Ferroptosis-Related Proteins Are Potential Diagnostic Molecular Markers for Patients with Preeclampsia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition and Processing of Gene Expression Data
2.2. Screening of the Differentially Expressed Genes (DEGs)
2.3. Analysis of Functional Enrichment and Pathway
2.4. Construction of the Protein–Protein Interaction Network
2.5. Biochemical Measurement of Blood Samples
2.6. Statistic
3. Results
3.1. Results
3.1.1. Clinical Features of the Participants
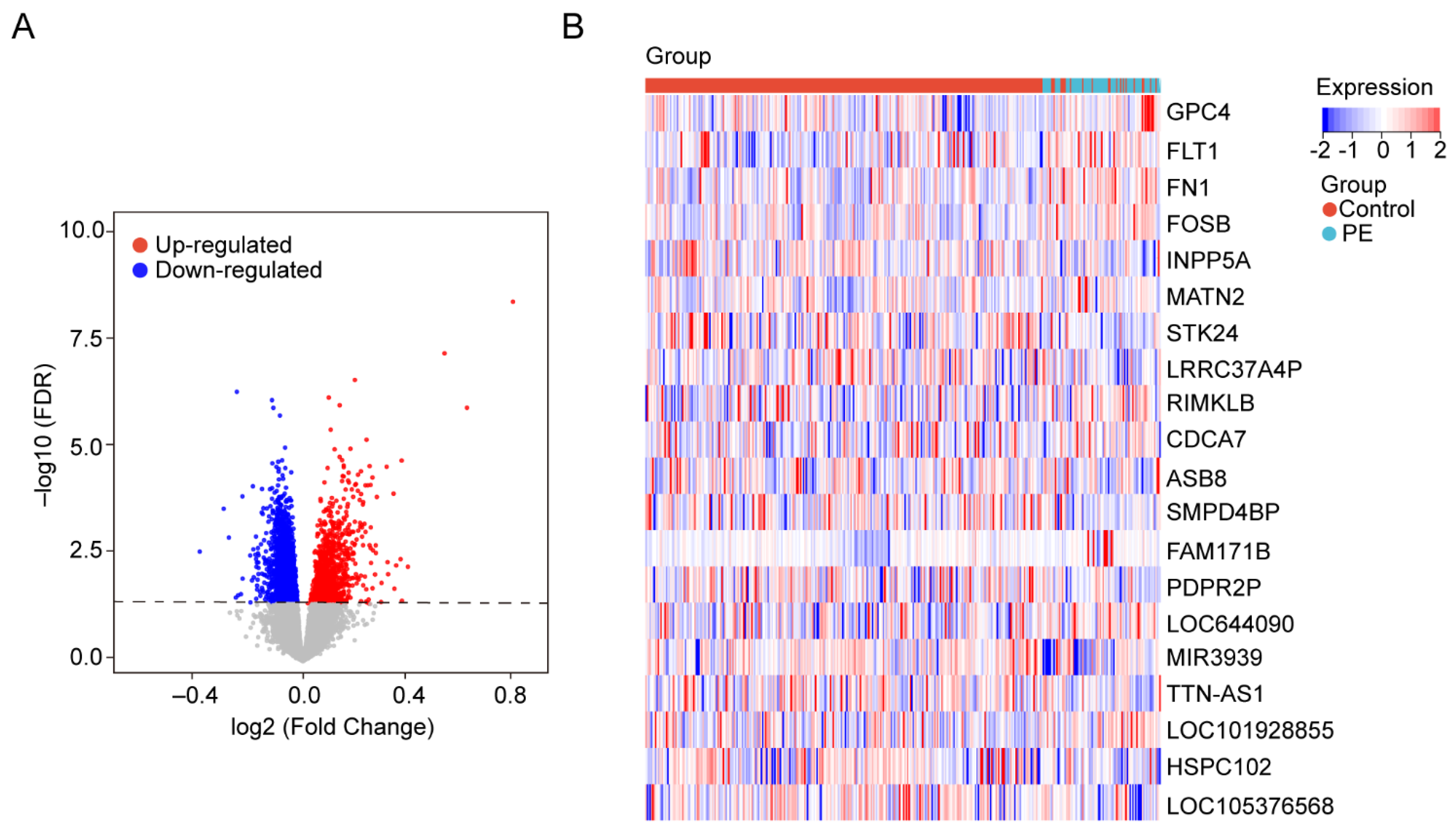
3.1.2. Identification of DEGs in Blood Samples
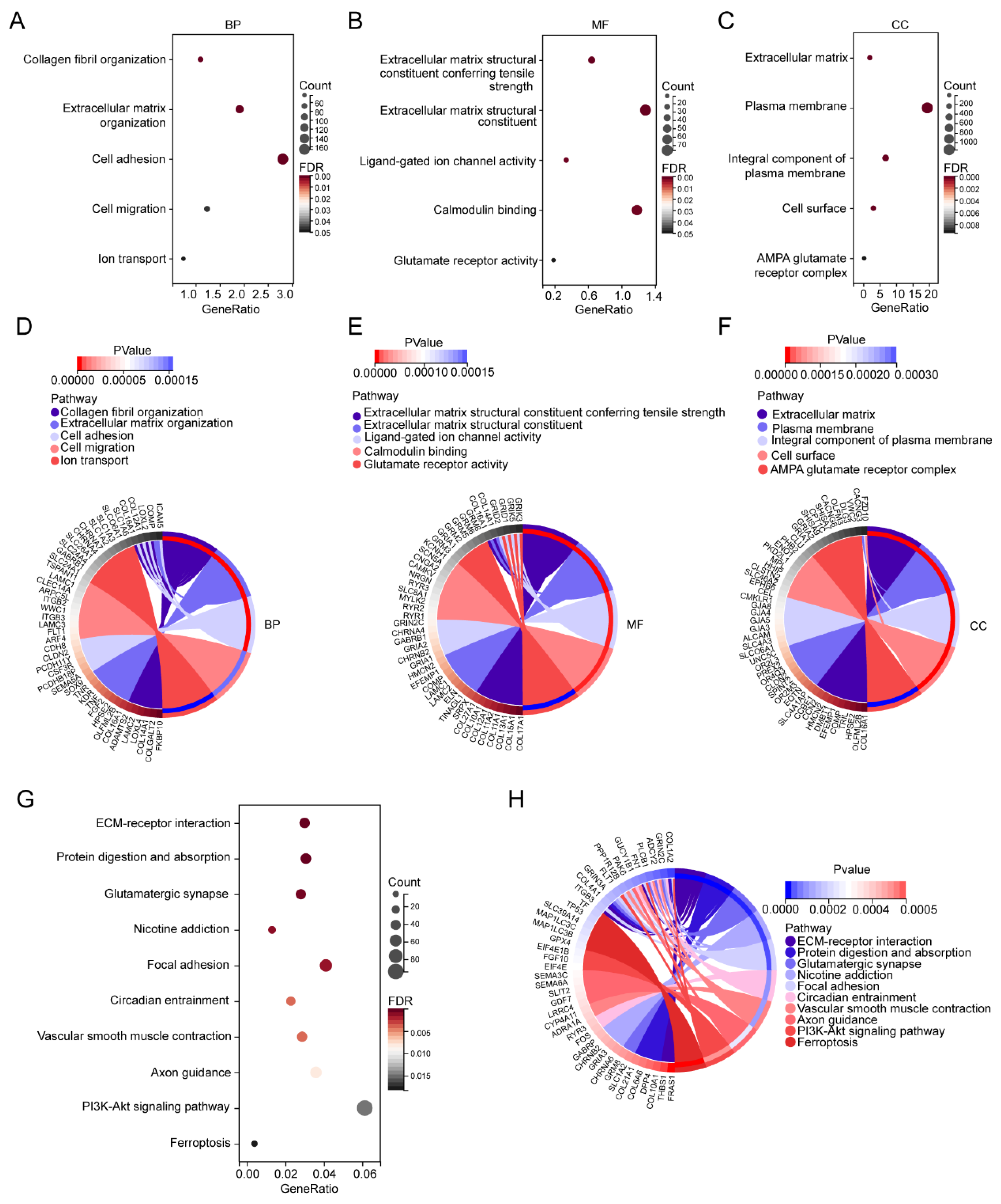
3.1.3. GO and KEGG Pathway Enrichment Analysis of the DEGs
3.1.4. Hub Gene Expression and Correlation with MAP
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cirkovic, A.; Garovic, V.; Milin Lazovic, J.; Milicevic, O.; Savic, M.; Rajovic, N.; Aleksic, N.; Weissgerber, T.; Stefanovic, A.; Stanisavljevic, D.; et al. Systematic review supports the role of DNA methylation in the pathophysiology of preeclampsia: A call for analytical and methodological standardization. Biol. Sex Differ. 2020, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, N.; Ikezaki, M.; Nishitsuji, K.; Yamamoto, M.; Matsuzaki, I.; Kato, N.; Takaoka, N.; Taniguchi, M.; Murata, S.-I.; Ino, K.; et al. Extracellularly Released Calreticulin Induced by Endoplasmic Reticulum Stress Impairs Syncytialization of Cytotrophoblast Model BeWo Cells. Cells 2021, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Kelemu, T.; Erlandsson, L.; Seifu, D.; Abebe, M.; Teklu, S.; Storry, J.R.; Hansson, S.R. Association of Maternal Regulatory Single Nucleotide Polymorphic CD99 Genotype with Preeclampsia in Pregnancies Carrying Male Fetuses in Ethiopian Women. Int. J. Mol. Sci. 2020, 21, 5837. [Google Scholar] [CrossRef]
- Zhang, J.; Klebanoff, M.A.; Roberts, J.M. Prediction of adverse outcomes by common definitions of hypertension in pregnancy. Obstet. Gynecol. 2001, 97, 261–267. [Google Scholar] [PubMed]
- Lawlor, D.A.; Macdonald-Wallis, C.; Fraser, A.; Nelson, S.M.; Hingorani, A.; Davey Smith, G.; Sattar, N.; Deanfield, J. Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: Findings from the Avon Longitudinal Study of Parents and Children. Eur. Heart J. 2012, 33, 335–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, C.; Park, S.; Seok, J.; Jang, H.Y.; Bang, Y.J.; Kim, G.I.J. Altered expression of ADM and ADM2 by hypoxia regulates migration of trophoblast and HLA-G expression. Biol. Reprod. 2021, 104, 159–169. [Google Scholar] [CrossRef]
