Neurons for Ejaculation and Factors Affecting Ejaculation
Abstract
Simple Summary
Abstract
1. Introduction
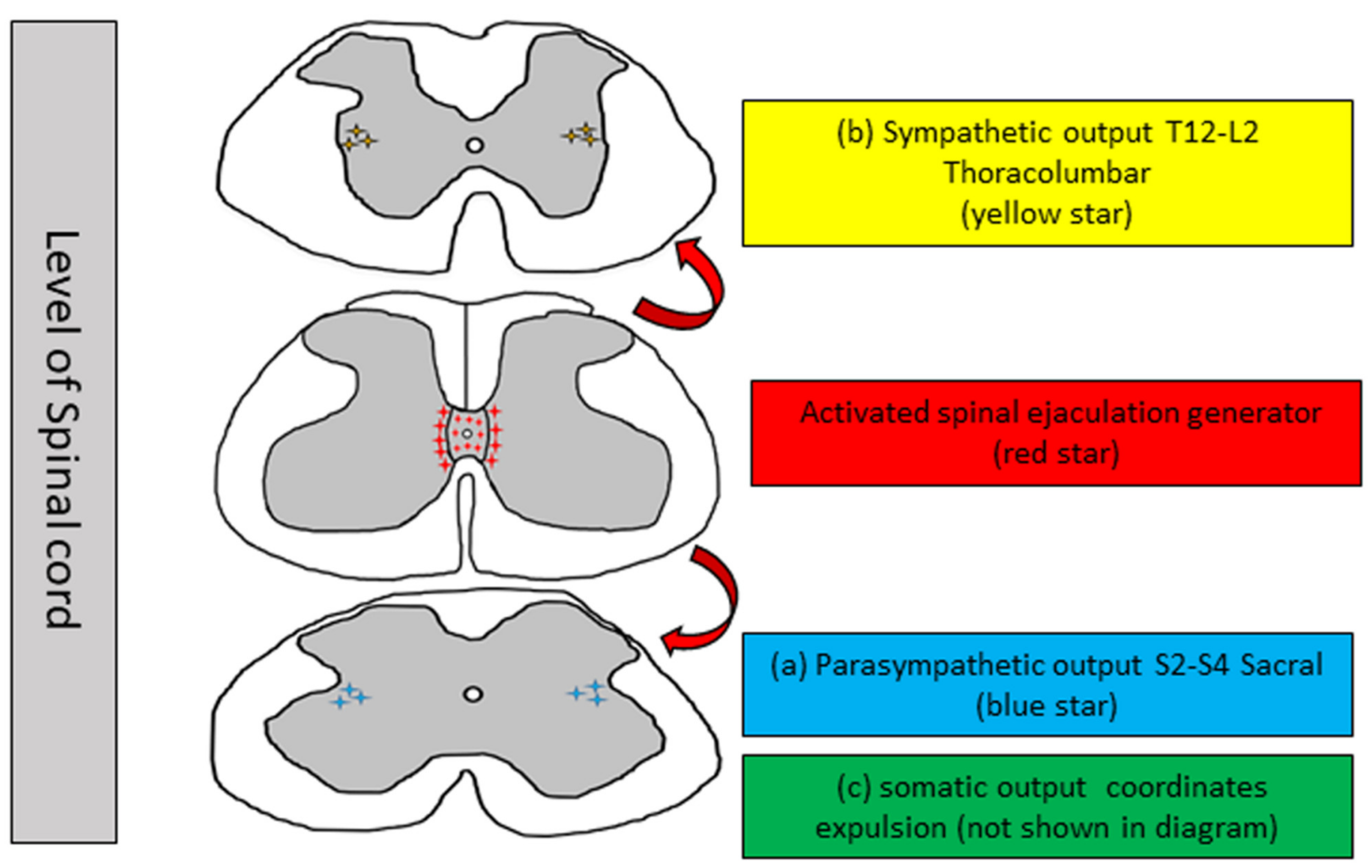
2. Neurophysiology of Ejaculation
2.1. Neurons for Ejaculation in Non-Mammalians: Corazonin
2.2. Emission
2.3. Expulsion [Anterograde Ejaculation]
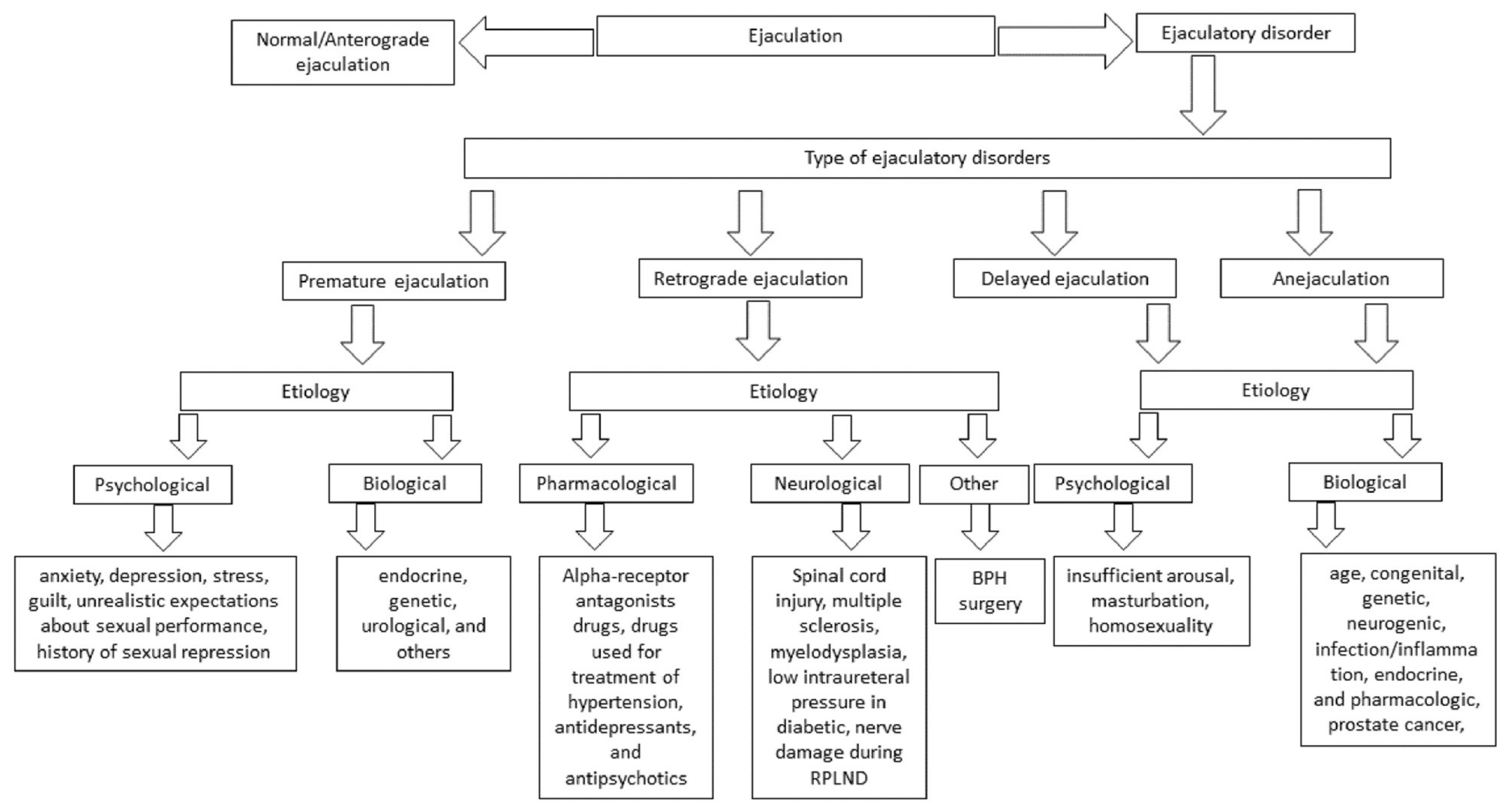
3. Ejaculatory Dysfunction
4. Premature Ejaculation
4.1. Psychological
4.2. Biological
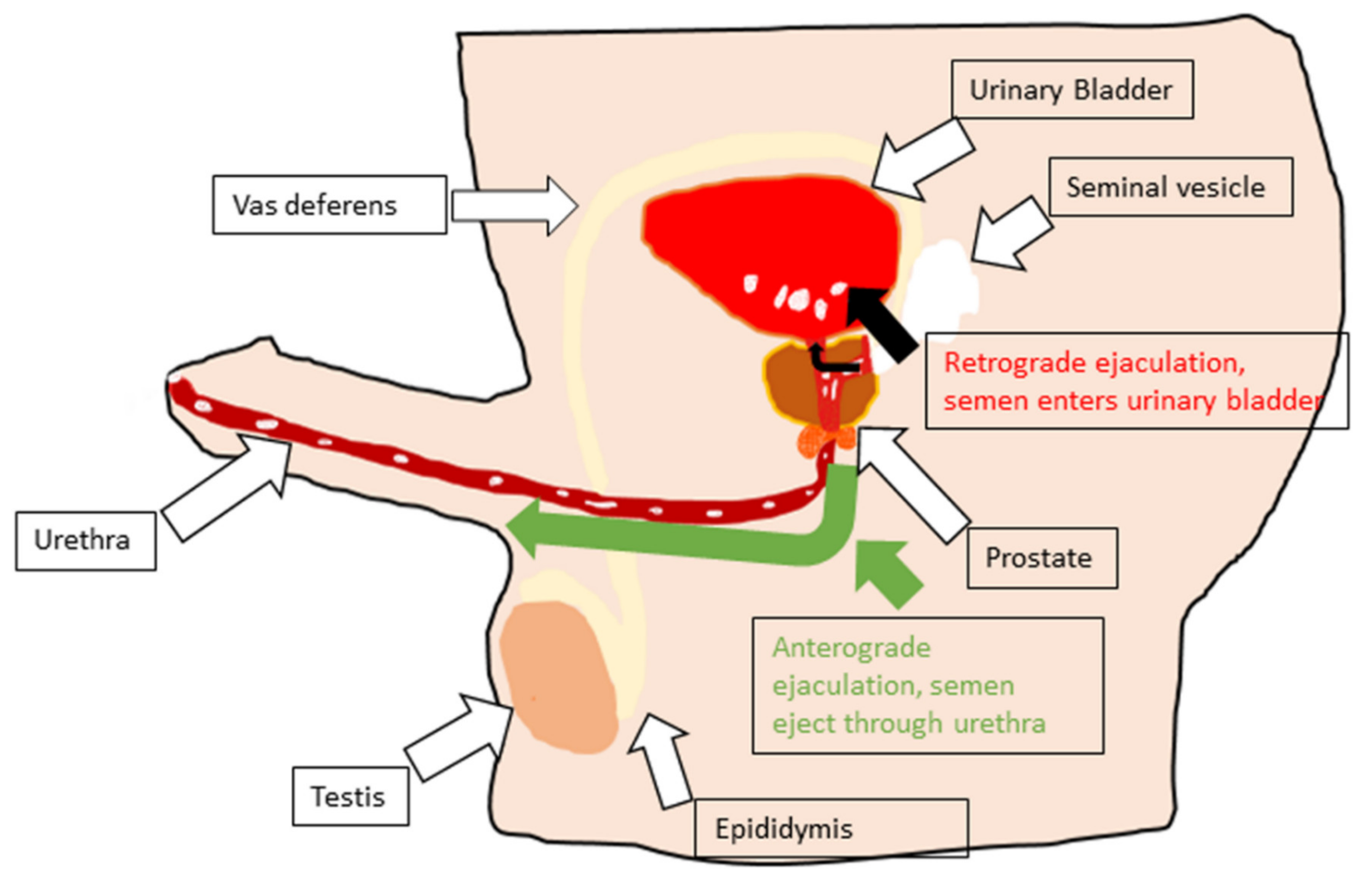
5. Retrograde Ejaculation
5.1. Pharmacologic Factors
5.2. Neurogenic Factors
5.3. Other Factors
6. Delayed Ejaculation and Anejaculation
6.1. Psychological Factors Include Religious Factors, Insufficient Arousal, Masturbation, and Homosexuality
6.2. Biological Factors Include Age, Race, Genetic, Congenital, Endocrine, Neurogenic, Infection/Inflammation, and Pharmacological Factors
7. Future Directions and Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clement, P.; Giuliano, F. Physiology and pharmacology of ejaculation. Basic Clin. Pharmacol. Toxicol. 2016, 119 (Suppl. 3), 18–25. [Google Scholar] [CrossRef] [PubMed]
- Revenig, L.; Leung, A.; Hsiao, W. Ejaculatory physiology and pathophysiology: Assessment and treatment in male infertility. Transl. Androl. Urol. 2014, 3, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Everaert, K.; de Waard, W.I.; Van Hoof, T.; Kiekens, C.; Mulliez, T.; D’Herde, C. Neuroanatomy and neurophysiology related to sexual dysfunction in male neurogenic patients with lesions to the spinal cord or peripheral nerves. Spinal Cord 2010, 48, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Shafik, A. Response of the urethral and intracorporeal pressures to cavernosus muscle stimulation: Role of the muscles in erection and ejaculation. Urology 1995, 46, 85–88. [Google Scholar] [CrossRef]
- Nemec, E.D.; Mansfield, L.; Kennedy, J.W. Heart rate and blood pressure responses during sexual activity in normal males. Am. Heart J. 1976, 92, 274–277. [Google Scholar] [CrossRef]
- Kruger, T.; Exton, M.S.; Pawlak, C.; von zur Muhlen, A.; Hartmann, U.; Schedlowski, M. Neuroendocrine and cardiovascular response to sexual arousal and orgasm in men. Psychoneuroendocrinology 1998, 23, 401–411. [Google Scholar] [CrossRef]
- Rowland, D.L. Genital and heart rate response to erotic stimulation in men with and without premature ejaculation. Int. J. Impot. Res. 2010, 22, 318–324. [Google Scholar] [CrossRef]
- Coolen, L.M.; Allard, J.; Truitt, W.A.; McKenna, K.E. Central regulation of ejaculation. Physiol. Behav. 2004, 83, 203–215. [Google Scholar] [CrossRef]
- Wibowo, E.; Wassersug, R.J. Multiple orgasms in men-what we know so far. Sex Med. Rev. 2016, 4, 136–148. [Google Scholar] [CrossRef]
- Arafa, M.M.; Zohdy, W.A.; Shamloul, R. Prostatic massage: A simple method of semen retrieval in men with spinal cord injury. Int. J. Androl. 2007, 30, 170–173. [Google Scholar] [CrossRef]
- Sipski, M.; Alexander, C.J.; Gomez-Marin, O. Effects of level and degree of spinal cord injury on male orgasm. Spinal Cord 2006, 44, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Cui, Y.; Li, X.; Zhang, H.; Hu, J.; Liu, J.; Chen, Z.J. Sperm retrieval in anejaculatory diabetic men who failed in drug treatment and penile vibratory stimulation during blood sugar under control. Andrologia 2014, 46, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Alwaal, A.; Breyer, B.N.; Lue, T.F. Normal male sexual function: Emphasis on orgasm and ejaculation. Fertil. Steril. 2015, 104, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Barazani, Y.; Stahl, P.J.; Nagler, H.M.; Stember, D.S. Management of ejaculatory disorders in infertile men. Asian J. Androl. 2012, 14, 525–529. [Google Scholar] [CrossRef]
