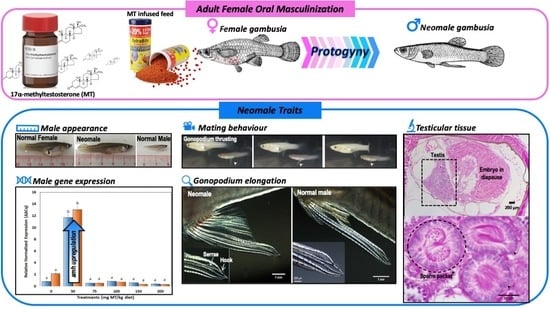
Masculinization of Adult Gambusia holbrooki: A Case of Recapitulation of Protogyny in a Gonochorist?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Wild Specimens
2.2. Experimental Fish and Rearing Conditions
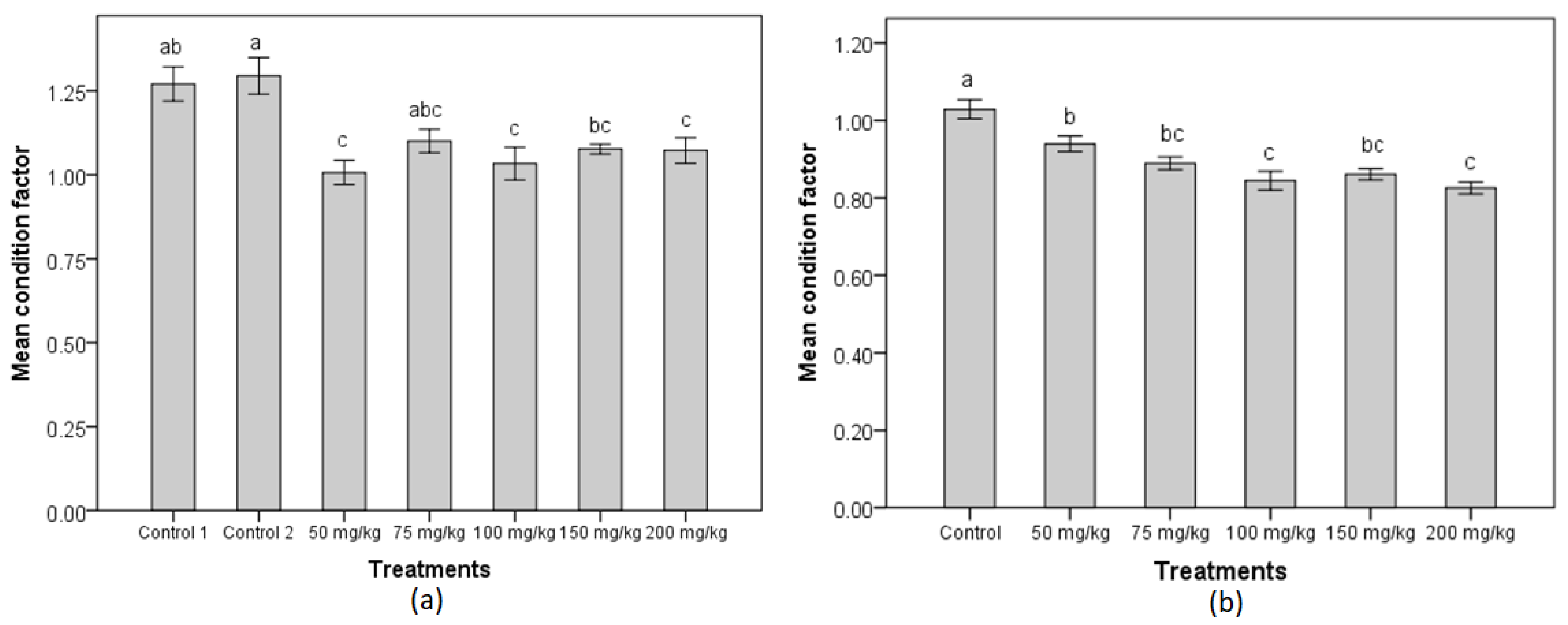
2.3. Condition Factor
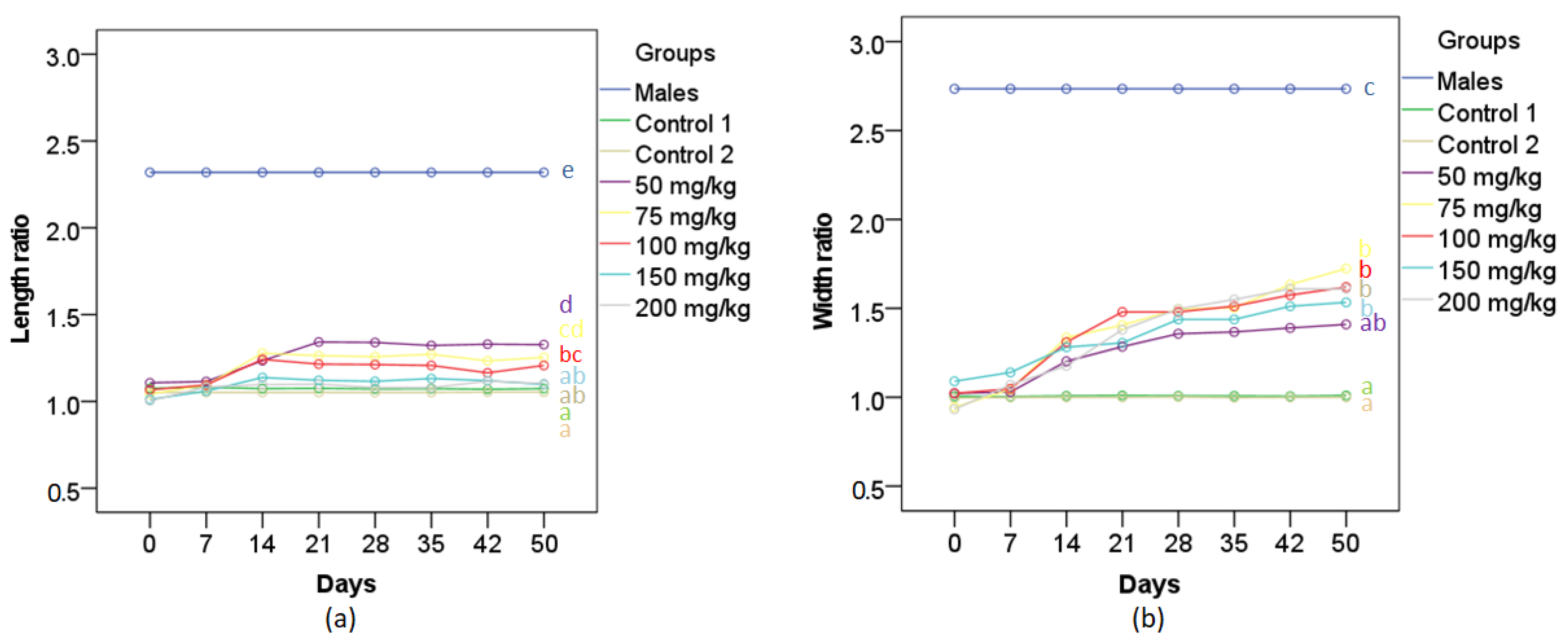
2.4. Photography and IMAGE Analysis
2.5. Gonad Histology
2.6. Gene Expression
2.6.1. Total RNA Extraction, gDNA Removal and Reverse Transcription
2.6.2. Gene Expression Analysis
2.7. Behavioural Interactions
2.8. Statistical Analysis
3. Results
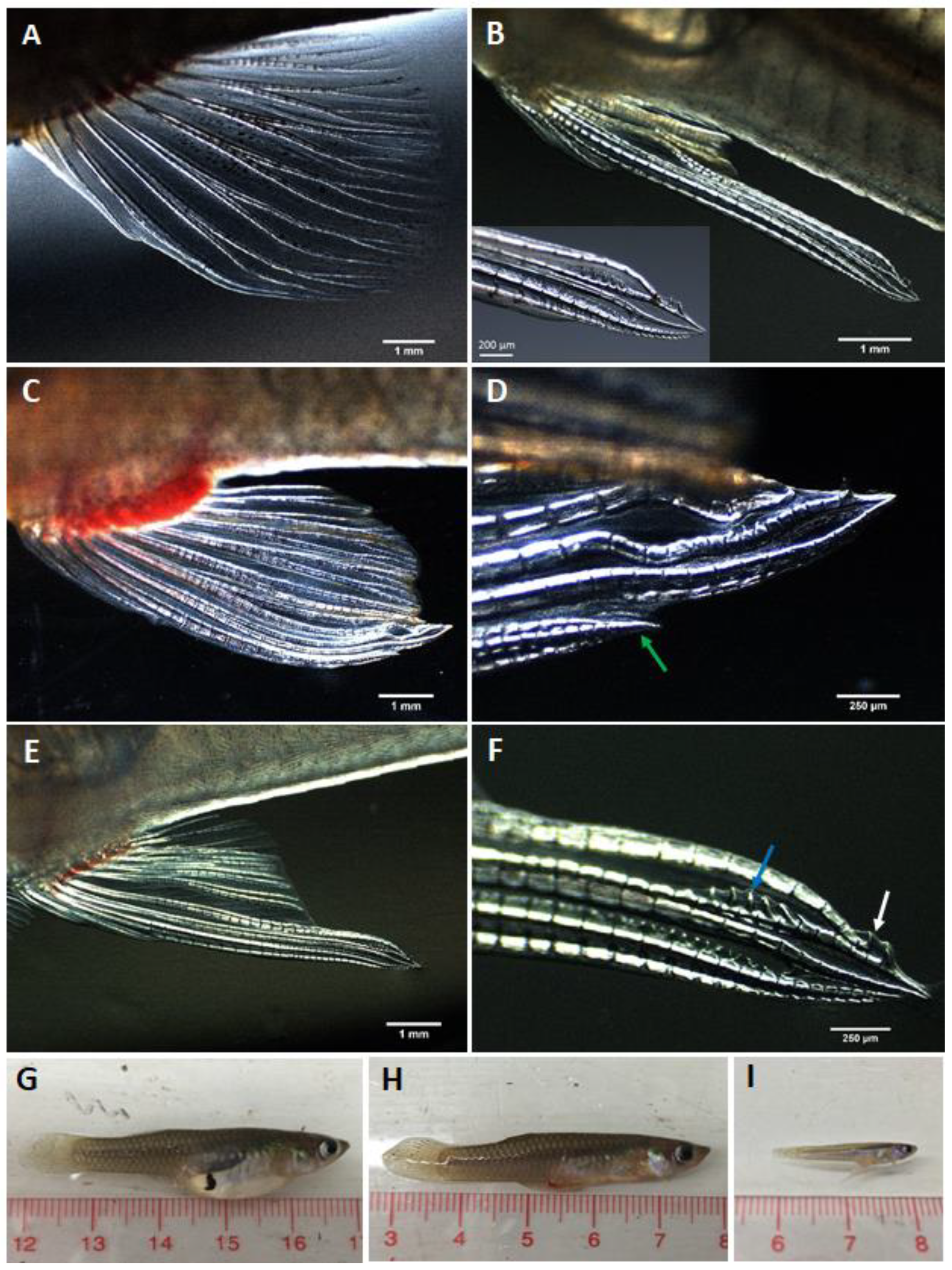
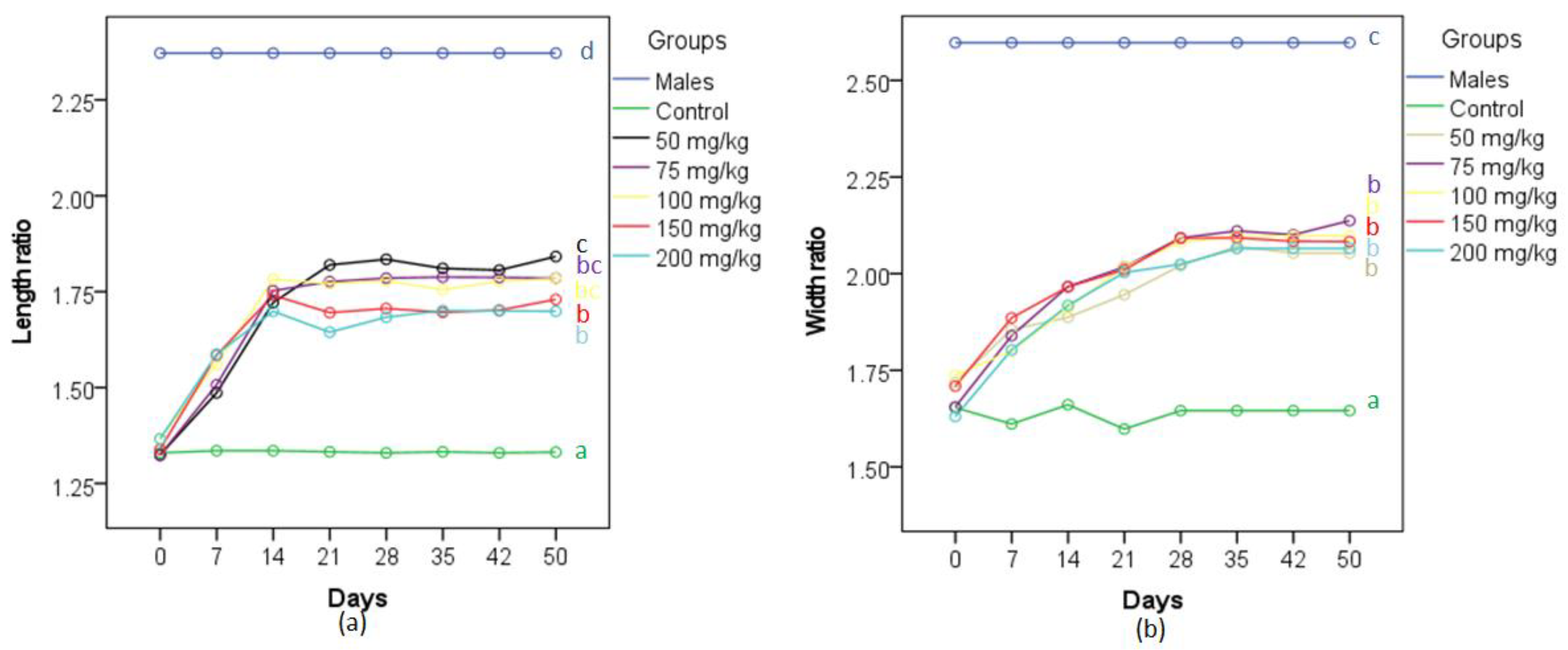
3.1. Gonopodial and Body Morphology of Adult Females Following MT Treatment
3.1.1. Repeat Gravid Females Treated with MT
3.1.2. Maiden Gravid Females Treated with MT
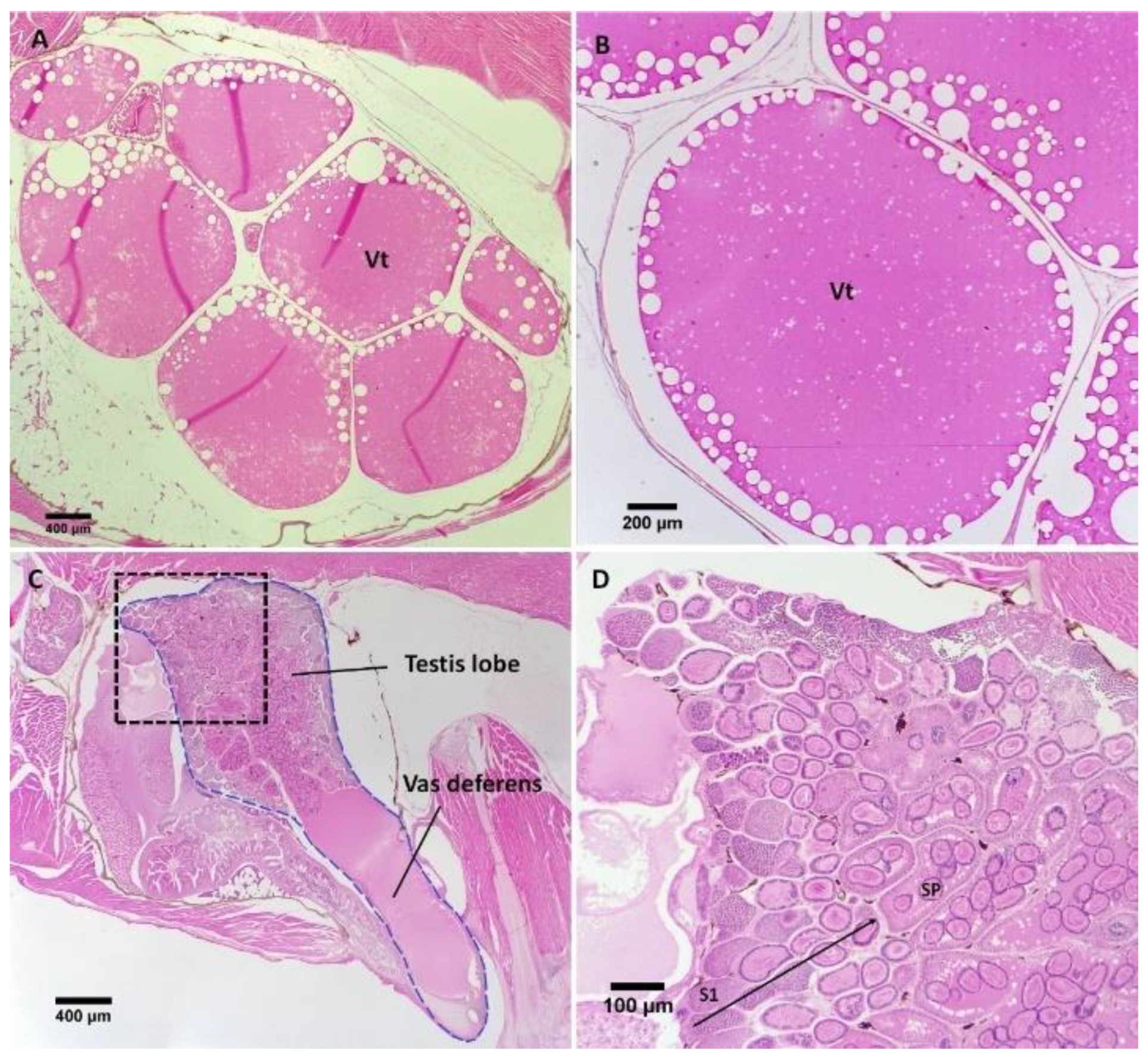
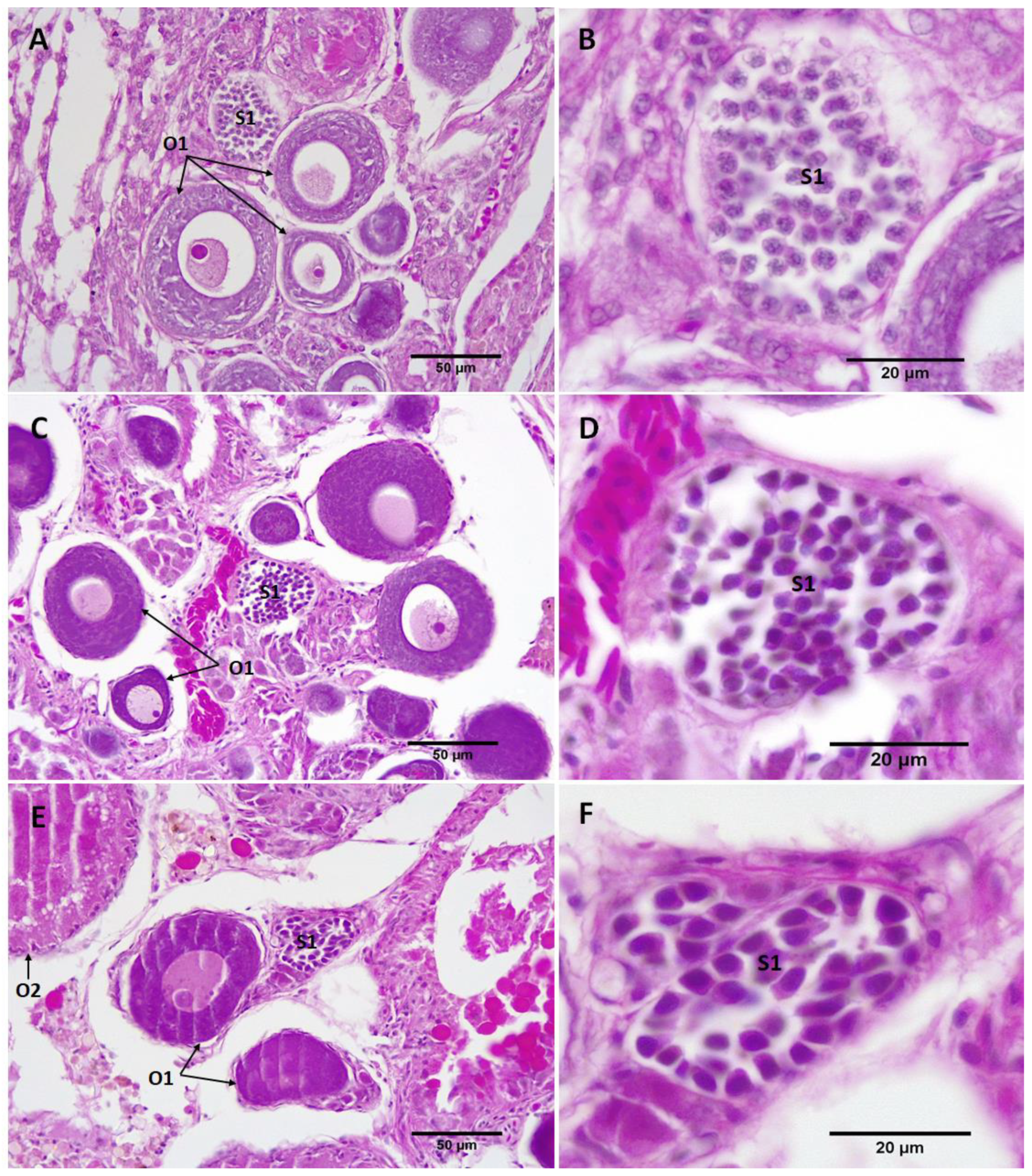
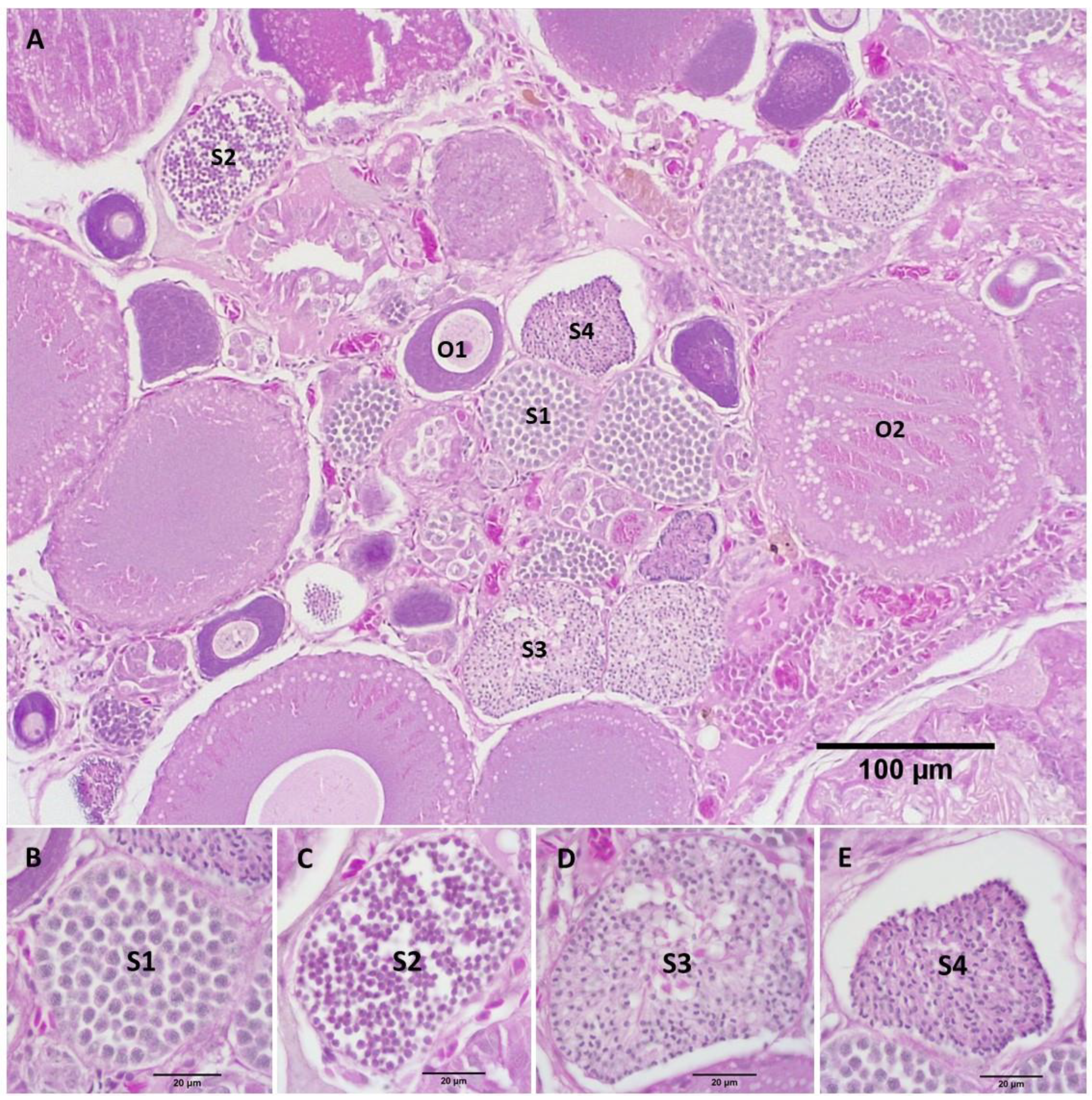
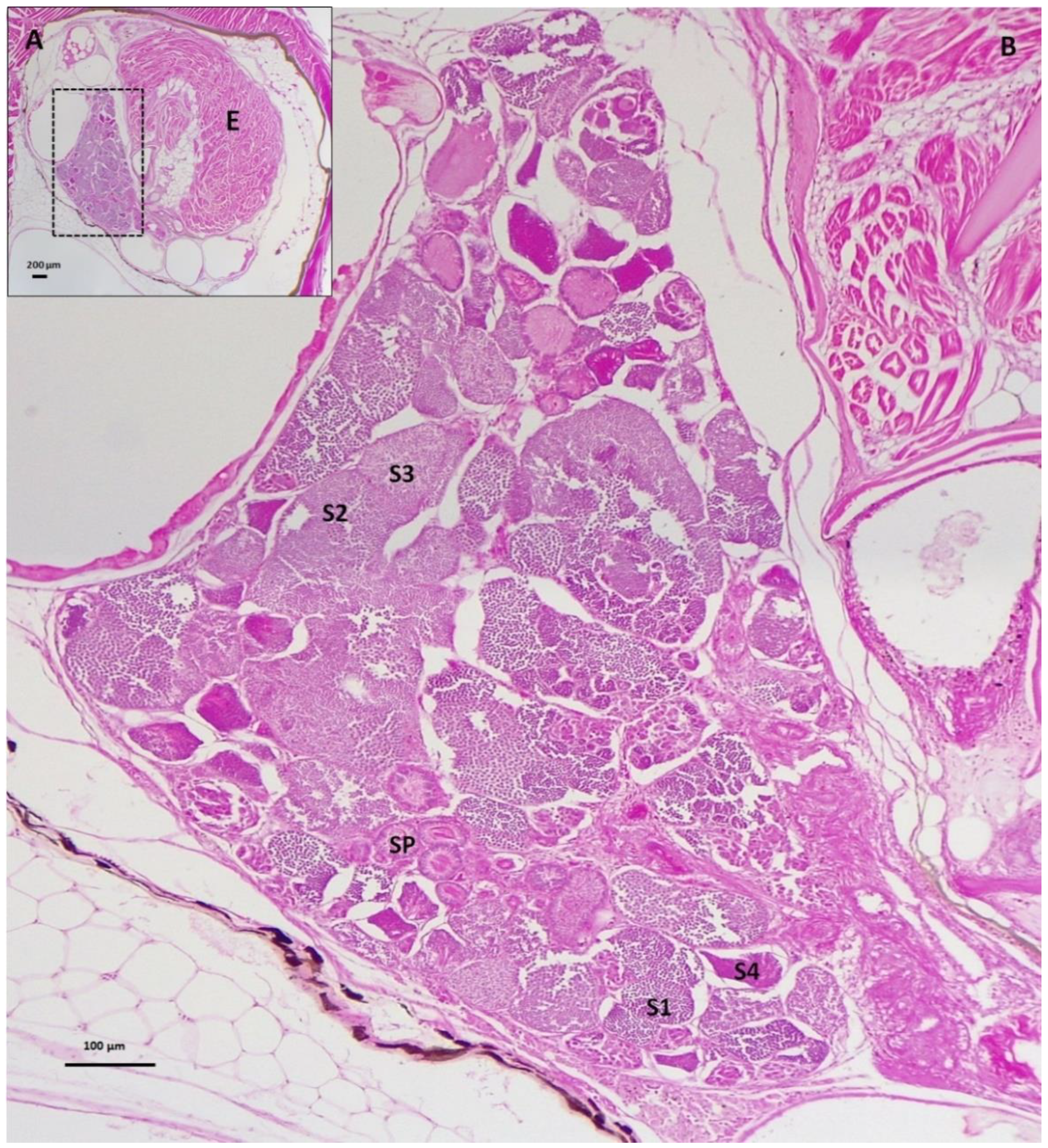
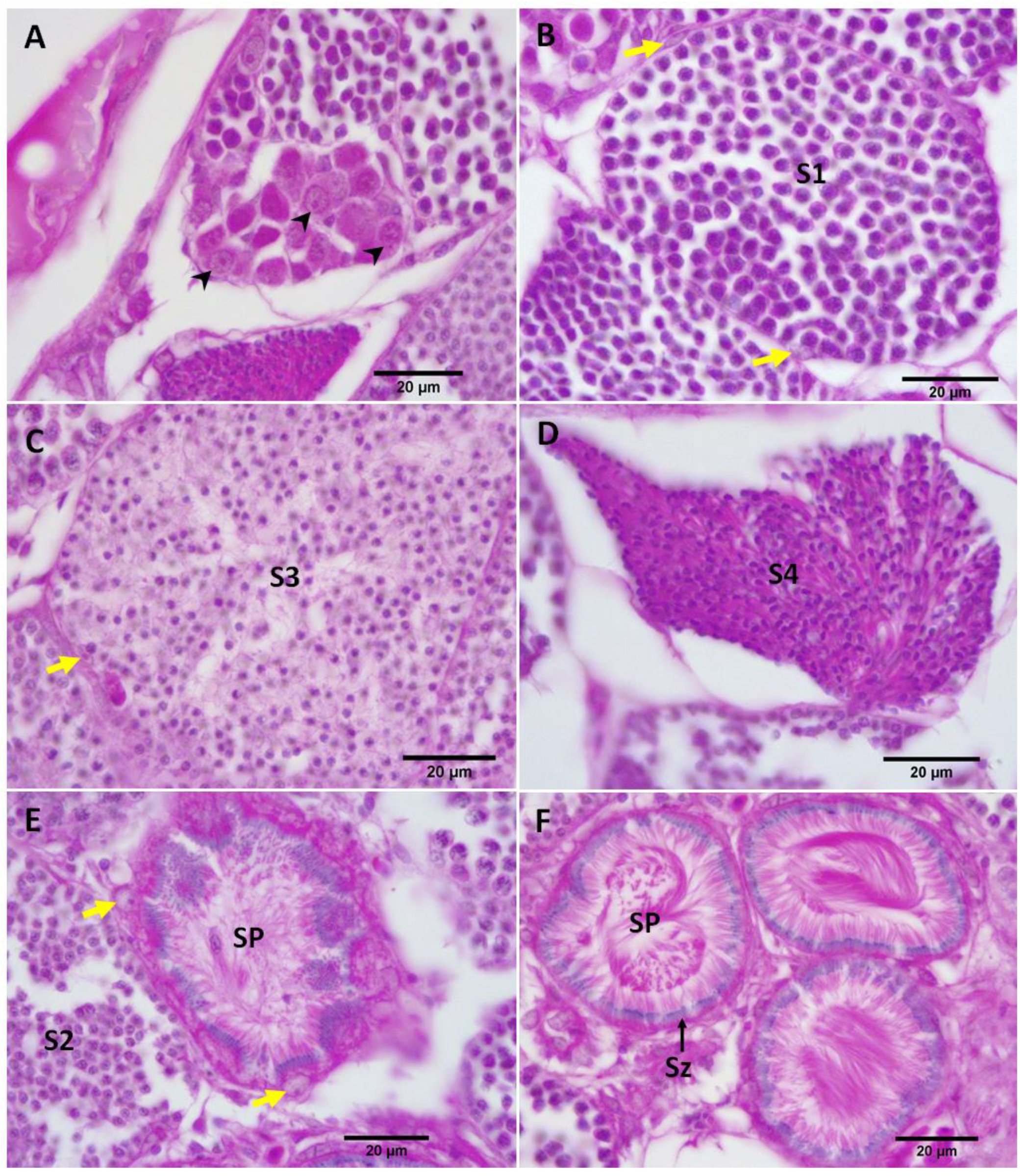
3.2. Gonad Histology
3.2.1. Control Female and Male Gonads
3.2.2. Repeat Gravid Females Treated with MT
3.2.3. Maiden Gravid Females Treated with MT
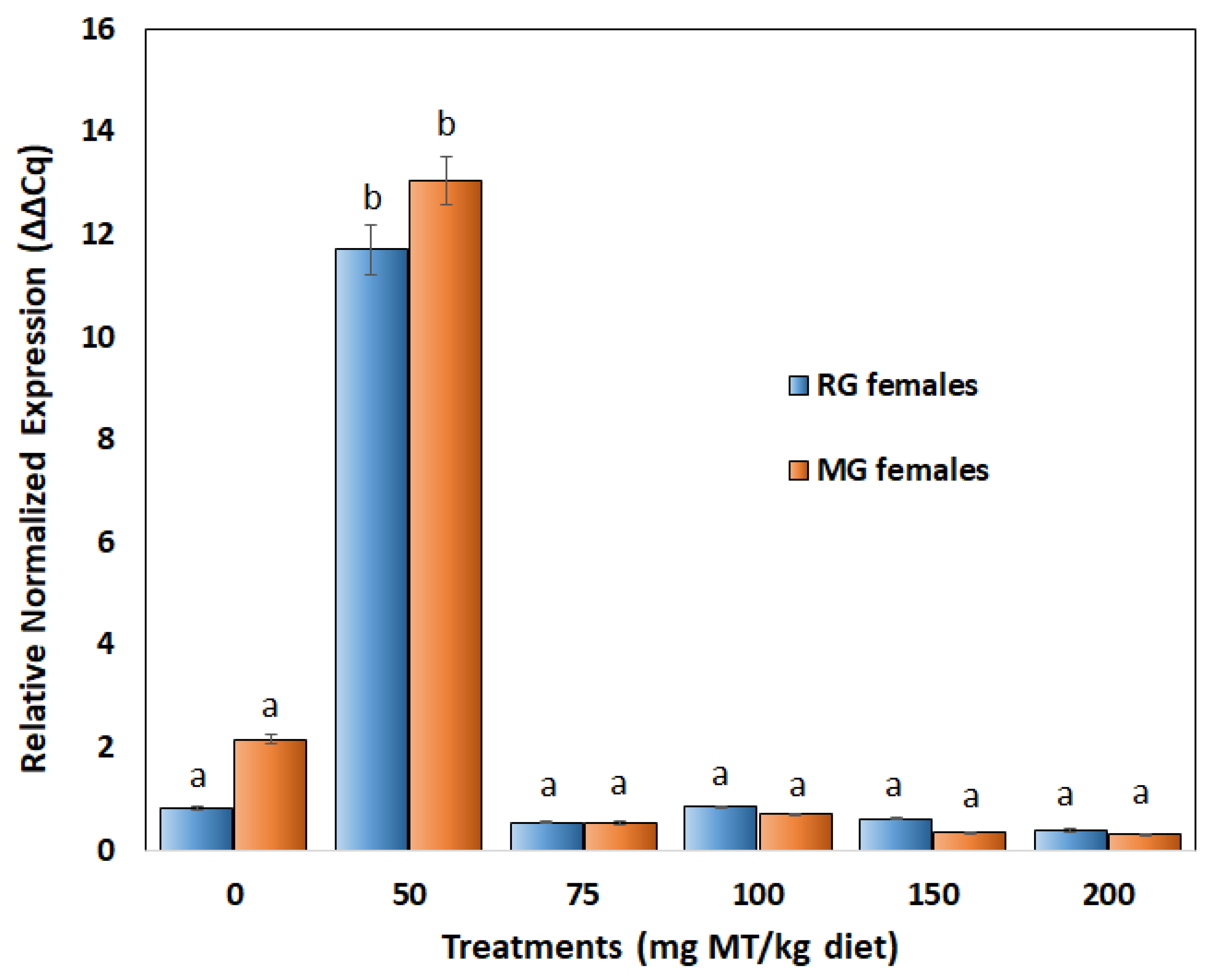
3.3. Expression of Amh in Ovaries of Treated Fish
3.4. Behavioral Interactions
3.5. Stability of Sex Reversal
4. Discussion
4.1. MT Exposure Had a Masculinizing Effect on Anal Fin, Body Shape and Gonad Morphology
4.2. The Amh Response Suggests a Narrow Physiological Window of Susceptibility for Sex Reversal of Adult Females
4.3. Treated Adult Females Mimic the Behavior of Normal Males
4.4. Stability of Sex Reversal
