Feasibility of Ultrasound Attenuation Imaging for Assessing Pediatric Hepatic Steatosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. MRI Acquisition
2.3. ATI Acquisition
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Results of the Ordinal Logistic Regression Test for the Fatty Liver Groups
3.3. Results of the Linear Regression Test for ATI Values
3.4. Diagnostic Performance of ATI Values
3.5. Factors Related to Measurement Failure with ATI in Pediatric Participants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Schwimmer, J.B.; Sundaram, S.S.; et al. Naspghan clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: Recommendations from the expert committee on nafld (econ) and the north american society of pediatric gastroenterology, hepatology and nutrition (naspghan). J. Pediatric Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar]
- Nobili, V.; Mantovani, A.; Cianfarani, S.; Alisi, A.; Mosca, A.; Sartorelli, M.R.; Maffeis, C.; Loomba, R.; Byrne, C.D.; Targher, G. Prevalence of prediabetes and diabetes in children and adolescents with biopsy-proven non-alcoholic fatty liver disease. J. Hepatol. 2019, 71, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Park, G.; Lee, H.S.; Lee, M.; Lee, H.I.; Choi, H.S.; Suh, J.; Kwon, A.; Kim, H.S.; Chae, H.W. Comparison of the triglyceride glucose index and modified triglyceride glucose indices to predict nonalcoholic fatty liver disease in youths. J. Pediatrics 2022, 242, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Mustafa, G.; Alam, M.; Ahmad, N. Insulin resistance in development and progression of nonalcoholic fatty liver disease. World J. Gastrointest. Pathophysiol. 2016, 7, 211–217. [Google Scholar] [CrossRef]
