Simple Summary
Currently, polymeric biomaterials are the choice for the design of scaffolds for the regeneration of peripheral nerves. Polyhydroxybutyrate (PHB) is a polymer belonging to the class of polyesters that are produced naturally in nature by microorganisms. To gain a better understanding of the efficacy of therapeutic approaches involving PHB scaffolds for peripheral nerve regeneration, we conducted a systematic review of the literature with the aim of discussing the current knowledge of PHB scaffolds applied to nerve regeneration. The use of PHB as a biomaterial to prepare tubular scaffolds for nerve regeneration was shown to be promising. The incorporation of additives appears to be a trend that improves nerve regeneration.
Abstract
In the last two decades, artificial scaffolds for nerve regeneration have been produced using a variety of polymers. Polyhydroxybutyrate (PHB) is a natural polyester that can be easily processed and offer several advantages; hence, the purpose of this review is to provide a better understanding of the efficacy of therapeutic approaches involving PHB scaffolds in promoting peripheral nerve regeneration following nerve dissection in animal models. A systematic literature review was performed following the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) criteria. The revised databases were: Pub-Med/MEDLINE, Web of Science, Science Direct, EMBASE, and SCOPUS. Sixteen studies were included in this review. Different animal models and nerves were studied. Extension of nerve gaps reconnected by PHB scaffolds and the time periods of analysis were varied. The additives included in the scaffolds, if any, were growth factors, neurotrophins, other biopolymers, and neural progenitor cells. The analysis of the quality of the studies revealed good quality in general, with some aspects that could be improved. The analysis of the risk of bias revealed several weaknesses in all studies. The use of PHB as a biomaterial to prepare tubular scaffolds for nerve regeneration was shown to be promising. The incorporation of additives appears to be a trend that improves nerve regeneration. One of the main weaknesses of the reviewed articles was the lack of standardized experimentation on animals. It is recommended to follow the currently available guidelines to improve the design, avoid the risk of bias, maximize the quality of studies, and enhance translationality.
1. Introduction
Peripheral nerve injury is a problem of high incidence that implies loss of both sensibility and motor function [,]. When the injury causes neurotmesis—i.e., discontinuity of the nerve—surgical techniques must be applied in an attempt to repair the damage []. The most commonly used techniques consist of directly suturing the ends of the cut nerve or closing the gap with an autograft—i.e., placing a piece of nerve harvested from the same individual—or an allograft—i.e., a piece of nerve harvested from another individual of the same species [,]. The ends of the nerve can only be sutured when the gap is short [,]. The use of autografts or allografts to close wider gaps has the disadvantage of causing denervation or requiring a donor [,]. An interesting alternative to close extensive gaps is the placement of an artificial scaffold with the characteristics of promoting nerve regeneration [].
Scaffolds are biomaterials designed to perform the following functions during tissue regeneration: (i) promote cell-biomaterial interaction, cell adhesion, and extracellular matrix deposition, (ii) allow the passage of gases, nutrients, and regulatory factors to allow cell survival, proliferation, and differentiation, (iii) biodegrade to a controllable rate similar to the rate of tissue regeneration under the conditions of interest, and (iv) cause minimal toxicity in vivo []. The design of the scaffold is very important since it determines its properties, causing it to be more efficient in specific applications []. One of the major challenges in developing a scaffold lies primarily in the choice of a biomaterial with suitable properties [].
Different promising alternatives for building scaffolds have been described in the literature, including peptides [], glycoderivatives [], and synthetic materials such as polyrotaxanes []. Polymeric biomaterials are widely preferred when it comes to scaffold design for peripheral nerve regeneration []. In the last two decades, artificial scaffolds for nerve regeneration have been produced using a variety of polymers, both natural and synthetic, and biodegradable and non-biodegradable []. Among the biodegradable and biocompatible polymers, polyhydroxyalkanoates (PHAs; Figure 1) stand out as they are a family of polyhydroxyesters of 3-, 4-, 5- and 6-hydroxyalkanoic acids, which are produced by a variety of bacterial species under nutrient-limiting conditions with carbon excess and are found as discrete cytoplasmic inclusions in bacterial cells. For this reason, they are considered green plastics and have a positive social and environmental impact [,].
Poly-3-hydroxybutyrate (P3HB) is a very promising biodegradable polymer of the polyhydroxyalkanoate (PHA) family for making nerve scaffolds [,,,,,,,,,,,,,,,,,,,,,]. The advantages of P3HB over other polymeric materials that give it many possibilities of being an ideal material include their synthesis from renewable sources, good tensile strength, good flexibility, and excellent biocompatibility and biodegradability properties, properties necessary in biomedical applications [,,,]. It is also a versatile natural polymer that can be extruded, molded, spun into fibers, made into films, and mixed with other polymers to produce heteropolymers. This has made the selection of the P3HB biopolymer a natural choice to create 3D structures suitable for fabricating tissue engineering scaffolds [].
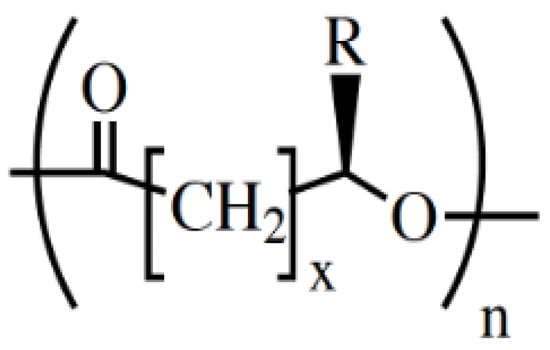
Figure 1.
