Full-Length Transcriptome Reconstruction Reveals the Genetic Mechanisms of Eyestalk Displacement and Its Potential Implications on the Interspecific Hybrid Crab (Scylla serrata ♀ × S. paramamosain ♂)
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Collection, RNA Extraction and Sequencing
2.2. SMRT Sequencing Data Processing
2.3. Collapsing Redundant Transcripts Isoforms
2.4. Completeness and Characteristics Analysis of Reconstructed Transcriptomes
2.5. Gene Functional Annotation
2.6. Alternative Splicing (AS) Events Analysis
2.7. Quantification of Identified Transcripts
2.8. Differential Alternative Splicing (DAS) Events, Differential Expressed Transcripts (DETs), and Their Enrichment Analysis
2.9. Validation of AS Events and Differential Expressed Genes
3. Results
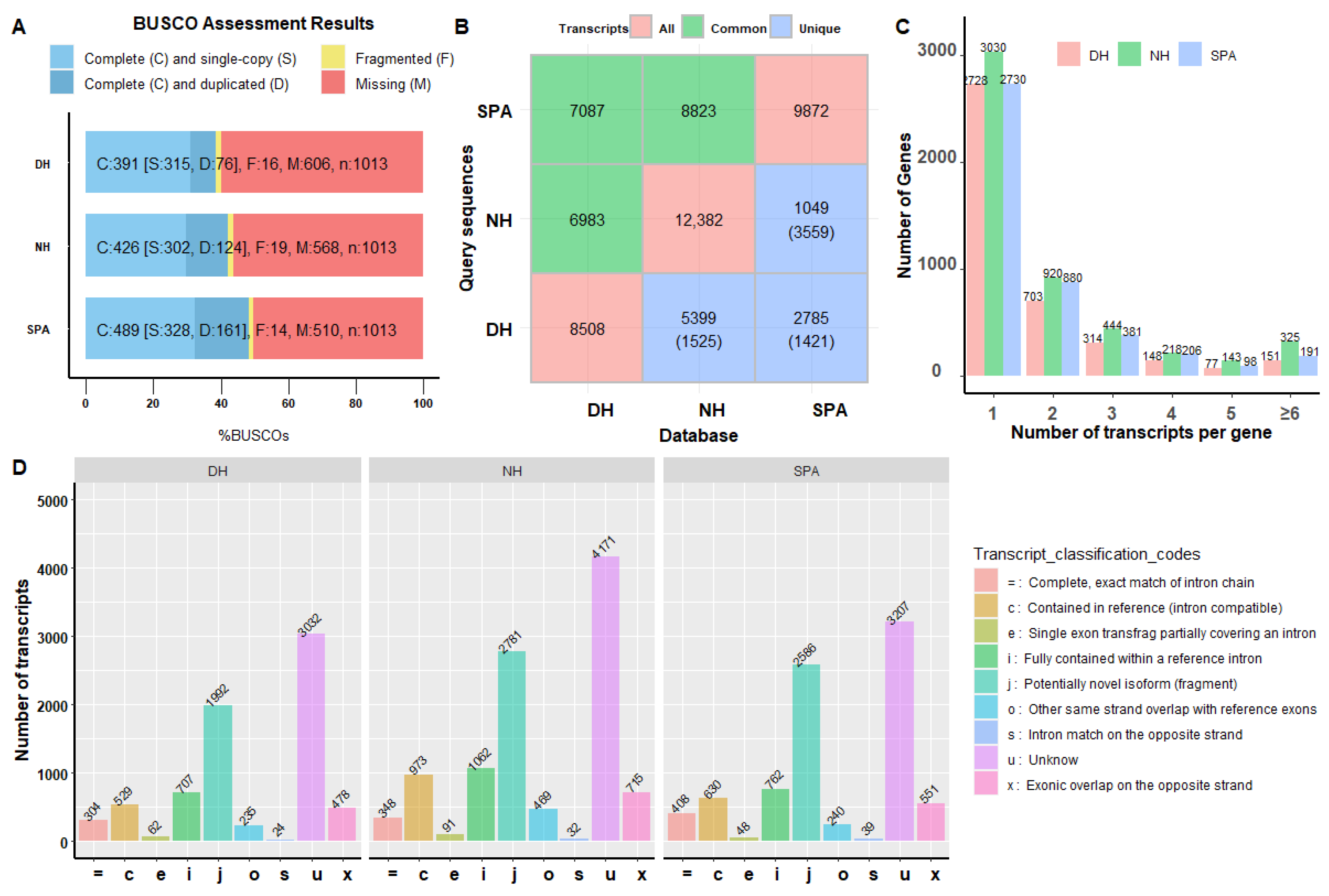
3.1. Summary of PacBio Iso-Seq Data
3.2. Collapsing Redundant Isoforms
3.3. Evaluation of Reconstructed Transcriptomes
3.4. Functional Annotation
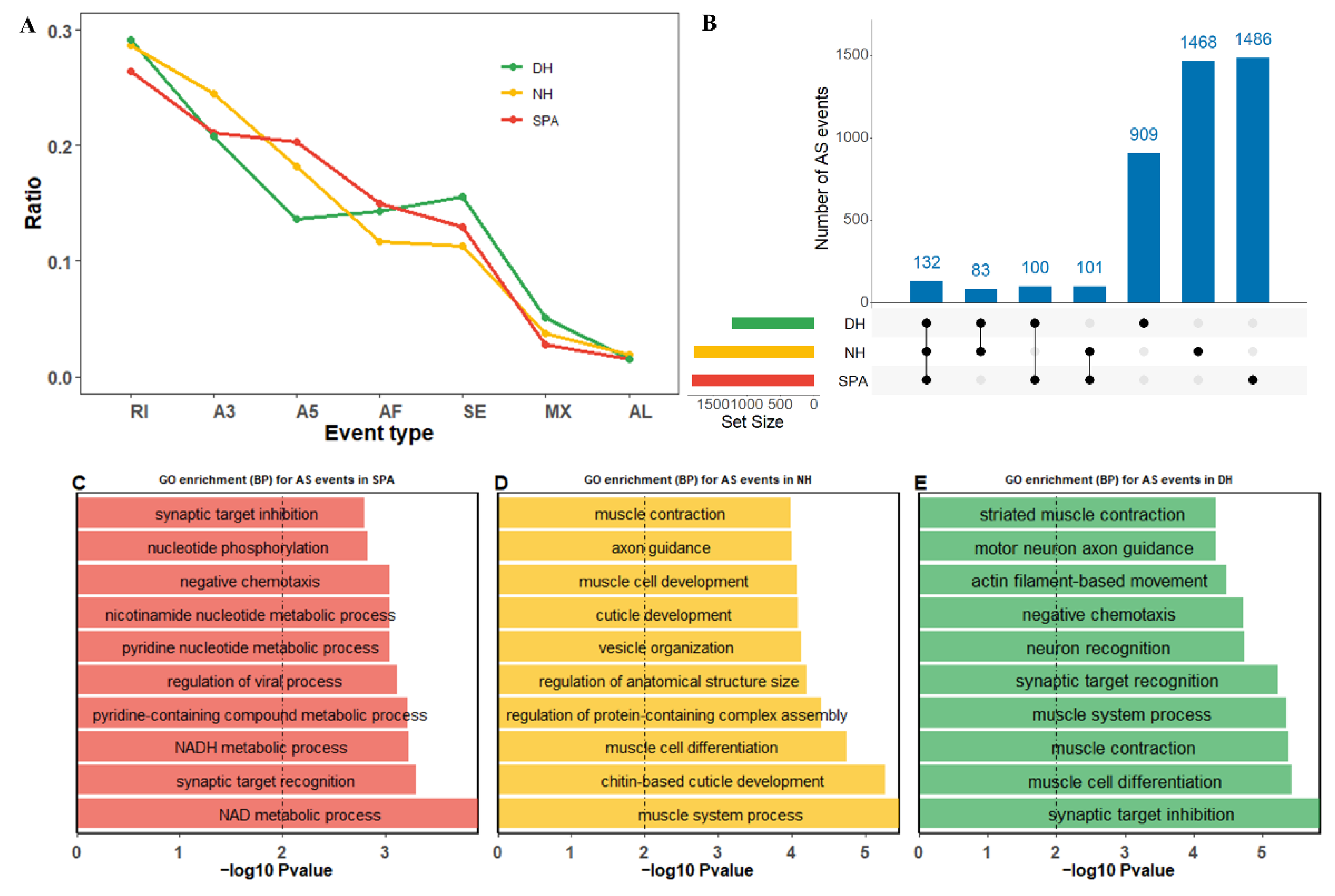
3.5. Alternative Splicing (AS) Events
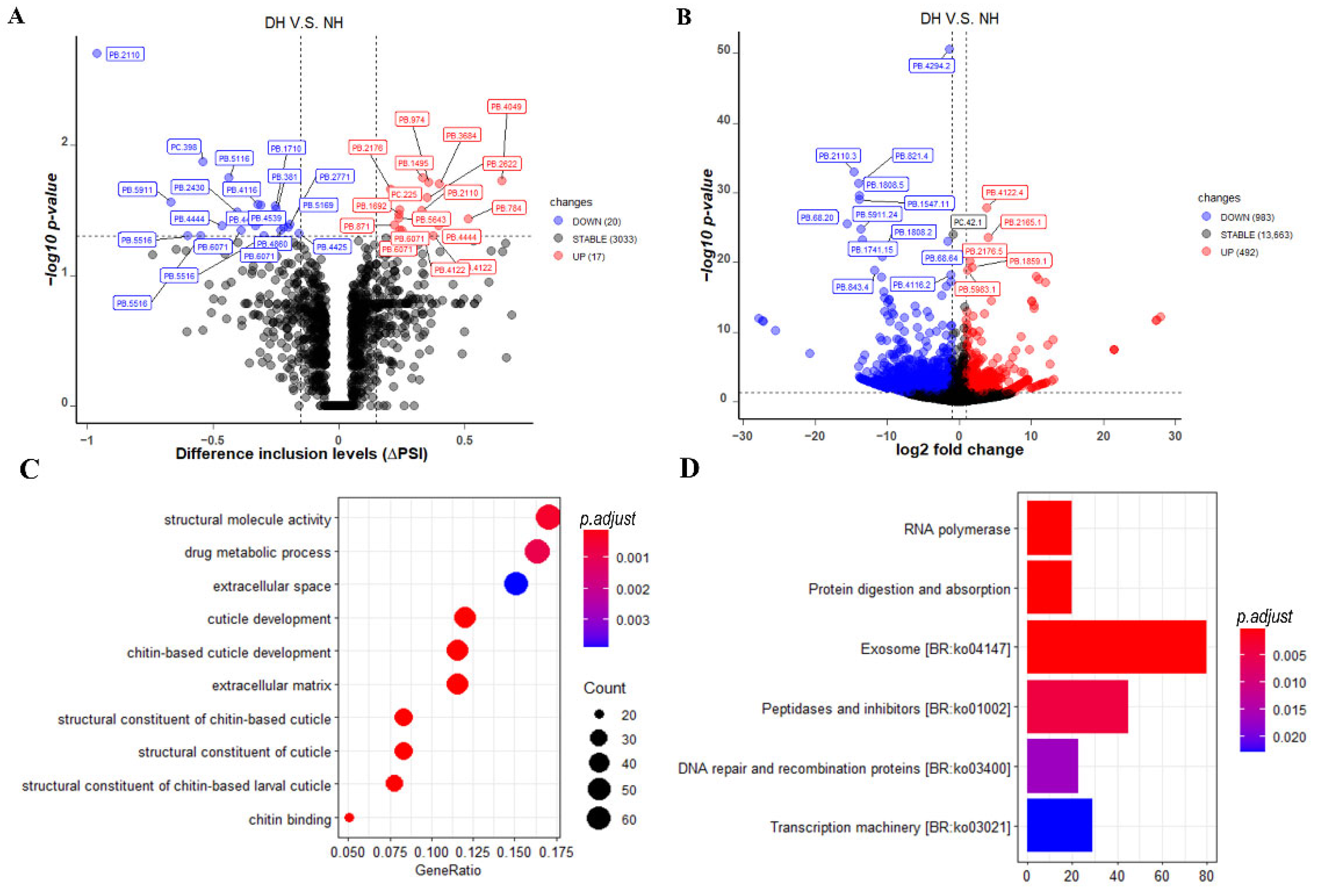
3.6. Differential Alternative Splicing (DAS) Events, Differential Expressed Transcripts (DETs), and Their Enrichment Analysis
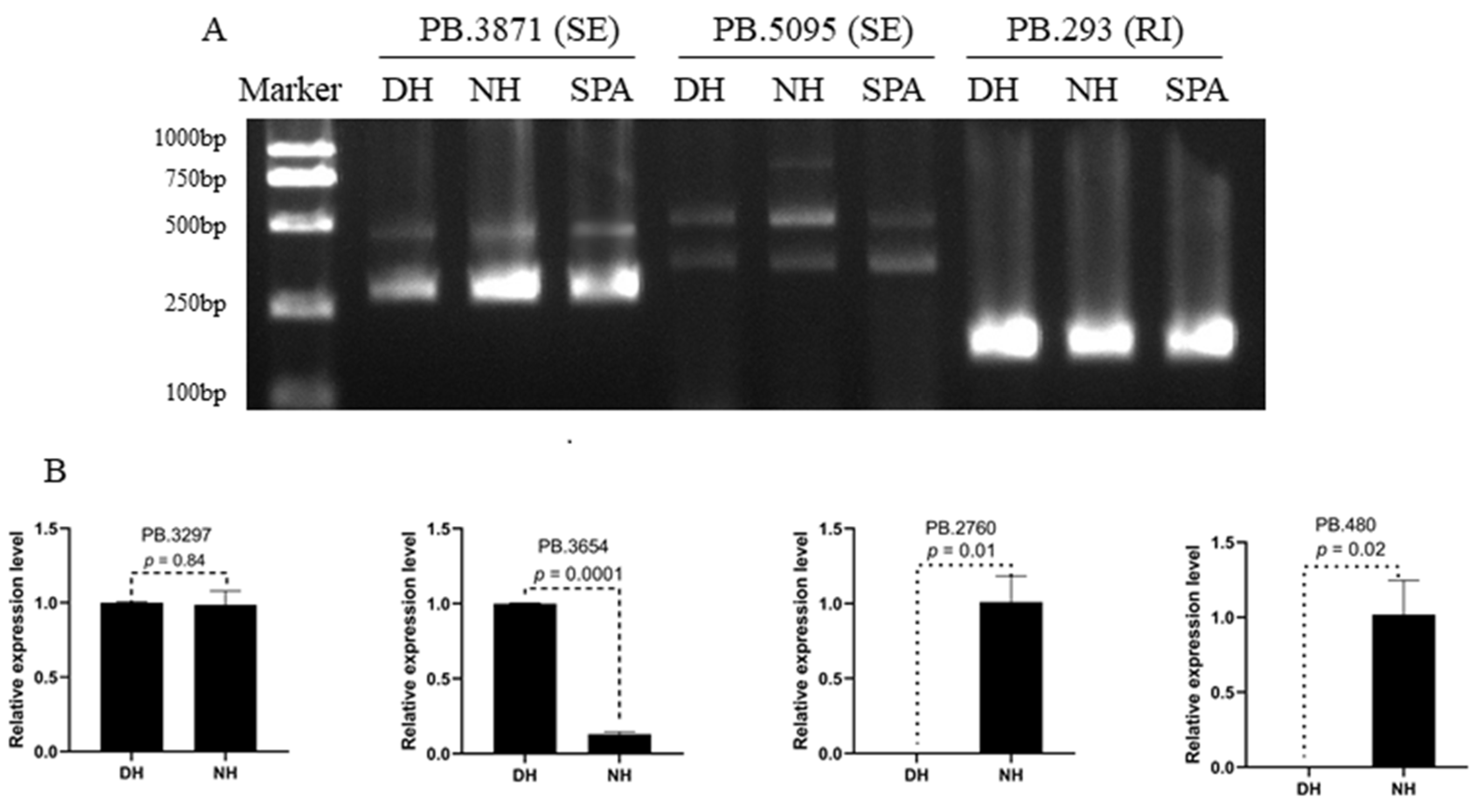
3.7. Validation of Alternative Splicing (AS) Events and Significant Differential Expression Transcripts (DGEs)
4. Discussions
4.1. Reconstructed Transcriptomes Based on the Non-Hybrid Correction Methods and Two-Step Collapsing Strategy
4.2. The Genetic Mechanisms of Eyestalk Displacement and Its Potential Implications on the Novel Interspecific Hybrid Crab
4.3. The Transcriptomes Difference between Purebred (SPA) and Crossbred (NH and DH) Mud Crabs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waiho, K.; Fazhan, H.; Quinitio, E.T.; Baylon, J.C.; Fujaya, Y.; Azmie, G.; Wu, Q.; Shi, X.; Ikhwanuddin, M.; Ma, H. Larval rearing of mud crab (Scylla): What lies ahead. Aquaculture 2018, 493, 37–50. [Google Scholar] [CrossRef]
- Ma, H.; Ma, L.; Ma, C.; Cui, H. Novel Polymorphic Microsatellite Markers in Scylla paramamosain and Cross-Species Amplification in Related Crab Species. J. Crustacean Biol. 2010, 30, 441–444. [Google Scholar] [CrossRef]
- Fazhan, H.; Waiho, K.; Quinitio, E.; Baylon, J.C.; Fujaya, Y.; Rukminasari, N.; Azri, M.F.D.; Shahreza, M.S.; Ma, H.; Ikhwanuddin, M. Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ 2020, 8, e8066. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, A.; Fang, S.; Zhang, Y.; Cui, W.; Fang, H.; Ikhwanuddin, M.; Ma, H. The significant sex-biased expression pattern of Sp-Wnt4 provides novel insights into the ovarian development of mud crab (Scylla Paramamosain). Int. J. Biol. Macromol. 