- Song, K.; Lee, H.W.; Choi, H.S.; Park, G.; Lee, H.S.; Kim, S.J.; Lee, M.; Suh, J.; Kwon, A.; Kim, H.S.; et al. Comparison of the modified tyg indices and other parameters to predict non-alcoholic fatty liver disease in youth. Biology 2022, 11, 685. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, M.; Liu, Z.; Yuan, H.; Wu, X.; Shi, T.; Chen, X.; Zhang, T. Increasing prevalence of nafld/nash among children, adolescents and young adults from 1990 to 2017: A population-based observational study. BMJ Open 2021, 11, e042843. [Google Scholar] [CrossRef]
- Welsh, J.A.; Karpen, S.; Vos, M.B. Increasing prevalence of nonalcoholic fatty liver disease among united states adolescents, 1988-1994 to 2007-2010. J. Pediatrics 2013, 162, 496–500. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Park, G.; Lee, H.S.; Lee, M.; Lee, H.I.; Ahn, J.; Lee, E.; Choi, H.S.; Suh, J.; Kwon, A.; et al. Trends in prediabetes and non-alcoholic fatty liver disease associated with abdominal obesity among korean children and adolescents: Based on the korea national health and nutrition examination survey between 2009 and 2018. Biomedicines 2022, 10, 584. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, H.G.; Kim, M.J.; Koh, H.; Kim, H.Y.; Roh, Y.H.; Lee, M.J. Normal range of hepatic fat fraction on dual- and triple-echo fat quantification mr in children. PLoS ONE 2015, 10, e0117480. [Google Scholar] [CrossRef] [Green Version]
- Shah, J.; Okubote, T.; Alkhouri, N. Overview of updated practice guidelines for pediatric nonalcoholic fatty liver disease. Gastroenterol. Hepatol. 2018, 14, 407–414. [Google Scholar]
- Molleston, J.P.; Schwimmer, J.B.; Yates, K.P.; Murray, K.F.; Cummings, O.W.; Lavine, J.E.; Brunt, E.M.; Scheimann, A.O.; Unalp-Arida, A. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. J. Pediatrics 2014, 164, 707–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraioli, G.; Maiocchi, L.; Savietto, G.; Tinelli, C.; Nichetti, M.; Rondanelli, M.; Calliada, F.; Preda, L.; Filice, C. Performance of the attenuation imaging technology in the detection of liver steatosis. J. Ultrasound Med. 2021, 40, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Ajmera, V.; Loomba, R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in nafld. Nat. Rev. Endocrinol. 2022, 18, 55–66. [Google Scholar] [CrossRef]
