Reproductive Biology of Dry Grassland Specialist Ranunculus illyricus L. and Its Implications for Conservation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Vegetative Reproduction
2.3. Generative Reproduction
2.3.1. Pollen Quality (Production and Viability)
- A—number of pollen grains per anther,
- X—average number of pollen grains per counting field,
- Ve—total volume of the pollen grain solution in a 1 mL (1000 μL) Eppendorf tube,
- Vc—volume of the counting field of 0.1 mm3 (0.1 μL),
- n—number of stamens in the Eppendorf tube.
2.3.2. Seed Viability and Ability to Germinate
2.3.3. Development and Survival of Seed-Derived Plants under Ex Situ Conditions
2.4. Statistical Analysis
3. Results
3.1. Annual Development Cycle
3.2. Vegetative Reproduction
3.3. Generative Reproduction
3.3.1. Flowering and Fruit Setting
3.3.2. Seed Germination
3.3.3. Development of Seedlings under Ex Situ Conditions
4. Discussion
5. Conclusions
- This is the first time the annual developmental cycle of R. illyricus has been described, allowing us to present its ability to reproduce both generatively and vegetatively.
- The efficiency of vegetative propagation ex situ depended on the age of the tuber (clone) and indirectly on weather conditions. After three years, the best clones could produce up to 57 progeny clusters, which flowered in the first vegetative season, but the regeneration potential of the tubers started to decrease in the case of the two-year-old tubers.
- The high potential of R. illyricus for generative reproduction was limited by low seed-setting efficiency under ex situ conditions and difficulties with seed germination and seedling survival. In addition, the first flowering plant of seed origin was observed in the third year after planting.
- Vegetative reproduction was more effective than generative reproduction because more progeny clusters could be obtained during one season and they were able to propagate through both reproduction modes in the following season.
- The best way to increase the natural resources of this species would be ex situ generative propagation followed by vegetative propagation of the resulting plants.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chapin, F.S.; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef]