2021, 183, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Waiho, K.; Shi, X.; Fazhan, H.; Li, S.; Zhang, Y.; Zheng, H.; Liu, W.; Fang, S.; Ikhwanuddin, M.; Ma, H. High-density genetic linkage maps provide novel insights into ZW/ZZ sex determination system and growth performance in mud crab (Scylla paramamosain). Front. Genet. 2019, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Waiho, K.; Li, X.; Ikhwanuddin, M.; Miao, G.; Lin, F.; Zhang, Y.; Li, S.; Zheng, H.; Liu, W.; et al. Female-specific SNP markers provide insights into a WZ/ZZ sex determination system for mud crabs Scylla paramamosain, S. tranquebarica and S. serrata with a rapid method for genetic sex identification. BMC Genom. 2018, 19, 981. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Lu, J.; Wu, Q.; Waiho, K.; Aweya, J.J.; Fazhan, H.; Zhang, Y.; Li, S.; Zheng, H.; Lin, F.; et al. Comparative analysis of growth performance between female and male mud crab Scylla paramamosain crablets: Evidences from a four-month successive growth experiment. Aquaculture 2019, 505, 351–362. [Google Scholar] [CrossRef]
- Cui, W.; Fang, S.; Lv, L.; Huang, Z.; Lin, F.; Wu, Q.; Zheng, H.; Li, S.; Zhang, Y.; Ikhwanuddin, M.; et al. Evidence of Sex Differentiation Based on Morphological Traits During the Early Development Stage of Mud Crab Scylla paramamosain. Front. Vet. Sci. 2021, 8, 712942. [Google Scholar] [CrossRef]
- Wu, Q.; Shi, X.; Fang, S.; Xie, Z.; Guan, M.; Li, S.; Zheng, H.; Zhang, Y.; Ikhwanuddin, M.; Ma, H. Different biochemical composition and nutritional value attribute to salinity and rearing period in male and female mud crab Scylla paramamosain. Aquaculture 2019, 513, 734417. [Google Scholar] [CrossRef]
- Wu, Q.; Waiho, K.; Huang, Z.; Li, S.; Zheng, H.; Zhang, Y.; Ikhwanuddin, M.; Lin, F.; Ma, H. Growth performance and biochemical composition dynamics of ovary, hepatopancreas and muscle tissues at different ovarian maturation stages of female mud crab, Scylla paramamosain. Aquaculture 2020, 515, 734560. [Google Scholar] [CrossRef]
- Wang, S.; Tang, C.; Tao, M.; Qin, Q.; Zhang, C.; Luo, K.; Zhao, R.; Wang, J.; Ren, L.; Xiao, J.; et al. Establishment and application of distant hybridization technology in fish. Sci. China Life Sci. 2019, 62, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J. Molecular mechanisms of polyploidy and hybrid vigor. Trends Plant Sci. 2010, 15, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wu, Q.; Tan, H.; Lin, F. Establishment of Inter-Specific Hybridization Technique and Identification of Phenotypic and Genotypic Characters of Hybrids in Mud Crab(Scylla paramamosain and S. serrata). J. Shantou Univ. 2021, 36, 59–66. [Google Scholar] [CrossRef]
