Vitamin C Is Essential for the Maintenance of Skeletal Muscle Functions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
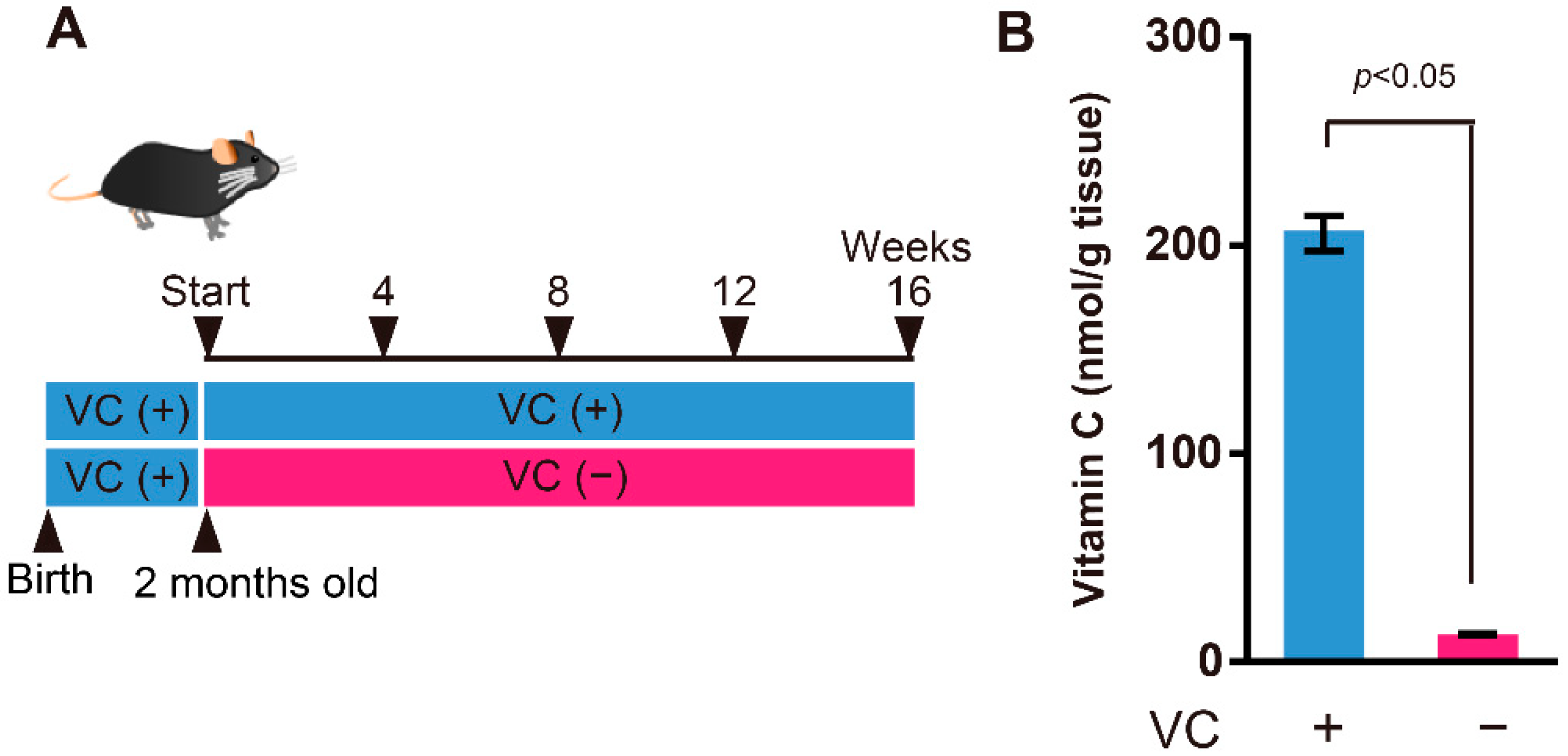
2.1. Animals
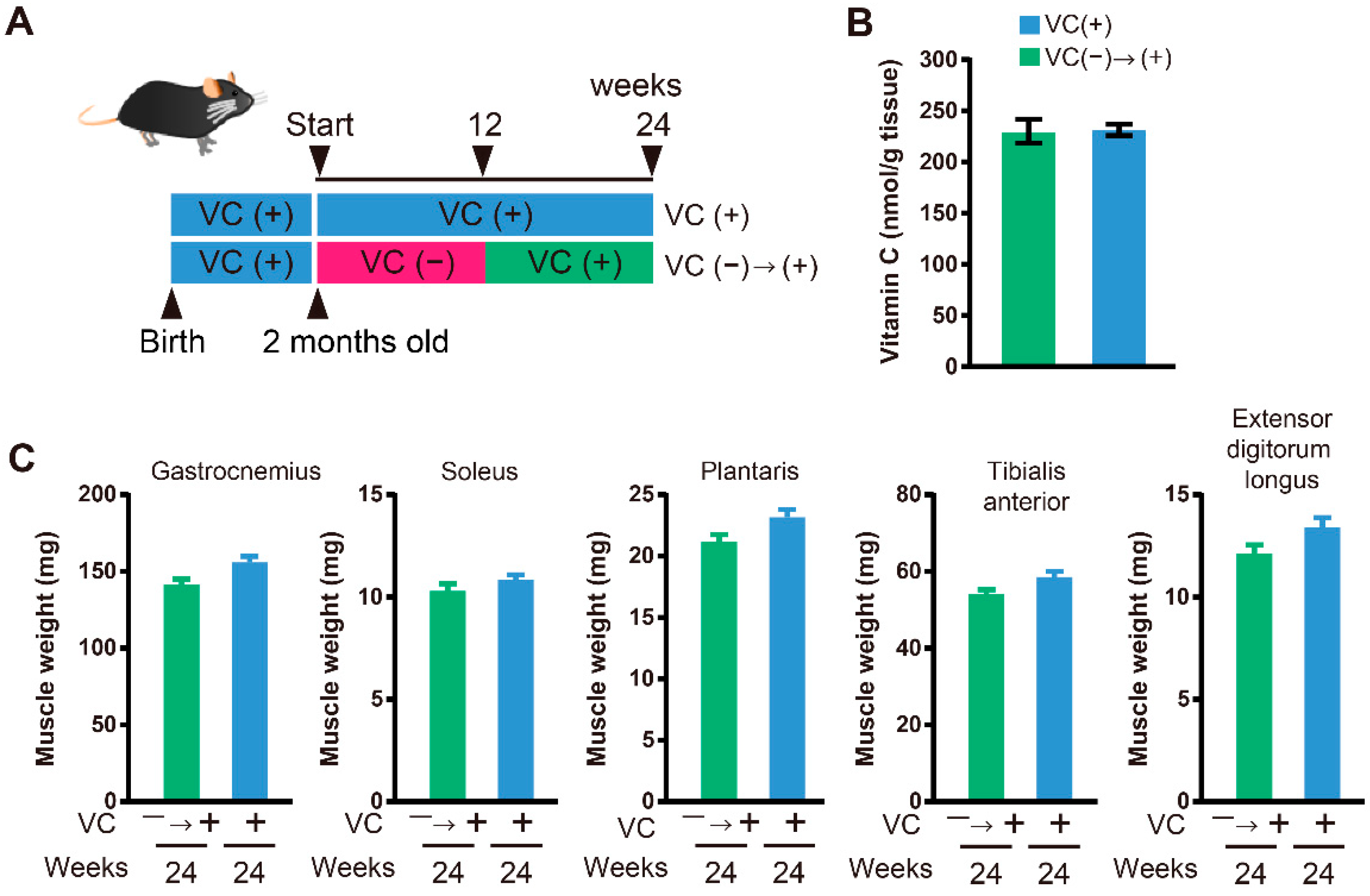
2.2. Measurement of VC Level
2.3. Behavioral Tests
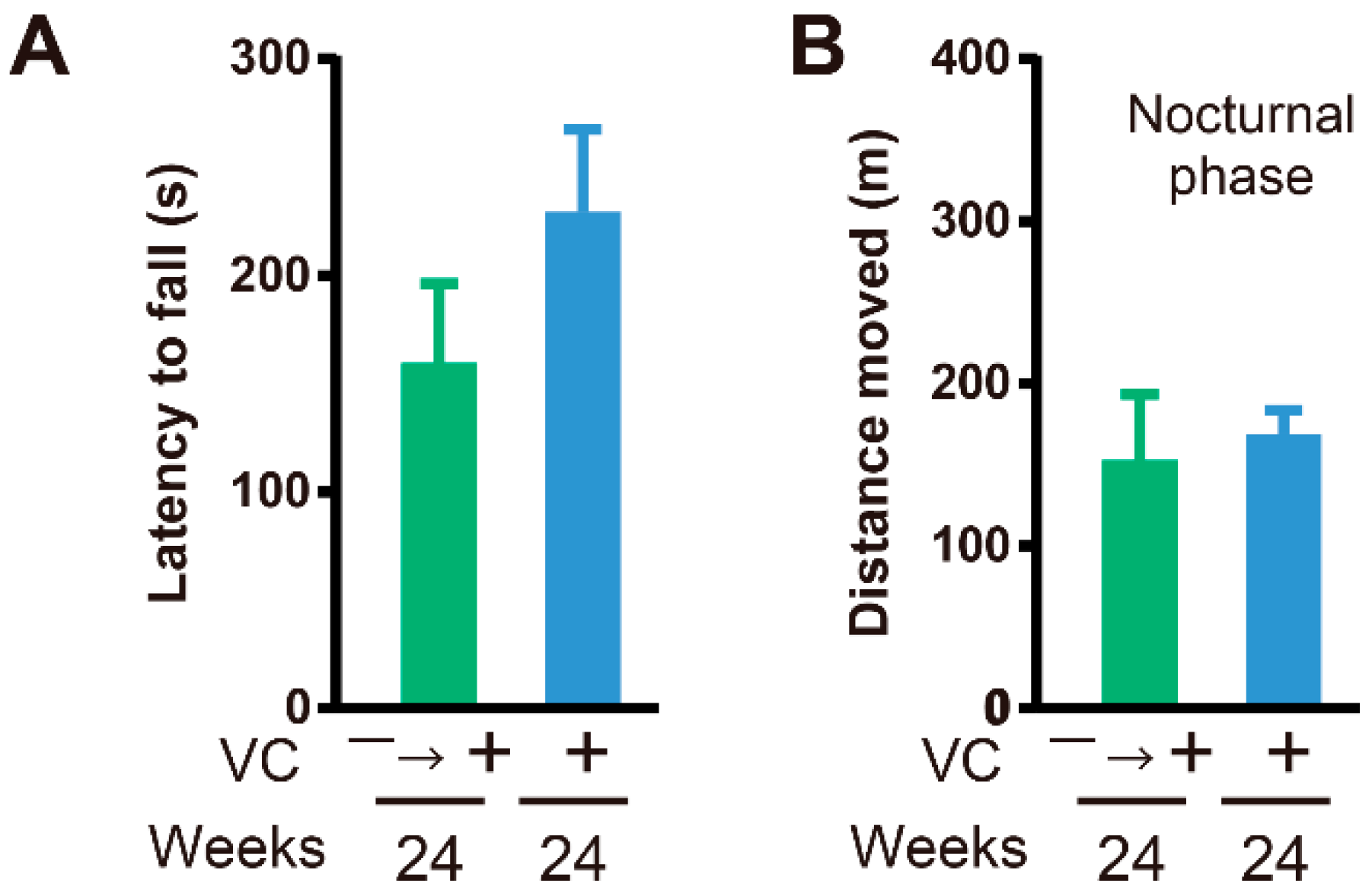
2.3.1. Physical Endurance
2.3.2. Grasping Strength
2.3.3. Activity in the Cage
2.4. Statistical Analysis
3. Results
3.1. VC Levels in the Gastrocnemius Muscles
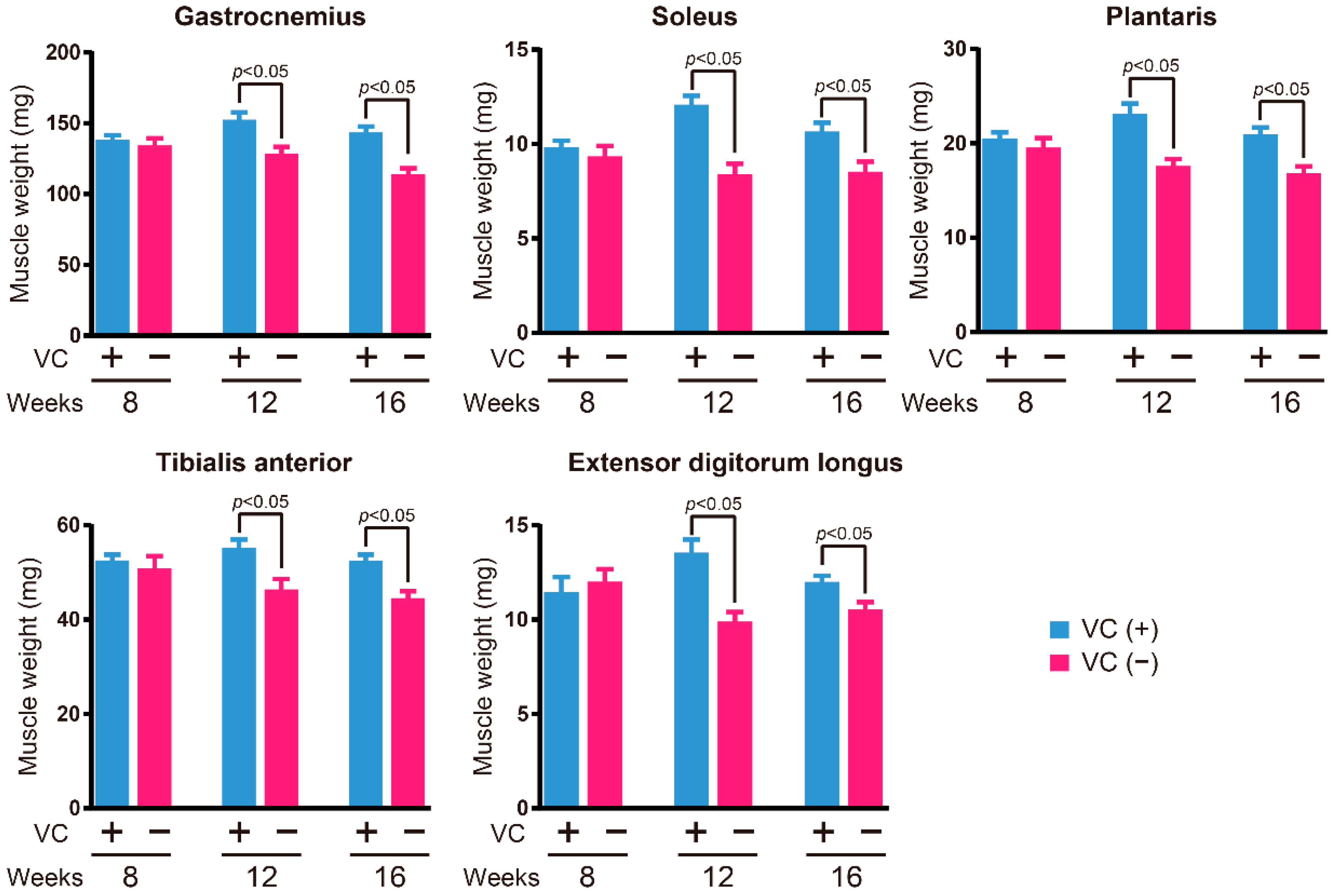
3.2. Skeletal Muscle Weight
3.3. Physical Ability
3.4. Functional Restoration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M. Oxidation of ascorbic acid with superoxide anion generated by the xanthine-xanthine oxidase system. Biochem. Biophys. Res. Commun. 1975, 63, 463–468. [Google Scholar] [CrossRef]
- Moritz, B.; Schmitz, A.E.; Rodrigues, A.L.S.; Dafre, A.L.; Cunha, M.P. The role of vitamin C in stress-related disorders. J. Nutr. Biochem. 2020, 85, 108459. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak-Baranska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Lane, D.J.; Lawen, A. Ascorbate and plasma membrane electron transport--enzymes vs efflux. Free Radic. Biol. Med. 2009, 47, 485–495. [Google Scholar] [CrossRef]