- Sugimoto, K.; Moriyasu, F.; Oshiro, H.; Takeuchi, H.; Abe, M.; Yoshimasu, Y.; Kasai, Y.; Sakamaki, K.; Hara, T.; Itoi, T. The role of multiparametric us of the liver for the evaluation of nonalcoholic steatohepatitis. Radiology 2020, 296, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.K.; Lee, J.M.; Joo, I.; Yoon, J.H.; Lee, D.H.; Lee, J.Y.; Han, J.K. Prospective evaluation of hepatic steatosis using ultrasound attenuation imaging in patients with chronic liver disease with magnetic resonance imaging proton density fat fraction as the reference standard. Ultrasound Med. Biol. 2019, 45, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Gatos, I.; Drazinos, P.; Yarmenitis, S.; Theotokas, I.; Koskinas, J.; Koullias, E.; Mitranou, A.; Manesis, E.; Zoumpoulis, P.S. Liver ultrasound attenuation: An ultrasound attenuation index for liver steatosis assessment. Ultrasound Q. 2022, 38, 124–132. [Google Scholar] [CrossRef]
- Ferraioli, G.; Berzigotti, A.; Barr, R.G.; Choi, B.I.; Cui, X.W.; Dong, Y.; Gilja, O.H.; Lee, J.Y.; Lee, D.H.; Moriyasu, F.; et al. Quantification of liver fat content with ultrasound: A wfumb position paper. Ultrasound Med. Biol. 2021, 47, 2803–2820. [Google Scholar] [CrossRef] [PubMed]
- Cailloce, R.; Tavernier, E.; Brunereau, L.; Fievet, A.; Falip, C.; Dujardin, F.; Willot, S.; Patat, F.; Morel, B. Liver shear wave elastography and attenuation imaging coefficient measures: Prospective evaluation in healthy children. Abdom. Radiol. 2021, 46, 4629–4636. [Google Scholar] [CrossRef]
- Bae, J.S.; Lee, D.H.; Suh, K.S.; Kim, H.; Lee, K.B.; Lee, J.Y.; Han, J.K. Noninvasive assessment of hepatic steatosis using a pathologic reference standard: Comparison of ct, mri, and us-based techniques. Ultrasonography 2022, 41, 344–354. [Google Scholar] [CrossRef]