- Beyer, C.; Contreras, J.L.; Morali, G.; Larsson, K. Effects of castration and sex steroid treatment on the motor copulatory pattern of the rat. Physiol. Behav. 1981, 27, 727–730. [Google Scholar] [CrossRef]
- Chehensse, C.; Facchinetti, P.; Bahrami, S.; Andrey, P.; Soler, J.M.; Chretien, F.; Bernabe, J.; Clement, P.; Denys, P.; Giuliano, F. Human spinal ejaculation generator. Ann. Neurol. 2017, 81, 35–45. [Google Scholar] [CrossRef]
- Pescatori, E.S.; Calabro, A.; Artibani, W.; Pagano, F.; Triban, C.; Italiano, G. Electrical stimulation of the dorsal nerve of the penis evokes reflex tonic erections of the penile body and reflex ejaculatory responses in the spinal rat. J. Urol. 1993, 149, 627–632. [Google Scholar] [CrossRef]
- Veening, J.G.; Coolen, L.M. Neural mechanisms of sexual behavior in the male rat: Emphasis on ejaculation-related circuits. Pharmacol. Biochem. Behav. 2014, 121, 170–183. [Google Scholar] [CrossRef]
- Sengul, G.; Puchalski, R.B.; Watson, C. Cytoarchitecture of the spinal cord of the postnatal (P4) mouse. Anat. Rec. 2012, 295, 837–845. [Google Scholar] [CrossRef]
- Mostafa, T.; Abdel-Hamid, I.A. Ejaculatory dysfunction in men with diabetes mellitus. World J. Diabetes 2021, 12, 954–974. [Google Scholar] [CrossRef]
- Morgan, C.; de Groat, W.C.; Nadelhaft, I. The spinal distribution of sympathetic preganglionic and visceral primary afferent neurons that send axons into the hypogastric nerves of the cat. J. Comp. Neurol. 1986, 243, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H. Sexually dimorphic nuclei in the spinal cord control male sexual functions. Front. Neurosci. 2014, 8, 184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Truitt, W.A.; Coolen, L.M. Identification of a potential ejaculation generator in the spinal cord. Science 2002, 297, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Oti, T.; Ueda, R.; Kumagai, R.; Nagafuchi, J.; Ito, T.; Sakamoto, T.; Kondo, Y.; Sakamoto, H. Sexual experience induces the expression of gastrin-releasing peptide and oxytocin receptors in the spinal ejaculation generator in rats. Int. J. Mol. Sci. 2021, 22, 10362. [Google Scholar] [CrossRef]
- Truitt, W.A.; Shipley, M.T.; Veening, J.G.; Coolen, L.M. Activation of a subset of lumbar spinothalamic neurons after copulatory behavior in male but not female rats. J. Neurosci. 2003, 23, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Ju, G.; Melander, T.; Ceccatelli, S.; Hokfelt, T.; Frey, P. Immunohistochemical evidence for a spinothalamic pathway co-containing cholecystokinin- and galanin-like immunoreactivities in the rat. Neuroscience 1987, 20, 439–456. [Google Scholar] [CrossRef]
- Xu, C.; Yaici, E.D.; Conrath, M.; Blanchard, P.; Leclerc, P.; Benoit, G.; Verge, D.; Giuliano, F. Galanin and neurokinin-1 receptor immunoreactive [corrected] spinal neurons controlling the prostate and the bulbospongiosus muscle identified by transsynaptic labeling in the rat. Neuroscience 2005, 134, 1325–1341. [Google Scholar] [CrossRef]
- Xu, C.; Giuliano, F.; Yaici, E.D.; Conrath, M.; Trassard, O.; Benoit, G.; Verge, D. Identification of lumbar spinal neurons controlling simultaneously the prostate and the bulbospongiosus muscles in the rat. Neuroscience 2006, 138, 561–573. [Google Scholar] [CrossRef]
- Sun, X.Q.; Xu, C.; Leclerc, P.; Benoit, G.; Giuliano, F.; Droupy, S. Spinal neurons involved in the control of the seminal vesicles: A transsynaptic labeling study using pseudorabies virus in rats. Neuroscience 2009, 158, 786–797. [Google Scholar] [CrossRef]