- Cui, W.; Guan, M.; Sadek, M.A.; Wu, F.; Wu, Q.; Tan, H.; Shi, X.; Ikhwanuddin, M.; Ma, H. Construction of a genetic linkage map and QTL mapping for sex indicate the putative genetic pattern of the F1 hybrid Scylla (Scylla serrata ♀ × S. paramamosain ♂). Aquaculture 2021, 545, 737222. [Google Scholar] [CrossRef]
- Farhadi, A.; Lv, L.; Song, J.; Zhang, Y.; Ye, S.; Zhang, N.; Zheng, H.; Li, S.; Zhang, Y.; Ikhwanuddin, M.; et al. Whole-transcriptome RNA sequencing revealed the roles of chitin-related genes in the eyestalk abnormality of a novel mud crab hybrid (Scylla serrata ♀ × S. paramamosain ♂). Int. J. Biol. Macromol. 2022, 208, 611–626. [Google Scholar] [CrossRef]
- Han, Z.; Li, X.; Li, X.; Xu, W.; Li, Y. Circadian rhythms of melatonin in haemolymph and optic lobes of Chinese mitten crab (Eriocheir sinensis) and Chinese grass shrimp (Palaemonetes sinensis). Biol. Rhythm Res. 2018, 50, 400–407. [Google Scholar] [CrossRef]
- Li, Y.; Han, Z.; She, Q.; Zhao, Y.; Wei, H.; Dong, J.; Xu, W.; Li, X.; Liang, S. Comparative transcriptome analysis provides insights into the molecular basis of circadian cycle regulation in Eriocheir sinensis. Gene 2019, 694, 42–49. [Google Scholar] [CrossRef]
- Mykles, D.L.; Chang, E.S. Hormonal control of the crustacean molting gland: Insights from transcriptomics and proteomics. Gen. Comp. Endocrinol. 2020, 294, 113493. [Google Scholar] [CrossRef]
- Magana-Gallegos, E.; Arevalo, M.; Cuzon, G.; Gaxiola, G. Effects of using the biofloc system and eyestalk ablation on reproductive performance and egg quality of Litopenaeus vannamei (Boone, 1931) (Decapoda: Dendrobranchiata: Penaeidae). Anim. Reprod. Sci. 2021, 228, 106749. [Google Scholar] [CrossRef]
- Chang, E.S. Comparative Endocrinology of Molting and Reproduction: Insects and Crustaceans. Annu. Rev. Entomol. 1993, 38, 161–180. [Google Scholar] [CrossRef]
- Amankwah, B.K.; Wang, C.; Zhou, T.; Liu, J.; Shi, L.; Wang, W.; Chan, S. Eyestalk Ablation, a Prerequisite for Crustacean Reproduction: A review. Isr. J. Aquac. Bamidgeh 2019, 71, 14. [Google Scholar]
- Nguyen, T.V.; Jung, H.; Rotllant, G.; Hurwood, D.; Mather, P.; Ventura, T. Guidelines for RNA-seq projects: Applications and opportunities in non-model decapod crustacean species. Hydrobiologia 2018, 825, 5–27. [Google Scholar] [CrossRef]
- Pop, M.; Salzberg, S.L. Bioinformatics challenges of new sequencing technology. Trends Genet. 2008, 24, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Wenger, A.M.; Peluso, P.; Rowell, W.J.; Chang, P.-C.; Hall, R.J.; Concepcion, G.T.; Ebler, J.; Fungtammasan, A.; Kolesnikov, A.; Olson, N.D.; et al. Accurate circular consensus long-read sequencing improves variant detection and assembly of a human genome. Nat. Biotechnol. 2019, 37, 1155–1162. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Jiang, H.; Li, L.; Ma, J.; Li, H.; Chen, J. Full-length transcriptome analysis of Litopenaeus vannamei reveals transcript variants involved in the innate immune system. Fish. Shellfish Immunol. 2019, 87, 346–359. [Google Scholar] [CrossRef]