- Shin, J.; Kim, M.J.; Shin, H.J.; Yoon, H.; Kim, S.; Koh, H.; Lee, M.J. Quick assessment with controlled attenuation parameter for hepatic steatosis in children based on mri-pdff as the gold standard. BMC Pediatr. 2019, 19, 112. [Google Scholar] [CrossRef] [Green Version]
- Bae, J.S.; Lee, D.H.; Lee, J.Y.; Kim, H.; Yu, S.J.; Lee, J.H.; Cho, E.J.; Lee, Y.B.; Han, J.K.; Choi, B.I. Assessment of hepatic steatosis by using attenuation imaging: A quantitative, easy-to-perform ultrasound technique. Eur. Radiol. 2019, 29, 6499–6507. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.K.; Choi, S.H.; Lee, J.S.; Kim, S.Y.; Lee, S.S.; Kim, K.W. Accuracy of the ultrasound attenuation coefficient for the evaluation of hepatic steatosis: A systematic review and meta-analysis of prospective studies. Ultrasonography 2022, 41, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H. Quantitative assessment of fatty liver using ultrasound attenuation imaging. J. Med. Ultrason. 2021, 48, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Kurosaki, M.; Yasui, Y.; Tsuchiya, K.; Izumi, N. Attenuation coefficient (att) measurement for liver fat quantification in chronic liver disease. J. Med. Ultrason. 2021, 48, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Podrug, K.; Sporea, I.; Lupusoru, R.; Pastrovic, F.; Mustapic, S.; Bâldea, V.; Bozin, T.; Bokun, T.; Salkic, N.; Șirli, R.; et al. Diagnostic performance of 2-d shear-wave elastography with propagation maps and attenuation imaging in patients with non-alcoholic fatty liver disease. Ultrasound Med. Biol. 2021, 47, 2128–2137. [Google Scholar] [CrossRef]
- Dioguardi Burgio, M.; Ronot, M.; Reizine, E.; Rautou, P.E.; Castera, L.; Paradis, V.; Garteiser, P.; Van Beers, B.; Vilgrain, V. Quantification of hepatic steatosis with ultrasound: Promising role of attenuation imaging coefficient in a biopsy-proven cohort. Eur. Radiol. 2020, 30, 2293–2301. [Google Scholar] [CrossRef]
- Runge, J.H.; van Giessen, J.; Draijer, L.G.; Deurloo, E.E.; Smets, A.; Benninga, M.A.; Koot, B.G.P.; Stoker, J. Accuracy of controlled attenuation parameter compared with ultrasound for detecting hepatic steatosis in children with severe obesity. Eur. Radiol. 2021, 31, 1588–1596. [Google Scholar] [CrossRef]