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Todd, E.V.; Liu, H.; Muncaster, S.; Gemmell, N.J. Bending Genders: The Biology of Natural Sex Change in Fish. Sex. Dev. 2016, 10, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T. Sex Differentiation. In Fish Physiology; Hoar, W.S., Randall, D.J., Eds.; Academic Press: Cambridge, MA, USA, 1969; Volume 3, pp. 117–175. [Google Scholar]
- Pandian, T. Genetic Sex Differentiation in Fish; CRC Press: Boca Raton, FL, USA, 2012; Volume 1. [Google Scholar]
- Kuwamura, T.; Sunobe, T.; Sakai, Y.; Kadota, T.; Sawada, K. Hermaphroditism in fishes: An annotated list of species, phylogeny, and mating system. Ichthyol. Res. 2020, 67, 341–360. [Google Scholar] [CrossRef]
- Rocha, M.; Arukwe, A.; Kapoor, B. Fish Reproduction; Science Publishers: Enfield, NH, USA, 2008. [Google Scholar]
- de Mitcheson, Y.S.; Liu, M. Functional hermaphroditism in teleosts. Fish Fish. 2008, 9, 1–43. [Google Scholar] [CrossRef]
- Orlando, E.F.; Davis, W.P.; Guillette, L.J., Jr. Aromatase activity in the ovary and brain of the eastern mosquitofish (Gambusia holbrooki) exposed to paper mill effluent. Environ. Health Perspect. 2002, 110, 429–433. [Google Scholar] [CrossRef] [Green Version]
- Golan, M.; Levavi-Sivan, B. Artificial masculinization in tilapia involves androgen receptor activation. Gen. Comp. Endocrinol. 2014, 207, 50–55. [Google Scholar] [CrossRef]
- Pandian, T.; Sheela, S. Hormonal induction of sex reversal in fish. Aquaculture 1995, 138, 1–22. [Google Scholar] [CrossRef]
- Beardmore, J.; Mair, G.; Lewis, R. Monosex male production in finfish as exemplified by tilapia: Applications, problems, and prospects. Reprod. Biotechnol. Finfish Aquac. 2001, 283–301. [Google Scholar] [CrossRef]
- Takahashi, H. Modification of the development of female reproductive organs in the guppy, Poecilia reticulata, following an androgen treatment in their juvenile period. Bull. Fac. Fish. Hokkaido Univ. 1974, 25, 174–199. [Google Scholar]
