Chronic Toxicity of Primary Metabolites of Chloroacetamide and Glyphosate to Early Life Stages of Marbled Crayfish Procambarus virginalis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Substances
2.2. Test Organism
2.3. Experimental Protocol
- AE: 4.0 µg/L acetochlor ESA
- AMPA: 4.0 µg/L aminomethylphosphonic acid
- AE + AMPA: (4.0 µg/L AE + 4.0 µg/L AMPA)
- Control just 20 mL tap water only.
2.4. Tested Parameters
2.4.1. Growth Rate
2.4.2. Early Ontogeny
2.4.3. Behaviour
2.4.4. Oxidative Stress and Antioxidant Biomarkers
2.4.5. Histology
2.5. Statistical Analysis
3. Results
3.1. Cumulative Mortality and Growth
3.2. Early Ontogeny
3.3. Behaviour
3.4. Oxidative Stress and Antioxidant Response
3.5. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koutnik, D.; Stara, A.; Velisek, J. The effect of selected triazines on fish: A review. Slov. Vet. Res. 2015, 52, 107–131. [Google Scholar]
- Sharma, S.; Dar, O.I.; Singh, K.; Kaur, A.; Faggio, C. Triclosan elicited biochemical and transcriptomic alternations in Labeo rohita larvae. Environ. Toxicol. Pharmacol. 2021, 88, 103748. [Google Scholar] [CrossRef] [PubMed]
- Yalsuyi, A.M.; Vajargah, M.F.; Hajimoradloo, A.; Galangash, M.M.; Prokić, M.D.; Faggio, C. Evaluation of behavioral changes and tissue damages of common carp (Cyprinus carpio) after exposure to herbicide-glyphosate. Vet. Sci. 2021, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Foley, M.E.; Sigler, V.; Gruden, C.L. A multiphasic characterization of the impact of the herbicide acetochlor on freshwater bacterial communities. ISME J. 2008, 2, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamun, A. Pesticide Degradations Resides and Environmental Concerns. In Pesticide Residue in Foods: Sources, Management, and Control; Khan, M.S., Rahman, M.S., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 87–102. [Google Scholar] [CrossRef]
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating Pesticide Degradation in the Environment: Blind Spots and Emerging Opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef] [Green Version]
- Vajargah, M.F.; Namin, J.I.; Mohsenpour, R.; Yalsuyi, A.M.; Prokić, M.D.; Faggio, C. Histological effects of sublethal concentrations of insecticide Lindane on intestinal tissue of grass carp (Ctenopharyngodon idella). Vet. Res. Commun. 2021, 45, 373–380. [Google Scholar] [CrossRef]
- Stara, A.; Pagano, M.; Albano, M.; Savoca, S.; Di Bella, G.; Albergamo, A.; Koutkova, Z.; Sandova, M.; Velisek, J.; Fabrello, J.; et al. Effects of long-term exposure of Mytilus galloprovincialis to thiacloprid: A multibiomarker approach. Environ. Pollut. 2021, 289, 117892. [Google Scholar] [CrossRef]
- Riahi, B.; Rafatpanah, H.; Mahmoudi, M.; Memar, B.; Brook, A.; Tabasi, N.; Karimi, G. Immunotoxicity of paraquat after subacute exposure to mice. Food Chem. Toxicol. 2010, 48, 1627–1631. [Google Scholar] [CrossRef]
- Ceyhun, S.B.; Senturk, M.; Ekinci, D.; Erdoğan, O.; Çiltas, A.; Kocaman, E.M. Deltamethrin attenuates antioxidant defense system and induces the expression of heat shock protein 70 in rainbow trout. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 215–223. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Kubec, J.; Zuskova, E.; Buric, M.; Kouba, A. Effects of metazachlor and its major metabolite metazachlor OA on early life stages of marbled crayfish. Sci. Rep. 2020, 10, 875–879. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Agriculture of Czech Republic. Register of Plant Protection Products. Available online: http://eagri.cz/public/app/eagriapp/POR/ (accessed on 12 April 2021).
- Minnesota Department of Agriculture. Acetochlor—General Information. Available online: https://www.mda.state.mn.us/acetochlor-general-information (accessed on 12 April 2021).
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent advances in glyphosate biodegradation. Appl. Microbiol. Biotechnol. 2018, 102, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. ARP GW Raw Data: Supporting and Related Materials; Document ID: EPA-HQ-OPP-2007-0725-0018; USEPA: Washington, DC, USA, 2007.
- United States Environmental Protection Agency. Raw Data for Graphs of Detections of Acetochlor in Drinking Water Wells: USEPA Document Type: Supporting and Related Materials; Document ID:EPA-HQ-OPP-2007-0725-0030; USEPA: Washington, DC, USA, 2007.