- Newton, B.W. A sexually dimorphic population of galanin-like neurons in the rat lumbar spinal cord: Functional implications. Neurosci. Lett. 1992, 137, 119–122. [Google Scholar] [CrossRef]
- Phan, D.C.; Newton, B.W. Cholecystokinin-8-like immunoreactivity is sexually dimorphic in a midline population of rat lumbar neurons. Neurosci. Lett. 1999, 276, 165–168. [Google Scholar] [CrossRef]
- Kozyrev, N.; Coolen, L.M. Activation of galanin and cholecystokinin receptors in the lumbosacral spinal cord is required for ejaculation in male rats. Eur. J. Neurosci. 2017, 45, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, A.P.; Zhang, X.; Hokfelt, T. An immunohistochemical investigation of the opioid cell column in lamina X of the male rat lumbosacral spinal cord. Neurosci. Lett. 1999, 270, 9–12. [Google Scholar] [CrossRef]
- Wiggins, J.W.; Sledd, J.E.; Coolen, L.M. Spinal cord injury causes reduction of galanin and gastrin releasing peptide mRNA expression in the spinal ejaculation generator of male rats. Front. Neurol. 2021, 12, 670536. [Google Scholar] [CrossRef]
- Sakamoto, H.; Matsuda, K.; Zuloaga, D.G.; Hongu, H.; Wada, E.; Wada, K.; Jordan, C.L.; Breedlove, S.M.; Kawata, M. Sexually dimorphic gastrin releasing peptide system in the spinal cord controls male reproductive functions. Nat. Neurosci. 2008, 11, 634–636. [Google Scholar] [CrossRef]
- Kozyrev, N.; Lehman, M.N.; Coolen, L.M. Activation of gastrin-releasing peptide receptors in the lumbosacral spinal cord is required for ejaculation in male rats. J. Sex Med. 2012, 9, 1303–1318. [Google Scholar] [CrossRef]
- Facchinetti, P.; Giuliano, F.; Laurin, M.; Bernabe, J.; Clement, P. Direct brain projections onto the spinal generator of ejaculation in the rat. Neuroscience 2014, 272, 207–216. [Google Scholar] [CrossRef]
- Veenstra, J.A. Isolation and structure of corazonin, a cardioactive peptide from the American cockroach. FEBS Lett. 1989, 250, 231–234. [Google Scholar] [CrossRef]
- Pan, Y.; Robinett, C.C.; Baker, B.S. Turning males on: Activation of male courtship behavior in Drosophila melanogaster. PLoS ONE 2011, 6, e21144. [Google Scholar] [CrossRef]
- Khan, Z.; Tondravi, M.; Oliver, R.; Vonhoff, F.J. Drosophila corazonin neurons as a hub for regulating growth, stress responses, ethanol-related behaviors, copulation persistence and sexually dimorphic reward pathways. J. Dev. Biol. 2021, 9, 26. [Google Scholar] [CrossRef]
- Zer-Krispil, S.; Zak, H.; Shao, L.; Ben-Shaanan, S.; Tordjman, L.; Bentzur, A.; Shmueli, A.; Shohat-Ophir, G. Ejaculation induced by the activation of crz neurons is rewarding to Drosophila males. Curr. Biol. 2018, 28, 1445–1452.e1443. [Google Scholar] [CrossRef] [PubMed]
- Tayler, T.D.; Pacheco, D.A.; Hergarden, A.C.; Murthy, M.; Anderson, D.J. A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 20697–20702. [Google Scholar] [CrossRef] [PubMed]
- Porst, H.; Burri, A. Fortacin spray for the treatment of premature ejaculation. Urologia 2017, 84, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Federighi, G.; Asteriti, S.; Cangiano, L. Lumbar spinal cord neurons putatively involved in ejaculation are sexually dimorphic in early postnatal mice. J. Comp. Neurol. 2020, 528, 624–636. [Google Scholar] [CrossRef]
- Master, V.A.; Turek, P.J. Ejaculatory physiology and dysfunction. Urol. Clin. N. Am. 2001, 28, 363–375. [Google Scholar] [CrossRef]