- Low, W.; Theo, S.; Lim, L.; Phang, C. Anabolic and androgenic effects of 17-methyltestosterone on guppies. Singap. J. Prim. Ind. 1994, 22, 81–89. [Google Scholar]
- Chakraborty, S.B.; Molnár, T.; Hancz, C. Effects of methyltestosterone, tamoxifen, genistein and Basella alba extract on masculinization of guppy (Poecilia reticulata). J. Appl. Pharm. Sci. 2012, 2, 48. [Google Scholar]
- Mousavi-Sabet, H.; Ghasemnezhad, H. Masculinization, mortality and growth rates of swordtail Xiphophorus hellerii (Poeciliidae) affected by methyltestosterone. Poeciliid Res. 2013, 3, 7–13. [Google Scholar]
- George, T.; Pandian, T. Dietary administration of androgens induces sterility in the female-heterogametic black molly, Poecilia sphenops (Cuvier & Valenciennes, 1846). Aquac. Res. 1998, 29, 167–175. [Google Scholar]
- Yanong, R.P.; Hill, J.E.; Daniels, C.J.; Watson, C.A. Efficacy of 17-α-methyltestosterone for expression of male secondary sexual characteristics in the green swordtail. N. Am. J. Aquac. 2006, 68, 224–229. [Google Scholar] [CrossRef]
- Amiri-Moghaddam, J.; Maniei, F.; Mahboobi-Soofiani, N.; Asadollah, S. Use of 17-α-methyltestosterone for production of male secondary sexual characteristics in the adult female green swordtail (Xiphophorus hellerii). Aquac. Aquar. Conserv. Legis.-Int. J. Bioflux Soc. (AACL Bioflux) 2010, 3, 1–8. [Google Scholar]
- Ramee, S.W.; Lipscomb, T.N.; Wood, A.L.; Watson, C.A.; DiMaggio, M.A. The effect of dietary17 alpha-methyltestosteroneadministration on secondary sex coloration in adult female Rosy Barbs and Dwarf Gouramis. J. World Aquac. Soc. 2020, 51, 1119–1131. [Google Scholar] [CrossRef]
- Steinbach, C.; Císař, P.; Šauer, P.; Klicnarová, J.; Schmidt-Posthaus, H.; Golovko, O.; Kroupová, H.K. Synthetic progestin etonogestrel negatively affects mating behavior and reproduction in Endler’s guppies (Poecilia wingei). Sci. Total Environ. 2019, 663, 206–215. [Google Scholar] [CrossRef]