- Ferraioli, G.; Maiocchi, L.; Raciti, M.V.; Tinelli, C.; De Silvestri, A.; Nichetti, M.; De Cata, P.; Rondanelli, M.; Chiovato, L.; Calliada, F.; et al. Detection of liver steatosis with a novel ultrasound-based technique: A pilot study using mri-derived proton density fat fraction as the gold standard. Clin. Transl. Gastroenterol. 2019, 10, e00081. [Google Scholar] [CrossRef]
- Sugimoto, K.; Moriyasu, F.; Oshiro, H.; Takeuchi, H.; Yoshimasu, Y.; Kasai, Y.; Itoi, T. Clinical utilization of shear wave dispersion imaging in diffuse liver disease. Ultrasonography 2020, 39, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.; Lee, J.M.; Joo, I.; Lee, D.H.; Yoon, J.H.; Kang, H.J.; Ahn, S.J. Reproducibility of ultrasound attenuation imaging for the noninvasive evaluation of hepatic steatosis. Ultrasonography 2020, 39, 121–129. [Google Scholar] [CrossRef] [Green Version]
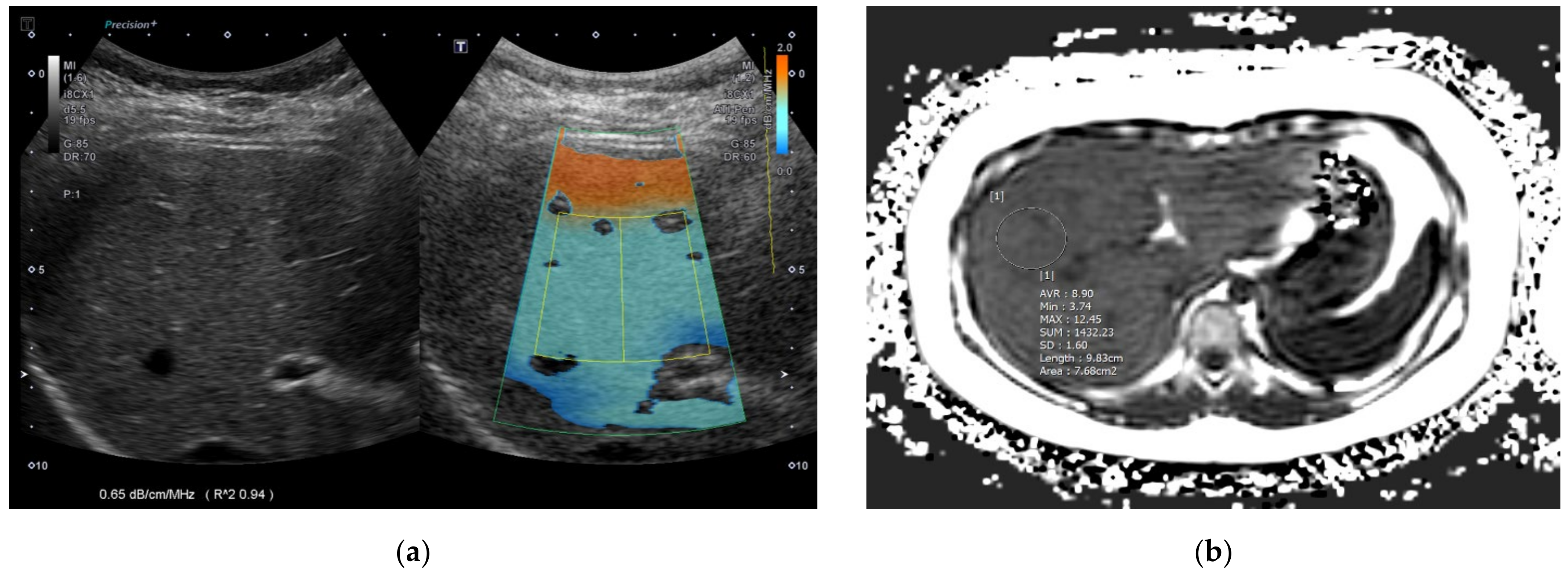
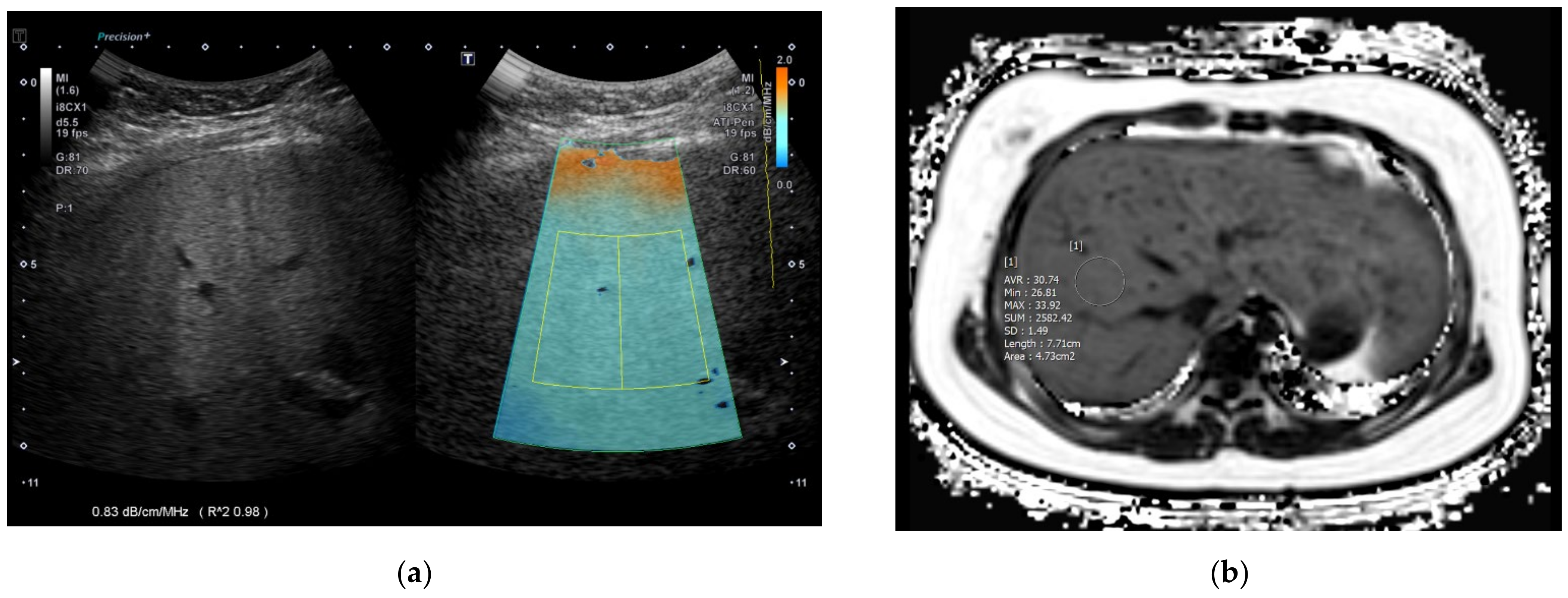
| Variables | Normal Liver Group 1 (n = 31) | Mild Fatty Liver Group 2 (n = 30) | Moderate–Severe Fatty Liver Group 3 (n = 27) | Overall p-Value | Corrected p-Value (1 vs. 2) | Corrected p-Value (2 vs. 3) | Corrected p-Value (1 vs. 3) | |
|---|---|---|---|---|---|---|---|---|
| Demographics | Age (years) | 9.4 ± 2.5 | 10.2 ± 2.4 | 11.8 ± 2.3 | 0.001 | 0.661 | 0.037 | 0.001 |
| Sex (M:F) | 13:18 | 15:15 | 19:08 | 0.086 * | 0.527 | 0.118 | 0.056 | |
| Height (cm) | 139.4 ± 12.7 | 145.1 ± 10.7 | 153.9 ± 14.4 | <0.001 | 0.253 | 0.03 | <0.001 | |
| Weight (kg) | 42.1 ± 10.5 | 51.4 ± 13.0 | 69.8 ± 19.6 | <0.001 | 0.044 | <0.001 | <0.001 | |
| BMI (kg/m2) | 21.5 ± 3.4 | 24.8 ± 3.2 | 27.9 ± 3.4 | <0.001 | 0.001 | 0.002 | <0.001 | |
| SBP (mmHg) | 112.3 ± 10.9 | 117.3 ± 10.9 | 120.2 ± 11.9 | 0.041 | 0.326 | 0.999 | 0.039 | |
| DBP (mmHg) | 64.8 ± 10.5 | 66.5 ± 9.2 | 68.8 ± 10.3 | 0.363 | 0.999 | 0.999 | 0.469 | |