- Fu, L.; Lu, X.; Tan, J.; Wang, L.; Chen, J. Multiresidue determination and potential risks of emerging pesticides in aquatic products from Northeast China by LC–MS/MS. J. Environ. Sci. (China) 2018, 63, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Y.; Yang, Y.; Tam, N.F.-Y.; Tao, R.; Dai, Y.-N. Pesticides in three rural rivers in Guangzhou, China: Spatiotemporal distribution and ecological risk. Environ. Sci. Pollut. Res. Int. 2019, 26, 3569–3577. [Google Scholar] [CrossRef]
- Battaglin, W.; Furlong, E.; Burkhardt, M.; Peter, C. Occurrence of sulfonylurea, sulfonamide, imidazolinone, and other herbicides in rivers, reservoirs and ground water in the Midwestern United States, 1998. Sci. Total Environ. 2000, 248, 123–133. [Google Scholar] [CrossRef]
- Boyd, R.A. Herbicides and herbicide degradates in shallow groundwater and the Cedar River a municipal well field, Cedar Rapids, Iowa. Sci. Total Environ. 2000, 248, 241–253. [Google Scholar] [CrossRef]
- Dictor, M.-C.; Baran, N.; Gautier, A.; Mouvet, C. Acetochlor mineralization and fate of its two major metabolites in two soils under laboratory conditions. Chemosphere 2008, 71, 663–670. [Google Scholar] [CrossRef]
- de Guzman, N.P.; Hendley, P.; Gustafson, D.I.; van Wesenbeeck, I.; Klein, A.J.; Fuhrman, J.D.; Travis, K.; Simmons, N.D.; Teskey, W.E.; Durham, R.B. The Acetochlor Registration Partnership State Ground Water Monitoring Program. J. Environ. Qual. 2005, 34, 793–803. [Google Scholar] [CrossRef]
- Janniche, G.S.; Mouvet, C.; Albrechtsen, H.-J. Acetochlor sorption and degradation in limestone subsurface and aquifers. Pest. Manag. Sci. 2010, 66, 1287–1297. [Google Scholar] [CrossRef]
- Kalkhoff, S.J.; Kolpin, D.W.; Thurman, E.M.; Ferrer, I.; Barcelo, D. Degradation of chloroacetanilide herbicides: The prevalance of sulfonic and oxanilic acid metabolites in lowa groundwaters and surface waters. Environ. Sci. Technol. 1998, 32, 1738–1740. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Thurman, E.M.; Linhart, S.M. The environmental occurrence of herbicides: The importance of degradates in ground water. Arch. Environ. Contam. Toxicol. 1998, 35, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.; Thurman, E.M.; Linhart, S. Finding minimal herbicide concentrations in ground water? Try looking for their degradates. Sci. Total Environ. 2000, 248, 115–122. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Y.; Zhang, X.; Zhao, S.; Qiu, J.; Li, N.; He, J. Anaerobic biodegradation and detoxification of chloroacetamide herbicides by a novel Proteiniclasticum sediminis BAD-10T. Environ. Res. 2022, 209, 112859. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.C.; Kolpin, D.W.; Scribner, E.A.; Thurman, E.M. Herbicides and Degradates in Shallow Aquifers of Illinois: Spatial and Temporal Trends. J. Am. Water Resour. Assoc. 2005, 41, 537–547. [Google Scholar] [CrossRef]
- Postle, J.K.; Rheineck, B.D.; Allen, P.E.; Baldock, J.O.; Cook, C.J.; Zogbaum, R.; Vandenbrook, J.P. Chloroacetanilide Herbicide Metabolites in Wisconsin Groundwater: 2001 Survey Results. Environ. Sci. Technol. 2004, 38, 5339–5343. [Google Scholar] [CrossRef] [PubMed]
- Satin, S. I V Roce 2016 Jsme Pili Vodu S Překročeným Limitem Pro Metabolit Pesticide Acetochlor ESA. Available online: https://zivotniprostrediotrokovic.webnode.cz/news/i-v-roce-2016-jsme-pili-vodu-s-prekrocenym-limitem-pro-metabolit-pesticitu-acetochlor-esa/ (accessed on 11 April 2021).
- Moulisová, A.; Bendakovská, L.; Kožíšek, F.; Vavrouš, A.; Jeligová, H.; Kotal, F. Pesticidy a jejich metabolity v pitné vodě. Jaký je současný stav v České Republice? Vodn. Hospodářství 2017, 68, 4–10. [Google Scholar]
- Kodeš, V.; Pesticidy v podzemních vodách ČR. Czech Hydrometeorological Institute: Department of Water Quality. Available online: https://www.crystalwater.sk/wp-content/uploads/sites/12/2017/11/chmu-pesticidy-podzemne-vody-cr.pdf (accessed on 12 April 2021).
- Crump, D.; Werry, K.; Veldhoen, N.; Van Aggelen, G.; Helbing, C.C. Exposure to the herbicide acetochlor alters thyroid hormone-dependent gene expression and metamorphosis in Xenopus Laevis. Environ. Health Perspect. 2002, 110, 1199–1205. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Sun, X.; Niu, L.; Yang, W.; Tu, W.; Lu, L.; Song, S.; Liu, W. Enantioselective thyroid disruption in zebrafish embryo-larvae via exposure to environmental concentrations of the chloroacetamide herbicide acetochlor. Sci. Total Environ. 2019, 653, 1140–1148. [Google Scholar] [CrossRef]
- Yang, M.; Hu, J.; Li, S.; Ma, Y.; Gui, W.; Zhu, G. Thyroid endocrine disruption of acetochlor on zebrafish (Danio rerio) larvae. J. Appl. Toxicol. 2016, 36, 844–852. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Guan, M.; Zhang, W.; Tian, H.; Jiang, C.; Tan, X.; Kang, W. Genotoxicity of chloroacetamide herbicides and their metabolites in vitro and In Vivo. Int. J. Mol. Med. 2021, 47, 103. [Google Scholar] [CrossRef]
- Wang, H.; Meng, Z.; Zhou, L.; Cao, Z.; Liao, X.; Ye, R.; Lu, H. Effects of acetochlor on neurogenesis and behaviour in zebrafish at early developmental stages. Chemosphere 2019, 220, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.M.; Mohamed, I.A.; Fathy, M.; Sayed, A.E.-D.H. Neuro-hepatopathological changes in juvenile Oreochromis niloticus exposed to sublethal concentrations of commercial herbicides. Environ. Toxicol. Pharmacol. 2022, 2022, 103871. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhao, L.; Liu, K.; Liu, W. Enantiomeric environmental behavior, oxidative stress and toxin release of harmful cyanobacteria Microcystis aeruginosa in response to napropamide and acetochlor. Environ. Pollut. 2019, 246, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xu, E.G.; Ren, Y.; Jin, S.; Zhang, T.; Liu, J.; Li, Z. Mixture Toxicity of Bensulfuron-Methyl and Acetochlor to Red Swamp Crayfish (Procambarus clarkii): Behavioral, Morphological and Histological Effects. Int. J. Environ. Res. Public Health 2017, 14, 1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Tu, W.; Deng, M.; Jin, Y.; Lu, B.; Zhang, C.; Lin, C.; Wu, Y.; Liu, W. Stereoselective induction of developmental toxicity and immunotoxicity by acetochlor in the early life stage of zebrafish. Chemosphere 2016, 164, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Minnesota Department of Health. Acetochlor ESA and Drinking Water. Available online: https://www.health.state.mn.us/communities/environment/risk/docs/guidance/gw/acetachloresainfo.pdf (accessed on 12 April 2021).
