Effect of Diet Consistency on Rat Mandibular Growth: A Geometric Morphometric and Linear Cephalometric Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet
2.2. Radiograph Acquisition
2.3. Data Processing
2.4. Statistical Analysis
3. Results
3.1. Weight Measurement
3.2. Geometric Morphometric Analysis
3.3. Length Measurement
3.4. Posterior Height Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dias, G.J.; Cook, R.B.; Mirhosseini, M. Influence of food consistency on growth and morphology of the mandibular condyle. Clin. Anat. 2011, 24, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Yang, L.Y.; Chen, K.T.; Chiu, W.C. The influence of masticatory hypofunction on developing rat craniofacial structure. Int. J. Oral Maxillofac. Surg. 2010, 39, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Proffit, W.R.; Fields, H.W.; Sarver, D.M. Contemporary orthodontic appliances. In Contemporary Orthodontics; Elsiever: St. Louis, MO, USA, 2012; pp. 348–350. [Google Scholar]
- Moss, M.L. The functional matrix hypothesis revisited. 1. The role of mechanotransduction. Am. J. Orthod. Dentofac. Orthop. 1997, 112, 8–11. [Google Scholar] [CrossRef]
- Moss, M.L. The functional matrix hypothesis revisited. 3. The genomic thesis. Am. J. Orthod. Dentofacial. Orthop. 1997, 112, 338–342. [Google Scholar] [CrossRef]
- Kiliaridis, S.; Shyu, B.C. Isometric muscle tension generated by masseter stimulation after prolonged alteration of the consistency of the diet fed to growing rats. Arch. Oral Biol. 1988, 33, 467–472. [Google Scholar] [CrossRef]
- Ravosa, M.J.; Klopp, E.B.; Pinchoff, J.; Stock, S.R.; Hamrick, M.W. Plasticity of mandibular biomineralization in myostatindeficient mice. J. Morphol. 2007, 268, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, F.D.S.; Diniz, P.; Carvalho, P.E.G.; Ferreira, E.C.; Paulon, A.S.R.; Ferreira-Santos, R. Effects of masticatory hypofunction on mandibular morphology, mineral density and basal bone area. Braz. J. Oral Sci. 2013, 12, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Takahashi, S.; Domon, T. Effects of a Liquid Diet on the Temporomandibular Joint of Growing Rats. Med. Princ. Pract. 2015, 24, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Kimmel, D.B. The effect of dietary consistency on bone mass and turnover in the growing rat mandible. Archs. Oral Biol. 1991, 2, 129–138. [Google Scholar] [CrossRef]
