Not Too Warm, Not Too Cold: Thermal Treatments to Slightly Warmer or Colder Conditions from Mother’s Origin Can Enhance Performance of Montane Butterfly Larvae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System
2.2. Temperature Treatments
2.3. Experimental Design
Diet
2.4. Statistical Analysis
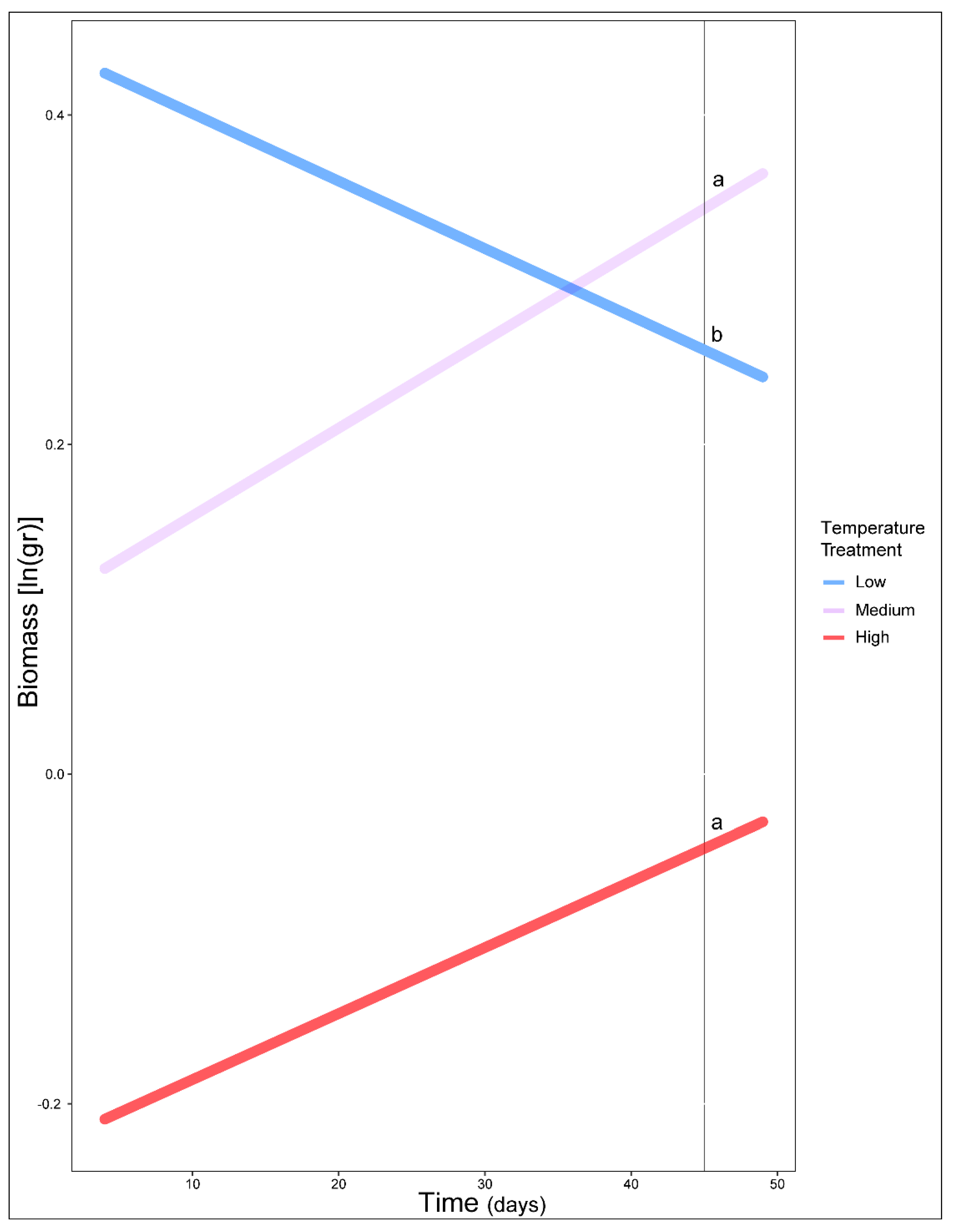
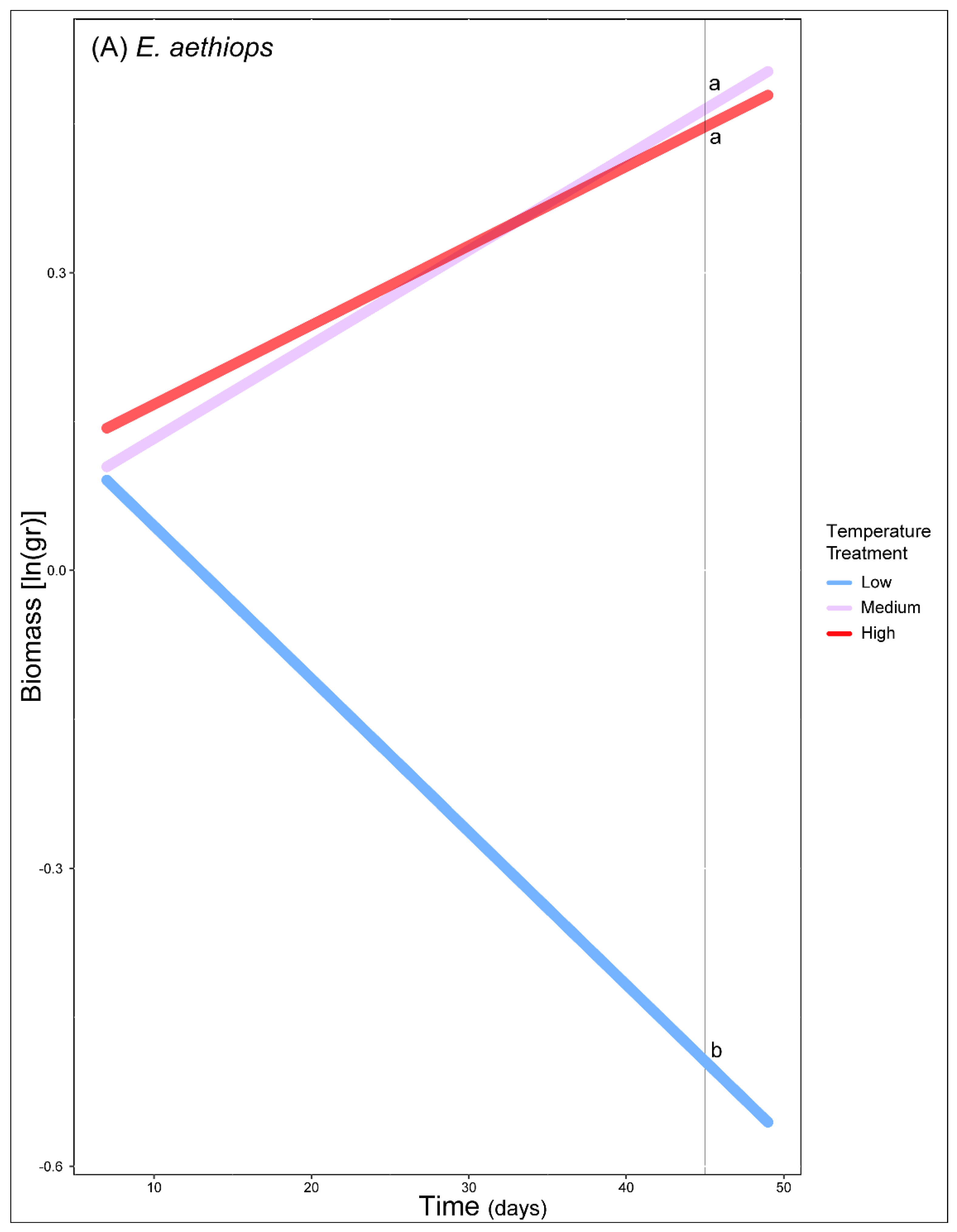
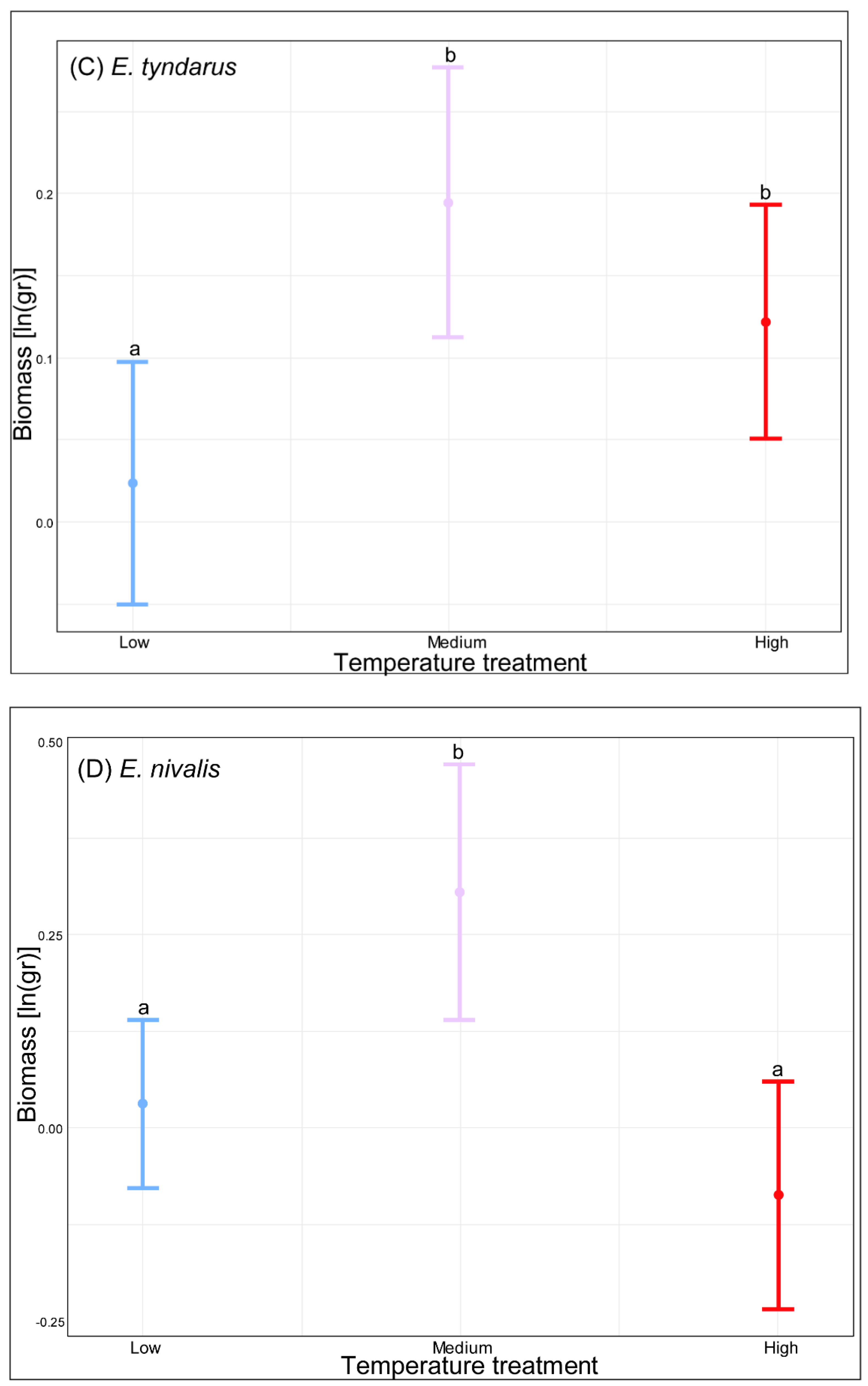
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kingsolver, J.G.; Shlichta, J.G.; Ragland, G.J.; Massie, K.R. Thermal reaction norms for caterpillar growth depend on diet. Evol. Ecol. Res. 2006, 8, 703–715. [Google Scholar]
- Ehl, S.; Dalstein, V.; Tull, F.; Gros, P.; Schmitt, T. Specialized or opportunistic—How does the high mountain endemic butterfly Erebia nivalis survive in its extreme habitats? Insect Sci. 2018, 25, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Lombardero, M.; Castedo-Dorado, F.; Ayres, M. Extreme climatic events affect populations of Asian chestnut gall wasps, Dryocosmus kuriphilus, but do not stop the spread. Agric. For. Entomol. 2021, 23, 473–488. [Google Scholar] [CrossRef]
- Vitasse, Y.; Ursenbacher, S.; Klein, G.; Bohnenstengel, T.; Chittaro, Y.; Delestrade, A.; Monnerat, C.; Rebetez, M.; Rixen, C.; Strebel, N.; et al. Phenological and elevational shifts of plants, animals and fungi under climate change in the European Alps. Biol. Rev. 2021, 96, 1816–1835. [Google Scholar] [CrossRef] [PubMed]
- Vrba, P.; Nedvěd, O.; Zahradníčková, H.; Konvička, M. More complex than expected: Cold hardiness and the concentration of cryoprotectants in overwintering larvae of five Erebia butterflies (Lepidoptera: Nymphalidae). Eur. J. Entomol. 2017, 114, 470–480. [Google Scholar] [CrossRef]