- Pimm, S.L.; Joppa, L.N. How many plant species are there, where are they, and at what rate are they going extinct? Ann. Mo. Bot. Gard. 2015, 100, 170–176. [Google Scholar] [CrossRef]
- Corlett, R.T. Plant diversity in a changing world: Status, trends, and conservation needs. Plant Divers. 2016, 38, 10–16. [Google Scholar] [CrossRef]
- Massey, J.R.; Whitson, P.D. Species biology, the key to plant preservation. Rhodora 1980, 82, 97–103. [Google Scholar]
- Hamrick, J.L.; Godt, M.W. Effects of life history traits on genetic diversity in plant species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 1291–1298. [Google Scholar] [CrossRef]
- Török, P.; Dembicz, I.; Dajic-Stevanovic, Z.; Kuzemko, A. Grasslands of Eastern Europe. In Encyclopedia of the World’s Biomes; Elsevier: Amsterdam, The Netherlands, 2020; Volume 3, pp. 703–713. [Google Scholar] [CrossRef]
- Dengler, J.; Biurrun, I.; Boch, S.; Dembicz, I.; Török, P. Grasslands of the Palaearctic biogeographic realm: Introduction and synthesis. In Encyclopedia of the World’s Biomes; Elsevier: Amsterdam, The Netherlands, 2020; Volume 3, pp. 617–637. [Google Scholar] [CrossRef]
- Hörandl, E.; Emadzade, K. Evolutionary classification: A case study on the diverse plant genus Ranunculus L. (Ranunculaceae). Perspect. Plant Ecol. Evol. Syst. 2012, 14, 310–324. [Google Scholar] [CrossRef]
- Erdős, L.; Tölgyesi, C.; Dénes, A.; Darányi, N.; Fodor, A.; Bátori, Z.; Tolnay, D. Comparative analysis of the natural and seminatural plant communities of Mt Nagy and other parts of the Villány Mts (south Hungary). Thaiszia J. Bot. 2014, 24, 1–21. [Google Scholar]
- Moskal-del Hoyo, M.; Mueller-Bieniek, A.; Alexandrowicz, W.P.; Wilczyński, J.; Wędzicha, S.; Kapcia, M.; Przybyła, M.M. The continuous persistence of open oak forests in the Miechów Upland (Poland) in the second half of the Holocene. Quat. Int. 2017, 458, 14–27. [Google Scholar] [CrossRef]
- Lachashvili, N.J.; Khachidze, M.N.; Eradze, N.V.; Khetsuriani, L.D. Steppe of Tbilisi environs (East Georgia, South Caucasus). Ann. Agrar. Sci. 2017, 15, 332–338. [Google Scholar] [CrossRef]
- Bede, Á.; Csathó, A.I. Complex characterization of kurgans in the Csanádi-hát region, Hungary. Tájökológiai Lapok 2019, 17, 131–145. [Google Scholar]
- Kovács, D. Adatok Magyarországflórájához I. (Data to the flora of Hungary I.). Kitaibelia 2014, 19, 254–259. [Google Scholar]