- Angus, R.A.; McNatt, H.B.; Howell, W.M.; Peoples, S.D. Gonopodium development in normal male and 11-ketotestosterone-treated female mosquitofish (Gambusia affinis): A quantitative study using computer image analysis. Gen. Comp. Endocrinol. 2001, 123, 222–234. [Google Scholar] [CrossRef]
- Turner, C.L. Morphogenesis of the gonopodium in Gambusia affinis affinis. J. Morphol. 1941, 69, 161–185. [Google Scholar] [CrossRef]
- Leusch, F.D.L.; Chapman, H.F.; Kay, G.W.; Gooneratne, S.R.; Tremblay, L.A. Anal fin morphology and gonadal histopathology in mosquitofish (Gambusia holbrooki) exposed to treated municipal sewage effluent. Arch. Environ. Contam. Toxicol. 2006, 50, 562–574. [Google Scholar] [CrossRef]
- Norazmi-Lokman, N.H.; Purser, G.J.; Patil, J.G. Gravid Spot Predicts Developmental Progress and Reproductive Output in a Livebearing Fish, Gambusia holbrooki. PLoS ONE 2016, 11, e0147711. [Google Scholar] [CrossRef]
- Turner, C.L. Gonopodial characteristics produced in the anal fins of females of Gambusia affinis affinis by treatment with ethinyl testosterone. Biol. Bull. 1941, 80, 371–383. [Google Scholar] [CrossRef]
- Bahamonde, P.A.; Munkittrick, K.R.; Martyniuk, C.J. Intersex in teleost fish: Are we distinguishing endocrine disruption from natural phenomena? Gen. Comp. Endocrinol. 2013, 192, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Antuofermo, E.; Ariu, R.; Burrai, G.P.; Polinas, M.; Sanna, M.A.; Esposito, G.; Prearo, M.; Pais, A. First evidence of intersex condition in extensively reared mullets from Sardinian lagoons (central–western Mediterranean, Italy). Ital. J. Anim. Sci. 2017, 16, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Honji, R.M.; Caneppele, D.; Pandolfi, M.; Lo Nostro, F.L.; Moreira, R.G. A case of intersex occurrence in Steindachneridion parahybae (Steindachner, 1877) (Siluriformes: Pimelodidae) under captivity condition: A cytogenetic and morphological study. Neotrop. Ichthyol. 2016, 14, 12. [Google Scholar] [CrossRef] [Green Version]