- Suggitt, A.J.; Stefanescu, C.; Páramo, F.; Oliver, T.; Anderson, B.J.; Hill, J.K.; Roy, D.B.; Brereton, T.; Thomas, C.D. Habitat associations of species show consistent but weak responses to climate. Biol. Lett. 2012, 8, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Konvička, M.; Beneš, J.; Čížek, O.; Kuras, T.; Klečková, I. Has the currently warming climate affected populations of the mountain ringlet butterfly, Erebia epiphron (Lepidoptera: Nymphalidae), in low-elevation mountains? Eur. J. Entomol. 2016, 113, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Rytteri, S.; Kuussaari, M.; Saastamoinen, M. Microclimatic variability buffers butterfly populations against increased mortality caused by phenological asynchrony between larvae and their host plants. Oikos 2021, 130, 753–765. [Google Scholar] [CrossRef]
- Scalercio, S.; Bonacci, T.; Mazzei, A.; Pizzolotto, R.; Brandmayr, P. Better up, worse down: Bidirectional consequences of three decades of climate change on a relict population of Erebia cassioides. J. Insect Conserv. 2014, 18, 643–650. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.C.; Guisan, A.; Vittoz, P.; Wohlgemuth, T.; Zimmermann, N.E.; Dullinger, S.; Pauli, H.; Willner, W.; Svenning, J.C. Going against the flow: Potential mechanisms for unexpected downslope range shifts in a warming climate. Ecography 2010, 33, 295–303. [Google Scholar] [CrossRef]
- Konvicka, M.; Beneš, J.; Schmitt, T. Ecological Limits Vis-à-vis Changing Climate: Relic Erebia Butterflies in Insular Sudeten Mountains. In Relict Species; Habel, J.C., Assmann, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 341–355. [Google Scholar]