General chemical structure of the PHAs. Typical values: x = 1 to 4; n = 1000 to 10,000; R = alkyl group (CmH2m+1) or functionalized alkyl group. Reused from [] with kind permission of John Wiley and Sons.
PHB in its simpler form, i.e., Poly(3HB) (Figure 2), became widely available at the beginning of the 1990s, which provided opportunities for its evaluation as a medical biomaterial []. At the beginning, it was not targeted as an implantable biomaterial and was thus lacking the quality for obtaining approval of drug administrators []. PHB causes prolonged and acute inflammatory responses, so the need was to produce PHB of high purity, check its biodegradation in vivo, conduct the fabrication of scaffolds and modify their surface []. The first film made of PHB for surgical applications was approved by the FDA in 2007 [].
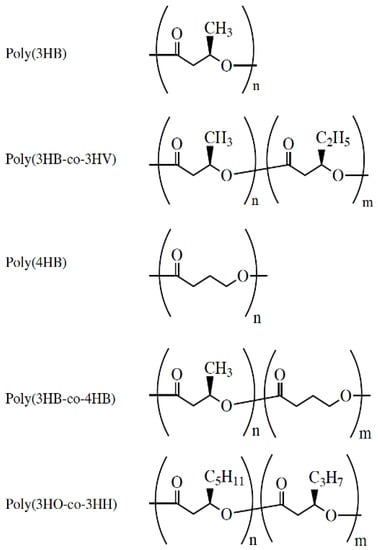
Figure 2.
Chemical structure of PHAs under medical investigation. Reused from [] with kind permission of John Wiley and Sons.
The advantages of using PH3B are inherent; however, one of the main challenges faced by researchers engineering tissues from natural polymers is the design of scaffolds with desirable physical and mechanical properties for the growth and proliferation of cells []. P3HB represents a great industrial and scientific advance in the search for new sustainable energy sources.
Based on this premise, this systematic review will discuss the current knowledge of PHB scaffolds applied to nerve regeneration. The focus is to provide a better understanding of the efficacy of therapeutic approaches involving PHB scaffolds in promoting peripheral nerve regeneration following nerve dissection in animal models.
2. Materials and Methods
2.1. Literature Search Strategy
A systematic literature review was performed following the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) criteria []. The revised databases were: PubMed/MEDLINE, Web of Science, Science Direct, EMBASE, and SCOPUS. The following query was used: (“PHB” OR “P3HB”) AND (“nerve injury” OR “nerve lesion” OR “nerve regeneration”). A manual search of the literature was carried out by reviewing the references in the articles found in the electronic search. The search was performed between September 2019 and April 2022.
2.2. Eligibility Criteria
This systematic review includes primary in vivo studies analyzing the use of PHB scaffolds applied to achieve peripheral nerve regeneration in lesions of the type of nerve resection. No publication date limit was selected. Studies in English conducted on animals were considered, as they were the most numerous in this topic and because they can be analyzed regarding their quality associated with methodology/results and risk of bias using available instruments (ARRIVE guidelines [] and SYRCLE RoB [] respectively).
2.3. Article Selection
The articles obtained in the systematic search were analyzed by reviewing the titles and abstracts. Those articles that met the eligibility criteria were analyzed in full text to confirm their relevance to the topic analyzed.
2.4. Data Extraction
Data were collected from full-text studies, in which relevant aspects of nerve injury and PHB nerve scaffolds were reported. The information collected was: Authors and year of publication; nerve (type)/gap size/time periods; animal model and sample size (n); PHB used; scaffold fabrication method; additives used (if any); methods used; conclusion or main outcome.
2.5. Analysis of Methodology and Results of Selected Studies
The analysis of the methodology and the results of the selected studies was performed by analyzing the full-text studies to determine whether some aspects of the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines were covered. The aspects considered were the ones relevant to the main focus of this review: Ethics, the authors declare following ethical guidelines for animal experimentation or having approval by a Scientific Ethical Committee; control, experimental protocol has a control group; control 2: experimental protocol has 2 control groups; PHB type: the authors declare the type of PHB used (e.g., Polyhydroxybutyrate, Poly-3-hydroxybutyrate, etc.); PHB origin: the authors declare the origin of the PHB used; scaffold fabrication method: the authors explain the method of scaffold fabrication; nerve gap size: the authors declare the size of the nervous gap; nerve studied: the authors declare which nerve was studied; time periods: the authors declare in which time periods were the evaluations carried out; surgical procedure: the authors explain the surgical procedures performed; euthanasia method: the authors explain the euthanasia method used; species: the authors declare the animal species studied; sex/weight: the authors declare sex and weight of animals at the beginning of the experimental protocol; group size and distribution: the authors declare the size of the experimental group and explain the distribution of animals in the groups; group size justification: the authors justify the sample size; statistics: the authors declare the statistical methods used; complete results: the authors present the complete results of the proposed methodology; precision measures: the author report the precision values of the quantitative data (e.g., SD, SEM, IQ distance); limitations: the authors state the limitations of the study; conclusion > objectives: the conclusion is consistent with the proposed objectives. The analysis using the ARRIVE guidelines was presented in the form of a table in order to show if the items analyzed were covered by each individual study or not. In the end, a score of the percentage of compliance was calculated for each study and included in the results table.
2.6. Analysis of the Risk of Bias of Selected Studies
The analysis of the risk of bias of the selected studies was carried out in the full-text documents using the SYRCLE RoB (Systematic Review Center for Laboratory Animal Experimentation—Risk of Bias) tool, which was also adapted to the main focus of this review. The dimensions analyzed for each study were: selection bias; performance bias; detection bias; attrition bias; reporting bias and other bias sources. The results of the risk of bias of the selected studies using the SYRCLE RoB tool were presented in the form of a table showing the fulfilled (Y), unfulfilled (N), and uncertain (U) dimensions. The ARRIVE and SYRCLE RoB analyses were performed by two independent examiners. In cases where there was disagreement between the examiners, a third examiner was consulted.