- Rodrigues, L.; Traina, A.A.; Nakamai, L.F.; Luz, J.G. Effects of the unilateral removal and dissection of the masseter muscle on the facial growth of young rats. Braz. Oral Res. 2009, 23, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Jurek, A.; Gozdowski, D.; Czochrowska, E.M.; Zadurska, M. Effect of Tooth Agenesis on Mandibular Morphology and Position. Int. J. Environ. Res. Public Health 2021, 18, 11876. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.S.; Fernandes, M.H. Rodent models in bone-related research: The relevance of calvarial defects in the assessment of bone regeneration strategies. Lab. Anim. 2011, 45, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Tsolakis, I.A.; Verikokos, C.; Perrea, D.; Bitsanis, E.; Tsolakis, A.I. Effects of diet consistency on mandibular growth. A review. J. Hell. Vet. Med. Soc. 2019, 70, 1603–1610. [Google Scholar] [CrossRef] [Green Version]
- Karamani, I.I.; Tsolakis, I.A.; Makrygiannakis, M.A.; Georgaki, M.; Tsolakis, A.I. Impact of Diet Consistency on the Mandibular Morphology: A Systematic Review of Studies on Rat Models. Int. J. Environ. Res. Public Health 2022, 19, 2706. [Google Scholar] [CrossRef] [PubMed]
- Dontas, I.; Tsolakis, A.I.; Khaldi, L.; Patra, E.; Lyriritis, G.P. Malocclusion in Aging Wistar Rats. J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 1–5. [Google Scholar]
- Nicholson, E.K.; Stock, S.R.; Hamrick, M.W.; Ravosa, M.J. Biomineralization and adaptive plasticity of the temporomandibular joint in myostatin knockout mice. Arch. Oral Biol. 2006, 51, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Kufley, S.; Scott, J.E.; Ramirez-Yanez, G. The effect of the physical consistency of the diet on the bone quality of the mandibular condyle in rats. Arch. Oral Biol. 2017, 77, 23–26. [Google Scholar] [CrossRef]
- Roach, I.H.; Mehta, G.; Oreffo, O.C.R.; Clarke, N.M.P.; Cooper, C. Temporal Analysis of Rat Growth Plates: Cessation of Growth with Age Despite Presence of a Physis. J. Histochem. Cytochem. 2003, 51, 373–383. [Google Scholar] [CrossRef] [Green Version]
- McCutcheon, J.E.; Marinelli, M. Age matters. Eur. J. Neurosci. 2009, 29, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Odman, A.; Mavropoulos, A.; Kiliaridis, S. Do masticatory functional changes influence the mandibular morphology in adult rats. Arch. Oral Biol. 2008, 53, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Sano, R.; Kawai, N.; Langenbach, C.E.J.A.; Brugman, P.; Tanne, K.; Van Eijden, T.M.G.J. Effect of Food Consistency on the Degree of Mineralization in the Rat Mandible. Ann. Biomed. Eng. 2007, 35, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Grünheid, T.; Langenbach, G.E.J.; Brugman, P.; Vincent Everts, V.; Zentner, A. The masticatory system under varying functionalload. Part 2: Effect of reduced masticatory load on the degree and distribution of mineralization in the rabbit mandible. Eur. J. Orthod. 