- Cui, W.; Yang, Q.; Zhang, Y.; Farhadi, A.; Fang, H.; Zheng, H.; Li, S.; Zhang, Y.; Ikhwanuddin, M.; Ma, H. Integrative Transcriptome Sequencing Reveals the Molecular Difference of Maturation Process of Ovary and Testis in Mud Crab Scylla paramamosain. Front. Mar. Sci. 2021, 8, 658091. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, W.; Zhang, F.; Ma, C.; Liu, Z.; Yang, M.H.; Chen, W.; Li, Q.; Cui, M.; Jiang, K.; et al. A chromosome-level genome of the mud crab (Scylla paramamosain Estampador) provides insights into the evolution of chemical and light perception in this crustacean. Mol. Ecol. Resour. 2021, 21, 1299–1317. [Google Scholar] [CrossRef]
- Huang, Y.; Niu, B.; Gao, Y.; Fu, L.; Li, W. CD-HIT Suite: A web server for clustering and comparing biological sequences. Bioinformatics 2010, 26, 680–682. [Google Scholar] [CrossRef]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Simao, F.A.; Zdobnov, E.M. BUSCO Update: Novel and Streamlined Workflows along with Broader and Deeper Phylogenetic Coverage for Scoring of Eukaryotic, Prokaryotic, and Viral Genomes. Mol. Biol. Evol. 2021, 38, 4647–4654. [Google Scholar] [CrossRef]
- Kriventseva, E.V.; Kuznetsov, D.; Tegenfeldt, F.; Manni, M.; Dias, R.; Simão, F.A.; Zdobnov, E.M. OrthoDB v10: Sampling the diversity of animal, plant, fungal, protist, bacterial and viral genomes for evolutionary and functional annotations of orthologs. Nucleic Acids Res. 2018, 47, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Pertea, G.; Pertea, M. GFF Utilities: GffRead and GffCompare. F1000Research 2020, 9, 304. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Forslund, K.; Coelho, L.P.; Szklarczyk, D.; Jensen, L.J.; von Mering, C.; Bork, P. Fast Genome-Wide Functional Annotation through Orthology Assignment by eggNOG-Mapper. Mol. Biol. Evol. 2017, 34, 2115–2122. [Google Scholar] [CrossRef]
- Trincado, J.L.; Entizne, J.C.; Hysenaj, G.; Singh, B.; Skalic, M.; Elliott, D.J.; Eyras, E. SUPPA2: Fast, accurate, and uncertainty-aware differential splicing analysis across multiple conditions. Genome Biol. 2018, 19, 40. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics-A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Feng, S.; Xu, M.; Liu, F.; Cui, C.; Zhou, B. Reconstruction of the full-length transcriptome atlas using PacBio Iso-Seq provides insight into the alternative splicing in Gossypium australe. BMC Plant Biol. 2019, 19, 365. [Google Scholar] [CrossRef]