- Molnár, A.V.; Löki, V.; Máté, A.; Molnár, A.; Takács, A.; Nagy, T.; Lovas-Kiss, Á.; Lukács, B.A.; Sramkó, G.; Tökölyi, J. The occurrence of Spiraea crenata and other rare steppe plant in Pannonian graveyards. Biologia 2017, 72, 500–509. [Google Scholar] [CrossRef]
- Tomović, G.; Randjelović, V.; Niketić, M.; Vukojičić, S.; Zlatković, B. New distribution data of some Pontic and submediterranean plant species in Serbia. Arch. Biol. Sci. 2003, 55, 45–54. [Google Scholar] [CrossRef]
- Kaźmierczakowa, R.; Towpasz, K. Ranunculus illyricus L.—Jaskier illiryjski. In Polish Red Data Book of Plants—Pteridophytes and Flowering Plants, 3rd ed.; Kaźmierczakowa, R., Zarzycki, K., Mirek, Z., Eds.; Polish Academy of Sciences, Institute of Nature Conservation: Cracow, Poland, 2014; pp. 196–197. (In Polish) [Google Scholar]
- Towpasz, K.; Cwener, A. Nowe stanowisko Ranunculus illyricus (Ranunculaceae) w Polsce. Fragmenta Floristica et Geobotanica Polonica 2002, 9, 370–372. [Google Scholar]
- Dembicz, J.; Kozub, Ł. Potwierdzenie występowania Ranunculus illyricus (Ranuncululaceae) w Skorocicach (Wyżyna Małopolska) (Confirmation of Ranunculus illyricus (Ranunculaceae) locality in Skorocice (Małopolska Upland)). Fragm. Florist. Geobot. Pol. 2015, 22, 381–384. [Google Scholar]
- PLADIAS. Database of the Czech Flora and Vegetation. Available online: www.pladias.cz (accessed on 10 March 2022).
- Mourad, M.M.; Hamed, K.A.; Al-Nowaihi, A.S. The morphology and anatomy of the achene in certain species of sub-family Ranunculoideae (Ranunculaceae) with special reference to the achene vasculature. Taeckholmia 2000, 20, 33–49. [Google Scholar] [CrossRef]
- Gherghişan, E. Research on blastogenesis process ex situ in some species of angiosperms in Macin Mountains National Park. J. Hortic. For. 2013, 17, 133–140. [Google Scholar]
- Aslam, M.S.; Choudhary, B.A.; Uzair, M.; Ijaz, A.S. The genus Ranunculus: A phytochemical and ethnopharmacological review. Int. J. Pharm. Pharm. Sci. 2012, 4, 15–22. [Google Scholar]
- Hachelaf, A.; Touil, A.; Zellagui, A.; Rhouati, S. Antioxidant and antibacterial activities of essential oil extracted from Ranunculus arvensis L. Der Pharma Chem. 2015, 7, 170–173. [Google Scholar]
- Kelemen, C.D.; Houdkova, M.; Urbanova, K.; Badarau, S.; Gurean, D.; Pamfil, D.; Kokoska, L. Chemical composition of the essential oils of aerial parts of Aconitum, Anemone and Ranunculus (Ranunculaceae) species from Romania. J. Essent. Oil-Bear. Plants 2019, 22, 728–745. [Google Scholar] [CrossRef]