- United States Environmental Protection Agency. Report of the Food Quality Protection act (FQPA) Tolerance Reassessment Progress and Risk Management Decision (TRED) for Acetochlor. Prevention, Pesticides and Toxic Substances, EPA 738-R-00-009. Available online: https://archive.epa.gov/pesticides/reregistration/web/pdf/acetochlor_tred.pdf (accessed on 3 February 2021).
- Bonansea, R.I.; Filippi, I.; Wunderlin, D.A.; Marino, D.J.G.; Amé, M.V. The Fate of Glyphosate and AMPA in a Freshwater Endorheic Basin: An Ecotoxicological Risk Assessment. Toxics 2018, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Levine, S.L.; von Mérey, G.; Minderhout, T.; Manson, P.; Sutton, P. Aminomethylphosphonic acid has low chronic toxicity to Daphnia Magna Pimephales Promelas. Environ. Toxicol. Chem. 2015, 34, 1382–1389. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Preliminary Ecological Risk Assessment in Support of the Registration Review of Glyphosate and Its Salts (Washington, DC, USA. EPA 20460). Available online: https://www.epa.gov/sites/default/files/2019-04/documents/glyphosate-signed-efed-rtc.pdf#:~:text=The%20Environmental%20Fate%20and%20Effects%20Division%20%28EFED%29%20has,103613%2C%20103605%29%20developed%20in%20support%20of%20Registration%20Review (accessed on 3 February 2021).
- Sun, M.; Li, H.; Jaisi, D.P. Degradation of glyphosate and bioavailability of phosphorus derived from glyphosate in a soil-water system. Water Res. 2019, 163, 114840. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Kolpin, D.W.; Scribner, E.A.; Kuivila, K.M.; Sandstorm, M.W. Glyphosate, other herbicides, and transformation products in midwestern streams. J. Am. Water Res. Assoc. 2005, 41, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and its gedradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. J. Am. Water Res. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- Coupe, R.H.; Kalkhoff, S.J.; Capel, P.D.; Gregoire, C. Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest. Manag. Sci. 2012, 68, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Thurman, E.M.; Lee, E.A.; Meyer, M.; Furlong, E.; Glassmeyer, S. Urban contributions of glyphosate and its degradate AMPA to streams in the United States. Sci. Total Environ. 2005, 354, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Scribner, E.A.; Battaglin, W.A.; Dietze, J.E.; Thurman, E.M. Reconnaissance Data for Glyphosate, Other Selected Herbicides, Their Degradation Products, and Antibiotics in 51 Streams in Nine Midwestern States, 2002; U.S. Geological Survey Open-File Report 03-217; U.S. Geological Survey: Reston, VA, USA, 2003; p. 101.
- Tresnakova, N.; Stara, A.; Velisek, J. Effects of Glyphosate and Its Major Metabolite AMPA on Aquatic Organisms. Appl. Sci. 2021, 11, 9004. [Google Scholar] [CrossRef]