| AST (IU/L) | 26.8 ± 14.2 | 25.7 ± 8.7 | 44.9 ± 26.5 | <0.001 | 0.999 | <0.001 | 0.001 | |
| ALT (IU/L) | 17.8 ± 11.9 | 28.4 ± 16.7 | 64.4 ± 52.4 | <0.001 | 0.564 | <0.001 | <0.001 | |
| MRI (mild: n = 5, moderate to severe: n = 15) | Liver longitudinal diameter (cm) | 16.0 ± 1.0 | 18.0 ± 2.3 | 0.089 ** | ||||
| PDFF (%) | 12.9 ± 10.4 | 25.2 ± 10.8 | 0.04 ** | |||||
| T2* (ms) | 19.0 ± 3.5 | 16.5 ± 2.5 | 0.087 ** | |||||
| MRE (kPa) | 2.1 ± 0.2 | 2.0 ± 0.3 | 0.805 ** | |||||
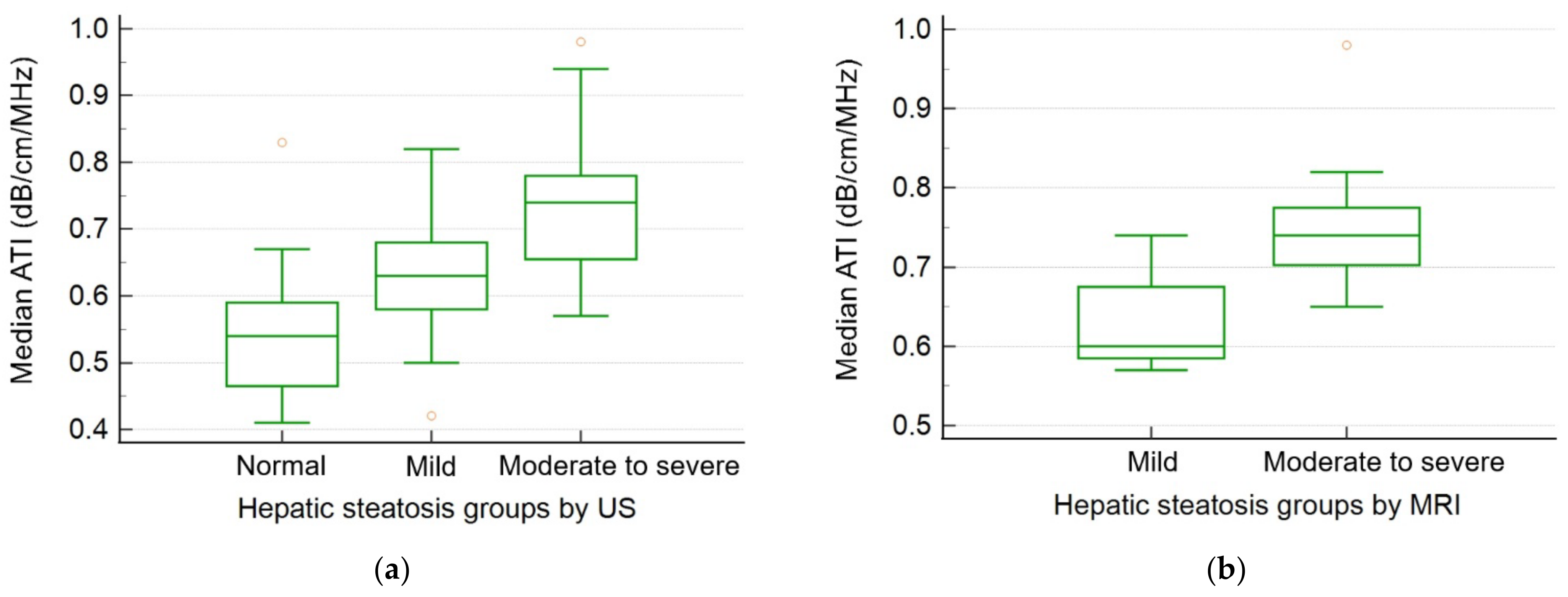
| US | ATI median (dB/cm/MHz) | 0.54 ± 0.09 | 0.63 ± 0.08 | 0.73 ± 0.11 | <0.001 | 0.001 | <0.001 | <0.001 |
| ATI IQR | 0.09 ± 0.1 | 0.06 ± 0.03 | 0.06 ± 0.03 | 0.125 | 0.287 | 0.999 | 0.201 | |
| ATI IQR/median | 0.14 ± 0.07 | 0.1 ± 0.06 | 0.08 ± 0.04 | 0.001 | 0.028 | 0.575 | 0.001 | |
| SWE median (m/s) | 1.19 ± 0.07 | 1.29 ± 0.16 | 1.26 ± 0.14 | 0.009 | 0.019 | 0.999 | 0.026 | |
| SWE IQR | 0.12 ± 0.06 | 0.12 ± 0.07 | 0.13 ± 0.09 | 0.995 | 0.999 | 0.999 | 0.999 | |
| SWE IQR/median | 0.10 ± 0.05 | 0.09 ± 0.06 | 0.09 ± 0.06 | 0.721 | 0.999 | 0.999 | 0.999 | |
| SWE median (kPa) | 4.39 ± 0.62 | 5.1 ± 1.46 | 5.04 ± 1.44 | 0.059 | 0.092 | 0.999 | 0.156 | |
| SWD median [(m/s)/kHz] | 12.39 ± 1.41 | 12.45 ± 1.10 | 12.57 ± 1.26 | 0.872 | 0.999 | 0.999 | 0.999 | |
| SWD IQR | 1.6 ± 0.82 | 1.76 ± 1.05 | 1.4 ± 0.77 | 0.327 | 0.999 | 0.999 | 0.999 | |
| SWD IQR/med | 0.13 ± 0.07 | 0.14 ± 0.08 | 0.11 ± 0.06 | 0.302 | 0.999 | 0.374 | 0.999 | |
| Variables | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | ||
| Demographics | Age (years) | 0.311 | 0.137–0.485 | <0.001 | 0.122 | −0.163–0.407 | 0.401 |
| Sex (male) | 0.854 | 0.065–1.643 | 0.034 | −0.792 | −2.120–0.535 | 0.242 | |
| (female) | (Reference) | (Reference) | |||||
| Height (cm) | 0.068 | 0.035–0.102 | <0.001 | ||||
| Weight (kg) | 0.09 | 0.056–0.123 | <0.001 | ||||
| BMI (kg/m2) | 0.385 | 0.247–0.524 | <0.001 | 0.453 | 0.197–0.709 | 0.001 | |