- Leingärtner, A.; Hoiss, B.; Krauss, J.; Steffan-Dewenter, I. Combined effects of extreme climatic events and elevation on nutritional quality and herbivory of alpine plants. PLoS ONE 2014, 9, e93881. [Google Scholar] [CrossRef] [PubMed]
- Konvicka, M.; Kuras, T.; Liparova, J.; Slezak, V.; Horázná, D.; Klečka, J.; Kleckova, I. Low winter precipitation, but not warm autumns and springs, threatens mountain butterflies in middle-high mountains. PeerJ 2021, 9, e12021. [Google Scholar] [CrossRef] [PubMed]
- Stuhldreher, G.; Fartmann, T. Oviposition-site preferences of a declining butterfly Erebia medusa (Lepidoptera: Satyrinae) in nutrient-poor grasslands. Eur. J. Entomol. 2015, 112, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Jaumann, S.; Snell-Rood, E.C. Trade-offs between fecundity and choosiness in ovipositing butterflies. Anim. Behav. 2017, 123, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Serruys, M.; Van Dyck, H. Development, survival, and phenotypic plasticity in anthropogenic landscapes: Trade-offs between offspring quantity and quality in the nettle-feeding peacock butterfly. Oecologia 2014, 176, 379–387. [Google Scholar] [CrossRef]
- Allen, M.R.; Dube, O.P.; Solecki, W.; Aragón-Durand, F.; Cramer, W.; Humphreys, S.; Kainuma, M.; Kala, J.; Mahowald, N.; Mulugetta, Y.; et al. Framing and Context. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019; p. 84. [Google Scholar]
- Wilson, R.J.; Gutiérrez, D. Effects of climate change on the elevational limits of species ranges. In Ecological Consequences of Climate Change: Mechanisms, Conservation, and Management; Beever, E.A., Belant, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 107–131. [Google Scholar]