- Ng, S.-W.; Norwitz, S.G.; Norwitz, E.R. The impact of iron overload and ferroptosis on reproductive disorders in humans: Implications for preeclampsia. Int. J. Mol. Sci. 2019, 20, 3283. [Google Scholar] [CrossRef] [Green Version]
- Bailey, L.J.; Alahari, S.; Tagliaferro, A.; Post, M.; Caniggia, I. Augmented trophoblast cell death in preeclampsia can proceed via ceramide-mediated necroptosis. Cell Death Dis. 2017, 8, e2590. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.; Wang, J.-X.; Chen, M.-H.; Xu, J.-J.; Jiang, M.-H.; Feng, Y.-L.; Gu, Y.-F. miR-30-5p-mediated ferroptosis of trophoblasts is implicated in the pathogenesis of preeclampsia. Redox Biol. 2020, 29, 101402. [Google Scholar] [CrossRef]
- Wang, P.; Cui, Y.; Ren, Q.; Yan, B.; Zhao, Y.; Yu, P.; Gao, G.; Shi, H.; Chang, S.; Chang, Y.-Z. Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death Dis. 2021, 12, 447. [Google Scholar] [CrossRef]
- Zhou, N.; Bao, J. FerrDb: A manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database 2020, 2020, baaa021. [Google Scholar] [CrossRef]
- Sui, S.; Zhang, J.; Xu, S.; Wang, Q.; Wang, P.; Pang, D. Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells. Cell Death Dis. 2019, 10, 331. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, W.K.; Bae, K.-H.; Lee, S.C.; Lee, E.-W.J.B. Lipid metabolism and ferroptosis. Biology 2021, 10, 184. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, S.-J.; Li, D.; Chu, B.; Gu, W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gu, W. p53 in ferroptosis regulation: The new weapon for the old guardian. Cell Death Differ. 2022, 29, 895–910. [Google Scholar] [CrossRef]
- Fang, K.; Du, S.; Shen, D.; Xiong, Z.; Jiang, K.; Liang, D.; Wang, J.; Xu, H.; Hu, L.; Zhai, X. SUFU Suppresses Ferroptosis Sensitivity in Breast Cancer Cells via Hippo/YAP Pathway. iScience 2022. [Google Scholar] [CrossRef]
- Ma, X.; Yan, W.; He, N. Lidocaine attenuates hypoxia/reoxygenation-induced inflammation, apoptosis and ferroptosis in lung epithelial cells by regulating the p38 MAPK pathway. Mol. Med. Rep. 2022, 25, 150. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Zou, N.; Chen, Q.; Wang, C.; Zhou, X.; Luo, L.; Qi, H.; Li, J.; Liu, Z.; et al. Lipocalin-2 silencing suppresses inflammation and oxidative stress of acute respiratory distress syndrome by ferroptosis via inhibition of MAPK/ERK pathway in neonatal mice. Bioengineered 2022, 13, 508–520. [Google Scholar] [CrossRef]