2011, 33, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Tsolakis, A.I.; Spyropoulos, M.N.; Katsavrias, E.; Alexandridis, K. Effects of altered mandibular function on mandibular growth after condylectomy. Eur. J. Orthod. 1997, 19, 9–19. [Google Scholar] [CrossRef]
- Kiliaridis, S.; Thilander, B.; Kjellberg, H.; Topouzelis, N.; Zafiriadis, A. Effect of low masticatory function on condylar growth: Amorphometric study in the rat. Am. J. Orthod. Dentofac. Orthop. 1999, 116, 121–125. [Google Scholar] [CrossRef]
- Kiliaridis, S.; Engstrdm, C.; Thilander, B. The relationship between masticatory function and craniofacial morphology I. A cephalometric longitudinal analysis in the growing rat fed a soft diet. Eur. J. Orthod. 1985, 7, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.; Nishioka, T.; Shioiri, E.; Takahashi, T.; Kimura, M. Effects of Dietary Consistency on the Mandible of Rats at the Growth Stage: Computed X-ray Densitometric and Cephalometric Analysis. Angle Orthod. 2002, 72, 468–475. [Google Scholar] [PubMed]
- Abed, G.S.; Buschang, P.H.; Taylor, R.; Hinton, R.J. Maturational and functional related differences in rat craniofacial growth. Arch. Oral Biol. 2007, 52, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Hichijo, N.; Kawai, N.; Mori, H.; Sano, R.; Ohnuki, Y.; Okumura, S.; Langenbach, G.E.J.; Tanaka, E. Effects of the masticatory demand on the rat mandibular development. J. Oral Rehabil. 2014, 41, 581–587. [Google Scholar] [CrossRef] [PubMed]
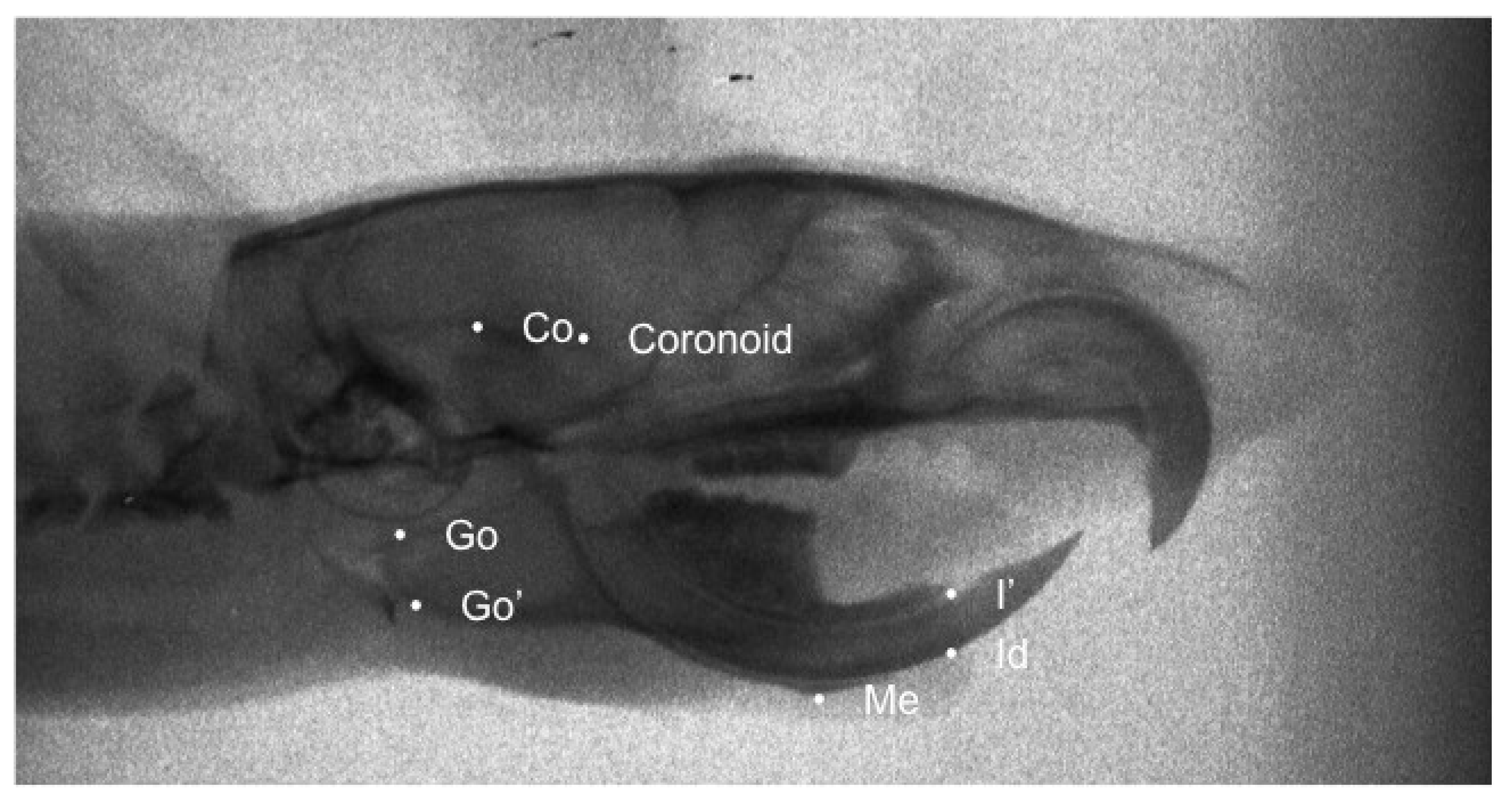
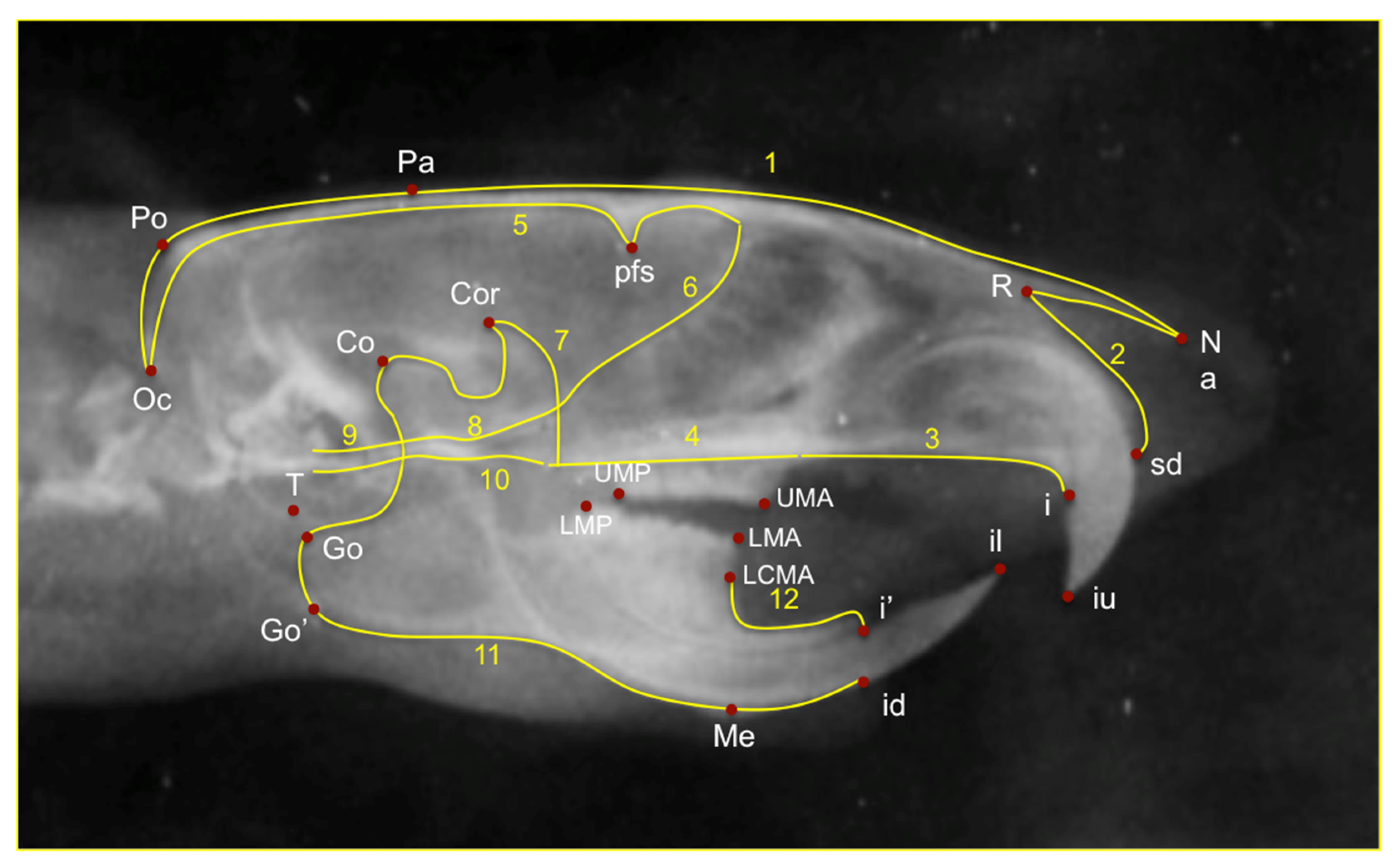

| Cephalometric Landmarks | Definition |
|---|---|
| Co | Most posterior-superior point on the mandibular condyle. |
| Go | Most posterior point of the angular process of the mandible |
| Go’ | Point on the most inferior contour of the angular process of the mandible |
| Coronoid | Most posterosuperior point of condylar process |
| Me | The most inferior and anterior point of the lower border of the mandible |
| Id | Most inferior and anterior point on the alveolar process of the mandible |
| I’ | The most anterior edge of the alveolar bone on the convexity of the lower incisor. |
| Structures | Cephalometric Measurements |
|---|---|
| Mandibular length | Co-Me |