- Stork, N.E. How Many Species of Insects and Other Terrestrial Arthropods Are There on Earth? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Prokosch, J.; Bernitz, Z.; Bernitz, H.; Erni, B.; Altwegg, R. Are animals shrinking due to climate change? Temperature-mediated selection on body mass in mountain wagtails. Oecologia 2019, 189, 841–849. [Google Scholar] [CrossRef]
- Wu, C.H.; Holloway, J.D.; Hill, J.K.; Thomas, C.D.; Chen, I.C.; Ho, C.K. Reduced body sizes in climate-impacted Borneo moth assemblages are primarily explained by range shifts. Nat. Commun. 2019, 10, 4612. [Google Scholar] [CrossRef] [Green Version]
- Stamp, N.E.; Casey, T.M. Caterpillars: Ecological and Evolutionary Constraints on Foraging; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Calosi, P.; Bilton, D.T.; Spicer, J.I.; Votier, S.C.; Atfield, A. What determines a species’ geographical range? Thermal biology and latitudinal range size relationships in European diving beetles (Coleoptera: Dytiscidae). J. Anim. Ecol. 2010, 79, 194–204. [Google Scholar] [CrossRef]
- Tewksbury, J.J.; Huey, R.B.; Deutsch, C.A. Putting the Heat on Tropical Animals. Science 2008, 320, 1296. [Google Scholar] [CrossRef]
- Bennett, J.M.; Sunday, J.; Calosi, P.; Villalobos, F.; Martínez, B.; Molina-Venegas, R.; Araújo, M.B.; Algar, A.C.; Clusella-Trullas, S.; Hawkins, B.A.; et al. The evolution of critical thermal limits of life on Earth. Nat. Commun. 2021, 12, 1198. [Google Scholar] [CrossRef] [PubMed]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Vrba, P.; Konvicǩa, M.; Nedvěd, O. Reverse altitudinal cline in cold hardiness among erebia butterflies. Cryo-Letters 2012, 33, 251–258. [Google Scholar]
- Sonderegger, P. The Erebia of Switzerland (Lepidoptera: Satyrinae, Genus Erebia); Peter Sonderegger: Biel/Bienne, Switzerland, 2005; p. 712. [Google Scholar]