- Antunes, A.M.; Rocha, T.L.; Pires, F.S.; de Freitas, M.A.; Leite, V.R.M.C.; Arana, S.; Moreira, P.C.; Sabóia-Morais, S.M.T. Gender-specific histopathological response in guppies Poecilia reticulata exposed to glyphosate or its metabolite aminomethylphosphonic acid. J. Appl. Toxicol. 2017, 37, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, C.; Demetrio, P.M.; Alonso, L.L.; Mac Loughlin, T.M.; Cerdá, E.; Sarandón, S.J.; Marino, D.J. Evidence for soil pesticide contamination of an agroecological farm from a neighboring chemical-based production system. Agric. Ecosyst. Environ. 2021, 313, 107341. [Google Scholar] [CrossRef]
- Pérez, D.J.; Okada, E.; De Gerónimo, E.; Menone, M.; Aparicio, V.C.; Costa, J.L. Spatial and temporal trends and flow dynamics of glyphosate and other pesticides within an agricultural watershed in Argentina. Environ. Toxicol. Chem. 2017, 36, 3206–3216. [Google Scholar] [CrossRef] [Green Version]
- Pérez, D.J.; Iturburu, F.G.; Calderon, G.; Oyesqui, L.A.; De Gerónimo, E.; Aparicio, V.C. Ecological risk assessment of current-use pesticides and biocides in soils, sediments and surface water of a mixed land-use basin of the Pampas region, Argentina. Chemosphere 2021, 263, 128061. [Google Scholar] [CrossRef]
- Székács, A.; Mörtl, M.; Darvas, B. Monitoring Pesticide Residues in Surface and Ground Water in Hungary: Surveys in 1990–2015. J. Chem. 2015, 2015, 717948. [Google Scholar] [CrossRef] [Green Version]
- Heras, R.D.L.; Rodríguez-Gil, J.L.; Sauto, J.S.S.; Sánchez, P.S.; Catalá, M. Analysis of lipid peroxidation in animal and plant tissues as field-based biomarker in Mediterranean irrigated agroecosystems (Extremadura, Spain). J. Environ. Sci. Health 2018, 9, 567–579. [Google Scholar] [CrossRef]
- Esser, H.O. A review of the correlation between physicochemical properties and bioaccumulation. Pestic. Sci. 1986, 17, 265–276. [Google Scholar] [CrossRef]
- Rhodes, L.D.; Gardner, G.R.; Van Beneden, R.J. Short-term tissue distribution, depuration and possible gene expression effects of [3H] TCDD exposure in soft-shell clams (Mya arenaria). Environ. Toxicol. Chem. 1997, 16, 1888–1894. [Google Scholar] [CrossRef]
- Uno, H.S.S.; Shiraishi, H.; Hatakeyama, S.; Otsuki, A.; Koyama, J.; Uno, S. Accumulative Characteristics of Pesticide Residues in Organs of Bivalves (Anodonta woodiana and Corbicula leana) Under Natural Conditions. Arch. Environ. Contam. Toxicol. 2001, 40, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, V.C.; De Gerónimo, E.; Marino, D.; Primost, J.; Carriquiriborde, P.; Costa, J.L. Environmental fate of glyphosate and aminomethylphosphonic acid in surface waters and soil of agricultural basins. Chemosphere 2013, 93, 1866–1873. [Google Scholar] [CrossRef]
- Yokley, R.A.; Mayer, L.C.; Huang, S.-B.; Vargo, J.D. Analytical Method for the Determination of Metolachlor, Acetochlor, Alachlor, Dimethenamid, and Their Corresponding Ethanesulfonic and Oxanillic Acid Degradates in Water Using SPE and LC/ESI-MS/MS. Anal. Chem. 2020, 74, 3754–3759. [Google Scholar] [CrossRef]