- Akbari, T.M. Bioecological and agrocultural properties of Ranunculus L. in the flora of Azerbaijan. Int. J. Curr. Res. Biosci. Plant. Biol. 2017, 4, 69–73. [Google Scholar] [CrossRef]
- Alexander, M.P. Diferential staining of aborted and nonaborted pollen. Stain Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Godini, A. Counting pollen grains of some almond cultivars by means of anhaemocytometer. Rivista Di Ortoflorofrutticoltura Italiana 1981, 65, 173–178. [Google Scholar]
- Peters, J. Tetrazolium Testing Handbook: Contribution No. 29 to the Handbook on Seed Testing, Revised 2000; AOSA: Las Cruces, NM, USA, 2000. [Google Scholar]
- International Seed Testing Association (ISTA). International rules for seed testing. Seed Sci. Technol. 1999, 27, 1–333. [Google Scholar]
- Troll, W. Vergleichende Morphologie der Höheren Pflanzen; Verlag von Gebrüder Bortraeger: Berlin-Zehlendorf, Germany, 1943; pp. 2646–2652. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993; Volume 1, pp. 269–286. [Google Scholar]
- Kocot, D.; Sitek, E.; Nowak, B.; Kołton, A.; Stachurska-Swakoń, A.; Towpasz, K. The Effectiveness of the Sexual Reproduction in Selected Clonal and Nonclonal Species of the Genus Ranunculus. Biology 2022, 11, 85. [Google Scholar] [CrossRef]
- Cursach, J.; Rita, J. Reproductive biology of Ranunculus weyleri (Ranunculaceae), a narrowly endemic plant from the Balearic Islands with disjunct populations. Flora Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 726–735. [Google Scholar] [CrossRef]
- Rita, J.; Cursach, J. Creating new populations of Apium bermejoi (Apiaceae), a critically endangered endemic plant on Menorca (Balearic Islands). An. Jard. Bot. Madr. 2013, 70, 27–38. [Google Scholar] [CrossRef]
- Leśniański, G.Z.; Szmalec, T. Stan Ochrony Gatunków Roślin w Polsce w Latach 2013–2018; Biuletyn Monitoringu Przyrody, Biblioteka Monitoringu Środowiska GIOŚ: Warszawa, Poland, 2021; Volume 23, pp. 1–155. [Google Scholar]
- Dembicz, I.; Moysiyenko, I.I.; Kozub, Ł.; Dengler, J.; Zakharova, M.; Sudnik-Wójcikowska, B. Steppe islands in a sea of fields: Where island biogeography meets the reality of a severely transformed landscape. J. Veg. Sci. 2021, 32, 1–11. [Google Scholar] [CrossRef]