- Abdel-moneim, A.; Coulter, D.P.; Mahapatra, C.T.; Sepúlveda, M.S. Intersex in fishes and amphibians: Population implications, prevalence, mechanisms and molecular biomarkers. J. Appl. Toxicol. 2015, 35, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.; Mahapatra, C.T.; Hatef, A.; Sepulveda, M.S. Ovarian structure protein 1: A sensitive molecular biomarker of gonadal intersex in female Japanese medaka after androgen exposure. Environ. Toxicol. Chem. 2015, 34, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Galbreath, P.F.; Adams, N.D.; Sherrill, L.W. Successful sex reversal of brook trout with 17 alpha-methyldihydrotestosterone treatments. N. Am. J. Aquac. 2003, 65, 235–239. [Google Scholar] [CrossRef]
- Atar, H.H.; Bekcan, S.; Dogankaya, L. Effects of different hormones on sex reversal of rainbow trout (Oncorhynchus mykiss) and production of all-female populations. Biotechnol. Biotechnol. Equip. 2009, 23, 1509–1514. [Google Scholar] [CrossRef] [Green Version]
- Kwan, T.N.; Patil, J.G. Sex biased expression of anti-Mullerian hormone (amh) gene in a live bearing fish, Gambusia holbrooki: Evolutionary implications and potential role in sex differentiation. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2019, 231, 59–66. [Google Scholar] [CrossRef]
- Cate, R.; Mattaliano, R.; Hession, C.; Tizard, R.; Farber, N.; Cheung, A.; Ninfa, E.; Frey, A.; Gash, D.; Chow, E. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell 1986, 45, 685–698. [Google Scholar] [CrossRef]
- Rodríguez-Marí, A.; Yan, Y.-L.; BreMiller, R.A.; Wilson, C.; Cañestro, C.; Postlethwait, J.H. Characterization and expression pattern of zebrafish anti-Müllerian hormone (amh) relative to sox9a, sox9b, and cyp19a1a, during gonad development. Gene Expr. Patterns 2005, 5, 655–667. [Google Scholar] [CrossRef]