- Longshaw, M.; Bateman, K.S.; Stebbing, P.; Stentiford, G.D.; Hockley, F.A. Disease risks associated with the importation and release of non-native crayfish species into mainland Britain. Aquat. Biol. 2012, 16, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Silveyra, G.R.; Silveyra, P.; Vatnick, I.; Medesani, D.A.; Rodríguez, E.M. Effects of atrazine on vitellogenesis, steroid levels and lipid peroxidation, in female red swamp crayfish Procambarus clarkii. Aquat. Toxicol. 2018, 197, 136–142. [Google Scholar] [CrossRef] [PubMed]
- OECD (Organization for Economic Cooperation and Development). Guideline for Testing of Chemicals 215; Fish Juvenile Growth Test: Paris, France, 2000. [Google Scholar]
- Vogt, G. The marbled crayfish: A new model organism for research on development, epigenetics and evolutionary biology. J. Zool. 2008, 276, 1–13. [Google Scholar] [CrossRef]
- Stara, A.; Zuskova, E.; Kouba, A.; Velisek, J. Effects of terbuthylazine-desethyl, a terbuthylazine degradation product, on red swamp crayfish (Procambarus clarkii). Sci. Total Environ. 2016, 566, 733–740. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Bagnyukova, T.V.; Husak, V.; Luzhna, L.I.; Lushchak, O.V.; Storey, K. Hyperoxia results in transient oxidative stress and an adaptive response by antioxidant enzymes in goldfish tissues. Int. J. Biochem. Cell Biol. 2005, 37, 1670–1680. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A Spectrophotometric Method for Measuring the Breakdown of Hydrogen Peroxide by Catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bradford, M.M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ceccaldi, H. Anatomy and physiology of digestive tract of Crustaceans Decapods reared in aquaculture. Adv. Trop. Aqua. 1989, 9, 243–259. [Google Scholar]
- Stancova, V.; Plhalova, L.; Bartoskova, M.; Zivna, D.; Prokes, M.; Marsalek, P.; Blahova, J.; Skoric, M.; Svobodova, Z. Effects of Mixture of Pharmaceuticals on Early Life Stages of Tench (Tinca tinca). BioMed Res. Int. 2014, 2014, 253468. [Google Scholar] [CrossRef] [Green Version]
- Stara, A.; Pagano, M.; Capillo, G.; Fabrello, J.; Sandova, M.; Albano, M.; Zuskova, E.; Velisek, J.; Matozzo, V.; Faggio, C. Acute effects of neonicotinoid insecticides on Mytilus galloprovincialis: A case study with the active compound thiacloprid and the commercial formulation calypso 480 SC. Ecotoxicol. Environ. Saf. 2020, 203, 110980. [Google Scholar] [CrossRef]
- West, M.E.J.; Moore, P.A. Bt Proteins Exacerbate Negative Growth Effects in Juvenile Rusty (F. rusticus) Crayfish Fed Corn Diet. Arch. Environ. Contam. Toxicol. 2009, 77, 452–460. [Google Scholar] [CrossRef]
- Rodrigues, L.D.B.; Costa, G.G.; Thá, E.L.; da Silva, L.R.; de Oliveira, R.; Leme, D.M.; Cestari, M.M.; Grisolia, C.K.; Valadares, M.C.; de Oliveira, G.A.R. Impact of the glyphosate-based commercial herbicide, its components and its metabolite AMPA on non-target aquatic organisms. Mutat. Res. Gen. Toxicol. Environ. Mutagen. 2019, 842, 94–101. [Google Scholar] [CrossRef]