- Zhao, L.; Bachelier, J.B.; Chang, H.L.; Tian, X.H.; Ren, Y. Inflorescence and floral development in Ranunculus and three allied genera in Ranunculeae (Ranunculoideae, Ranunculaceae). Plant Syst. Evol. 2012, 298, 1057–1071. [Google Scholar] [CrossRef]
- Linkies, A.; Leubner-Metzger, G. Beyond gibberellins and abscisic acid: How ethylene and jasmonates control seed germination. Plant Cell Rep. 2012, 31, 253–270. [Google Scholar] [CrossRef]
- Golmohammadzadeh, S.; Zaefarian, F.; Rezvani, M. Effects of some chemical factors, prechilling treatments and interactions on the seed dormancy-breaking of two Papaver species. Weed Biol. Manag. 2015, 15, 11–19. [Google Scholar] [CrossRef]
- Nowak, B.; Sitek, E.; Augustynowicz, J. Sourcing and Propagation of Pontechium maculatum for Horticulture and Species Restoration. Biology 2020, 9, 317. [Google Scholar] [CrossRef]
- Sitek, E.; Nowak, B.; Fecowicz, M.; Gajewski, Z.; Dańda, P.; Kapała, K.; Kozik-Dąbek, B. Application of horticultural and tissue culture methods for ex situ conservation of endangered Primula farinosa L. Acta Soc. Bot. Pol. 2020, 89, 1–15. [Google Scholar] [CrossRef]
- Karami, A.; Khosh-Khui, M. Presence of double dormancy in Wild Persian Buttercup (Ranunculus asiaticus L.). Int. J. Agric. Res. 2010, 2, 97–101. [Google Scholar] [CrossRef][Green Version]
- Czarnecka, B. The dynamics of the population of a steppe perennial Senecio macrophyllus M. BIEB. during xerothermic grassland overgrowing. Acta Soc. Bot. Pol. 2009, 78, 247–256. [Google Scholar] [CrossRef][Green Version]
- James, J.J.; Svejcar, T.J.; Rinella, M.J. Demographic processes limiting seedling recruitment in arid grassland restoration. J. Appl. Ecol. 2011, 48, 961–969. [Google Scholar] [CrossRef]
- Partzsch, M.; Faulhaber, M.; Meier, T. The effect of the dominant grass Festuca rupicola on the establishment of rare forbs in semi-dry grasslands. Folia Geobot. 2018, 53, 103–113. [Google Scholar] [CrossRef]
- Harper, J.L.; White, J. The demography of plants. Annu. Rev. Ecol. Evol. Syst. 1974, 5, 419–463. [Google Scholar] [CrossRef]
- Beruto, M.; Rabaglio, M.; Viglione, S.; Van Labeke, M.C.; Dhooghe, E. Ranunculus. In Ornamental Crops. Handbook of Plant Breeding; Van Huylenbroeck, J., Ed.; Springer: Cham, Switzerland, 2018; Volume 11, pp. 649–671. [Google Scholar] [CrossRef]