- Pfennig, F.; Standke, A.; Gutzeit, H.O. The role of Amh signaling in teleost fish–multiple functions not restricted to the gonads. Gen. Comp. Endocrinol. 2015, 223, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Parks, L.G.; Lambright, C.S.; Orlando, E.F.; Guillette, L.J.; Ankley, G.T.; Gray, L.E. Masculinization of Female Mosquitofish in Kraft Mill Effluent-Contaminated Fenholloway River Water Is Associated with Androgen Receptor Agonist Activity. Toxicol. Sci. 2001, 62, 257–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, W.M.; Black, D.A.; Bortone, S.A. Abnormal expression of secondary sex characters in a population of mosquitofish, Gambusia affinis holbrooki: Evidence for environmentally-induced masculinization. Copeia 1980, 1980, 676–681. [Google Scholar] [CrossRef] [Green Version]
- Brockmeier, E.K.; Ogino, Y.; Iguchi, T.; Barber, D.S.; Denslow, N.D. Effects of 17β-trenbolone on Eastern and Western mosquitofish (Gambusia holbrooki and G. affinis) anal fin growth and gene expression patterns. Aquat. Toxicol. 2013, 128, 163–170. [Google Scholar] [CrossRef]
- Patil, J.G.; Norazmi-Lokman, N.H.; Kwan, T.N. Reproductive viability of paradoxically masculinized Gambusia holbrooki generated following diethylstilbestrol (DES) treatment. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 248, 110468. [Google Scholar] [CrossRef]
- de Castro, P.L.; Patil, J.G. Comparative gonad histology and semen quality of normal (XY) and neo-males (XX) of Atlantic salmon (Salmo salar). Aquac. Res. 2019, 50, 3171–3180. [Google Scholar] [CrossRef]
- Gutierrez, J.B.; Teem, J.L. A model describing the effect of sex-reversed YY fish in an established wild population: The use of a Trojan Y chromosome to cause extinction of an introduced exotic species. J. Theor. Biol. 2006, 241, 333–341. [Google Scholar] [CrossRef]