3. Results
Study Selection
The article search and selection process is summarized in Figure 3. The total number of articles found in the databases was 193 citations, of which 82 articles were duplicates. After the initial reading by title and abstract, 71 articles were ruled out for being unrelated to the review topic. After reading full-text articles (40 in total), 24 were excluded. Finally, in this review, 16 articles were included that corresponded to experimental studies in vivo that met the previously defined criteria.
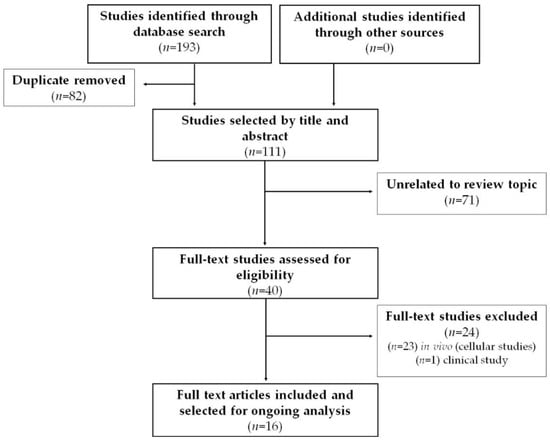
Figure 3.
Systematic search flow diagram.
Data were extracted from studies in selected animals using Table 1 which considers information relevant to nerve regeneration studies using tubular scaffolds prepared with PHB.

Table 1.
Evidence extracted from animal studies selected.
Studies using PHB as a biomaterial for the preparation of tubular scaffolds used in peripheral nerve regeneration have been developed since the 1990s [,,,]. Several animal models have been used, with most studies carried out on rodents and rabbits. Likewise, different nerves have been studied, with the sciatic nerve being the most studied of all. It is also noted that the sizes of nerve gaps reconnected by PHB tubular scaffolds and the periods of analysis were varied.
The most common PHB form used in the selected studies was “poly-3-hydroxybutyrate.” The most common way of manufacturing the scaffolds was rolling a purchased PHB sheet to form a tube (Figure 4 and Figure 5). The sheets were made by Astra Tech AB (Mölndal, Sweden), had a molecular weight of approximately 150 kD, and unidirectional fiber orientation. Only three studies manufactured the PHB scaffolds autonomously [,,]. Regarding the additives included in the scaffolds, a trend was repeated in almost all studies from 2003 to 2018: the adding of growth factors, other biopolymers, and neural progenitor cells.
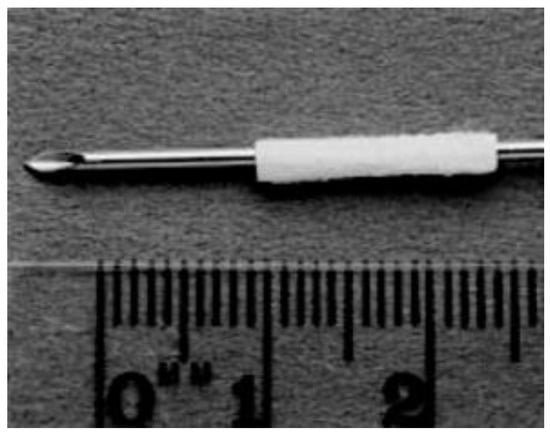
Figure 4.
Unidirectional fiber orientated sheet of PHB (8 × 14 mm) rolled around a 16 G cannula to form a tube 14 mm long and with an internal diameter of 1.6 mm. Reused from (Hazari et al., 1999b) [] with kind permission of Elsevier.
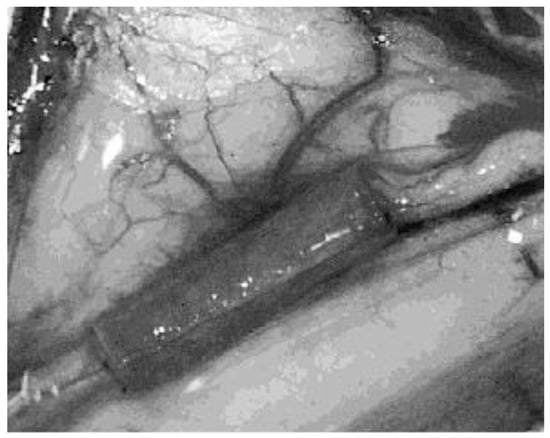
Figure 5.
Superficial radial nerve of a cat sectioned and wrapped in a PHB conduit sealed by fibrin glue. Magnification 6×. Reused from (Ljungberg et al., 1999) [] with kind permission of John Wiley and Sons.
Most of the selected studies analyzed morphological characteristics using different methods—e.g., histology, immunohistochemistry, and ultrastructure. Functional aspects of nerve recovery were analyzed only in the two most recent studies [,].
The use of PHB as a fabrication material for tubular nerve scaffolds (NC, NGC) seems to be promising for peripheral nerve regeneration [,] in cases of long-gap nerve injury [] generating a rapid reconnection of these nerve gaps []. The studies reported adequate properties of PHB in terms of mechanical strength, biodegradability, biocompatibility, and support for the proliferation and growth of neurons [,]. However, in some cases, no differences were noted with the standard surgical treatment of epineural repair (epineural suture) [,]. In other cases, other materials, such as fibrin, obtained better results []; even PHB without additives inhibited peripheral nerve regeneration []. The inclusion of additives such as GGF [,], rhLIF [], and cells [,,] in the PHB scaffolds suggest an improvement in the nerve regeneration results obtained.