- Yang, N.; Wang, Q.; Ding, B.; Gong, Y.; Wu, Y.; Sun, J.; Wang, X.; Liu, L.; Zhang, F.; Du, D.; et al. Expression profiles and functions of ferroptosis-related genes in the placental tissue samples of early- and late-onset preeclampsia patients. BMC Pregnancy Childbirth 2022, 22, 87. [Google Scholar]
- El-Khalik, S.R.A.; Ibrahim, R.R.; Ghafar, M.T.A.; Shatat, D.; El-Deeb, O.S. Novel insights into the SLC7A11-mediated ferroptosis signaling pathways in preeclampsia patients: Identifying pannexin 1 and toll-like receptor 4 as innovative prospective diagnostic biomarkers. J. Assist. Reprod. Genet. 2022, 39, 1115–1124. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Y.; Lu, L.; Zhou, Y.; Wu, D.; Brännström, M.; Shao, L.R.; Billig, H. Overactivation of the androgen receptor exacerbates gravid uterine ferroptosis via interaction with and suppression of the NRF2 defense signaling pathway. FEBS Lett. 2022, 596, 806–825. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Q.; Wu, J. Adiponectin ameliorates placental injury in gestational diabetes mice by correcting fatty acid oxidation/peroxide imbalance-induced ferroptosis via restoration of CPT-1 activity. Endocrine 2022, 75, 781–793. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, D.; Liu, S.; Dong, Z.; Zhou, J.; Yin, Y.; Wan, D. Maternal iron supplementation during pregnancy affects placental function and iron status in offspring. J. Trace Elem. Med. Biol. 2022, 71, 126950. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, X.; Xie, F.; Zhang, L.; Yan, H.; Huang, J.; Zhang, C.; Zhou, F.; Chen, J.; Zhang, L. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun. 2022, 42, 88–116. [Google Scholar] [CrossRef]
- Cheng, Z.; Akatsuka, S.; Li, G.H.; Mori, K.; Takahashi, T.; Toyokuni, S. Ferroptosis resistance determines high susceptibility of murine A/J strain to iron-induced renal carcinogenesis. Cancer Sci. 2022, 113, 65–78. [Google Scholar] [CrossRef]
- Zhou, Q.; Ruan, D.J.M.H. SIRT1-autophagy axis may inhibit oxidative stress-induced ferroptosis in human nucleus pulposus cells. Med. Hypotheses 2022, 159, 110757. [Google Scholar] [CrossRef]