| Coronoid-Me | |
| Go-Me | |
| Go’-Me | |
| Co-Id | |
| Co-I’ | |
| Posterior mandibular height | Co-Go |
| Co-Go’ |
| Mean Weight (g) | SD | p Value | |
|---|---|---|---|
| Soft Diet | 248.4 | 5.1 | p = 0.074 |
| Hard Diet | 247.7 | 5.5 |
| Variables | Cochran’s Alpha | |
|---|---|---|
| Linear measurements | Go’-Me | 0.833 |
| Go-Me | 0.857 | |
| Coronoid-Me | 0.859 | |
| Co-Me | 0.822 | |
| Co-Id | 0.934 | |
| Co-I’ | 0.944 | |
| Co-Go | 0.923 | |
| Co-Go’ | 0.911 |
| Linear Measurements | Diet S | Diet H | |
|---|---|---|---|
| (n = 12) | (n = 12) | ||
| Mean (SD) | Mean (SD) | S-H | |
| Go’-Me | 15.80 (1.86) | 18.65 (1.28) | <0.001 |
| Go-Me | 17.38 (1.32) | 20.59 (1.42) | <0.001 |
| Coronoid-Me | 13.98 (1.11) | 15.74 (1.42) | 0.001 |
| Co-Me | 17.43 (1.24) | 20.74 (1.56) | <0.001 |
| Co-Id | 20.59 (1.17) | 24.47 (1.53) | <0.001 |
| Co-I’ | 19.84 (1.16) | 23.42 (1.47) | <0.001 |
| Co-Go | 5.91 (0.49) | 7.65 (0.55) | <0.001 |
| Co-Go’ | 6.97 (0.69) | 8.84 (0.61) | <0.001 |
| Study design | 1 | a. In the first group the female Wistar were fed a soft diet and in the second group, they were fed a hard diet b. Animals were kept in separate cages. The bedding material of the cages of this group was sifted to exclude large particles that could stimulate extra gnawing activity. Page 2,3 |
| Sample size | 2 | a. A total sample of 24 female Wistar aged 30 days was used in this study. (12 for each group) b. Sample size was decided according to previous similar research. Page 2 |
| Inclusion and exclusion criteria | 3 | a. The soft diet group received the ordinary diet, ground and mixed with water in standardized proportions. The hard diet groups were fed the ordinary diet for rats in hard pellet form b. Soft diet group (n = 12), Hard diet group (n = 12) Page 2,3 |
| Randomization | 4 | a. They were computer-generated randomized and equally separated into two groups Page 2 |
| Blinding | 5 | a. The operator for linear and geometric morphometric analysis was blinded. It was given a random number for each wistar that only the rest of the authors knew to which group corresponds. Page 3 |
| Outcome measures | 6 | a. Mandibular morphology Page 4 |
| Statistical methods | 7 | a. Differences related to diet, were assessed using regression analyses. Each measurement was regressed on diet and their interaction. When the normality assumption for the residuals was violated, quantile regression was used. Comparisons were adjusted using the Bonferroni method for multiple comparisons. Analysis was performed on α = 5% level of statistical significance. For statistical analysis of morphometric measurements, a permutation test was used (10,000 permutations without replacement). Page 3 |