- Peterkofsky, B.; Udenfriend, S. Enzymatic Hydroxylation of Proline in Microsomal Polypeptide Leading to Formation of Collagen. Proc. Natl. Acad. Sci. USA 1965, 53, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Bei, R.; Mistretta, A.; Marventano, S.; Calabrese, G.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Galvano, F.; Gazzolo, D. Effects of vitamin C on health: A review of evidence. Front. Biosci. (Landmark Ed.) 2013, 18, 1017–1029. [Google Scholar] [CrossRef]
- Patak, P.; Willenberg, H.S.; Bornstein, S.R. Vitamin C is an important cofactor for both adrenal cortex and adrenal medulla. Endocr. Res. 2004, 30, 871–875. [Google Scholar] [CrossRef]
- Linster, C.L.; Van Schaftingen, E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007, 274, 1–22. [Google Scholar] [CrossRef]
- Pohanka, M.; Pejchal, J.; Snopkova, S.; Havlickova, K.; Karasova, J.Z.; Bostik, P.; Pikula, J. Ascorbic acid: An old player with a broad impact on body physiology including oxidative stress suppression and immunomodulation: A review. Mini Rev. Med. Chem. 2012, 12, 35–43. [Google Scholar] [CrossRef]
- Nishikimi, M.; Kawai, T.; Yagi, K. Guinea pigs possess a highly mutated gene for L-gulono-gamma-lactone oxidase, the key enzyme for L-ascorbic acid biosynthesis missing in this species. J. Biol. Chem. 1992, 267, 21967–21972. [Google Scholar] [CrossRef]
- Nishikimi, M.; Yagi, K. Molecular basis for the deficiency in humans of gulonolactone oxidase, a key enzyme for ascorbic acid biosynthesis. Am. J. Clin. Nutr. 1991, 54, 1203S–1208S. [Google Scholar] [CrossRef]