- Ali, A.; Thorgaard, G.H.; Salem, M. PacBio Iso-Seq Improves the Rainbow Trout Genome Annotation and Identifies Alternative Splicing Associated with Economically Important Phenotypes. Front. Genet. 2021, 12, 683408. [Google Scholar] [CrossRef]
- Wang, B.; Kumar, V.; Olson, A.; Ware, D. Reviving the Transcriptome Studies: An Insight into the Emergence of Single-Molecule Transcriptome Sequencing. Front. Genet. 2019, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Sedlazeck, F.J.; Rescheneder, P.; Smolka, M.; Fang, H.; Nattestad, M.; von Haeseler, A.; Schatz, M.C. Accurate detection of complex structural variations using single-molecule sequencing. Nat. Methods 2018, 15, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Koren, S.; Schatz, M.C.; Walenz, B.P.; Martin, J.; Howard, J.T.; Ganapathy, G.; Wang, Z.; Rasko, D.A.; McCombie, W.R.; Jarvis, E.D.; et al. Hybrid error correction and de novo assembly of single-molecule sequencing reads. Nat. Biotechnol. 2012, 30, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Eid, J.; Fehr, A.; Gray, J.; Luong, K.; Lyle, J.; Otto, G.; Peluso, P.; Rank, D.; Baybayan, P.; Bettman, B.; et al. Real-time DNA sequencing from single polymerase molecules. Science 2009, 323, 133–138. [Google Scholar] [CrossRef]
- Fu, S.; Wang, A.; Au, K.F. A comparative evaluation of hybrid error correction methods for error-prone long reads. Genome Biol. 2019, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jain, C.; Aluru, S. A comprehensive evaluation of long read error correction methods. BMC Genom. 2020, 21, 889. [Google Scholar] [CrossRef]
- Yang, X.; Ikhwanuddin, M.; Li, X.; Lin, F.; Wu, Q.; Zhang, Y.; You, C.; Liu, W.; Cheng, Y.; Shi, X.; et al. Comparative Transcriptome Analysis Provides Insights into Differentially Expressed Genes and Long Non-Coding RNAs between Ovary and Testis of the Mud Crab (Scylla paramamosain). Mar. Biotechnol. 2018, 20, 20–34. [Google Scholar] [CrossRef]
- Liu, S.; Chen, G.; Xu, H.; Zou, W.; Yan, W.; Wang, Q.; Deng, H.; Zhang, H.; Yu, G.; He, J.; et al. Transcriptome analysis of mud crab (Scylla paramamosain) gills in response to Mud crab reovirus (MCRV). Fish. Shellfish Immunol. 2017, 60, 545–553. [Google Scholar] [CrossRef]
- Wan, H.; Jia, X.; Zou, P.; Zhang, Z.; Wang, Y. The Single-molecule long-read sequencing of Scylla paramamosain. Sci. Rep. 2019, 9, 12401. [Google Scholar] [CrossRef]
- Lin, J.L.; Shi, X.; Fang, S.B.; Zhang, Y.; You, C.H.; Ma, H.Y.; Lin, F. Comparative transcriptome analysis combining SMRT and NGS sequencing provides novel insights into sex differentiation and development in mud crab (Scylla paramamosain). Aquaculture 2019, 513, 734447. [Google Scholar] [CrossRef]
- Johnson, B.R.; Atallah, J.; Plachetzki, D.C. The importance of tissue specificity for RNA-seq: Highlighting the errors of composite structure extractions. BMC Genom. 2013, 14, 586. [Google Scholar] [CrossRef] [PubMed]
