doc. Environ. Toxicol. Chem. 1995, 14, 1687–1695. [Google Scholar] [CrossRef]
- Frontalini, F.; Curzi, D.; Cesarini, E.; Canonico, B.; Giordano, F.M.; De Matteis, R.; Bernhard, J.M.; Pieretti, N.; Gu, B.; Eskelsen, J.R.; et al. Mercury-pollution induction of intracellular lipid accumulation and lysosomal compartment amplification in the benthic foraminifer ammonia parkinsoniana. PLoS ONE 2016, 11, e0162401. [Google Scholar] [CrossRef] [PubMed]
- Nigam, R.; Linshy, V.N.; Kurtarkar, S.R.; Saraswat, R. Effects of sudden stress due to heavy metal mercury on benthic foraminifer Rosalina leei: Laboratory culture experiment. Mar. Poll. Bull. 2009, 59, 362–368. [Google Scholar] [CrossRef]
- US EPA. National Recommended Water Quality Criteria—Aquatic Life Criteria Table [WWW Document]. 2015. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table (accessed on 2 March 2022).
- Registration Dossier—European Chemicals Agency. [WWW Document]. 2022. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/5169/6/1 (accessed on 2 March 2022).
- Singaram, G.; Harikrishnan, T.; Chen, F.-Y.; Bo, J.; Giesy, J.P. Modulation of immune-associated parameters and antioxidant responses in the crab (Scylla serrata) exposed to mercury. Chemosphere 2013, 90, 917–928. [Google Scholar] [CrossRef]
- Sıkdokur, E.; Belivermiş, M.; Sezer, N.; Pekmez, M.; Bulan, Ö.K.; Kılıç, Ö. Effects of microplastics and mercury on manila clam Ruditapes philippinarum: Feeding rate, immunomodulation, histopathology and oxidative stress. Environ. Poll. 2020, 262, 114247. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Okado-Matsumoto, A.; Fridovich, I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J. Biol. Chem. 2001, 276, 38388–38393. [Google Scholar] [CrossRef] [PubMed]
- Akerboom, T.P.; Sies, H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981, 77, 373–382. [Google Scholar] [CrossRef]
- Canesi, L.; Viarengo, A. Age-related differences in glutathione metabolism in mussel tissue (Mytilus edulis L.). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 116B, 217–221. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, C.; Kandemir, F.M.; Darendelioğlu, E.; Yıldırım, S.; Kucukler, S.; Dortbudak, M.B. Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J. Trace Elem. Med. Biol. 2019, 56, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, M.; Usuki, F. Methylmercury-mediated oxidative stress and activation of the cellular protective system. Antioxidants 2020, 9, 1004. [Google Scholar] [CrossRef]
- Farina, M.; Rocha, J.B.T.; Aschner, M. Mechanisms of methylmercury-induced neurotoxicity: Evidence from experimental studies. Life Sci 2011, 89, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Tollefsen, K.E.; Song, Y.; Høgåsen, T.; Øverjordet, I.B.; Altin, D.; Hansen, B.H. Mortality and transcriptional effects of inorganic mercury in the marine copepod Calanus finmarchicus. J. Toxicol. Environ. Health A 2017, 80, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Quig, D. Cysteine metabolism and metal toxicity. Altern. Med. Rev. 1998, 3, 262–270. [Google Scholar] [PubMed]
- Sharaf, A.; De Michele, R.; Sharma, A.; Fakhari, S.; Oborník, M. Transcriptomic analysis reveals the roles of detoxification systems in response to mercury in Chromera velia. Biomolecules 2019, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, A.; Wei, Y.Y.; Meng, Q.; Zheng, Q.; Yang, Z.M. Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicology 2010, 19, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, M.; Shireen, K.F.; Atkinson, A.; Khan, A.T. Lipid peroxidation and antioxidant enzyme activity in different organs of mice exposed to low level of mercury. J. Environ. Sci. Health B 2001, 36, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Unsal, V. Natural phytotherapeutic antioxidants in the treatment of mercury intoxication—A review. Adv. Pharm. Bull. 2018, 8, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, H.; Yim, B.; Rhee, J.S.; Won, E.J.; Lee, Y.M. Identification and molecular characterization of two Cu/Zn-SODs and Mn-SOD in the marine ciliate Euplotes crassus: Modulation of enzyme activity and transcripts in response to copper and cadmium. Aquat. Toxicol. 