The analysis of the quality of the studies through the application of the ARRIVE guidelines adapted for this review is summarized in Table 2. Some of the analyzed aspects showed a low level of compliance in the studies. For example, “sample size justification” was not met in any study; the inclusion of a “second control group” was present in three [,,] of the sixteen studies; the limitations were declared in four [,,,]; euthanasia methods in six [,,,,]; and the sex/weight of the animals in eight [,,,,,,,]. In addition, six studies [,,,,] present a conclusion that disagrees with the objectives set and four [,,,] of the included studies did not inform of the statistical methods used.

Table 2.
Quality analysis of animal studies using ARRIVE guidelines [] adapted for nerve regeneration treated with PHB scaffolds.
The risk of bias analyzed by applying the SYRCLE RoB tool (Table 3) showed that the criteria of the dimensions “Performance” and “Detection” were “Unclear” in all parameters. In the “Selection” dimension, only the “Baseline” parameter was met in half of the studies. In practically all selected studies, it was not clear if the “Allocation” and “Sequence” parameters were covered. Four studies fulfilled the “Attrition” parameter because they declared that there were no animal losses and/or explained the losses that occurred [,,,]. Five studies did not clearly explain animal losses, but reported the postoperative characteristics of the experimental protocols [,,,,]; and seven studies did not deliver clear information for animal losses occurred in the experiments [,,,,,,]. In the “Reporting” dimension, only nine studies declared the positive and negative results of the experiments performed [,,,,,,,,].

Table 3.
Analysis of the risk of bias in animal studies using the SYRCLE RoB tool [] scale adapted for nerve damage and treated with PHB scaffolds.
Finally, seven studies showed other possible sources of bias (Other/Free of Other Problems). Borkenhagen et al. did not state a clear objective or hypothesis of the study []. Ljungberg et al. showed a correlation that is not described in the methodology []. Hazari et al. presented data on controls selectively []. In addition, six studies showed a close relationship with the company that developed the PHB sheets used [,,,,,]. Furthermore, in two of them, an author was affiliated with the company [,]; in another two, the authors declared being financed by the company [,]; and finally, the PHB used was donated by the company in two other studies [,].
4. Discussion
The present systematic review gathered and analyzed evidence on the use of polyhydroxybutyrate biopolymer (PHB), a type of polyhydroxyalkanoate (PHA), in peripheral nerve regeneration. PHB is a biopolymer of microbial origin [] that have several positive properties in their use as a material in the construction of nerve scaffolds, such as adequate flexibility and tensile strength, excellent biocompatibility, and biodegradability [,,,].
This systematic review focused mainly on PHB because it is the most used PHA for this type of application. After the initial literature search, several in vitro and animal studies and only one clinical study were found []. Thus, the authors decided to focus on animal studies due to the number of studies available and the existence of well-known tools to analyze quality (ARRIVE) and risk of bias (SYRCLE RoB) for these types of studies.
The in vitro studies that evaluated the use of PHB as a biomaterial for the construction of peripheral nerve scaffolds are of fundamental importance in this topic, mainly helping to understand cellular behaviors in obtaining the best regenerative results [,,,,]. However, these studies show high heterogeneity in their methods and designs, which makes it difficult to compare their results. In recent studies, it was noted that Schwann cells, the glial cells supporting the peripheral nervous system, were the most studied cell type [,,]. PC12 [], neuronal [], and fibroblast lineage [] cells that produce the collagen that makes up the endo and perineurium region were also studied with the intention of understanding the interaction of PHB with peripheral nerve regeneration.
The method of fabrication of PHB scaffolds chosen by these studies was preferentially electrospinning [,,,,]. The cell analysis strategies used were mainly viability [], differentiation [,] proliferation [,,], migration [], adhesion [], and cell growth orientation [,,]. Better results were observed in the cases where PHB was associated with some approaches to improve the properties of this material, such as the mixture/blend of other polymers (PHBV [,], P(3HO), and P(3HB) []) and polyaniline [], in addition to the functionalization of the PHB scaffolds using oxygen plasma printing [] and naturally occurring peptides in the ECM [].
The ARRIVE and SYRCLE RoB scores revealed a good quality of studies carried out in animals with the tubular PHB nerve scaffold. However, weaknesses were noted in these studies. The ARRIVE application revealed a weakness present in all selected studies: the lack of justification for the sample size, which is a factor that directly affects the statistical power associated with the study and, therefore, the reliability of the conclusions. In addition, only four studies declared their limitations [,,,], six studies declared the method of euthanasia applied [,,,,,] and eight studies [,,,,,,,] stated the weight and sex of the animals. This lack of information also influences the validity and mainly the reproducibility of the studies carried out. Finally, it was noted that six studies [,,,,,] presented a conclusion that did not answer clearly the objective of the study and four studies [,,,] did not state clearly the statistical methods used. This lack of information may raise doubts about the results of these studies.
The application of the SYRCLE RoB tool to assess the risk of bias in the selected studies revealed that many of the criteria and dimensions evaluated did not present sufficiently clear data or information. The parameter of “Baseline” were fulfilled by seven studies [,,,,,,]. The “Reporting bias” dimension was met by nine studies [,,,,,,,,] and “Attrition bias” was met in only four studies [,,,]. A fact that drew attention in the analysis of the risk of bias was the close relationship between authors and the company Astratech in six studies [,,,,,]. Astratech developed the PHB sheets used in the studies and provided funding. In all these cases, the results of using this biomaterial in nerve regeneration were positive or promising.