| SBP (mmHg) | 0.048 | 0.010–0.086 | 0.013 | −0.029 | −0.091–0.034 | 0.364 | |
| DBP (mmHg) | 0.032 | −0.010–0.074 | 0.136 | ||||
| AST (IU/L) | 0.055 | 0.023–0.088 | 0.001 | 0.085 | −0.058–0.227 | 0.246 | |
| ALT (IU/L) | 0.06 | 0.034–0.087 | <0.001 | 0.007 | −0.063–0.076 | 0.852 | |
| US | ATI median (dB/cm/MHz) | 15.536 | 10.186–20.886 | <0.001 | 22.09 | 11.949–32.230 | <0.001 |
| ATI IQR | −10.53 | −22.387–1.326 | 0.082 | ||||
| ATI IQR/median | −13.46 | −21.190–−5.729 | 0.001 | ||||
| SWE median (m/s) | 3.746 | 0.511–6.980 | 0.023 | 0.725 | −3.773–5.224 | 0.752 | |
| SWE IQR | 0.099 | −4.682–4.879 | 0.968 | ||||
| SWE IQR/median | −2.252 | −8.895–4.390 | 0.506 | ||||
| SWE median (kPa) | 0.302 | −0.045–0.649 | 0.088 | ||||
| SWD median [(m/s)/kHz] | 0.089 | −0.225–0.402 | 0.579 | ||||
| SWD IQR | −0.157 | −0.596–0.282 | 0.483 | ||||
| SWD IQR/median | −2.369 | −7.961–3.223 | 0.406 | ||||
| Variables | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| Estimate | Standard Error | p-Value | Estimate | Standard Error | p-Value | ||
| Demographics | Age (years) | 0.008 | 0.005 | 0.131 | |||
| Sex | −0.044 | 0.025 | 0.089 | ||||
| Height (cm) | 0.001 | 0.001 | 0.122 | ||||
| Weight (kg) | 0.002 | 0.001 | 0.006 | ||||
| BMI (kg/m2) | 0.008 | 0.003 | 0.013 | −0.019 | 0.009 | 0.047 | |
| SBP (mmHg) | 0.003 | 0.001 | 0.011 | 0.002 | 0.002 | 0.314 | |
| DBP (mmHg) | 0.002 | 0.001 | 0.08 | ||||
| AST (IU/L) | 0.002 | 0.001 | 0.002 | ||||
| ALT (IU/L) | 0.001 | 0.001 | <0.001 | 0.001 | 0.001 | 0.998 | |
| MRI (mild: n = 5, moderate to severe: n = 15) | Liver longitudinal diameter (cm) | −0.012 | 0.01 | 0.235 | |||
| PDFF (%) | 0.005 | 0.002 | 0.014 | 0.007 | 0.005 | 0.147 | |
| T2* (ms) | −0.019 | 0.007 | 0.012 | 0.009 | 0.019 | 0.653 | |
| MRE (kPa) | −0.017 | 0.098 | 0.863 | ||||
| US | SWE median (m/s) | 0.067 | 0.093 | 0.473 | |||
| SWD median [(m/s)/kHz] | −0.003 | 0.011 | 0.765 | ||||
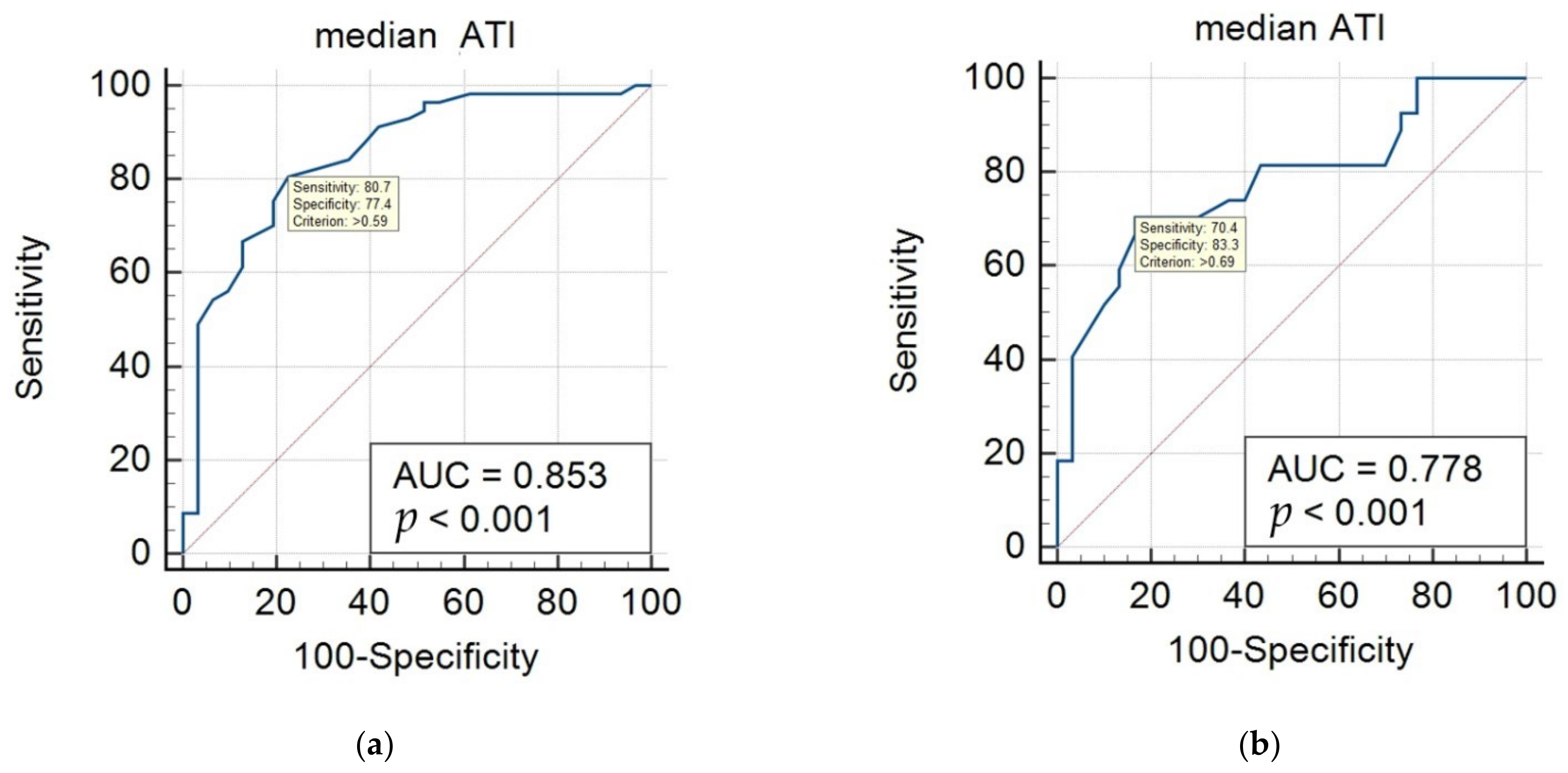