- Frey, M.N.; Schmit, J.P. Early-Season Treatment of Fig Buttercup (Ranunculus ficaria). Invasive Plant Sci. Manag. 2017, 10, 191–200. [Google Scholar] [CrossRef]
- Eriksson, O. Seedling dynamics and life histories in clonal plants. Oikos 1989, 55, 231–238. [Google Scholar] [CrossRef]
- Pirożnikow, E. Life cycle of herbaceous plants in disturbed and undisturbed sites of oak-linden-hornbeam forest (Tilio-Carpinetum). Ekol. Pol. 1998, 46, 157–168. [Google Scholar]
- Kamenetsky, R.; Peterson, L.R.; Melville, L.H.; Machado, C.F.; Bewley, J.D. Seasonal adaptations of the tuberous roots of Ranunculus asiaticus to desiccation and resurrection by changes in cell structure and protein content. New Phytol. 2005, 166, 193–204. [Google Scholar] [CrossRef]
- Dhooghe, E.; Grunewald, W.; Reheul, D.; Goetghebeur, P.; Van Labeke, M.C. Floral characteristics and gametophyte development of Anemone coronaria L. and Ranunculus asiaticus L. (Ranunculaceae). Sci. Hortic. 2012, 138, 73–80. [Google Scholar] [CrossRef]
- Godefroid, S.; Rivière, S.; Waldren, S.; Boretos, N.; Eastwood, R.; Vanderborght, T. To what extent are threatened European plant species conserved in seed banks? Biol. Conserv. 2011, 144, 1494–1498. [Google Scholar] [CrossRef]
- Coppi, A.; Lastrucci, L.; Carta, A.; Foggi, B. Analysis of genetic structure of Ranunculus baudotii in a Mediterranean wetland. Implications for selection of seeds and seedlings for conservation. Aquat. Bot. 2015, 126, 25–31. [Google Scholar] [CrossRef]
- Luijten, S.H.; Kéry, M.; Oostermeijer, J.G.B.; Den Nijs, H.(J.)C.M. Demographic consequences of inbreeding and outbreeding in Arnica montana: A field experiment. J. Ecol. 2002, 90, 593–603. [Google Scholar] [CrossRef]
- Scobie, A.R.; Wilcock, C.C. Limited mate availability decreases reproductive success of fragmented populations of Linnaea borealis, a rare, clonal self-incompatible plant. Ann. Bot. 2009, 103, 835–846. [Google Scholar] [CrossRef]
- Gavin-Smyth, N.; Kramer, A.T.; Urbina-Casanova, R.; Vitt, P.; Fant, J.B. Genetic rescue reduces mate limitation in a threatened, clonal, and self, clonal, and plant species. Restor. Ecol. 2021, 29, e13458. [Google Scholar] [CrossRef]
- Kunz, M.; Buchanan, M.F.; Randall, J.L.; Wall, W.A.; Hohmann, M.G. Life cycle, vegetative propagation, and reintroduction of federally endangered rough-leaved loosestrife, Lysimachia asperulifolia. Castanea 2014, 79, 18–26. [Google Scholar] [CrossRef]