- Ishigami, A.; Fujita, T.; Handa, S.; Shirasawa, T.; Koseki, H.; Kitamura, T.; Enomoto, N.; Sato, N.; Shimosawa, T.; Maruyama, N. Senescence marker protein-30 knockout mouse liver is highly susceptible to tumor necrosis factor-alpha- and Fas-mediated apoptosis. Am. J. Pathol. 2002, 161, 1273–1281. [Google Scholar] [CrossRef]
- Kondo, Y.; Inai, Y.; Sato, Y.; Handa, S.; Kubo, S.; Shimokado, K.; Goto, S.; Nishikimi, M.; Maruyama, N.; Ishigami, A. Senescence marker protein 30 functions as gluconolactonase in L-ascorbic acid biosynthesis, and its knockout mice are prone to scurvy. Proc. Natl. Acad. Sci. USA 2006, 103, 5723–5728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M. Vitamin C: Effects of exercise and requirements with training. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 125–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef] [Green Version]
- Saito, K.; Yokoyama, T.; Yoshida, H.; Kim, H.; Shimada, H.; Yoshida, Y.; Iwasa, H.; Shimizu, Y.; Kondo, Y.; Handa, S.; et al. A significant relationship between plasma vitamin C concentration and physical performance among Japanese elderly women. J Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 295–301. [Google Scholar] [CrossRef]
- Martin, H.; Aihie Sayer, A.; Jameson, K.; Syddall, H.; Dennison, E.M.; Cooper, C.; Robinson, S. Does diet influence physical performance in community-dwelling older people? Findings from the Hertfordshire Cohort