| Experimental animals | 8 | a. Female Wistar rats b. Healthy female wistar rats Page 2 |
| Experimental procedures | 9 | a. Each group was fed daily for a 30 days period time with the appropriate diet. b. Afterwards the sedated animals were hung via their upper incisors from the horizontal post and the ear posts were placed in a position corresponding to the animal’s earholes at the plastic vertical projections. c. The X-ray films were manually developed with a developing time of 5 min and a fixing time of 8 min. The films were washed for 1 h after fixation and air-dried. d. In order to digitize the X-rays, we scanned them by using the Epson V750 Pro. e. Once the films were scanned they were uploaded to the Viewbox software and then digitally traced. Page 2,3 |
| Results | 10 | a. They were significant differences between all soft diet groups when they were compared with the hard diet groups in all linear measurements. (Go’-Me, Go-Me, Coronoid-Me, Co-Me, Co-Id, Co-I’, Co-Go, Co-Go’) b. The superimposition of the morphometric means of each group showed differences on the condyle, the angle of the mandible, and the body of the mandible. Page 5,6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsolakis, I.A.; Verikokos, C.; Perrea, D.; Alexiou, K.; Gizani, S.; Tsolakis, A.I. Effect of Diet Consistency on Rat Mandibular Growth: A Geometric Morphometric and Linear Cephalometric Study. Biology 2022, 11, 901. https://doi.org/10.3390/biology11060901
Tsolakis IA, Verikokos C, Perrea D, Alexiou K, Gizani S, Tsolakis AI. Effect of Diet Consistency on Rat Mandibular Growth: A Geometric Morphometric and Linear Cephalometric Study. Biology. 2022; 11(6):901. https://doi.org/10.3390/biology11060901
Chicago/Turabian StyleTsolakis, Ioannis A., Christos Verikokos, Despoina Perrea, Konstantina Alexiou, Sotiria Gizani, and Apostolos I. Tsolakis. 2022. "Effect of Diet Consistency on Rat Mandibular Growth: A Geometric Morphometric and Linear Cephalometric Study" Biology 11, no. 6: 901. https://doi.org/10.3390/biology11060901
APA StyleTsolakis, I. A., Verikokos, C., Perrea, D., Alexiou, K., Gizani, S., & Tsolakis, A. I. (2022). Effect of Diet Consistency on Rat Mandibular Growth: A Geometric Morphometric and Linear Cephalometric Study. Biology, 11(6), 901. https://doi.org/10.3390/biology11060901