- Deeh Defo, P.B.; Asongu, E.; Wankeu, M.N.; Ngadjui, E.; Bonsou Fazin, G.R.; Kemka, F.X.; Carro-Juarez, M.; Kamanyi, A.; Kamtchouing, P.; Watcho, P. Guibourtia tessmannii-induced fictive ejaculation in spinal male rat: Involvement of D1, D2-like receptors. Pharm. Biol. 2017, 55, 1138–1143. [Google Scholar] [CrossRef]
- Comarr, A.E. Sexual function among patients with spinal cord injury. Urol. Int. 1970, 25, 134–168. [Google Scholar] [CrossRef]
- El-Hamd, M.A.; Saleh, R.; Majzoub, A. Premature ejaculation: An update on definition and pathophysiology. Asian J..Androl. 2019, 21, 425–432. [Google Scholar] [CrossRef]
- Bettocchi, C.; Verze, P.; Palumbo, F.; Arcaniolo, D.; Mirone, V. Ejaculatory disorders: Pathophysiology and management. Nat. Clin. Pract. Urol. 2008, 5, 93–103. [Google Scholar] [CrossRef]
- Carro-Juarez, M.; Rodriguez-Manzo, G.; de Lourdes Rodriguez Pena, M.; Franco, M.A. Rhythmic motor patterns accompanying ejaculation in spinal cord-transected male rats. Int. J. Impot. Res. 2014, 26, 191–195. [Google Scholar] [CrossRef]
- Gerstenberg, T.C.; Levin, R.J.; Wagner, G. Erection and ejaculation in man. Assessment of the electromyographic activity of the bulbocavernosus and ischiocavernosus muscles. Br. J. Urol. 1990, 65, 395–402. [Google Scholar] [CrossRef]
- Sung, H.H.; Kim, J.J.; Han, D.H.; Kang, S.J.; Chae, M.R.; Kim, C.Y.; Park, J.K.; Lee, S.W. New methodology for investigating ejaculation dysfunction: Measuring intraluminal seminal vesicle pressure in rats with a telemetric device. J. Sex Med. 2015, 12, 2134–2140. [Google Scholar] [CrossRef]
- Puppo, V.; Puppo, G. Comprehensive review of the anatomy and physiology of male ejaculation: Premature ejaculation is not a disease. Clin. Anat. 2016, 29, 111–119. [Google Scholar] [CrossRef]
- Masugi-Tokita, M.; Tomita, K.; Kobayashi, K.; Yoshida, T.; Kageyama, S.; Sakamoto, H.; Kawauchi, A. Metabotropic glutamate receptor subtype 7 is essential for ejaculation. Mol. Neurobiol. 2020, 57, 5208–5218. [Google Scholar] [CrossRef]
- Abdel-Hamid, I.A.; Ali, O.I. Delayed ejaculation: Pathophysiology, diagnosis, and treatment. World J. Mens Health 2018, 36, 22–40. [Google Scholar] [CrossRef]
- Gray, M.; Zillioux, J.; Khourdaji, I.; Smith, R.P. Contemporary management of ejaculatory dysfunction. Transl. Androl. Urol. 2018, 7, 686–702. [Google Scholar] [CrossRef]
- Hu, J.; Nagao, K.; Tai, T.; Kobayashi, H.; Nakajima, K. Randomized crossover trial of amoxapine versus vitamin B12 for retrograde ejaculation. Int. Braz. J. Urol. 2017, 43, 496–504. [Google Scholar] [CrossRef]
- Jannini, E.A.; Lenzi, A. Ejaculatory disorders: Epidemiology and current approaches to definition, classification and subtyping. World J. Urol. 2005, 23, 68–75. [Google Scholar] [CrossRef]
- Wolters, J.P.; Hellstrom, W.J. Current concepts in ejaculatory dysfunction. Rev. Urol. 2006, 8 (Suppl. 4), S18–S25. [Google Scholar]
- Lee, S.W.; Lee, J.H.; Sung, H.H.; Park, H.J.; Park, J.K.; Choi, S.K.; Kam, S.C. The prevalence of premature ejaculation and its clinical characteristics in Korean men according to different definitions. Int. J. Impot. Res. 2013, 25, 12–17. [Google Scholar] [CrossRef]
- Tiefer, L. Medicine, sexual norms, and the role of the DSM. Virtual Mentor 2014, 16, 923–927. [Google Scholar] [CrossRef]
- Althof, S.E.; McMahon, C.G.; Waldinger, M.D.; Serefoglu, E.C.; Shindel, A.W.; Adaikan, P.G.; Becher, E.; Dean, J.; Giuliano, F.; Hellstrom, W.J.; et al. An update of the international society of sexual medicine’s guidelines for the giagnosis and treatment of premature ejaculation (PE). Sex Med. 2014, 2, 60–90. [Google Scholar] [CrossRef]