- Jason, S. Swiss Butterflies, 2nd ed.; Charaxes Publications: Hull, UK, 2017; p. 35. [Google Scholar]
- Tennent, J. A checklist of the satyrine genus Erebia (Lepidoptera) (1758–2006). Zootaxa 2008, 1900, 1. [Google Scholar] [CrossRef]
- Peña, C.; Witthauer, H.; Klečková, I.; Fric, Z.; Wahlberg, N. Adaptive radiations in butterflies: Evolutionary history of the genus Erebia (Nymphalidae: Satyrinae). Biol. J. Linn. Soc. 2015, 116, 449–467. [Google Scholar] [CrossRef] [Green Version]
- Franco, A.M.A.; Hill, J.K.; Kitschke, C.; Collingham, Y.C.; Roy, D.B.; Fox, R.; Huntley, B.; Thomas, C.D. Impacts of climate warming and habitat loss on extinctions at species’ low-latitude range boundaries. Glob. Chang. Biol. 2006, 12, 1545–1553. [Google Scholar] [CrossRef]
- Grill, A.; Polic, D.; Guarento, E.; Fiedler, K. Permeability of habitat edges for Ringlet butterflies (Lepidoptera, Nymphalidae, Erebia Dalman 1816) in an alpine landscape. Nota Lepidopterol. 2020, 43, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Kleckova, I.; Konvicka, M.; Klecka, J. Thermoregulation and microhabitat use in mountain butterflies of the genus Erebia: Importance of fine-scale habitat heterogeneity. J. Therm. Biol. 2014, 41, 50–58. [Google Scholar] [CrossRef]
- Kraaijeveld, A.R.; Godfray, H.C.J. Potential life-history costs of parasitoid avoidance in Drosophila melanogaster. Evol. Ecol. Res. 2003, 5, 1251–1261. [Google Scholar]
- Kleckova, I.; Vrba, P.; Konvicka, M. Quantitative evidence for spatial variation in the biennial life cycle of the mountain butterfly Erebia euryale (Lepidoptera: Nymphalidae) in the Czech Republic. Eur. J. Entomol. 2015, 112, 114–119. [Google Scholar] [CrossRef]