The application of the two tools (ARRIVE and SYRCLE) for the analysis of studies carried out in animals revealed the weaknesses of these studies in relation to their methodology, results, and risk of bias. This lack of success to comply with all parameters and dimensions analyzed by both tools does not necessarily completely invalidate the results of these studies. Most of the studies selected in this review were published prior to ARRIVE (2010) and SYRCLE (2014). However, that does not mean that they were exempted from complying with the conditions needed to reach reliable results. There must be future efforts to comply with the parameters of tools like ARRIVE and SYRCLE in order to favor the standardization, quality, and, therefore, relevance of studies in animals. The studies in animals have evolved to favor their translationality to clinical studies in the last years [,,]. The data extracted from the selected studies showed that the use of PHB as a biomaterial for preparing nervous scaffolds dates back to the 1990s [,,,]. Most of the reviewed articles were published between 1995 and 2010. Then, the number of articles started to drop and only two of them were published in the last 10 years.
The model of nerve injury studied in all cases is that of nerve resection, in which one gap is generated in the nerve, which can be considered the most serious type of nerve injury because it generates a total nerve interruption (neurotmesis) with the problem of lack of biological structure for its reconnection. However, it was noted that the studied nerve, the gap size, and the recovery times were varied among studies. In most cases, the studied nerve was classified as “mixed”—e.g., sciatic, radial, peroneal, recurrent laryngeal—which favors the analysis of both motor and sensory recovery.
The size of the gap ranged from 2–3 mm in cats [,] to 40 mm in rabbits [,], with the 10 mm gap in rats being the most evaluated model [,,,,,,,,]. This could indicate a possible standardization of the gap among the most recent studies carried out in rats. The post-nerve injury analysis times were also very varied, from 7–30 days [] to 6–12 months [,] without an apparent pattern or trend in the selected studies. Previous studies have shown that the larger the size of the nerve gap, the worse the prognosis of nerve regeneration [].
Regarding the manufacture of the scaffolds, 13 studies used commercial PHB sheets made by electrospinning [,,,,,,,,,,,,]. Only three studies manufactured their own custom-made scaffolds for nerve regeneration by the methods of melt extrusion [], dipping-leaching [], and electrospinning []. The own manufacture of the scaffolds may not be as standardized as the use of PHB sheets industrially assembled by electrospinning; however, it would allow the customization of the characteristics of the scaffolds in order to improve regeneration results, especially with the intention of preparing an environment favorable to nerve cells because it resembles the extracellular matrix. The preparation of scaffolds by the electrospinning method allows obtaining a structure similar to the extracellular matrix, which would help to promote nerve regeneration []. Most of the studies did not discuss critical characteristics of the material such as molecular mass and purity. Further characterization of the material used is needed in order to understand its impact on the treatment success. PHB can cause prolonged and acute inflammatory responses, so the need is to produce PHB of high purity, check its biodegradation in vivo, improve the fabrication of scaffolds and modify their surface [].
Most of the included studies analyzed improvement of nerve regeneration by morphological methodologies exclusively, such as macroscopic morphology, histology, microscopic morphometry, immunohistochemistry, and ultrastructural analysis [,,,,,,,,,,,,,,,,,,,,,,]. These methods are known for their application in studies of changes in peripheral nerves, allowing some prediction of functional characteristics [,]. The main objective of using PHB scaffolds would be the restoration of the function lost by peripheral nerve damage, which remains one of the main challenges in nerve regeneration [,,,]. Studies analyzing sensory and motor function by direct methods are necessary to assess the effectiveness of nerve regeneration achieved by the use of this type of biomaterial. However, this was done only in two of the most recent studies [,].
The main results of the selected studies revealed in most studies that the use of PHB for peripheral nerve regeneration was positive and or promising, with the adequate features of mechanical strength, biocompatibility and proliferation of neurons [,,,,,,,,,,,,]. Studies carried out in rodents and those that included additives showed practically only positive or promising results from the use of PHB as a biomaterial for nerve regeneration. The inclusion of additives seems to be a trend that has been observed in most of the more recent studies [,,,,,,,], which seems to reveal that PHB nerve scaffolds would serve more as a vehicle for other nerve regeneration strategies, such as the adding of growth factors and cells.
Within the selected studies, the lack of control groups in five studies drew attention [,,,,]. The use of control groups was proposed in 1801 and is currently standard practice in basic and clinical studies []. The control group consists of a group with the same configuration as the experimental groups except for the applied/studied variable []. The lack of control groups makes the quality of these studies questionable, as a good control group validates the experiment carried out and provides the basis for evaluating the effects of treatments [] that are necessary when studying peripheral nerve regeneration using scaffolds. In addition, in these cases, two simultaneous control groups could be used, the negative control groups—i.e., lack of intervention—and positive control groups—i.e., intervention with expected effect []—in order to establish the effect of using PHB scaffolds in a more solid and objective way. However, among the selected studies only three recent studies used two control groups in their methodology [,,].
Only one clinical study was found on this topic, Åberg, et al. [], which compared the use of PHB tubular scaffolds with the epineural suture in 12 individuals through sensory and motor clinical, neuropsychological, morphological, and psychological tests after 2 weeks, 3, 6, 9, 12 and 18 months after surgery. The study revealed that most tests showed no differences between treatments, but suggested an improvement in sensory recovery in patients who received the PHB scaffold, although it would need confirmation in a larger clinical study. This result agrees in part with the animal studies analyzed in the present review; in addition, it is worth mentioning that the scaffold used in this clinical study performed by Åberg, et al. [] was not associated with any additive, which could change its results [].