- Waldinger, M.D.; Olivier, B. Animal models of premature and retarded ejaculation. World J. Urol. 2005, 23, 115–118. [Google Scholar] [CrossRef]
- Andersson, G.; Larsson, K. Effects of FG 5893, a new compound with 5-HT1A receptor agonistic and 5-HT2 receptor antagonistic properties, on male rat sexual behavior. Eur. J. Pharmacol. 1994, 255, 131–137. [Google Scholar] [CrossRef]
- Haensel, S.M.; Slob, A.K. Flesinoxan: A prosexual drug for male rats. Eur. J. Pharmacol. 1997, 330, 1–9. [Google Scholar] [CrossRef]
- Waldinger, M.D. Premature ejaculation: Definition and drug treatment. Drugs 2007, 67, 547–568. [Google Scholar] [CrossRef]
- Wincze, J.P. Psychosocial aspects of ejaculatory dysfunction and male reproduction. Fertil. Steril. 2015, 104, 1089–1094. [Google Scholar] [CrossRef]
- Waldinger, M.D. Recent advances in the classification, neurobiology and treatment of premature ejaculation. Adv. Psychosom. Med. 2008, 29, 50–69. [Google Scholar] [CrossRef]
- Coskuner, E.R.; Ozkan, B. Premature ejaculation and endocrine disorders: A literature review. World J. Mens Health 2022, 40, 38–51. [Google Scholar] [CrossRef]
- Xia, Y.; Li, J.; Shan, G.; Qian, H.; Wang, T.; Wu, W.; Chen, J.; Liu, L. Relationship between premature ejaculation and depression: A PRISMA-compliant systematic review and meta-analysis. Medicine 2016, 95, e4620. [Google Scholar] [CrossRef]
- Mourikis, I.; Antoniou, M.; Matsouka, E.; Vousoura, E.; Tzavara, C.; Ekizoglou, C.; Papadimitriou, G.N.; Vaidakis, N.; Zervas, I.M. Anxiety and depression among Greek men with primary erectile dysfunction and premature ejaculation. Ann. Gen. Psychiatry 2015, 14, 34. [Google Scholar] [CrossRef][Green Version]
- Son, H.; Song, S.H.; Lee, J.Y.; Paick, J.S. Relationship between premature ejaculation and depression in Korean males. J. Sex Med. 2011, 8, 2062–2070. [Google Scholar] [CrossRef]
- Wang, J.; Yan, C.; Zhao, Z.; Chen, H.; Shi, Z.; Du, Q.; Zhang, Y.; Qiu, Y.; Lang, Y.; Kong, L.; et al. Sexual dysfunction in patients with myasthenia gravis. J. Neuroimmunol. 2021, 358, 577669. [Google Scholar] [CrossRef]
- Fiala, L.; Lenz, J.; Konecna, P.; Zajicova, M.; Cerna, J.; Sajdlova, R. Premature ejaculation and stress. Andrologia 2021, 53, e14093. [Google Scholar] [CrossRef]
- Cihan, A.; Murat, N.; Demir, O.; Aslan, G.; Demir, T.; Gidener, S.; Esen, A.A. An experimental approach to the interrelationship between hyperthyroidism and ejaculation latency time in male rats. J. Urol. 2009, 181, 907–912. [Google Scholar] [CrossRef]
- Carani, C.; Isidori, A.M.; Granata, A.; Carosa, E.; Maggi, M.; Lenzi, A.; Jannini, E.A. Multicenter study on the prevalence of sexual symptoms in male hypo- and hyperthyroid patients. J. Clin. Endocrinol. Metab. 2005, 90, 6472–6479. [Google Scholar] [CrossRef]
- Cihan, A.; Demir, O.; Demir, T.; Aslan, G.; Comlekci, A.; Esen, A. The relationship between premature ejaculation and hyperthyroidism. J. Urol. 2009, 181, 1273–1280. [Google Scholar] [CrossRef]
- Cihan, A.; Esen, A.A. Systematic review and meta-analysis for the value of thyroid disorder screening in men with ejaculatory dysfunction. Int. J. Clin. Pract. 2021, 75, e14419. [Google Scholar] [CrossRef]
- Corona, G.; Jannini, E.A.; Lotti, F.; Boddi, V.; De Vita, G.; Forti, G.; Lenzi, A.; Mannucci, E.; Maggi, M. Premature and delayed ejaculation: Two ends of a single continuum influenced by hormonal milieu. Int. J. Androl. 2011, 34, 41–48. [Google Scholar] [CrossRef]
- Ozturk, M.I.; Koca, O.; Tuken, M.; Keles, M.O.; Ilktac, A.; Karaman, M.I. Hormonal evaluation in premature ejaculation. Urol. Int. 2012, 88, 454–458. [Google Scholar] [CrossRef]