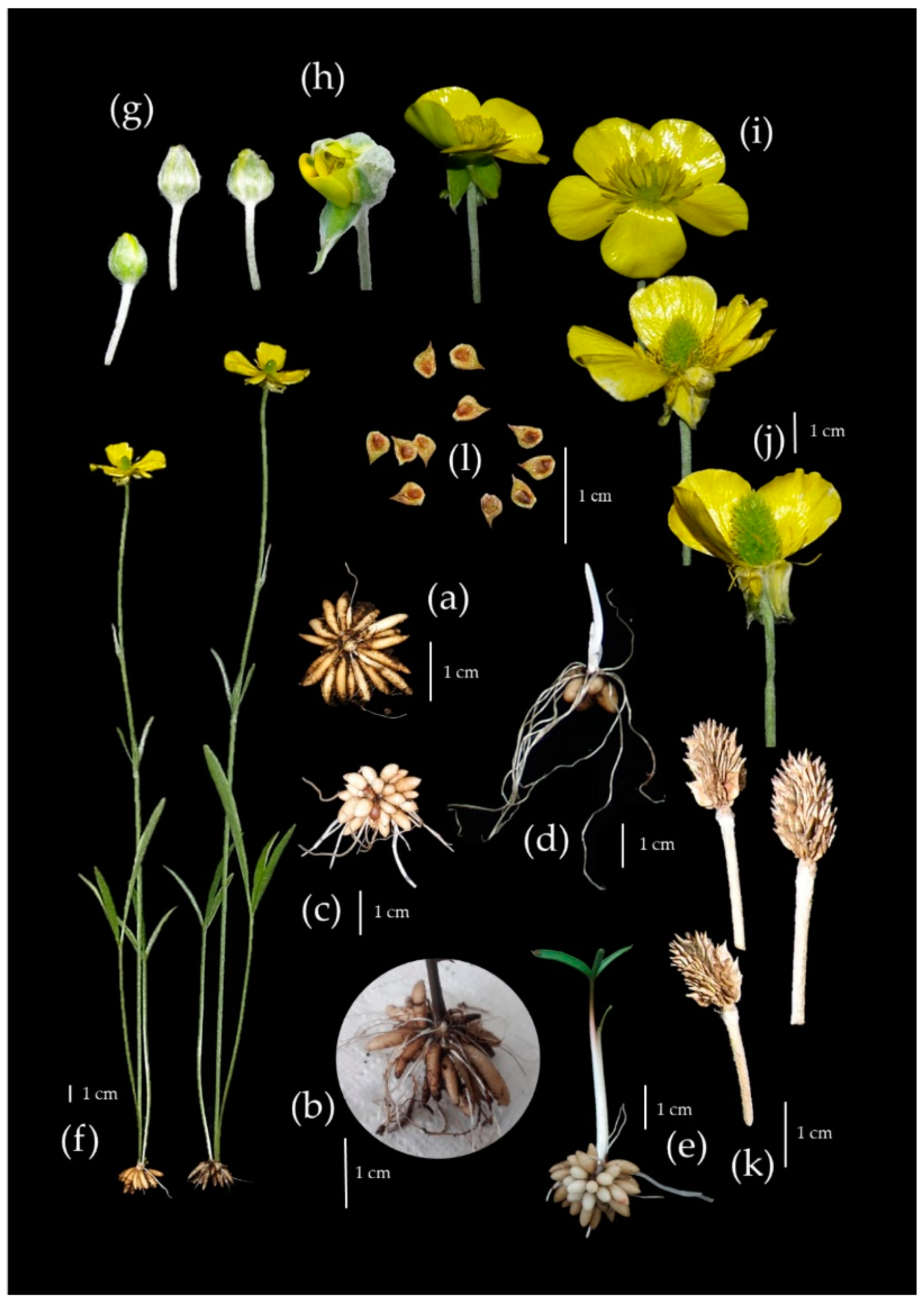

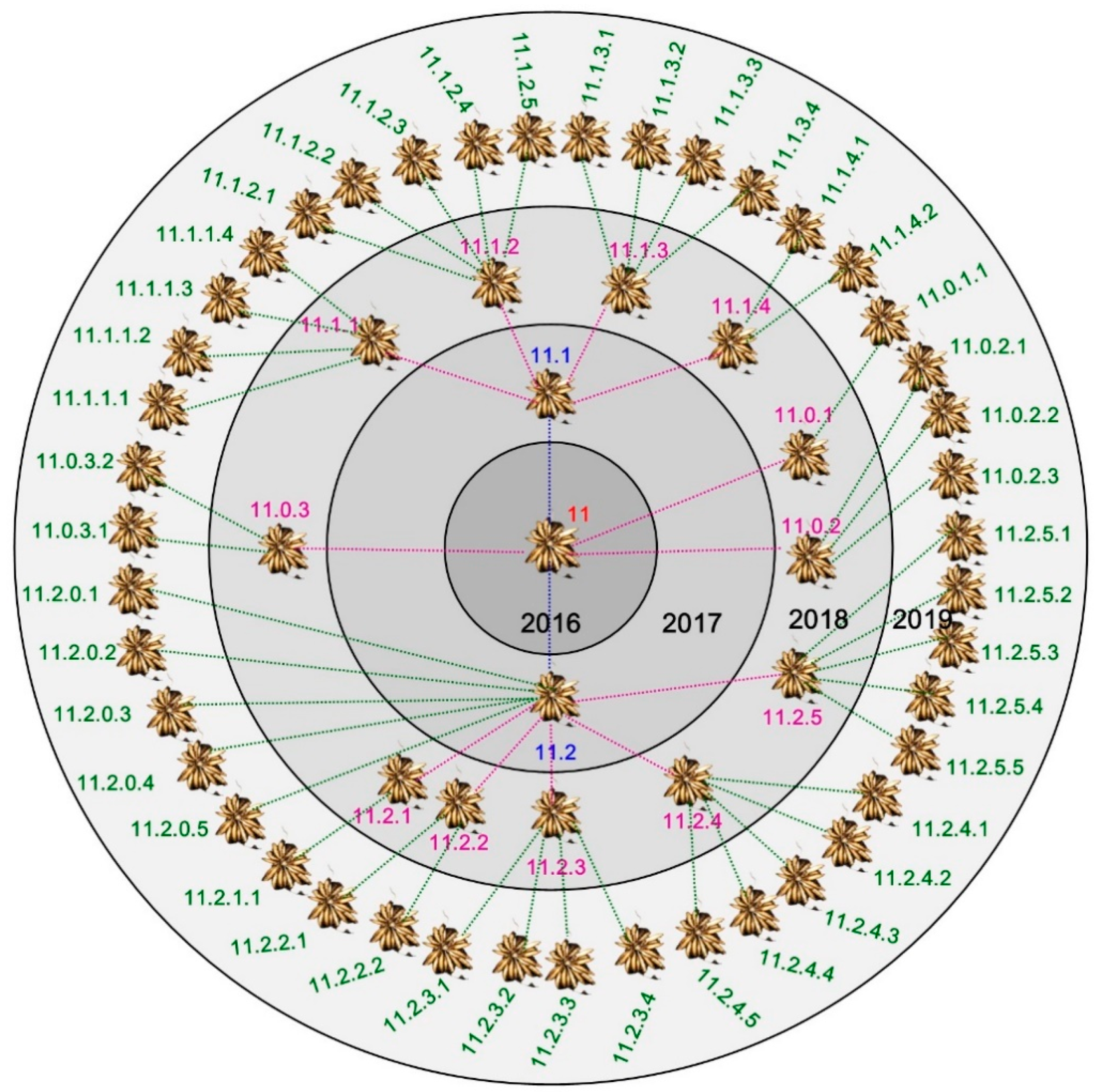
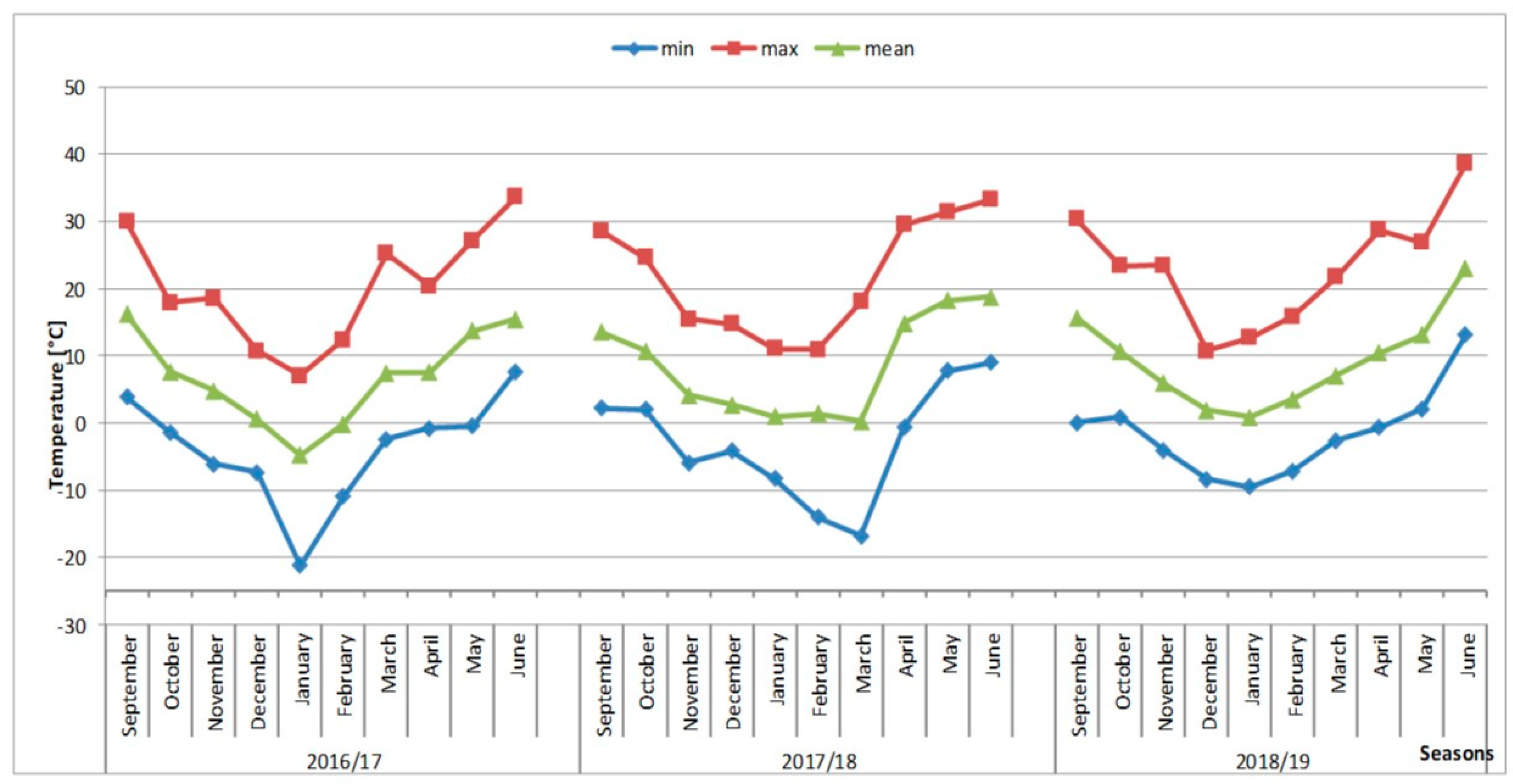
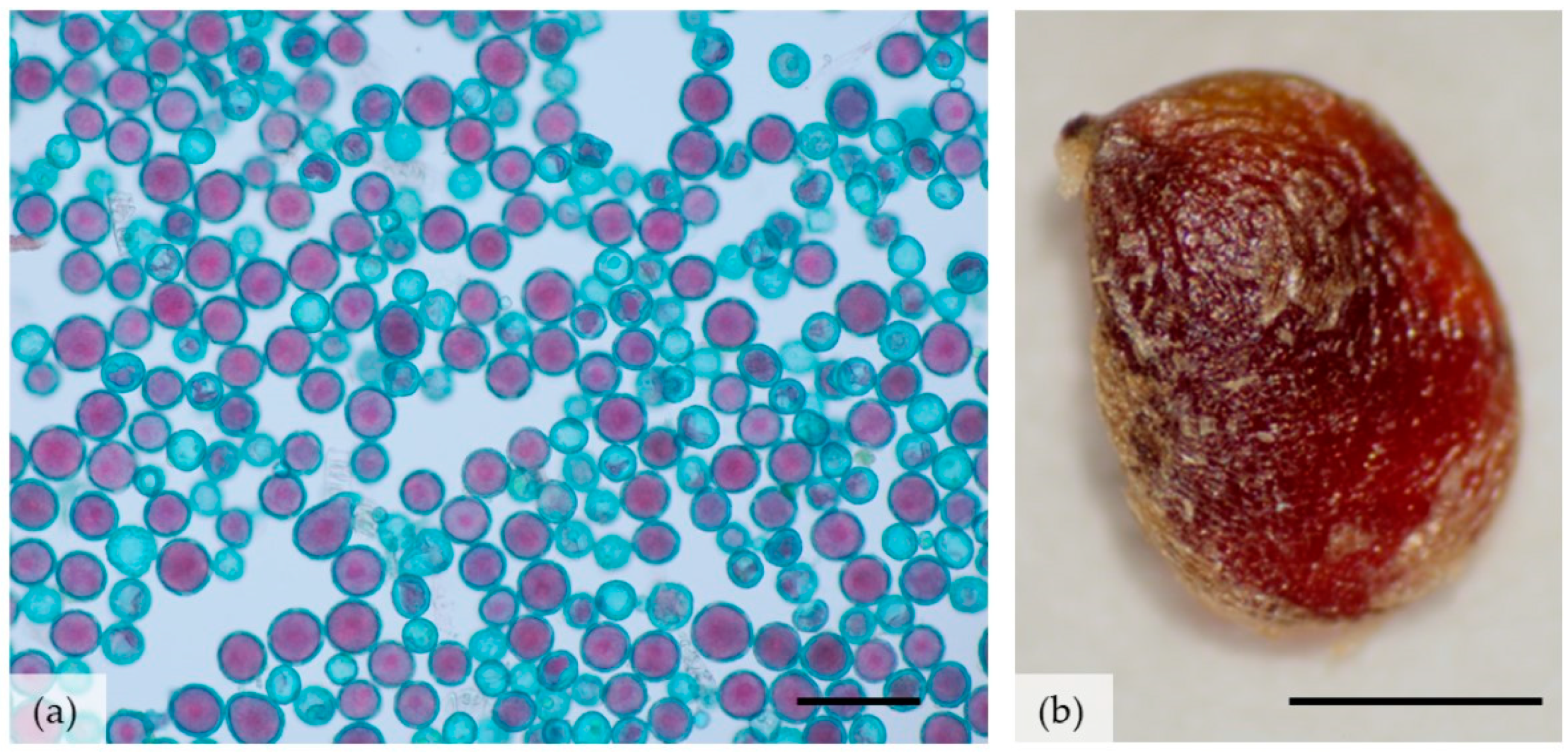

| Age of Cluster | Number of Clusters | FM of Tuber Cluster ± SE [mg] | Number of Tubers in One Cluster ± SE | Number of PC * Produced by One MC ** |
|---|---|---|---|---|
| New | 377 | 573.5 ± 16.9 b *** | 13.9 ± 0.21 a | Have not produced PC yet |
| One year | 85 | 682.3 ± 43.0 b | 22.1 ± 0.99 b | 2.23 b |
| Two years | 32 | 392.3 ± 46.5 a | 16.5 ± 1.15 a | 1.78 a |
| Kruskal–Wallis and Dunn’s test | Kruskal–Wallis and Dunn’s test | Student t-test |
| Age of Cluster | Number of Clusters | Number of Flowers per Cluster ± SE |
|---|---|---|
| New | 0 | Have not flowered yet |
| One year | 284 | 1.7 ± 0.08 b * |
| Two years | 105 | 1.3 ± 0.13 a |
| Mann–Whitney U test |
| Mode of Reproduction in 2018 | VG * | V | G | VG | V | G |
|---|---|---|---|---|---|---|
| Assessed Parameter | Clusters | |||||
| “New” 2017 (n = 64) | “One-Year” 2017 (n = 31) | |||||
| Number of tubers in cluster in 2017 | 16.0 a ** | 15.0 a | 15.5 a | 26.9 a | 24.1 a | 26.2 a |
| Fresh matter of clusters in 2017 [mg] | 1020 ab | 700 a | 1100 b | 1740 a | 1150 a | 1010 a |
| “One-year” 2018 | “Two-year” 2018 | |||||
| Number of flowers in 2018 | 2.2 a | × | 1.5 a | 1.7 a | × | 1.4 a |
| Percentage of flowers setting fruits in 2018 | 79.1 a | × | 50 a | 56.1 a | × | 50 a |
| Height of flowering stem in 2018 [cm] | 30.4 a | × | 19.9 a | 25.7 a | × | 25.2 a |
| Number of tubers in clusters in 2018 | 26.7 b | 19.9 a | × | 16.9 a | 14.7 a | × |
| Fresh matter of clusters in 2018 [mg] | 850 b | 560 a | × | 430 a | 300 a | × |
| Mean Temperatures | ||||
|---|---|---|---|---|
| March | April | May | June | |
| Number of tubers in cluster | r = 0.2551 p = 0.000 | r = −0.2560 p = 0.000 | r = −0.2448 p = 0.000 | r = 0.0016 p = 0.975 |
| Fresh matter of cluster | r = 0.4120 p = 0.000 | r = −0.4624 p = 0.000 | r = −0.3812 p = 0.000 | r = −0.1722 p = 0.000 |
| Year of Evaluation | Mean ± SE | Test | |
|---|---|---|---|
| Number of pollen grains in one anther [pcs] | 2019 | 2106.3 ± 990.8 | - |
| Number of stamens in one flower [pcs] | 2019 | 66.3 ± 1.6 | - |
| Number of pollen grains in one flower [pcs] | 2019 | 139,644 ± 69,822 | - |