Among the limitations of this systematic review, we can mention searching for studies published only in English, not reviewing published or unpublished studies such as theses and dissertations, or even in different databases from those consulted. In addition, this review was focused only on animal studies, which was the authors’ decision as they found only one clinical study on this topic.
5. Conclusions
The use of PHB as a biomaterial to prepare tubular scaffolds for nerve regeneration was shown to be promising. The incorporation of additives appears to be a trend that improves nerve regeneration.
One of the main weaknesses of the reviewed articles was the lack of standardized experimentation on animals. It is recommended to follow current guidelines, such as ARRIVE and SYRCLE RoB, to improve the design, avoid the risk of bias, maximize the quality of studies, and enhance translationality. In particular, control groups should be defined more carefully.
The experimental methodologies found in the literature were shown to be highly heterogeneous. To achieve more conclusive results, it would be recommended to standardize the injuries and the time periods analyzed in future studies. It would also be recommended to conduct functional analysis in order to directly assess the effectiveness of the treatments regarding function recovery.
Further efforts must be made in order to refine the fabrication of PHB mats intended for medical use. Chemical characteristics of the material should be addressed in future in vitro and in vivo studies in order to correlate them with the results. Critical aspects such as purity should be taken into account. Further characterization of the material used is needed in order to understand its impact on treatment success. Clinical studies should be conducted after proven success of the material in both in vitro and in vivo studies in animals.
Author Contributions
M.F.L.: Investigation, formal analysis, data curation and writing—original draft; G.Á.: Investigation, formal analysis, data curation and writing—original draft; P.C.: Review conceptualization, formal analysis and data curation; K.G.: Investigation and formal analysis; J.A.: Investigation and formal analysis; F.A.: Conceptualization and writing—original draft; I.G.: Formal analysis, data curation and supervision; F.J.D.: Conceptualization, writing—original draft, funding acquisition, supervision and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
ANID Agencia Nacional de Investigación y Desarrollo de Chile/Fondo Nacional de Desarrollo Científico y Tecnológico—Grant Number: FONDECYT 11190300.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data sharing is not applicable (Systematic Review of Literature).
Conflicts of Interest
All authors declare no conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Campbell, W.W. Evaluation and management of peripheral nerve injury. Clin. Neurophysiol. 2008, 119, 1951–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modrak, M.; Talukder, M.H.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2019, 98, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Seddighi, A.; Nikouei, A.; Seddighi, A.S.; Zali, A.R.; Tabatabaei, S.M.; Sheykhi, A.R.; Yourdkhani, F.; Naeimian, S. Peripheral nerve injury: A review article. Int. Clin. Neurosci. J. 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Poly. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Bitar, K.N.; Zakhem, E. Design strategies of biodegradable scaffolds for tissue regeneration. Biomed. Eng. Comp. Biol. 2014, 6, 13–20. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J. Biomed. Sci. 2009, 16, 108. [Google Scholar] [CrossRef] [Green Version]
- Diaferia, C.; Rosa, E.; Balasco, N.; Sibillano, T.; Morelli, G.; Giannini, C.; Vitagliano, L.; Accardo, A. The Introduction of a Cysteine Residue Modulates The Mechanical Properties of Aromatic Based Solid Aggregates and Self-Supporting Hydrogels. Chem. A Eur. J. 2021, 27, 14886–14898. [Google Scholar] [CrossRef]
- Yao, S.; Brahmi, R.; Portier, F.; Putaux, J.; Chen, J.; Halila, S. Hierarchical Self-Assembly of Amphiphilic β-C-Glycosylbarbiturates into Multiresponsive Alginate-Like Supramolecular Hydrogel Fibers and Vesicle Hydrogel. Chem. A Eur. J. 2021, 27, 16716–16721. [Google Scholar] [CrossRef]
- Cho, I.S.; Ooya, T. Cell-Encapsulating Hydrogel Puzzle: Polyrotaxane-Based Self-Healing Hydrogels. Chem. A Eur. J. 2019, 26, 913–920. [Google Scholar] [CrossRef]
- Chiono, V.; Tonda-Turo, C.; Ciardelli, G. Artificial scaffolds for peripheral nerve reconstruction. Int. Rev. Neurobiol. 2009, 87, 173–198. [Google Scholar] [CrossRef]
- Sinha Ray, S. Environmentally friendly polymer nanocomposites using polymer matrices from renewable sources. Environ. Friendly Polym. Nanocomposites 2013, 2013, 89–156. [Google Scholar] [CrossRef]
- Sanhueza, C.; Acevedo, F.; Rocha, S.; Villegas, P.; Seeger, M.; Navia, R. Polyhydroxyalkanoates as biomaterial for electrospun scaffolds. Int. J. Biol. Macromol. 2019, 124, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Borkenhagen, M.; Stoll, R.C.; Neuenschwander, P.; Suter, U.W.; Aebischer, P. In vivo performance of a new biodegradable polyester urethane system used as a nerve guidance channel. Biomaterials 1998, 19, 2155–2165. [Google Scholar] [CrossRef]