- Xu, Y.; Li, K.; Zhao, Y.; Zhou, L.; Liu, Y.; Zhao, J. Role of Ferroptosis in Stroke. Cell Mol. Neurobiol. 2022, 1–18. [Google Scholar] [CrossRef]
- Qu, W.; Cheng, Y.; Peng, W.; Wu, Y.; Rui, T.; Luo, C.; Zhang, J. Targeting iNOS Alleviates Early Brain Injury after Experimental Subarachnoid Hemorrhage via Promoting Ferroptosis of M1 Microglia and Reducing Neuroinflammation. Mol. Neurobiol. 2022, 59, 3124–3139. [Google Scholar] [CrossRef]
- Tian, H.; Xiong, Y.; Zhang, Y.; Leng, Y.; Tao, J.; Li, L.; Qiu, Z.; Xia, Z. Activation of NRF2/FPN1 pathway attenuates myocardial ischemia-reperfusion injury in diabetic rats by regulating iron homeostasis and ferroptosis. Cell Stress Chaperones 2021, 27, 149–164. [Google Scholar] [CrossRef]
- Beharier, O.; Kajiwara, K.; Sadovsky, Y. Ferroptosis, trophoblast lipotoxic damage, and adverse pregnancy outcome. Placenta 2021, 108, 32–38. [Google Scholar] [CrossRef]
- Ruiz-de-Angulo, A.; Bilbao-Asensio, M.; Cronin, J.; Evans, S.J.; Clift, M.J.D.; Llop, J.; Feiner, I.V.J.; Beadman, R.; Bascarán, K.Z.; Mareque-Rivas, J.C. Chemically Programmed Vaccines: Iron Catalysis in Nanoparticles Enhances Combination Immunotherapy and Immunotherapy-Promoted Tumor Ferroptosis. iScience 2020, 23, 101499. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.-L.; Huang, Z.-J.; Lin, Z.-T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Liao, T.; Xu, X.; Ye, X.; Yan, J. DJ-1 upregulates the Nrf2/GPX4 signal pathway to inhibit trophoblast ferroptosis in the pathogenesis of preeclampsia. Sci. Rep. 2022, 12, 2934. [Google Scholar] [CrossRef]
- Tian, P.; Xu, Z.; Guo, J.; Zhao, J.; Wang, R.; Chen, W.; Yang, Y.; Huang, W.; Mi, C.; Zhang, H. BPDE induces human trophoblast cell ferroptosis by up-regulating iron metabolism and promoting GPX4 proteasomal degradation. Ecotoxicol. Environ. Saf. 2021, 228, 113028. [Google Scholar] [CrossRef]
- Eaves, L.A.; Nguyen, H.T.; Rager, J.E.; Sexton, K.G.; Howard, T.; Smeester, L.; Freedman, A.N.; Aagaard, K.M.; Shope, C.; Lefer, B.; et al. Identifying the Transcriptional Response of Cancer and Inflammation-Related Genes in Lung Cells in Relation to Ambient Air Chemical Mixtures in Houston, Texas. Environ. Sci. Technol. 2020, 54, 13807–13816. [Google Scholar] [CrossRef]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.-J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Gnanapradeepan, K.; Basu, S.; Barnoud, T.; Budina-Kolomets, A.; Kung, C.-P.; Murphy, M.E. The p53 Tumor Suppressor in the Control of Metabolism and Ferroptosis. Front. Endocrinol. 2018, 9, 124. [Google Scholar] [CrossRef] [Green Version]