- Naumova, O.Y.; Lee, M.; Rychkov, S.Y.; Vlasova, N.V.; Grigorenko, E.L. Gene expression in the human brain: The current state of the study of specificity and spatiotemporal dynamics. Child. Dev. 2013, 84, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, A.; Honma, K.; Sharma, A.; Kuramitsu, H.K. Multiple functions of the leucine-rich repeat protein LrrA of Treponema denticola. Infect. Immun. 2004, 72, 4619–4627. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vidya, R.; Makesh, M.; Purushothaman, C.S.; Chaudhari, A.; Gireesh-Babu, P.; Rajendran, K.V. Report of leucine-rich repeats (LRRs) from Scylla serrata: Ontogeny, molecular cloning, characterization and expression analysis following ligand stimulation, and upon bacterial and viral infections. Gene 2016, 590, 159–168. [Google Scholar] [CrossRef]
- Sriphaijit, T.; Senapin, S. High expression of a novel leucine-rich repeat protein in hemocytes and the lymphoid organ of the black tiger shrimp Penaeus mouodon. Fish. Shellfish Immunol. 2007, 22, 264–271. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Wang, F.; Xiang, J.; Li, F. Identification and functional study of an LRR domain containing membrane protein in Litopenaeus vannamei. Dev. Comp. Immunol. 2020, 109, 103713. [Google Scholar] [CrossRef]
- Liu, C.I.; Cheng, T.L.; Chen, S.Z.; Huang, Y.C.; Chang, W.T. LrrA, a novel leucine-rich repeat protein involved in cytoskeleton remodeling, is required for multicellular morphogenesis in Dictyostelium discoideum. Dev. Biol. 2005, 285, 238–251. [Google Scholar] [CrossRef][Green Version]
- Kume, K.; Kubota, S.; Koyano, T.; Kanai, M.; Mizunuma, M.; Toda, T.; Hirata, D. Fission yeast leucine-rich repeat protein Lrp1 is essential for cell morphogenesis as a component of the morphogenesis Orb6 network (MOR). Biosci. Biotechnol. Biochem. 2013, 77, 1086–1091. [Google Scholar] [CrossRef]
- Graham, P.L.; Anderson, W.R.; Brandt, E.A.; Xiang, J.; Pick, L. Dynamic expression of Drosophila segmental cell surface-encoding genes and their pair-rule regulators. Dev. Biol. 2019, 447, 147–156. [Google Scholar] [CrossRef]
- Wang, J.; Lee, J.; Liem, D.; Ping, P. HSPA5 Gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum. Gene 2017, 618, 14–23. [Google Scholar] [CrossRef]
- Gething, M.J. Role and regulation of the ER chaperone BiP. Semin. Cell Dev. Biol. 1999, 10, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, P.; Zhao, C.; Qiu, L. The anti-stresses capability of GRP78 in Penaeus monodon: Evidence from in vitro and in vivo studies. Fish. Shellfish Immunol. 2018, 72, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, A.; Miao, Y.; Liao, S.; Ye, C.; Lin, Q. Comparative proteomic identification of the hepatopancreas response to cold stress in white shrimp, Litopenaeus vannamei. Aquaculture 2016, 454, 27–34. [Google Scholar] [CrossRef]