- Ljungberg, C.; Johansson-Ruden, G.; Boström, K.J.; Novikov, L.; Wiberg, M. Neuronal survival using a resorbable synthetic conduit as an alternative to primary nerve repair. Microsurgery 1999, 19, 259–264. [Google Scholar] [CrossRef]
- Hazari, A.; Johansson-Rudén, G.; Junemo-Bostrom, K.; Ljungberg, C.; Terenghi, G.; Green, C.; Wiberg, M. A new resorbable wrap-around implant as an alternative nerve repair technique. J. Hand Surg. Br. 1999, 24, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Hazari, A.; Wiberg, M.; Johansson-Rudén, G.; Green, C.; Terenghi, G. A resorbable nerve conduit as an alternative to nerve autograft in nerve gap repair. Br. J. Plast. Surg. 1999, 52, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Young, R.C.; Terenghi, G.; Wiberg, M. Poly-3-hydroxybutyrate (PHB): A resorbable conduit for long-gap repair in peripheral nerves. Br. J. Plast. Surg. 2002, 55, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Mohanna, P.N.; Young, R.C.; Wiberg, M.; Terenghi, G. A composite poly-hydroxybutyrate–glial growth factor conduit for long nerve gap repairs. J. Anat. 2003, 203, 553–565. [Google Scholar] [CrossRef]
- Hart, A.M.; Wiberg, M.; Terenghi, G. Exogenous leukaemia inhibitory factor enhances nerve regeneration after late secondary repair using a bioartificial nerve conduit. Br. J. Plast. Surg. 2003, 56, 444–450. [Google Scholar] [CrossRef]
- Birchall, M.; Idowu, B.; Murison, P.; Jones, A.; Burt, R.; Ayling, S.; Stokes, C.; Pope, L.; Terenghi, G. Laryngeal abductor muscle reinnervation in a pig model. Acta Oto-Laryngol. 2004, 124, 839–846. [Google Scholar] [CrossRef]
- Mohanna, P.N.; Terenghi, G.; Wiberg, M. Composite PHB-GGF conduit for long nerve gap repair: A long-term evaluation. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2005, 39, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kalbermatten, D.F.; Erba, P.; Mahay, D.; Wiberg, M.; Pierer, G.; Terenghi, G. Schwann cell strip for peripheral nerve repair. J. Hand Surg. Eur. 2008, 33, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Kalbermatten, D.F.; Kingham, P.J.; Mahay, D.; Mantovani, C.; Pettersson, J.; Raffoul, W.; Balcin, H.; Pierer, G.; Terenghi, G. Fibrin matrix for suspension of regenerative cells in an artificial nerve conduit. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 669–675. [Google Scholar] [CrossRef]
- Kalbermatten, D.F.; Pettersson, J.; Kingham, P.J.; Pierer, G.; Wiberg, M.; Terenghi, G. New fibrin conduit for peripheral nerve repair. J. Reconstr. Microsurg. 2009, 25, 27–33. [Google Scholar] [CrossRef]
- Bian, Y.Z.; Wang, Y.; Aibaidoula, G.; Chen, G.Q.; Wu, Q. Evaluation of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials 2009, 30, 217–225. [Google Scholar] [CrossRef]
- Åberg, M.; Ljungberg, C.; Edin, E.; Millqvist, H.; Nordh, E.; Theorin, A.; Terenghi, G.; Wiberg, M. Clinical evaluation of a resorbable wrap-around implant as an alternative to nerve repair: A prospective, assessor-blinded, randomised clinical study of sensory, motor and functional recovery after peripheral nerve repair. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 1503–1509. [Google Scholar] [CrossRef]
- Durgam, H.; Sapp, S.; Deister, C.; Khaing, Z.; Chang, E.; Luebben, S.; Schmidt, C.E. Novel degradable co-polymers of polypyrrole support cell proliferation and enhance neurite out-growth with electrical stimulation. J. Biomater. Sci. Polym. Ed. 2019, 21, 1265–1282. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Mirmohammadi, S.A.; Irani, S. Polyhydroxybutyrate (PHB) scaffolds as a model for nerve tissue engineering application: Fabrication and in vitro assay. Int. J. Polym. Mater. 2011, 60, 562–575. [Google Scholar] [CrossRef]
- Genchi, G.G.; Ciofani, G.; Polini, A.; Liakos, I.; Iandolo, D.; Athanassiou, A.; Pisignano, D.; Mattoli, V.; Menciassi, A. PC12 neuron-like cell response to electrospun poly (3-hydroxybutyrate) substrates. J. Tissue Eng. Regen. Med. 2015, 9, 151–161. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Wang, C.T. Neuronal differentiation of induced pluripotent stem cells in hybrid polyester scaffolds with heparinized surface. Colloids Surf. B 2012, 100, 9–15. [Google Scholar] [CrossRef]
- Chan, R.T.; Russell, R.A.; Marçal, H.; Lee, T.H.; Holden, P.J.; Foster, J.R. BioPEGylation of polyhydroxybutyrate promotes nerve cell health and migration. Biomacromolecules 2014, 15, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Daranarong, D.; Chan, R.T.; Wanandy, N.S.; Molloy, R.; Punyodom, W.; Foster, L.J. Electrospun polyhydroxybutyrate and poly (L-lactide-co-ε-caprolactone) composites as nanofibrous scaffolds. BioMed. Res. Int. 2014, 2014, 741408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaakxs, D.; Kalbermatten, D.F.; Pralong, E.; Raffoul, W.; Wiberg, M.; Kingham, P.J. Poly-3-hydroxybutyrate strips seeded with regenerative cells are effective promoters of peripheral nerve repair. J. Tissue Eng. Regen. Med. 2017, 11, 812–821. [Google Scholar] [CrossRef]