- Canat, L.; Erbin, A.; Canat, M.; Dinek, M.; Caskurlu, T. Assessment of hormonal activity in patients with premature ejaculation. Int. Braz. J. Urol. 2017, 43, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Pirke, K.M.; Kockott, G.; Aldenhoff, J.; Besinger, U.; Feil, W. Pituitary gonadal system function in patients with erectile impotence and premature ejaculation. Arch. Sex Behav. 1979, 8, 41–48. [Google Scholar] [CrossRef]
- Corona, G.; Jannini, E.A.; Mannucci, E.; Fisher, A.D.; Lotti, F.; Petrone, L.; Balercia, G.; Bandini, E.; Chiarini, V.; Forti, G.; et al. Different testosterone levels are associated with ejaculatory dysfunction. J. Sex Med. 2008, 5, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.G.; Hosseini, S.R.; Alizadeh, F.; Rangzan, N. Serum testosterone and gonadotropins levels in patients with premature ejaculation: A comparison with normal men. Adv. Biomed Res. 2014, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.T. Sexual function in altered physiological states: Comparison of effects of hypertension, diabetes, hyperprolactinemia, and others to “normal” aging in male rats. Neurosci. Biobehav. Rev. 1995, 19, 279–302. [Google Scholar] [CrossRef]
- Scarano, W.R.; Messias, A.G.; Oliva, S.U.; Klinefelter, G.R.; Kempinas, W.G. Sexual behaviour, sperm quantity and quality after short-term streptozotocin-induced hyperglycaemia in rats. Int. J. Androl. 2006, 29, 482–488. [Google Scholar] [CrossRef]
- De, A.; Singh, M.F.; Singh, V.; Ram, V.; Bisht, S. Treatment effect of l-norvaline on the sexual performance of male rats with streptozotocin induced diabetes. Eur. J. Pharmacol. 2016, 771, 247–254. [Google Scholar] [CrossRef]
- Shi, G.J.; Zheng, J.; Wu, J.; Qiao, H.Q.; Chang, Q.; Niu, Y.; Sun, T.; Li, Y.X.; Yu, J.Q. Protective effects of Lycium barbarum polysaccharide on male sexual dysfunction and fertility impairments by activating hypothalamic pituitary gonadal axis in streptozotocin-induced type-1 diabetic male mice. Endocr. J. 2017, 64, 907–922. [Google Scholar] [CrossRef]
- Li, Z.M.; Liu, N.; Jiang, Y.P.; Yang, J.M.; Zheng, J.; Sun, M.; Li, Y.X.; Sun, T.; Wu, J.; Yu, J.Q. Vitexin alleviates streptozotocin-induced sexual dysfunction and fertility impairments in male mice via modulating the hypothalamus-pituitary-gonadal axis. Chem. Biol. Interact. 2019, 297, 119–129. [Google Scholar] [CrossRef]
- Pontes, D.A.; Fernandes, G.S.; Piffer, R.C.; Gerardin, D.C.; Pereira, O.C.; Kempinas, W.G. Ejaculatory dysfunction in streptozotocin-induced diabetic rats: The role of testosterone. Pharmacol. Rep. 2011, 63, 130–138. [Google Scholar] [CrossRef]
- Lert-Amornpat, T.; Maketon, C.; Fungfuang, W. Effect of Kaempferia parviflora on sexual performance in streptozotocin-induced diabetic male rats. Andrologia 2017, 49, e12770. [Google Scholar] [CrossRef]
- Ghaheri, M.; Miraghaee, S.; Babaei, A.; Mohammadi, B.; Kahrizi, D.; Saivosh Haghighi, Z.M.; Bahrami, G. Effect of Stevia rebaudiana Bertoni extract on sexual dysfunction in streptozotocin-induced diabetic male rats. Cell Mol. Biol. 2018, 64, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Minaz, N.; Razdan, R.; Hammock, B.D.; Mujwar, S.; Goswami, S.K. Impact of diabetes on male sexual function in streptozotocin-induced diabetic rats: Protective role of soluble epoxide hydrolase inhibitor. Biomed. Pharm. 2019, 115, 108897. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, A.; Ebiko, M.; Yoshizumi, M.; Ise, S.N.; Watanabe, C.; Mizoguchi, H.; Iwasaki, M.; Kimura, Y.; Sakurada, S. Effects of insulin replacement on ejaculatory dysfunction in streptozotocin-induced diabetic rats. Int. J. Urol. 2009, 16, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, P.A.; Brotcke, T.P.; Burrell, C.L.; Belis, J.A. Comparison of the effects of castration and streptozotocin-induced diabetes mellitus on contractile responses of the rat vas deferens. Pharmacology 1989, 38, 253–262. [Google Scholar] [CrossRef]