- Luan, W.; Li, F.; Zhang, J.; Wang, B.; Xiang, J. Cloning and expression of glucose regulated protein 78 (GRP78) in Fenneropenaeus chinensis. Mol. Biol. Rep. 2009, 36, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.-Z.; Ma, K.-S. Molecular cloning and expression analysis of glucose-regulated protein 78 (GRP78) gene in silkworm Bombyx mori. Biologia 2013, 68, 559–564. [Google Scholar] [CrossRef]
- Zhao, X.; Gou, X.; Liu, W.; Ma, E.; Moussian, B.; Li, S.; Zhu, K.; Zhang, J. The wing-specific cuticular protein LmACP7 is essential for normal wing morphogenesis in the migratory locust. Insect Biochem Mol. Biol 2019, 112, 103206. [Google Scholar] [CrossRef]
- Rocha, J.; Garcia-Carreño, F.L.; Muhlia-Almazán, A.; Peregrino-Uriarte, A.B.; Yépiz-Plascencia, G.; Córdova-Murueta, J.H. Cuticular chitin synthase and chitinase mRNA of whiteleg shrimp Litopenaeus vannamei during the molting cycle. Aquaculture 2012, 330–333, 111–115. [Google Scholar] [CrossRef]
- Hardardottir, H.M.; Male, R.; Nilsen, F.; Eichner, C.; Dondrup, M.; Dalvin, S. Chitin synthesis and degradation in Lepeophtheirus salmonis: Molecular characterization and gene expression profile during synthesis of a new exoskeleton. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2019, 227, 123–133. [Google Scholar] [CrossRef]
- Barriga-Montoya, C.; de la O-Martínez, A.; Fuentes-Pardo, B.; Gomez-Lagunas, F. Desensitization and recovery of crayfish photoreceptors. Dependency on circadian time, and pigment-dispersing hormone. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 203, 297–303. [Google Scholar] [CrossRef]
- Paschold, A.; Jia, Y.; Marcon, C.; Lund, S.; Larson, N.B.; Yeh, C.T.; Ossowski, S.; Lanz, C.; Nettleton, D.; Schnable, P.S.; et al. Complementation contributes to transcriptome complexity in maize (Zea mays L.) hybrids relative to their inbred parents. Genome Reserch 2012, 22, 2445–2454. [Google Scholar] [CrossRef]
- Xiao, Q.; Huang, Z.; Shen, Y.; Gan, Y.; Wang, Y.; Gong, S.; Lu, Y.; Luo, X.; You, W.; Ke, C. Transcriptome analysis reveals the molecular mechanisms of heterosis on thermal resistance in hybrid abalone. BMC Genom. 2021, 22, 650. [Google Scholar] [CrossRef] [PubMed]

| Sample | Types | Numbers of Sequences | Length of Isoforms | N50 9 | ||
|---|---|---|---|---|---|---|
| Min | Mean | Max | ||||
| SPA 1 | Subreads | 12,705,473 | 51 | 1636.8 | 211,052 | 1962 |
| CCS 4 | 265,745 | 104 | 1904 | 14,128 | 2177 | |
| FL 5 | 226,748 | 99 | 1814.2 | 10,492 | 2109 | |
| FLCN 6 | 221,222 | 68 | 1754.5 | 9608 | 2051 | |
| HQ 7 | 11,643 | 72 | 1780.5 | 6442 | 2053 | |