- Ozer, H.; Bozkurt, H.; Bozkurt, G.; Demirbilek, M. Regenerative potential of chitosan-coated poly-3-hydroxybutyrate conduits seeded with mesenchymal stem cells in a rat sciatic nerve injury model. Int. J. Neurosci. 2018, 128, 828–834. [Google Scholar] [CrossRef]
- Cheng, G.Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Valappil, S.P.; Roy, I.; Boccaccini, A.R. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules 2006, 7, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.F.; Martin, D.P. Applications of PHAs in medicine and pharmacy. Biopolymers 2002, 4, 91–127. [Google Scholar]
- Ray, S.; Kalia, V.C. Biomedical applications of polyhydroxyalkanoates. Indian, J. Microbiol. 2017, 57, 261–269. [Google Scholar] [CrossRef]
- Riaz, S.; Raza, Z.A.; Majeed, M.I.; Jan, T. Synthesis of zinc sulfide nanoparticles and their incorporation into poly (hydroxybutyrate) matrix in the formation of a novel nanocomposite. Mater. Res. Express 2018, 5, 055027. [Google Scholar] [CrossRef]
- Tanideh, N.; Azarpira, N.; Sarafraz, N.; Zare, S.; Rowshanghiyas, A.; Farshidfar, N.; Iraji, A.; Zarei, M.; El Fray, M. Poly(3-Hydroxybutyrate)-Multiwalled Carbon Nanotubes Electrospun Scaffolds Modified with Curcumin. Polymers 2020, 12, 2588. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Masaeli, E.; Morshed, M.; Nasr-Esfahani, M.H.; Sadri, S.; Hilderink, J.; van Apeldoorn, A.; van Blitterswijk, C.A.; Moroni, L. Fabrication, characterization and cellular compatibility of poly(hydroxy alkanoate) composite nanofibrous scaffolds for nerve tissue engineering. PLoS ONE 2013, 8, e57157. [Google Scholar] [CrossRef] [PubMed]
- Masaeli, E.; Wieringa, P.A.; Morshed, M.; Nasr-Esfahani, M.H.; Sadri, S.; van Blitterswijk, C.A.; Moroni, L. Peptide functionalized polyhydroxyalkanoate nanofibrous scaffolds enhance Schwann cells activity. Nanomedicine 2014, 10, 1559–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lizarraga-Valderrama, L.R.; Taylor, C.S.; Claeyssens, F.; Haycock, J.W.; Knowles, J.C.; Roy, I. Unidirectional neuronal cell growth and differentiation on aligned polyhydroxyalkanoate blend microfibres with varying diameters. J. Tissue Eng. Regen. Med. 2019, 13, 1581–1594. [Google Scholar] [CrossRef]
- Zamanifard, M.; Khorasani, M.T.; Daliri, M.; Parvazinia, M. Preparation and modeling of electrospun polyhydroxybutyrate/polyaniline composite scaffold modified by plasma and printed by an inkjet method and its cellular study. J. Biomater. Sci. Polym. Ed. 2020, 31, 1515–1537. [Google Scholar] [CrossRef]
- Austin, C.P. Opportunities and challenges in translational science. Clin. Transl. Sci. 2021, 14, 1629–1647. [Google Scholar] [CrossRef]
- Dalamagkas, K.; Tsintou, M.; Seifalian, A.; Seifalian, A.M. Translational Regenerative Therapies for Chronic Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 1776. [Google Scholar] [CrossRef] [Green Version]
- Djurhuus, J.C.; Wu, H.Y.; Fossum, M. A conversation on how animal experimental studies allies with translational medicine. J. Pediatr. Urol. 2021, 17, 622–629. [Google Scholar] [CrossRef]
- Bellamkonda, R.V. Peripheral nerve regeneration: An opinion on channels, scaffolds and anisotropy. Biomaterials 2006, 27, 3515–3518. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Murugan, R.; Ramakrishna, S.; Wang, X.; Ma, Y.X.; Wang, S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials 2004, 25, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Kemp, S.W.; Weber, C.; Borschel, G.H.; Gordon, T. Outcome measures of peripheral nerve regeneration. Ann. Anat. 2011, 193, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Fornaro, M.; Di Scipio, F.; Ronchi, G.; Giacobini-Robecchi, M.G.; Geuna, S. Chapter 5: Methods and protocols in peripheral nerve regeneration experimental research: Part II-morphological techniques. Int. Rev. Neurobiol. 2009, 87, 81–103. [Google Scholar] [CrossRef]
- Gordon, T.; Sulaiman, O.; Boyd, J.G. Experimental strategies to promote functional recovery after peripheral nerve injuries. J. Peripher. Nerv. Syst. 2003, 8, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Momeni, A.; Pyles, M.N.; Cha, J.Y.; Maan, Z.N.; Duscher, D.; Jew, O.S.; Siemers, F.; van Schoonhoven, J. The Role of Current Techniques and Concepts in Peripheral Nerve Repair. Plast. Surg. Int. 2016, 2016, 4175293. [Google Scholar] [CrossRef] [Green Version]
- Eggers, R.; de Winter, F.; Tannemaat, M.R.; Malessy, M.J.A.; Verhaagen, J. GDNF Gene Therapy to Repair the Injured Peripheral Nerve. Front. Bioeng. Biotechnol. 2020, 8, 583184. [Google Scholar] [CrossRef]
- Gordon, T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020, 2, 8652. [Google Scholar] [CrossRef]
- Edwards, J.; Thomas, R.; Guilliatt, R. Regenerative medicine: From the laboratory looking out. Palgrave Commun. 2017, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pithon, M.M. Importance of the control group in scientific research. Dental. Press, J. Orthod. 2013, 18, 13–14. [Google Scholar] [CrossRef] [Green Version]
- Moser, P. Out of Control? Managing Baseline Variability in Experimental Studies with Control Groups. Handb. Exp. Pharmacol. 2020, 257, 101–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).