Study. Age Ageing 2011, 40, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.; Wang, Y.; Padayatty, S.J.; Morrow, J. A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl. Acad. Sci. USA 2001, 98, 9842–9846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, R.; Kurokawa, T.; Kobayasi, T.; Tero-Kubota, S. Influences of sex and age on serum ascorbic acid. Tohoku J. Exp. Med. 1983, 140, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, W.J. Vitamin E, vitamin C, and exercise. Am. J. Clin. Nutr. 2000, 72, 647S–652S. [Google Scholar] [CrossRef] [PubMed]
- Bryer, S.C.; Goldfarb, A.H. Effect of high dose vitamin C supplementation on muscle soreness, damage, function, and oxidative stress to eccentric exercise. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Vina, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takisawa, S.; Funakoshi, T.; Yatsu, T.; Nagata, K.; Aigaki, T.; Machida, S.; Ishigami, A. Vitamin C deficiency causes muscle atrophy and a deterioration in physical performance. Sci. Rep. 2019, 9, 4702. [Google Scholar] [CrossRef] [Green Version]
- Tiidus, P.M.; Bombardier, E.; Hidiroglou, N.; Madere, R. Gender and exercise influence on tissue antioxidant vitamin status in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 1999, 45, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Panda, A.K.; Ruth, R.P.; Padhi, S.N. Effect of age and sex on the ascorbic acid content of kidney, skeletal muscle and pancreas of common Indian toad, Bufo melanostictus. Exp. Gerontol. 