- Fiorino, E.; Sehonova, P.; Plhalova, L.; Blahova, J.; Svobodova, Z.; Faggio, C. Effects of glyphosate on early life stages: Comparison between Cyprinus carpio and Danio rerio. Environ. Sci. Pollut. Res. 2018, 25, 8542–8549. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Koutnik, D.; Zuskova, E.; Kouba, A. Effect of prometryne on early life stages of marbled crayfish (Procambarus falllax f. virginalis). Neuroendocrinol. Lett. 2014, 35, 93–98. [Google Scholar] [PubMed]
- Velisek, J.; Stara, A.; Machova, J.; Dvorak, P.; Zuskova, E.; Prokes, M.; Svobodova, Z. Effect of terbutryn at environmental concentrations on early life stages of common carp (Cyprinus carpio L.). Pestic. Biochem. Physiol. 2012, 102, 102–108. [Google Scholar] [CrossRef]
- Bengtsson, B.-E. Effect of Zinc on Growth of the Minnow Phoxinus phoxinus. Oikos 1974, 25, 370–373. [Google Scholar] [CrossRef]
- Durmaz, H.; Sevgiler, Y.; Üner, N. Tissue-specific antioxidative and neurotoxic responses to diazinon in Oreochromis niloticus. Pestic. Biochem. Physiol. 2006, 84, 215–226. [Google Scholar] [CrossRef]
- Woltering, D.M. The growth response in fish chronic and early life stage toxicity tests: A critical review. Aquat. Toxicol. 1984, 5, 1–21. [Google Scholar] [CrossRef]
- Wang, S.; Seiwert, B.; Kästner, M.; Miltner, A.; Schäffer, A.; Reemtsma, T.; Yang, Q.; Nowak, K.M. (Bio)degradation of glyphosate in water-sediment microcosms—A stable isotope co-labeling approach. Water Res. 2016, 99, 91–100. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Zuskova, E.; Kubec, J.; Buric, M.; Kouba, A. Chronic toxicity of metolachlor OA on growth, ontogenetic development, antioxidant biomarkers and histopathology of early life stages of marbled crayfish. Sci. Total Environ. 2018, 643, 1456–1463. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Zuskova, E.; Kubec, J.; Buric, M.; Kouba, A. Effects of s-metolachlor on early life stages of marbled crayfish. Pestic. Biochem. Physiol. 2019, 153, 87–94. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Zuskova, E.; Kouba, A. Effects of three triazine metabolites and their mixture at environmentally relevant concentrations on early life stages of marbled crayfish (Procambarus fallax f. virginalis). Chemosphere 2017, 175, 440–445. [Google Scholar] [CrossRef]
- Gherardi, F. Behaviour. In Biology of Freshwater Crayfish; Blackwell Science: Oxford, UK, 2002; pp. 258–290. [Google Scholar]
- Kubec, J.; Kouba, A.; Buřič, M. Communication, behaviour, and decision making in crayfish: A review. Zool. Anz. 2019, 278, 28–37. [Google Scholar] [CrossRef]
- Manning, A.; Dawkins, M.S. An Introduction to Animal Behavior, 6th ed.; Cambridge University Press: Cambridge, MA, USA, 2012; p. 458. [Google Scholar]