- Kang, R.; Kroemer, G.; Tang, D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic. Biol. Med. 2019, 133, 162–168. [Google Scholar] [CrossRef]
- Sharp, A.N.; Heazell, A.E.P.; Baczyk, D.; Dunk, C.E.; Lacey, H.A.; Jones, C.J.P.; Perkins, J.E.; Kingdom, J.C.P.; Baker, P.N.; Crocker, I.P. Preeclampsia is associated with alterations in the p53-pathway in villous trophoblast. PLoS ONE 2014, 9, e87621. [Google Scholar] [CrossRef]
- Zhao, J.; Peng, W.; Ran, Y.; Ge, H.; Zhang, C.; Zou, H.; Ding, Y.; Qi, H. Dysregulated expression of ACTN4 contributes to endothelial cell injury via the activation of the p38-MAPK/p53 apoptosis pathway in preeclampsia. J. Physiol. Biochem. 2019, 75, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Zhuang, C.; Jiao, Y.; Yang, L. The possible involvement of circRNA DMNT1/p53/JAK/STAT in gestational diabetes mellitus and preeclampsia. Cell Death Discov. 2022, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Romero, R.; Yeo, L.; Gomez-Lopez, N.; Chaemsaithong, P.; Jaovisidha, A.; Gotsch, F.; Erez, O. Gynecology, The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S844–S866. [Google Scholar] [CrossRef] [PubMed]
- Lukey, M.J.; Greene, K.S.; Erickson, J.W.; Wilson, K.F.; Cerione, R.A. The oncogenic transcription factor c-Jun regulates glutaminase expression and sensitizes cells to glutaminase-targeted therapy. Nat. Commun. 2016, 7, 11321. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.H.; Zheng, Y.; Tang, L.J.; Zhou, S.T.; Zhu, J.N. SHARPIN regulates cell proliferation of cutaneous basal cell carcinoma via inactivation of the transcriptional factors GLI2 and c-JUN. Mol. Med. Rep. 2020, 21, 1799–1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappelmann, M.; Bosserhoff, A.; Kuphal, S. AP-1/c-Jun transcription factors: Regulation and function in malignant melanoma. Eur. J. Cell Biol. 2014, 93, 76–81. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, G.; Liu, Y.; Wu, Q.; Zhang, X.; Bian, Z.; Zhang, Y.; Pan, Q.; Sun, F. O-GlcNAcylated c-Jun antagonizes ferroptosis via inhibiting GSH synthesis in liver cancer. Cell Signal. 2019, 63, 109384. [Google Scholar] [CrossRef]