1984, 19, 95–100. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, H.; Yan, J.; Wang, L.; Huang, Y.; Liu, X.; Williams, R.W.; Lu, L.; Wang, Y.; Gu, W. Genome-wide gene expression profiles in antioxidant pathways and their potential sex differences and connections to vitamin C in mice. Int. J. Mol. Sci. 2013, 14, 10042–10062. [Google Scholar] [CrossRef]
- Kondo, Y.; Sakuma, R.; Ichisawa, M.; Ishihara, K.; Kubo, M.; Handa, S.; Mugita, H.; Maruyama, N.; Koga, H.; Ishigami, A. Potato chip intake increases ascorbic acid levels and decreases reactive oxygen species in SMP30/GNL knockout mouse tissues. J. Agric. Food Chem. 2014, 62, 9286–9295. [Google Scholar] [CrossRef]
- Morrison, D.; Hughes, J.; Della Gatta, P.A.; Mason, S.; Lamon, S.; Russell, A.P.; Wadley, G.D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015, 89, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Tonon, E.; Ferretti, R.; Shiratori, J.H.; Santo Neto, H.; Marques, M.J.; Minatel, E. Ascorbic acid protects the diaphragm muscle against myonecrosis in mdx mice. Nutrition 2012, 28, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Duran, B.O.S.; Goes, G.A.; Zanella, B.T.T.; Freire, P.P.; Valente, J.S.; Salomao, R.A.S.; Fernandes, A.; Mareco, E.A.; Carvalho, R.F.; Dal-Pai-Silva, M. Ascorbic acid stimulates the in vitro myoblast proliferation and migration of pacu (Piaractus mesopotamicus). Sci. Rep. 2019, 9, 2229. [Google Scholar] [CrossRef] [PubMed]