2018, 199, 296–304. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Otto, D.M.E.; Moon, T.W. Phase I and II enzymes and antioxidant responses in different tissues of brown bullheads from relatively polluted and non-polluted systems. Arch. Environ. Contam. Toxicol. 1996, 31, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Oestreicher, J.; Morgan, B. Glutathione: Subcellular distribution and membrane transport 1. Biochem. Cell Biol. 2019, 97, 270–289. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Scholz, R.W.; Reddy, P.V.; Wynn, M.K.; Graham, K.S.; Liken, A.D.; Gumpricht, E.; Reddy, C.C. Glutathione-dependent factors and inhibition of rat liver microsomal lipid peroxidation. Free Radic. Biol. Med. 1997, 23, 815–828. [Google Scholar] [CrossRef]
- Dickinson, D.A.; Forman, H.J. Cellular glutathione and thiols metabolism. Biochem. Pharmacol. 2002, 64, 1019–1026. [Google Scholar] [CrossRef]
- Lund, B.O.; Miller, D.M.; Woods, J.S. Studies on Hg(II)-induced H2O2 formation and oxidative stress in vivo and in vitro in rat kidney mitochondria. Biochem. Pharmacol. 1993, 45, 2017–2024. [Google Scholar] [CrossRef]
- Miller, D.M.; Lund, B.O.; Woods, J.S. Reactivity of Hg(II) with superoxide: Evidence for the catalytic dismutation of superoxide by Hg(II). J. Biochem. Toxicol. 1991, 6, 293–298. [Google Scholar] [CrossRef]
- Benlaifa, M.; Djebar, M.-R.; Berredjem, H.; Benamara, M.; Ouali, K.; Djebar, H. Stress induced by cadmium: Its effects on growth respiratory metabolism, antioxidant enzymes and reactive oxygen species (ROS) of Paramecium sp. Int. J. Pharm. Sci. Rev. Res. 2016, 38, 276–281. [Google Scholar]
- Wallace, S.J.; Leclerc, A.J.A.; Prosser, R.; de Solla, S.R.; Balakrishnan, V.; Langlois, V.S. Sub-lethal effects of calcium dinonylnaphthalenesulfonate on Western clawed frog embryos. Comp. Biochem. Physiol. D Genom. Proteom. 2020, 34, 100658. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, C.Y.T.; Kong, A.N.T. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 2005, 28, 249–268. [Google Scholar] [CrossRef]
- Lee, K.-W.; Raisuddin, S.; Rhee, J.-S.; Hwang, D.-S.; Yu, I.T.; Lee, Y.-M.; Park, H.G.; Lee, J.-S. Expression of glutathione S-transferase (GST) genes in the marine copepod Tigriopus japonicus exposed to trace metals. Aquat. Toxicol. 2008, 89, 158–166. [Google Scholar] [CrossRef]
- Lee, J.S.; Kang, H.M.; Jeong, C.B.; Han, J.; Park, H.G.; Lee, J.S. Protective role of freshwater and marine rotifer glutathione S-transferase sigma and omega isoforms transformed into heavy metal-exposed Escherichia coli. Environ. Sci. Technol. 2019, 53, 7840–7850. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kang, H.M.; Kim, D.H.; Wang, M.; Jeong, C.B.; Lee, J.S. Adverse effects of methylmercury (MeHg) on life parameters, antioxidant systems, and MAPK signaling pathways in the copepod Tigriopus japonicus. Aquat. Toxicol. 2017, 184, 133–141. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, D.H.; Kang, H.M.; Wang, M.; Jeong, C.-B.; Lee, J.S. Adverse effects of methylmercury (MeHg) on life parameters, antioxidant systems, and MAPK signaling pathways in the rotifer Brachionus koreanus and the copepod Paracyclopina nana. Aquat. Toxicol. 2017, 190, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Velki, M.; Meyer-Alert, H.; Seiler, T.-B.; Hollert, H. Enzymatic activity and gene expression changes in zebrafish embryos and larvae exposed to pesticides diazinon and diuron. Aquat. Toxicol. 2017, 193, 187–200. [Google Scholar] [CrossRef]
- Kim, S.H.; Jung, M.Y.; Lee, Y.M. Effect of heavy metals on the antioxidant enzymes in the marine ciliate Euplotes crassus. Toxicol. Environ. Health Sci. 2011, 3, 213–219. [Google Scholar] [CrossRef]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family—An evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef]