- Gao, D.; Huang, Y.; Sun, X.; Yang, J.; Chen, J.; He, J.J.J.o.C.; Medicine, M. Overexpression of c-Jun inhibits erastin-induced ferroptosis in Schwann cells and promotes repair of facial nerve function. J. Cell. Mol. Med. 2022, 26, 2191–2204. [Google Scholar] [CrossRef]
- Yu, Y.; Xie, Y.; Cao, L.; Yang, L.; Yang, M.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol. Cell. Oncol. 2015, 2, e1054549. [Google Scholar] [CrossRef] [Green Version]



| Variable | Control Group (n = 60) | PE Group (n = 60) | p Value |
|---|---|---|---|
| Maternal age, y | 29 (26–33) | 29 (27–33) | 0.819 |
| Body mass index, kg/m2 | 21 (19–22) | 22 (20–25) | 0.006 |
| Parity, n (%) | 0.355 | ||
| Primipara | 32 (53) | 38 (63) | |
| Multipara | 28 (47) | 22 (37) | |
| History of the PE, n (%) | 3 (5) | 4 (7) | 1.000 |
| Chronic hypertension, n (%) | 0 (0) | 2 (3) | 0.496 |
| PGDM, n (%) | 2 (3) | 2 (3) | 1.000 |
| Antiphospholipid syndrome, n (%) | 1 (2) | 2 (3) | 1.000 |
| Preexisting kidney disease, n (%) | 0 (0) | 2 (3) | 0.496 |
| Familiar risk, n (%) | 1 (2) | 3 (5) | 0.619 |
| Systolic pressure, mmHg | 117 (112–126) | 161 (146−170) | <0.001 |
| Diastolic pressure, mmHg | 75 (71–79) | 98 (92–114) | <0.001 |
| MAP, mmHg | 89 (86–93) | 119 (111–132) | <0.001 |
| 24-h proteinuria, g/24 h | 0.05 (0.04–0.12) | 1.33 (0.64–3.43) | <0.001 |
| Gestational age at birth, d | 266 (255–276) | 259 (244–267) | <0.001 |
| Fetal birth weight, g | 3194 (2949–3275) | 2900 (2200–3200) | 0.002 |
| RDS, n (%) | 1 (2) | 16 (27) | <0.001 |
| NICU, n (%) | 1 (2) | 16 (27) | <0.001 |
| Gene Symbol | Log2FC | p Value |
|---|---|---|
| MIR3939 | 0.681836871 | 0.0000000065 |
| LOC105376568 | 0.459679751 | 0.0000000968 |
| TTN-AS1 | 0.168772851 | 0.0000003906 |
| STK24 | 0.084107410 | 0.0000009831 |
| INPP5A | 0.119524431 | 0.0000014759 |
| HSPC102 | 0.532569188 | 0.0000016817 |
| SMPD4BP | 0.090404584 | 0.0000053122 |
| LRRC37A4P | 0.206650798 | 0.0000090054 |
| PDPR2P | 0.154363661 | 0.0000144329 |
| ASB8 | 0.103036862 | 0.0000148492 |
| GLYR1 | 0.119425589 | 0.0000221570 |
| SLC25A6P2 | 0.127278093 | 0.0000262861 |
| ERV3-1 | 0.320543342 | 0.0000268127 |
| TOR1A | 0.132429450 | 0.0000346933 |
| WHAMMP1 | 0.216623800 | 0.0000359022 |
| LRRC37A17P | 0.271869224 | 0.0000371765 |
| DHDDS-AS1 | 0.132565620 | 0.0000379569 |
| MAX | 0.080250037 | 0.0000396618 |