- Diao, Z.; Matsui, T.; Funaba, M. Stimulation of myogenesis by ascorbic acid and capsaicin. Biochem. Biophys. Res. Commun. 2021, 568, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Lis, D.M.; Jordan, M.; Lipuma, T.; Smith, T.; Schaal, K.; Baar, K. Collagen and Vitamin C Supplementation Increases Lower Limb Rate of Force Development. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 65–73. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takisawa, S.; Takino, Y.; Lee, J.; Machida, S.; Ishigami, A. Vitamin C Is Essential for the Maintenance of Skeletal Muscle Functions. Biology 2022, 11, 955. https://doi.org/10.3390/biology11070955
Takisawa S, Takino Y, Lee J, Machida S, Ishigami A. Vitamin C Is Essential for the Maintenance of Skeletal Muscle Functions. Biology. 2022; 11(7):955. https://doi.org/10.3390/biology11070955
Chicago/Turabian StyleTakisawa, Shoko, Yuka Takino, Jaewon Lee, Shuichi Machida, and Akihito Ishigami. 2022. "Vitamin C Is Essential for the Maintenance of Skeletal Muscle Functions" Biology 11, no. 7: 955. https://doi.org/10.3390/biology11070955
APA StyleTakisawa, S., Takino, Y., Lee, J., Machida, S., & Ishigami, A. (2022). Vitamin C Is Essential for the Maintenance of Skeletal Muscle Functions. Biology, 11(7), 955. https://doi.org/10.3390/biology11070955