- Duffy, J.E.; Cardinale, B.J.; France, K.E.; McIntyre, P.B.; Thébault, E.; Loreau, M. The functional role of biodiversity in ecosystems: Incorporating trophic complexity. Ecol. Lett. 2007, 10, 522–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitz, O.J. Predator Diversity and Trophic Interactions. Ecology 2007, 88, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- El Hajam, M.; Plavan, G.-I.; Kandri, N.I.; Dumitru, G.; Nicoara, M.N.; Zerouale, A.; Faggio, C. Evaluation of softwood and hardwood sawmill wastes impact on the common carp “Cyprinus carpio” and its aquatic environment: An oxidative stress study. Environ. Toxicol. Pharmacol. 2020, 75, 103327. [Google Scholar] [CrossRef] [PubMed]
- Sehonova, P.; Tokanova, N.; Hodkovicova, N.; Kroupova, H.K.; Tumova, J.; Blahova, J.; Marsalek, P.; Plhalova, L.; Doubkova, V.; Dobsikova, R.; et al. Oxidative stress induced by fluoroquinolone enrofloxacin in zebrafish (Danio rerio) can be ameliorated after a prolonged exposure. Environ. Toxicol. Pharmacol. 2019, 67, 87–93. [Google Scholar] [CrossRef]
- Mensah, P.K.; Palmer, C.G.; Muller, W.J.M. Lethal and Sublethal effects of pesticides on aquatic organisms: The cas of a freshwater shrimp exposure to Roundup®. In Pesticides Toxic Aspects; Larramendy, M.L., Soloneski, S., Eds.; In Tech: Rijeka, Croatia, 2014; pp. 163–185. [Google Scholar]
- Geret, F.; Serafim, A.; Bebianno, M.J. Antioxidant enzyme activities, metallothioneins and lipid peroxidation as biomarkers in Ruditapes decussatus? Ecotoxicology 2003, 12, 417–426. [Google Scholar] [CrossRef]
- Modesto, K.A.; Martinez, C.B. Roundup® causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 2010, 78, 294–299. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Zuskova, E.; Chabera, J.; Kubec, J.; Buric, M.; Kouba, A. Effects of chloridazon on early life stages of marbled crayfish. Chemosphere 2020, 257, 127189. [Google Scholar] [CrossRef]
- Crestani, M.; Menezes, C.; Glusczak, L.; Miron, D.D.S.; Spanevello, R.; Silveira, A.; Gonçalves, F.F.; Zanella, R.; Loro, V. Effect of clomazone herbicide on biochemical and histological aspects of silver catfish (Rhamdia quelen) and recovery pattern. Chemosphere 2007, 67, 2305–2311. [Google Scholar] [CrossRef]
- Glusczak, L.; Miron, D.D.S.; Moraes, B.S.; Simões, R.R.; Schetinger, M.R.C.; Morsch, V.M.; Loro, V. Acute effects of glyphosate herbicide on metabolic and enzymatic parameters of silver catfish (Rhamdia quelen). Comp. Biochem. Physiol. 2007, 146, 519–524. [Google Scholar] [CrossRef]
- Glusczak, L.; Loro, V.L.; Pretto, A.; Moraes, B.S.; Raabe, A.; Duarte, M.F.; da Fonseca, M.B.; de Menezes, C.C.; Valladão, D.M.D.S. Acute Exposure to Glyphosate Herbicide Affects Oxidative Parameters in Piava (Leporinus obtusidens). Arch. Environ. Contam. Toxicol. 2011, 61, 624–630. [Google Scholar] [CrossRef]
- Miron, D.D.S.; Pretto, A.; Crestani, M.; Glusczak, L.; Schetinger, M.R.; Loro, V.L.; Morsch, V.M. Biochemical effects of clomazone herbicide on piava (Leporinus obtusidens). Chemosphere 2008, 74, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Negro, L.; Senkman, L.; Montagna, M.; Collins, P. Freshwater decapods and pesticides: An unavoidable relation in the modern world. In Pesticides in the Modern World—Risks and Benefits; Stoytcheva, M., Ed.; In Tech: Rijeka, Croatia, 2011; pp. 198–199. [Google Scholar]