- Kaschube, M.; Moller-Hartmann, H.; Zetler, G. The field-stimulated vas deferens of the streptozotocin-diabetic mouse: Effects of prazosin, alpha, beta-methylene ATP, and variation of stimulation parameters. J. Neural Transm. 1989, 77, 171–180. [Google Scholar] [CrossRef]
- Kamata, K.; Kirisawa, H. Changes in electrophysiological properties and noradrenaline response in vas deferens of diabetic rats. Eur. J. Pharmacol. 1998, 350, 237–241. [Google Scholar] [CrossRef]
- Gunes, A.; Ceylan, A.; Sarioglu, Y.; Stefek, M.; Bauer, V.; Karasu, C. Antioxidants in diabetes-induced complications study group. Reactive oxygen species mediate abnormal contractile response to sympathetic nerve stimulation and noradrenaline in the vas deferens of chronically diabetic rats: Effects of in vivo treatment with antioxidants. Fundam. Clin. Pharmacol. 2005, 19, 73–79. [Google Scholar] [CrossRef]
- Tsounapi, P.; Honda, M.; Dimitriadis, F.; Shimizu, S.; Shiomi, T.; Hikita, K.; Saito, M.; Tomita, S.; Sofikitis, N.; Takenaka, A. Antioxidant treatment ameliorates diabetes-induced dysfunction of the vas deferens in a rat model. Andrologia 2018, 50, e12795. [Google Scholar] [CrossRef]
- Sandrini, M.; Vitale, G.; Vergoni, A.V.; Ottani, A.; Bertolini, A. Streptozotocin-induced diabetes provokes changes in serotonin concentration and on 5-HT1A and 5-HT2 receptors in the rat brain. Life Sci. 1997, 60, 1393–1397. [Google Scholar] [CrossRef]
- Abraham, P.M.; Anju, T.R.; Jayanarayanan, S.; Paulose, C.S. Serotonergic receptor upregulation in cerebral cortex and down regulation in brainstem of streptozotocin induced diabetic rats: Antagonism by pyridoxine and insulin. Neurosci. Lett. 2010, 483, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Martin-Tuite, P.; Shindel, A.W. Management options for premature ejaculation and delayed ejaculation in men. Sex Med. Rev. 2020, 8, 473–485. [Google Scholar] [CrossRef]
- Dunsmuir, W.D.; Holmes, S.A. The aetiology and management of erectile, ejaculatory, and fertility problems in men with diabetes mellitus. Diabet. Med. 1996, 13, 700–708. [Google Scholar] [CrossRef]
- Majzoub, A.; Arafa, M.; Al-Said, S.; Dabbous, Z.; Aboulsoud, S.; Khalafalla, K.; Elbardisi, H. Premature ejaculation in type II diabetes mellitus patients: Association with glycemic control. Transl. Androl. Urol. 2016, 5, 248–254. [Google Scholar] [CrossRef]
- El-Sakka, A.I. Premature ejaculation in non-insulin-dependent diabetic patients. Int. J. Androl. 2003, 26, 329–334. [Google Scholar] [CrossRef]
- Hammarsten, J.; Peeker, R. Urological aspects of the metabolic syndrome. Nat. Rev. Urol. 2011, 8, 483–494. [Google Scholar] [CrossRef]
- Lee, R.K.; Chung, D.; Chughtai, B.; Te, A.E.; Kaplan, S.A. Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms. BJU Int. 2012, 110, 540–545. [Google Scholar] [CrossRef]
- Salama, N.; Eid, A.; Swedan, A.; Hatem, A. Increased prevalence of premature ejaculation in men with metabolic syndrome. Aging Male 2017, 20, 89–95. [Google Scholar] [CrossRef]
- Bolat, D.; Kocabas, G.U.; Gunlusoy, B.; Aydogdu, O.; Aydin, M.E. The relationship between acquired premature ejaculation and metabolic syndrome: A prospective, comparative study. Int. J. Impot. Res. 2017, 29, 105–109. [Google Scholar] [CrossRef]