- Patil, J.G. An Adaptive Management Plan for Eradication of Gambusia Holbrooki from Tasmania, Australia; Inland Fisheries Service Tasmania: New Norfolk, TAS, Australia, 2012; p. 30. [Google Scholar]
- Norazmi-Lokman, N.H.; Purser, G.J.; Patil, J.G. Efficacy of estradiol in feminising the eastern mosquitofish, Gambusia holbrooki: Advance towards developing a genetic control option. Mar. Freshw. Res. 2021, 72, 1657–1666. [Google Scholar] [CrossRef]
- Hagenaars, A.; Knapen, D.; Meyer, I.; Van der Ven, K.; Hoff, P.; De Coen, W. Toxicity evaluation of perfluorooctane sulfonate (PFOS) in the liver of common carp (Cyprinus carpio). Aquat. Toxicol. 2008, 88, 155–163. [Google Scholar] [CrossRef]
- Rätz, H.-J.; Lloret, J. Variation in fish condition between Atlantic cod (Gadus morhua) stocks, the effect on their productivity and management implications. Fish. Res. 2003, 60, 369–380. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turan, F.; Çek, Ş.; Atik, E. Production of monosex male guppy, Poecilia reticulata, by 17α-methyltestosterone. Aquac. Res. 2006, 37, 200–203. [Google Scholar] [CrossRef]
- Peden, A.E. The function of gonopodial parts and behavioral pattern during copulation by Gambusia (Poeciliidae). Can. J. Zool. 1972, 50, 955–968. [Google Scholar] [CrossRef]
- Vega-Trejo, R.; O’Dea, R.E.; Jennions, M.D.; Head, M.L. The effects of familiarity and mating experience on mate choice in mosquitofish, Gambusia holbrooki. Behav. Ecol. 2014, 25, 1205–1211. [Google Scholar] [CrossRef] [Green Version]
- Laerd Statistics. Two-Way Mixed ANOVA Using SPSS Statistics. Available online: https://statistics.laerd.com/spsstutorials/two-way-repeated-measures-anova-using-spss-statistics.php (accessed on 3 July 2020).