| Viability of pollen grains [%] | 2017 | 61.8 ± 1.2 ab * | ANOVA and Tukey test p = 0.05 |
| 2018 | 68.5 ± 2.1 b | ||
| 2019 | 53.6 ± 4.9 a | ||
| Number of pistils in one flower [pcs] | 2017 | 138 ± 7.6 a | ANOVA and Tukey test p = 0.213 |
| 2018 | 154.7 ± 8.3 a | ||
| 2019 | 148.0 ± 3.4 a | ||
| Number of achenes in one flower [pcs] | 2017 | 18.9 ± 3.0 b | Kruskal–Wallis and Dunn’s test p = 0.0000 |
| 2018 | 6.0 ± 1.6 a | ||
| 2019 | 13.3 ± 1.8 b | ||
| Effectiveness of fruit set [%] | 2017 | 12.8 ± 1.7 c | Kruskal–Wallis and Dunn’s test p = 0.0000 |
| 2018 | 3.7 ± 0.9 a | ||
| 2019 | 8.9 ± 1.1 b |
| Temperature of Germination | Hot Stratification | Means for Temperature of Germination | |
|---|---|---|---|
| Without Hot Stratification | Hot Stratification (50 °C) | ||
| 20 ± 2 °C | 0.0 ± 0.0 a * | 0.0 ± 0.0 a | 0.0 ± 0.0 A |
| 10 °C | 40.0 ± 8.1 c | 20.0 ± 8.1 b | 30.0 ± 6.5 B |
| Means for stratification | 20.0 ± 8.5 A | 10.0 ± 1.8 A | |
| Application of GA3 | Cold Stratification | Means for GA3 Application | |
|---|---|---|---|
| Without Stratification | Cold Stratification (4 °C) | ||
| −GA3 | 0.0 ± 0.0 a * | 15 ± 9.6 ab | 7.5 ± 6.9 A |
| +GA3 | 5.0 ± 5 a | 30 ± 12.9 b | 17.5 ± 11.25 A |
| Means for stratification | 2.5 ± 3.5 A | 22.5 ± 11.3 B | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocot, D.; Sitek, E.; Nowak, B.; Kołton, A.; Towpasz, K. Reproductive Biology of Dry Grassland Specialist Ranunculus illyricus L. and Its Implications for Conservation. Biology 2022, 11, 873. https://doi.org/10.3390/biology11060873
Kocot D, Sitek E, Nowak B, Kołton A, Towpasz K. Reproductive Biology of Dry Grassland Specialist Ranunculus illyricus L. and Its Implications for Conservation. Biology. 2022; 11(6):873. https://doi.org/10.3390/biology11060873
Chicago/Turabian StyleKocot, Dawid, Ewa Sitek, Barbara Nowak, Anna Kołton, and Krystyna Towpasz. 2022. "Reproductive Biology of Dry Grassland Specialist Ranunculus illyricus L. and Its Implications for Conservation" Biology 11, no. 6: 873. https://doi.org/10.3390/biology11060873
APA StyleKocot, D., Sitek, E., Nowak, B., Kołton, A., & Towpasz, K. (2022). Reproductive Biology of Dry Grassland Specialist Ranunculus illyricus L. and Its Implications for Conservation. Biology, 11(6), 873. https://doi.org/10.3390/biology11060873