| SELP | 0.188509826 | 0.0000471182 |
| RPS2P7 | 0.148369736 | 0.0000508025 |
| NQO2 | 0.178847959 | 0.0000565570 |
| FAM153A | 0.129231134 | 0.0000603660 |
| TNFSF14 | 0.132533146 | 0.0000616615 |
| PDPR | 0.225867791 | 0.0000652686 |
| SLC4A10 | 0.146662353 | 0.0000768642 |
| TAB3 | 0.127250551 | 0.0000785952 |
| COLQ | 0.070045308 | 0.0000844558 |
| PDLIM1 | 0.149533133 | 0.0000849043 |
| OR6K3 | 0.172019882 | 0.0000919229 |
| NFE4 | 0.219922283 | 0.0000964147 |
| Gene Symbol | Log2FC | p Value |
|---|---|---|
| FAM171B | −0.214300119 | 0.0000007277 |
| FOSB | −0.100125939 | 0.0000011258 |
| FLT1 | −0.095896809 | 0.0000016907 |
| FN1 | −0.074430719 | 0.0000025301 |
| RIMKLB | −0.057612207 | 0.0000136380 |
| MATN2 | −0.067397889 | 0.0000264791 |
| GPC4 | −0.079923918 | 0.0000285482 |
| LOC101928855 | −0.099017279 | 0.0000309617 |
| LOC644090 | −0.085740243 | 0.0000373470 |
| CDCA7 | −0.056959849 | 0.0000398187 |
| LOC101059954 | −0.078430951 | 0.0000413221 |
| LINC02487 | −0.078594043 | 0.0000481066 |
| ELOC | −0.037768067 | 0.0000496455 |
| NHLH2 | −0.066026726 | 0.0000582698 |
| LOC100506489 | −0.064018314 | 0.0000697048 |
| LOC107986383 | −0.077890143 | 0.0000722787 |
| TUBB6 | −0.080615791 | 0.0000987167 |
| PRG1 | −0.162206377 | 0.0001033506 |
| LINC00309 | −0.054808819 | 0.0001114693 |
| TCN2 | −0.099952456 | 0.0001141195 |
| LOC112267901 | −0.132601517 | 0.0001190145 |
| SERINC2 | −0.106660347 | 0.0001205236 |
| IER5L-AS1 | −0.084976169 | 0.0001325151 |
| LOC284912 | −0.091842494 | 0.0001444731 |
| CHRNB3 | −0.058923918 | 0.0001519665 |
| NR4A2 | −0.063301192 | 0.0001692061 |
| YEATS4 | −0.196128294 | 0.0001758740 |
| LINC02953 | −0.069851232 | 0.0001776020 |
| LOC101928517 | −0.077337704 | 0.0001810568 |
| SNORD114-7 | −0.075879892 | 0.0001837738 |
| Gene Symbol | Log2FC | p Value |
|---|---|---|
| CS | 0.088024037 | 0.0013860456 |
| GPX4 | 0.127606332 | 0.0033606329 |
| DPP4 | 0.115610006 | 0.0041819650 |
| ANO6 | 0.092445408 | 0.0056013748 |
| NCF2 | 0.102692749 | 0.0106923591 |
| OXSR1 | 0.073276793 | 0.0132039673 |
| RPL8 | 0.070519563 | 0.0163624365 |
| EIF2AK4 | 0.051333877 | 0.0219376884 |
| TP53 | 0.061276552 | 0.0227802882 |
| ALOX12 | 0.090642503 | 0.0238840276 |
| G6PD | 0.06437918 | 0.0245358858 |
| ELAVL1 | 0.047106006 | 0.0257012638 |
| BECN1 | 0.054079622 | 0.0360843333 |
| SLC7A5 | 0.141674054 | 0.0444067288 |