- Roos, P.; Arnscheid, W. Aspekte der Ökologie und Zoogeographie der europaischen Erebien. Atalanta 1979, 10, 298–308. [Google Scholar]
- Loertscher, M. Population biology of two satyrine butterflies, Erebia meolans (de Prunner, 1798) and Erebia aethiops (Esper, 1777) (Lepidoptera: Satyridae). Nota Lepidopterol. 1991, 2 (Suppl. S2), 22–31. [Google Scholar]
- Broadway, R.M.; Colvin, A.A. Influence of cabbage proteinase inhibitors in situ on the growth of larval Trichoplusia ni and Pieris rapae. J. Chem. Ecol. 1992, 18, 1009–1024. [Google Scholar] [CrossRef]
- Grill, A.; Cerny, A.; Fiedler, K.; Biesmeijer, J.C. Hot summers, long life: Egg laying strategies of Maniola butterflies are affected by geographic provenance rather than adult diet. Contrib. Zool. 2013, 82, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Huey, R.B.; Berrigan, D. Temperature, demography, and ectotherm fitness. Am. Nat. 2001, 158, 204–210. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Ragland, G.J.; Shlichta, J.G. Quantitative genetics of continuous reaction norms: Thermal sensitivity of caterpillar growth rates. Evolution 2004, 58, 1521–1529. [Google Scholar] [CrossRef]
- Molleman, F.; Halali, S.; Kodandaramaiah, U. Oviposition preference maximizes larval survival in the grass-feeding butterfl y Melanitis leda (Lepidoptera: Nymphalidae). Eur. J. Entomol. 2020, 117, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Zuur, A.F. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: http://www.R-project.org/ (accessed on 1 May 2020).
- Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.6.0. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 1 May 2020).
- Lüdecke, D. ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 1 June 2020).