- Laerd Statistics. Kruskal-Wallis H Test Using SPSS Statistics. Available online: https://statistics.laerd.com/spsstutorials/kruskal-wallis-h-test-using-spss-statistics.php (accessed on 4 July 2020).
- Fishelson, L. Protogynous sex reversal in the fish Anthias squamipinnis (Teleostei, Anthiidae) regulated by the presence or absence of a male fish. Nature 1970, 227, 90–91. [Google Scholar] [CrossRef]
- Turner, C.L. Morphogenesis of the gonopodial suspensorium in Gambusia affinis and the induction of male suspensorial characters in the female by androgenic hormones. J. Exp. Zool. 1942, 91, 167–193. [Google Scholar] [CrossRef]
- Nguyen, H.; Bell, J.D.; Patil, J.G. Daily ageing to delineate population dynamics of the invasive fish Gambusia holbrooki: Implications for management and control. Biol. Invasions 2021, 23, 2261–2270. [Google Scholar] [CrossRef]
- Margolisnunno, H.; Halpernsebold, L.; Schreibman, M.P. Immunocytochemical changes in serotonin in the forebrain and pituitary of aging fish. Neurobiol. Aging 1986, 7, 17–21. [Google Scholar]
- Wang, B.; Wang, X.L.; Zou, Y.Y. Association between hormone receptors and HER-2/neu is age-related. Int. J. Clin. Exp. Pathol. 2015, 8, 8472–8479. [Google Scholar]
- Sridevi, P.; Chaitanya, R.K.; Prathibha, Y.; Balakrishna, S.L.; Dutta-Gupta, A.; Senthilkumaran, B. Early Exposure of 17 alpha-Ethynylestradiol and Diethylstilbestrol Induces Morphological Changes and Alters Ovarian Steroidogenic Pathway Enzyme Gene Expression in Catfish, Clarias gariepinus. Environ. Toxicol. 2015, 30, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Norazmi-Lokman, N.H. Hormonal Feminization and Associated Reproductive Impacts in the Eastern Mosquitofish Gambusia holbrooki. Ph.D. Thesis, The University of Tasmania, Launceston, Australia, 2016. [Google Scholar]
- Candi, G.; Castriota, L.; Andaloro, F.; Finoia, M.G.; Marino, G. Reproductive cycle and sex inversion in razor fish, a protogynous labrid in the southern Mediterranean Sea. J. Fish Biol. 2004, 64, 1498–1513. [Google Scholar] [CrossRef]
- Meffe, G.K.; Snelson, F. Ecology and Evolution of Livebearing Fishes; Prentice Hall: Englewood Cliffs, NJ, USA, 1989. [Google Scholar]
- Ogino, Y.; Katoh, H.; Yamada, G. Androgen dependent development of a modified anal fin, gonopodium, as a model to understand the mechanism of secondary sexual character expression in vertebrates. FEBS Lett. 2004, 575, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalfe, N.; Van Leeuwen, T.; Killen, S. Does individual variation in metabolic phenotype predict fish behaviour and performance? J. Fish Biol. 2016, 88, 298–321. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.Q.; Nguyen, A.T.; Kjorsvik, E.; Nguyen, M.D.; Arukwe, A. Seasonal reproductive cycle of Waigieu seaperch (Psammoperca waigiensis). Aquac. Res. 2012, 43, 815–830. [Google Scholar] [CrossRef]
- Josso, N.; Racine, C.; di Clemente, N.; Rey, R.; Xavier, F. The role of anti-Müllerian hormone in gonadal development. Mol. Cell. Endocrinol. 1998, 145, 3–7. [Google Scholar] [CrossRef]
- Grier, H.J. Comparative organization of Sertoli cells including the Sertoli cell barrier. In The Sertoli Cells; Rusell, L.D., Griswold, M.D., Eds.; Cache River Press: Clearwater, FL, USA, 1993; pp. 703–739. [Google Scholar] [CrossRef]
- Toft, G.; Guillette, L.J. Decreased sperm count and sexual behavior in mosquitofish exposed to water from a pesticide-contaminated lake. Ecotoxicol. Environ. Saf. 2005, 60, 15–20. [Google Scholar] [CrossRef]
- Frankel, T.E.; Meyer, M.T.; Orlando, E.F. Aqueous exposure to the progestin, levonorgestrel, alters anal fin development and reproductive behavior in the eastern mosquitofish (Gambusia holbrooki). Gen. Comp. Endocrinol. 2016, 234, 161–169. [Google Scholar] [CrossRef] [Green Version]
| Treatment Doses | Tissue Area (µm2) | ||
|---|---|---|---|
| (mg MT/kg Diet) | Total Gonad | Ovarian Tissue (%) | Testicular Tissue (%) |
| Control 1 | 21,544 | 21,544 (100) | 0 (0) |
| Control 2 | 36,244 | 36,244 (100) | 0 (0) |
| 50 | 26,037 | 26,006 (99.9) | 31 (0.1) |
| 75 | 6902 | 6865 (99.5) | 37 (0.5) |
| 100 | 11,613 | 11,613 (100) | 0 (0) |
| 150 | 14,009 | 13,985 (99.8) | 24 (0.2) |
| 200 | 14,226 | 13,062 (91.8) | 1164 (8.2) |
| Treatment Doses | Tissue Area (µm2) | ||
|---|---|---|---|
| (mg MT/kg Diet) | Total Gonad | Ovarian Tissue (%) | Testicular Tissue (%) |
| Control female | 22,790 | 22,790 (100) | 0 (0) |
| Control male | 30,113 | 0 (0) | 30,113 (100) |
| 50 | 16,274 | 8905 (55) | 7369 (45) |
| 75 | 11,347 | 11,347 (100) | 0 (0) |
| 100 | 16,450 | 16,450 (100) | 0 (0) |
| 150 | 5454 | 5454 (100) | 0 (0) |
| 200 | 18,599 | 18,599 (100) | 0 (0) |
| Number with No Gravid Spot (%) | Number with Gravid Spot (%) | Number Parturiated (%) | |
|---|---|---|---|
| Before treatment | 23 (19.8) | 93 (80.2) | - - |
| After treatment | 116 (100) | 0 (0) | 2 (1.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, N.K.; Kwan, T.N.; Purser, J.; Patil, J.G. Masculinization of Adult Gambusia holbrooki: A Case of Recapitulation of Protogyny in a Gonochorist? Biology 2022, 11, 694. https://doi.org/10.3390/biology11050694
Tran NK, Kwan TN, Purser J, Patil JG. Masculinization of Adult Gambusia holbrooki: A Case of Recapitulation of Protogyny in a Gonochorist? Biology. 2022; 11(5):694. https://doi.org/10.3390/biology11050694
Chicago/Turabian StyleTran, Ngoc Kim, Tzu Nin Kwan, John Purser, and Jawahar G. Patil. 2022. "Masculinization of Adult Gambusia holbrooki: A Case of Recapitulation of Protogyny in a Gonochorist?" Biology 11, no. 5: 694. https://doi.org/10.3390/biology11050694
APA StyleTran, N. K., Kwan, T. N., Purser, J., & Patil, J. G. (2022). Masculinization of Adult Gambusia holbrooki: A Case of Recapitulation of Protogyny in a Gonochorist? Biology, 11(5), 694. https://doi.org/10.3390/biology11050694