- Zou, E.; Stueben, B. Acute exposure to naphthalene reduces oxyregulating capacity of the brown shrimp, Penaeus aztecus, subjected to progressive hypoxia. Mar. Biol. 2006, 149, 1411–1415. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
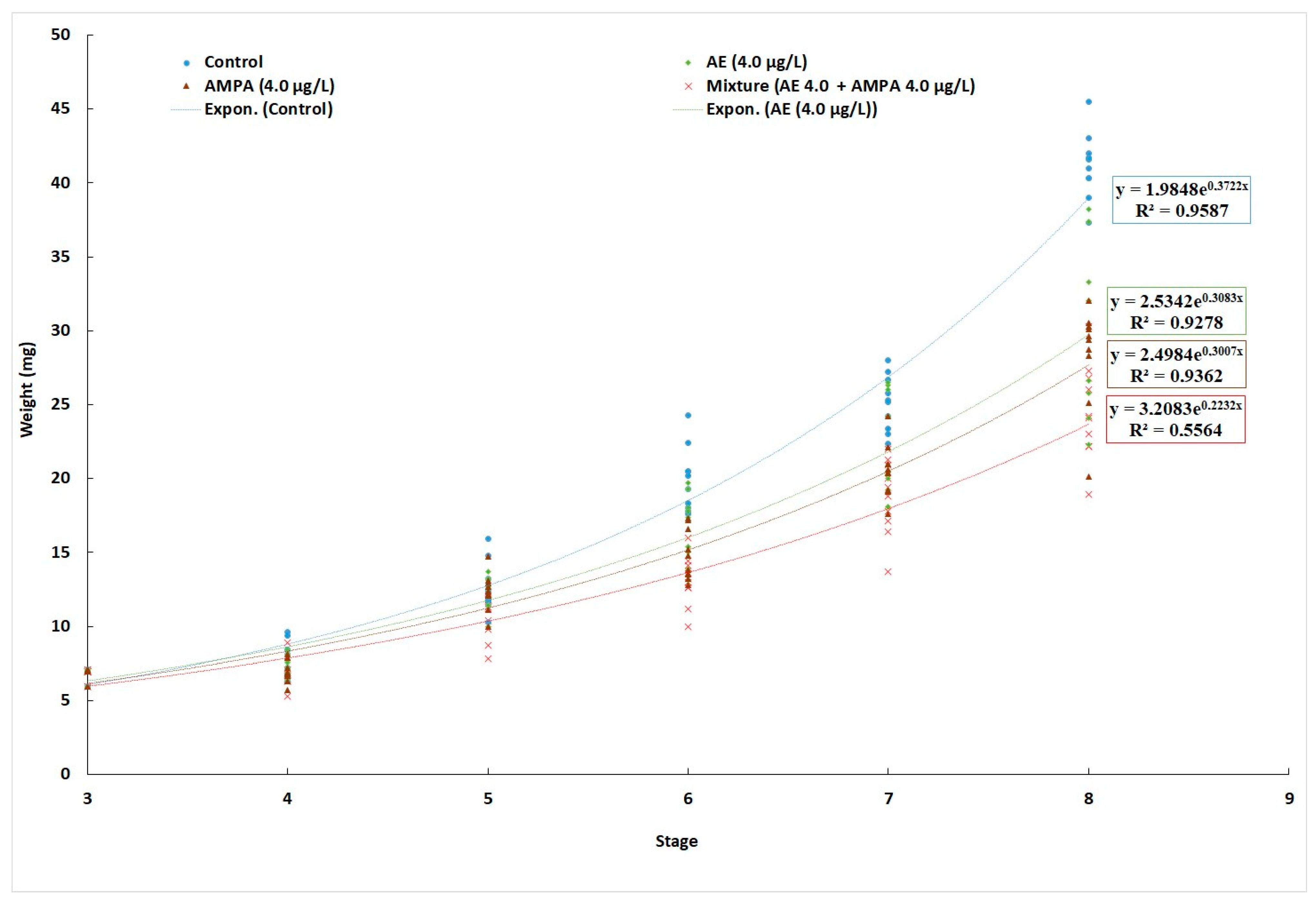
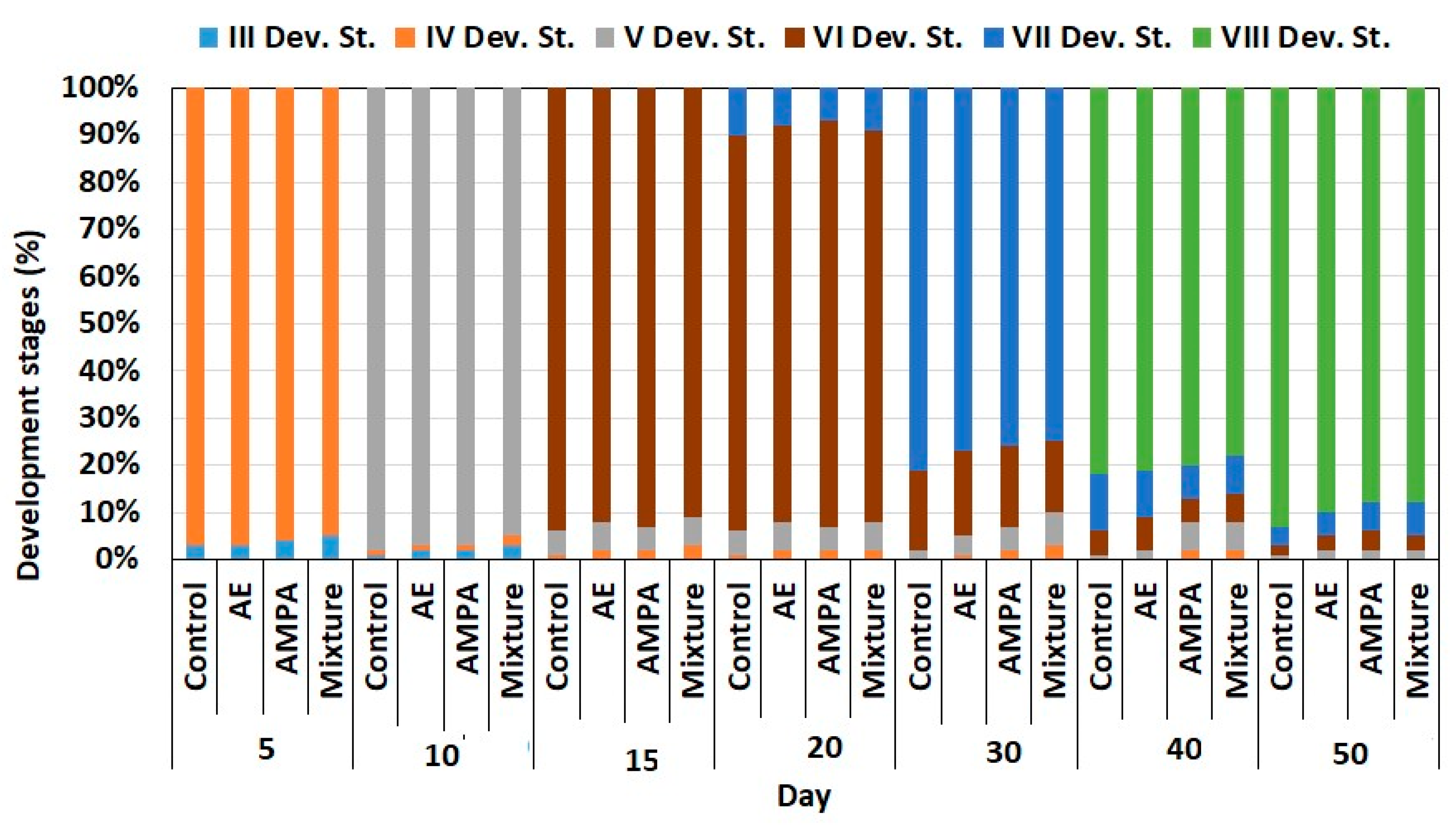
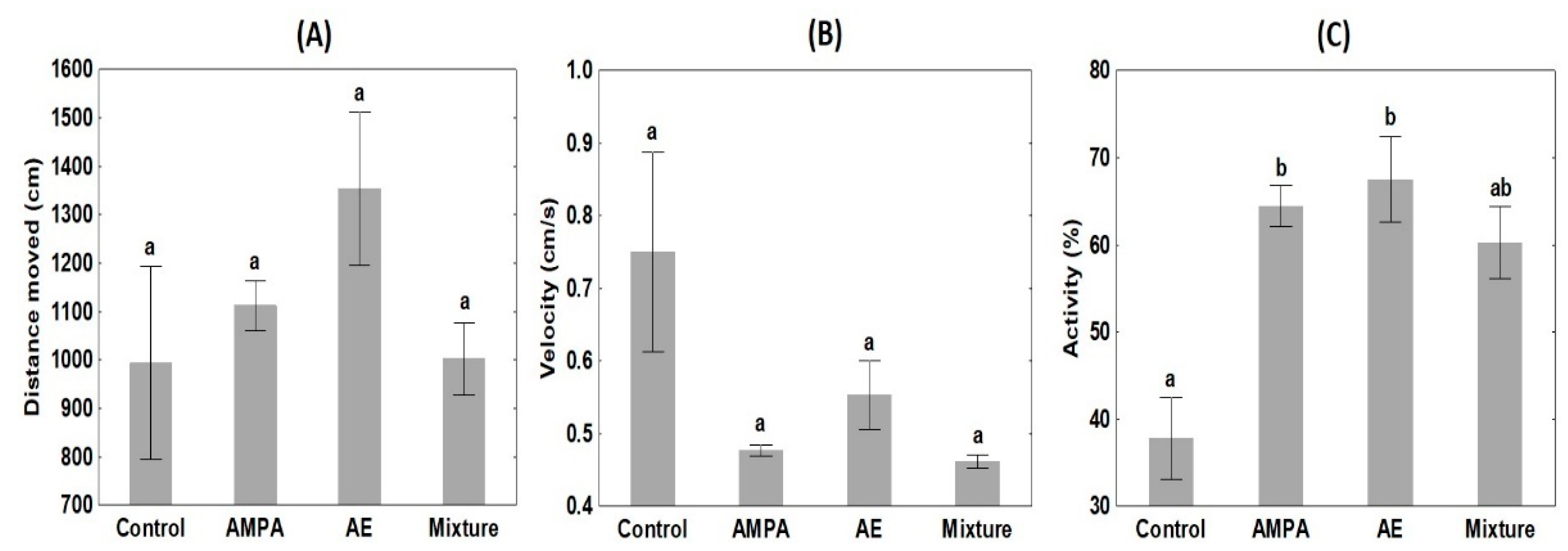
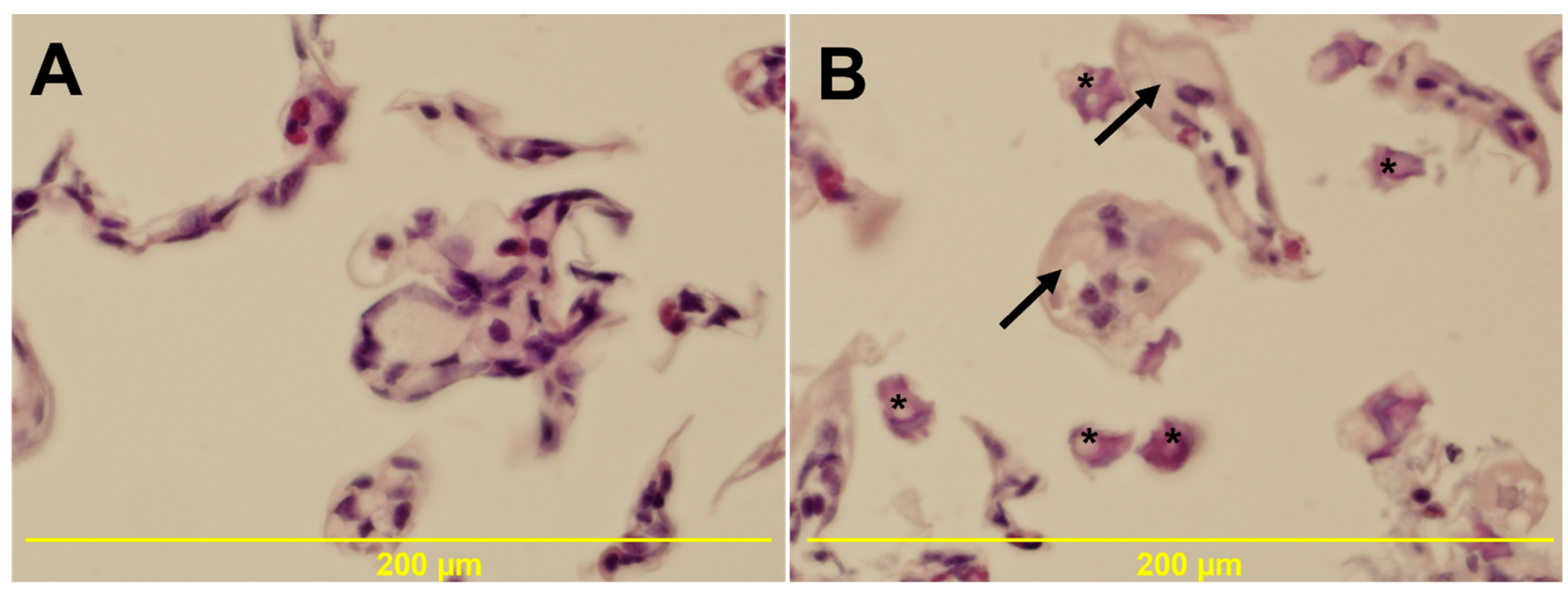
| Treatment | Control (0 µg/L) | AE (4.0 µg/L) | AMPA (4.0 µg/L) | AE + AMPA (4.0 µg/L + 4.0 µg/L) |
|---|---|---|---|---|
| M5 (Mean ± SD), mg | 7.94 ± 1.05 a | 7.55 ± 0.81 a | 7.66 ± 1.24 a | 7.40 ± 1.10 a |
| M50 (Mean ± SD), mg | 45.33 ± 3.52 a | 37.73 ± 7.96 b | 36.18 ± 4.50 b | 34.11 ± 8.74 b |
| SGR | 4.38 ± 0.08 a | 3.98 ± 0.10 b | 3.90 ± 0.09 b | 3.77 ± 0.12 b |
| Treatment | Control (0 µg/L) | AE (4.0 µg/L) | AMPA (4.0 µg/L) | AE + AMPA (4.0 µg/L + 4.0 µg/L) |
|---|---|---|---|---|
| TBARS (nmol/mg protein) | 0.214 ± 0.006 a | 0.246 ± 0.018 a | 0.247 ± 0.017 a | 0.219 ± 0.036 a |
| SOD (nmol/min/mg protein) | 0.060 ± 0.031 a | 0.199 ± 0.037 b | 0.205 ± 0.054 b | 0.136 ± 0.046 b |
| CAT (µmol/min/mg protein) | 0.326 ± 0.047 a | 0.406 ± 0.031 a | 0.424 ± 0.080 a | 0.387 ± 0.058 a |
| GST (nmol/min/mg protein) | 1.109 ± 0.038 a | 1.384 ± 0.229 ab | 1.416 ± 0.187 ab | 1.762 ± 0.576 b |
| AChE (nmol/min/mg protein) | 8.753 ± 1.921 a | 9.176 ± 4.658 a | 5.358 ± 1.794 a | 10.663 ± 3.270 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tresnakova, N.; Kubec, J.; Stara, A.; Zuskova, E.; Faggio, C.; Kouba, A.; Velisek, J. Chronic Toxicity of Primary Metabolites of Chloroacetamide and Glyphosate to Early Life Stages of Marbled Crayfish Procambarus virginalis. Biology 2022, 11, 927. https://doi.org/10.3390/biology11060927
Tresnakova N, Kubec J, Stara A, Zuskova E, Faggio C, Kouba A, Velisek J. Chronic Toxicity of Primary Metabolites of Chloroacetamide and Glyphosate to Early Life Stages of Marbled Crayfish Procambarus virginalis. Biology. 2022; 11(6):927. https://doi.org/10.3390/biology11060927
Chicago/Turabian StyleTresnakova, Nikola, Jan Kubec, Alzbeta Stara, Eliska Zuskova, Caterina Faggio, Antonin Kouba, and Josef Velisek. 2022. "Chronic Toxicity of Primary Metabolites of Chloroacetamide and Glyphosate to Early Life Stages of Marbled Crayfish Procambarus virginalis" Biology 11, no. 6: 927. https://doi.org/10.3390/biology11060927
APA StyleTresnakova, N., Kubec, J., Stara, A., Zuskova, E., Faggio, C., Kouba, A., & Velisek, J. (2022). Chronic Toxicity of Primary Metabolites of Chloroacetamide and Glyphosate to Early Life Stages of Marbled Crayfish Procambarus virginalis. Biology, 11(6), 927. https://doi.org/10.3390/biology11060927