| LQ 8 | 14 | 321 | 1883.8 | 3922 | 1839 | |
| NH 2 | Subreads | 15,425,110 | 50 | 1491.4 | 115,220 | 1656 |
| CCS | 420,743 | 67 | 1755.3 | 11,556 | 1939 | |
| FL | 345,977 | 102 | 1613.7 | 9605 | 1735 | |
| FLCN | 308,364 | 51 | 1495.2 | 9573 | 1534 | |
| HQ | 16,587 | 70 | 1630.5 | 6778 | 2012 | |
| LQ | 12 | 452 | 857.1 | 1617 | 951 | |
| DH 3 | Subreads | 15,459,279 | 50 | 1437.4 | 118,301 | 1590 |
| CCS | 367,638 | 70 | 1782.2 | 14,497 | 1994 | |
| FL | 253,545 | 104 | 1683.4 | 11,344 | 1926 | |
| FLCN | 221,371 | 50 | 1568.1 | 9804 | 1769 | |
| HQ | 10,336 | 69 | 1661.6 | 6526 | 2132 | |
| LQ | 9 | 115 | 604.1 | 1880 | 654 | |
| Samples | Numbers of Transcript Isoforms after Collapsing Redundant Isoforms | Length of Collapsing Redundant Isoforms | N50 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Reference Genome | Fake Genome | Unmap-Ped | Merge | Min | Max | Mean | ||
| SPA 1 | 9427 | 466 | 2 | 9872 | 92 | 6442 | 1798.9 | 2079 |
| NH 2 | 11,639 | 752 | 3 | 12,382 | 118 | 6778 | 1663.6 | 2041 |
| DH 3 | 7858 | 661 | 3 | 8508 | 80 | 6526 | 1685.5 | 2161 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, S.; Yu, X.; Chen, H.; Zhang, Y.; Wu, Q.; Tan, H.; Song, J.; Saqib, H.S.A.; Farhadi, A.; Ikhwanuddin, M.; et al. Full-Length Transcriptome Reconstruction Reveals the Genetic Mechanisms of Eyestalk Displacement and Its Potential Implications on the Interspecific Hybrid Crab (Scylla serrata ♀ × S. paramamosain ♂). Biology 2022, 11, 1026. https://doi.org/10.3390/biology11071026
Ye S, Yu X, Chen H, Zhang Y, Wu Q, Tan H, Song J, Saqib HSA, Farhadi A, Ikhwanuddin M, et al. Full-Length Transcriptome Reconstruction Reveals the Genetic Mechanisms of Eyestalk Displacement and Its Potential Implications on the Interspecific Hybrid Crab (Scylla serrata ♀ × S. paramamosain ♂). Biology. 2022; 11(7):1026. https://doi.org/10.3390/biology11071026
Chicago/Turabian StyleYe, Shaopan, Xiaoyan Yu, Huiying Chen, Yin Zhang, Qingyang Wu, Huaqiang Tan, Jun Song, Hafiz Sohaib Ahmed Saqib, Ardavan Farhadi, Mhd Ikhwanuddin, and et al. 2022. "Full-Length Transcriptome Reconstruction Reveals the Genetic Mechanisms of Eyestalk Displacement and Its Potential Implications on the Interspecific Hybrid Crab (Scylla serrata ♀ × S. paramamosain ♂)" Biology 11, no. 7: 1026. https://doi.org/10.3390/biology11071026
APA StyleYe, S., Yu, X., Chen, H., Zhang, Y., Wu, Q., Tan, H., Song, J., Saqib, H. S. A., Farhadi, A., Ikhwanuddin, M., & Ma, H. (2022). Full-Length Transcriptome Reconstruction Reveals the Genetic Mechanisms of Eyestalk Displacement and Its Potential Implications on the Interspecific Hybrid Crab (Scylla serrata ♀ × S. paramamosain ♂). Biology, 11(7), 1026. https://doi.org/10.3390/biology11071026