| KLHL24 | 0.05860919 | 0.0484929326 |
| SLC1A4 | −0.046713571 | 0.0025230647 |
| CDO1 | −0.042790266 | 0.0021886411 |
| ALOXE3 | −0.052995436 | 0.0044745713 |
| ANGPTL7 | −0.053769764 | 0.0069292813 |
| JUN | −0.049405558 | 0.0081302033 |
| SOCS1 | −0.087858572 | 0.0098413330 |
| NOX4 | −0.027259999 | 0.0153337346 |
| NOS2 | −0.039584599 | 0.0194259690 |
| CDKN2A | −0.033970203 | 0.0216855291 |
| TSC22D3 | −0.044797939 | 0.0219355680 |
| MUC1 | −0.045560811 | 0.0225179167 |
| AURKA | −0.051276727 | 0.0233380462 |
| CHAC1 | −0.050305866 | 0.0235809423 |
| NQO1 | −0.036099395 | 0.0257095124 |
| TP63 | −0.026632072 | 0.0308774916 |
| GPT2 | −0.043220744 | 0.0311273224 |
| AIFM2 | −0.036737201 | 0.0329521820 |
| MT3 | −0.04429197 | 0.0335564152 |
| ACSF2 | −0.028855554 | 0.0337071772 |
| EGFR | −0.032015545 | 0.0353011283 |
| GDF15 | −0.0688667 | 0.0360222189 |
| ALOX12B | −0.04162569 | 0.0379310322 |
| MIOX | −0.039351283 | 0.0409310117 |
| NNMT | −0.044199194 | 0.0411587687 |
| TF | −0.027938216 | 0.0417271472 |
| CEBPG | −0.039507462 | 0.0428706382 |
| RRM2 | −0.061984651 | 0.0429017623 |
| AKR1C1 | −0.044489901 | 0.0430243813 |
| PROM2 | −0.037538817 | 0.0459449958 |
| DDIT4 | −0.044721922 | 0.0483266707 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Yang, X.; Ding, Y.; Sun, L.; Zhang, P.; Liu, M.; Han, X.; Huang, Z.; Li, R. Ferroptosis-Related Proteins Are Potential Diagnostic Molecular Markers for Patients with Preeclampsia. Biology 2022, 11, 950. https://doi.org/10.3390/biology11070950
Shi M, Yang X, Ding Y, Sun L, Zhang P, Liu M, Han X, Huang Z, Li R. Ferroptosis-Related Proteins Are Potential Diagnostic Molecular Markers for Patients with Preeclampsia. Biology. 2022; 11(7):950. https://doi.org/10.3390/biology11070950
Chicago/Turabian StyleShi, Meiting, Xiaofeng Yang, Yuzhen Ding, Lu Sun, Ping Zhang, Mengyuan Liu, Xiaoxue Han, Zhengrui Huang, and Ruiman Li. 2022. "Ferroptosis-Related Proteins Are Potential Diagnostic Molecular Markers for Patients with Preeclampsia" Biology 11, no. 7: 950. https://doi.org/10.3390/biology11070950
APA StyleShi, M., Yang, X., Ding, Y., Sun, L., Zhang, P., Liu, M., Han, X., Huang, Z., & Li, R. (2022). Ferroptosis-Related Proteins Are Potential Diagnostic Molecular Markers for Patients with Preeclampsia. Biology, 11(7), 950. https://doi.org/10.3390/biology11070950