| Reference Modalities | Hepatic Steatosis Groups | Cutoff Value of ATI (dB/cm/MHz) | AUC | 95% CI | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| Grayscale US | normal vs. mild/moderate/severe | 0.59 | 0.853 | 0.762–0.920 | 80.7 | 77.4 |
| mild vs. moderate/severe | 0.69 | 0.778 | 0.649–0.878 | 70.4 | 83.3 | |
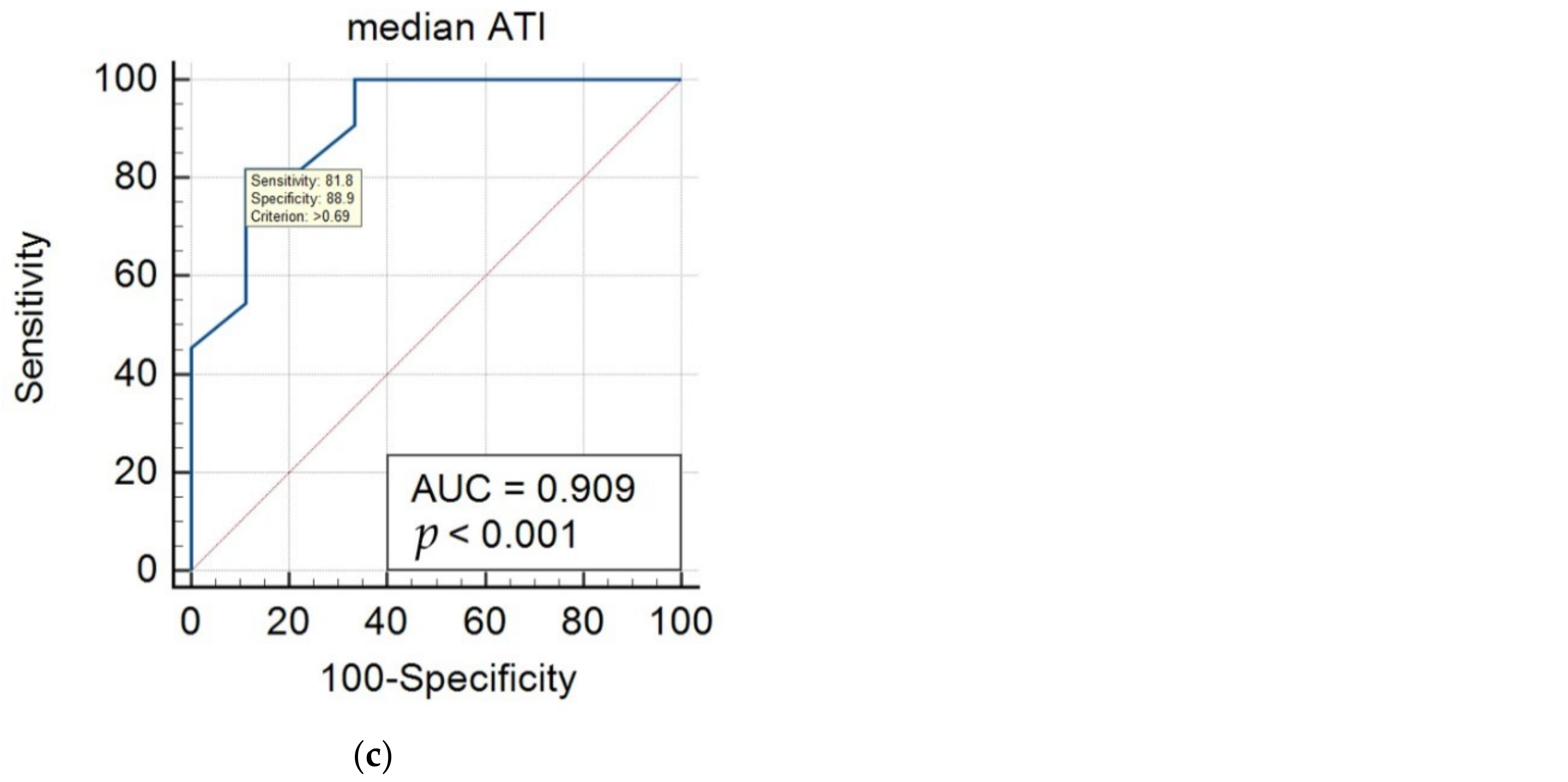
| MRI-PDFF | S1 (mild) vs. S2–3 (moderate–severe) | 0.69 | 0.909 | 0.695–0.990 | 81.8 | 88.9 |
| Success (n = 88) | Failure (n = 23) | p-Value * | OR ** | 95% CI ** | p-Value ** | |
|---|---|---|---|---|---|---|
| Age (years) | 10.4 ± 2.6 | 8.5 ± 1.4 | 0.065 | 1.638 | 1.042–2.576 | 0.033 |
| Sex | 47:41 | 5:18 | 0.007 | 0.242 | 0.083–0.710 | 0.01 |
| Height (cm) | 145.8 ± 13.8 | 134.7 ± 9.9 | 0.069 | 1.078 | 1.030–1.129 | 0.001 |
| Weight (kg) | 53.8 ± 18.4 | 36.4 ± 8.3 | <0.001 | 1.124 | 1.057–1.196 | <0.001 |
| BMI (kg/m2) | 24.6 ± 4.2 | 19.9 ± 3.6 | 0.421 | 1.361 | 1.168–1.585 | <0.001 |
| Liver echo on US (normal vs. abnormal) | 31:57 | 18:05 | <0.001 | 6.619 | 2.241–19.552 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.; Son, N.-H.; Chang, D.R.; Chae, H.W.; Shin, H.J. Feasibility of Ultrasound Attenuation Imaging for Assessing Pediatric Hepatic Steatosis. Biology 2022, 11, 1087. https://doi.org/10.3390/biology11071087
Song K, Son N-H, Chang DR, Chae HW, Shin HJ. Feasibility of Ultrasound Attenuation Imaging for Assessing Pediatric Hepatic Steatosis. Biology. 2022; 11(7):1087. https://doi.org/10.3390/biology11071087
Chicago/Turabian StyleSong, Kyungchul, Nak-Hoon Son, Dong Ryul Chang, Hyun Wook Chae, and Hyun Joo Shin. 2022. "Feasibility of Ultrasound Attenuation Imaging for Assessing Pediatric Hepatic Steatosis" Biology 11, no. 7: 1087. https://doi.org/10.3390/biology11071087
APA StyleSong, K., Son, N.-H., Chang, D. R., Chae, H. W., & Shin, H. J. (2022). Feasibility of Ultrasound Attenuation Imaging for Assessing Pediatric Hepatic Steatosis. Biology, 11(7), 1087. https://doi.org/10.3390/biology11071087