- Passaia, G.; Margis-Pinheiro, M. Glutathione peroxidases as redox sensor proteins in plant cells. Plant Sci. 2015, 234, 22–26. [Google Scholar] [CrossRef]
- Leonard, S.S.; Harris, G.K.; Shi, X. Metal-induced oxidative stress and signal transduction. Free Radic. Biol. Med. 2004, 37, 1921–1942. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- González-Rubio, G.; Fernández-Acero, T.; Martín, H.; Molina, M. Mitogen-activated protein kinase phosphatases (MKPs) in fungal signaling: Conservation, function, and regulation. Int. J. Mol. Sci. 2019, 20, 1709. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Igisu, H. Effects of heavy metals on mitogen-activated protein kinase pathways. Environ. Health Prev. Med. 2002, 6, 210–217. [Google Scholar] [CrossRef]
- Pan, J.; Chang, Q.; Wang, X.; Son, Y.; Zhang, Z.; Chen, G.; Luo, J.; Bi, Y.; Chen, F.; Shi, X. Reactive oxygen species-activated Akt/ASK1/p38 signaling pathway in nickel compound-induced apoptosis in BEAS 2B cells. Chem. Res. Toxicol. 2010, 23, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Li, Z.; Sun, S.; Fang, Y.; Wei, Z. TGF-β1 alleviates HgCl2 induced apoptosis via p38 MAPK signaling pathway in human trophoblast cells. Toxicol. In Vitro 2019, 61, 104626. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Hao, W.; Wei, X.; Xing, L.; Jiang, J.; Shang, L. The role of MAPK in the biphasic dose-response phenomenon induced by cadmium and mercury in HEK293 cells. Toxicol. In Vitro 2009, 23, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Paula, M.T.; Zemolin, A.P.; Vargas, A.P.; Golombieski, R.M.; Loreto, E.L.S.; Saidelles, A.P.; Picoloto, R.S.; Flores, E.M.M.; Pereira, A.B.; Rocha, J.B.T.; et al. Effects of Hg(II) exposure on MAPK phosphorylation and antioxidant system in D. melanogaster. Environ. Toxicol. 2014, 29, 621–630. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, G.E.; Buckley, B.A.; Place, S.P.; Zippay, M.L. Molecular chaperones in ectothermic marine animals: Biochemical function and gene expression. Integr. Comp. Biol. 2002, 42, 808–814. [Google Scholar] [CrossRef]
- Kiang, J.G. Inducible heat shock protein 70 kD and inducible nitric oxide synthase in hemorrhage/resuscitation-induced injury. Cell Res. 2004, 14, 450–459. [Google Scholar] [CrossRef]
- Somasundaram, S.; Abraham, J.S.; Maurya, S.; Makhija, S.; Gupta, R.; Toteja, R. Cellular and molecular basis of heavy metal-induced stress in ciliates. Curr. Sci. 2018, 114, 1858–1865. [Google Scholar] [CrossRef]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. CMLS Cell. Mol. Life Sci. 2005, 62, 670. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Papaconstantinou, A.D.; Brown, K.M.; Noren, B.T.; McAlister, T.; Fisher, B.R.; Goering, P.L. Mercury, cadmium, and arsenite enhance heat shock protein synthesis in chick embryos prior to embryotoxicity. Birth Defects Res. B Dev. Reprod. Toxicol. 2003, 68, 456–464. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciacci, C.; Betti, M.; Abramovich, S.; Cavaliere, M.; Frontalini, F. Mercury-Induced Oxidative Stress Response in Benthic Foraminifera: An In Vivo Experiment on Amphistegina lessonii. Biology 2022, 11, 960. https://doi.org/10.3390/biology11070960
Ciacci C, Betti M, Abramovich S, Cavaliere M, Frontalini F. Mercury-Induced Oxidative Stress Response in Benthic Foraminifera: An In Vivo Experiment on Amphistegina lessonii. Biology. 2022; 11(7):960. https://doi.org/10.3390/biology11070960
Chicago/Turabian StyleCiacci, Caterina, Michele Betti, Sigal Abramovich, Marco Cavaliere, and Fabrizio Frontalini. 2022. "Mercury-Induced Oxidative Stress Response in Benthic Foraminifera: An In Vivo Experiment on Amphistegina lessonii" Biology 11, no. 7: 960. https://doi.org/10.3390/biology11070960
APA StyleCiacci, C., Betti, M., Abramovich, S., Cavaliere, M., & Frontalini, F. (2022). Mercury-Induced Oxidative Stress Response in Benthic Foraminifera: An In Vivo Experiment on Amphistegina lessonii. Biology, 11(7), 960. https://doi.org/10.3390/biology11070960