- Chen, I.C.; Shiu, H.J.; Benedick, S.; Holloway, J.D.; Chey, V.K.; Barlow, H.S.; Hill, J.K.; Thomas, C.D. Elevation increases in moth assemblages over 42 years on a tropical mountain. Proc. Natl. Acad. Sci. USA 2009, 106, 1479–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- Konvicka, M.; Maradova, M.; Benes, J.; Fric, Z.; Kepka, P. Uphill shifts in distribution of butterflies in the Czech Republic: Effects of changing climate detected on a regional scale. Glob. Ecol. Biogeogr. 2003, 12, 403–410. [Google Scholar] [CrossRef] [Green Version]
- Moritz, C.; Patton, J.L.; Conroy, C.J.; Parra, J.L.; White, G.C.; Beissinger, S.R. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 2008, 322, 261–264. [Google Scholar] [CrossRef] [Green Version]
- Pörtner, H.O.; Bennett, A.F.; Bozinovic, F.; Clarke, A.; Lardies, M.A.; Lucassen, M.; Pelster, B.; Schiemer, F.; Stillman, J.H. Trade-offs in thermal adaptation: The need for a molecular to ecological integration. Physiol. Biochem. Zool. 2006, 79, 295–313. [Google Scholar] [CrossRef] [Green Version]
- Leather, S.; Walters, K.; Bale, J. The Ecology of Insect Overwintering; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Sehnal, F. Growth and life cycle. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Elmsford, NY, USA, 1985; Volume 2, pp. 1–86. [Google Scholar]
- Bozinovic, F.; Bastías, D.A.; Boher, F.; Clavijo-Baquet, S.; Estay, S.A.; Angilletta, M.J. The Mean and Variance of Environmental Temperature Interact to Determine Physiological Tolerance and Fitness. Physiol. Biochem. Zool. 2011, 84, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Pelini, S.L.; Dzurisin, J.D.K.; Prior, K.M.; Williams, C.M.; Marsico, T.D.; Sinclair, B.J.; Hellmann, J.J. Translocation experiments with butterflies reveal limits to enhancement of poleward populations under climate change. Proc. Natl. Acad. Sci. USA 2009, 106, 11160–11165. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, D. Effects of temperature on the size of aquatic ectotherms: Exceptions to the general rule. J. Therm. Biol. 1995, 20, 61–74. [Google Scholar] [CrossRef]
- Merckx, T.; Souffreau, C.; Kaiser, A.; Baardsen, L.F.; Backeljau, T.; Bonte, D.; Brans, K.I.; Cours, M.; Dahirel, M.; Debortoli, N.; et al. Body-size shifts in aquatic and terrestrial urban communities. Nature 2018, 558, 113–116. [Google Scholar] [CrossRef]
- Quenta, E.; Daza, A.; Lazzaro, X.; Jacobsen, D.; Dangles, O.; Cauvy-Fraunié, S. Aquatic biota responses to temperature in a high Andean geothermal stream. Freshw. Biol. 2021, 66, 1889–1900. [Google Scholar] [CrossRef]
- Stuhldreher, G.; Fartmann, T. Threatened grassland butterflies as indicators of microclimatic niches along an elevational gradient—Implications for conservation in times of climate change. Ecol. Indic. 2018, 94, 83–98. [Google Scholar] [CrossRef]
- Vrba, P.; Sucháčková Bartoňová, A.; Andres, M.; Nedvěd, O.; Šimek, P.; Konvička, M. Exploring Cold Hardiness within a Butterfly Clade: Supercooling Ability and Polyol Profiles in European Satyrinae. Insects 2022, 13, 369. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zografou, K.; Adamidis, G.C.; Sewall, B.J.; Grill, A. Not Too Warm, Not Too Cold: Thermal Treatments to Slightly Warmer or Colder Conditions from Mother’s Origin Can Enhance Performance of Montane Butterfly Larvae. Biology 2022, 11, 915. https://doi.org/10.3390/biology11060915
Zografou K, Adamidis GC, Sewall BJ, Grill A. Not Too Warm, Not Too Cold: Thermal Treatments to Slightly Warmer or Colder Conditions from Mother’s Origin Can Enhance Performance of Montane Butterfly Larvae. Biology. 2022; 11(6):915. https://doi.org/10.3390/biology11060915
Chicago/Turabian StyleZografou, Konstantina, George C. Adamidis, Brent J. Sewall, and Andrea Grill. 2022. "Not Too Warm, Not Too Cold: Thermal Treatments to Slightly Warmer or Colder Conditions from Mother’s Origin Can Enhance Performance of Montane Butterfly Larvae" Biology 11, no. 6: 915. https://doi.org/10.3390/biology11060915
APA StyleZografou, K., Adamidis, G. C., Sewall, B. J., & Grill, A. (2022). Not Too Warm, Not Too Cold: Thermal Treatments to Slightly Warmer or Colder Conditions from Mother’s Origin Can Enhance Performance of Montane Butterfly Larvae. Biology, 11(6), 915. https://doi.org/10.3390/biology11060915