- Pan, A.; Keum, N.; Okereke, O.I.; Sun, Q.; Kivimaki, M.; Rubin, R.R.; Hu, F.B. Bidirectional association between depression and metabolic syndrome: A systematic review and meta-analysis of epidemiological studies. Diabetes Care 2012, 35, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Skilton, M.R.; Moulin, P.; Terra, J.L.; Bonnet, F. Associations between anxiety, depression, and the metabolic syndrome. Biol. Psychiatry 2007, 62, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Mirzahosseini, S.; Karabelyos, C.; Dobozy, O.; Csaba, G. Changes in sexual behavior of adult male and female rats neonatally treated with vitamin D3. Hum. Exp. Toxicol. 1996, 15, 573–576. [Google Scholar] [CrossRef]
- Hartmann, U.; Schedlowski, M.; Kruger, T.H. Cognitive and partner-related factors in rapid ejaculation: Differences between dysfunctional and functional men. World J. Urol. 2005, 23, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Groves, N.J.; Kesby, J.P.; Eyles, D.W.; McGrath, J.J.; Mackay-Sim, A.; Burne, T.H. Adult vitamin D deficiency leads to behavioural and brain neurochemical alterations in C57BL/6J and BALB/c mice. Behav. Brain Res. 2013, 241, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhang, Y.; Wang, T.; Lin, Y.; Yu, J.; Xia, Q.; Zhu, P.; Zhu, D.M. Vitamin D supplementation improves anxiety but not depression symptoms in patients with vitamin D deficiency. Brain Behav. 2020, 10, e01760. [Google Scholar] [CrossRef]
- Yang, D.W.; Sun, J. Correlation of lifelong premature ejaculation with 5-HT system gene polymorphism. Zhonghua Nan Ke Xue 2021, 27, 748–752. [Google Scholar]
- Waldinger, M.D. The neurobiological approach to premature ejaculation. J. Urol. 2002, 168, 2359–2367. [Google Scholar] [CrossRef]
- Waldinger, M.D.; Rietschel, M.; Nothen, M.M.; Hengeveld, M.W.; Olivier, B. Familial occurrence of primary premature ejaculation. Psychiatr. Genet. 1998, 8, 37–40. [Google Scholar] [CrossRef]
- Liang, C.Z.; Hao, Z.Y.; Li, H.J.; Wang, Z.P.; Xing, J.P.; Hu, W.L.; Zhang, T.F.; Ge, W.W.; Zhang, X.S.; Zhou, J.; et al. Prevalence of premature ejaculation and its correlation with chronic prostatitis in Chinese men. Urology 2010, 76, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Jannini, E.A.; Simonelli, C.; Lenzi, A. Disorders of ejaculation. J. Endocrinol. Investig. 2002, 25, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Nikoobakht, M.; Aloosh, M.; Hasani, M. Seminal plasma magnesium and premature ejaculation: A case-control study. Urol. J. 2005, 2, 102–105. [Google Scholar] [CrossRef]
- Omu, A.E.; Al-Bader, A.A.; Dashti, H.; Oriowo, M.A. Magnesium in human semen: Possible role in premature ejaculation. Arch. Androl. 2001, 46, 59–66. [Google Scholar] [CrossRef]
- Aloosh, M.; Hassani, M.; Nikoobakht, M. Seminal plasma magnesium and premature ejaculation: A case-control study. BJU Int. 2006, 98, 402–404. [Google Scholar] [CrossRef]
- Soni, K.K.; Zhang, L.T.; Choi, B.R.; Karna, K.K.; You, J.H.; Shin, Y.S.; Lee, S.W.; Kim, C.Y.; Zhao, C.; Chae, H.J.; et al. Protective effect of MOTILIPERM in varicocele-induced oxidative injury in rat testis by activating phosphorylated inositol requiring kinase 1alpha (p-IRE1alpha) and phosphorylated c-Jun N-terminal kinase (p-JNK) pathways. Pharm. Biol. 2018, 56, 94–103. [Google Scholar] [CrossRef]
- Lotti, F.; Corona, G.; Mancini, M.; Biagini, C.; Colpi, G.M.; Innocenti, S.D.; Filimberti, E.; Gacci, M.; Krausz, C.; Sforza, A.; et al. The association between varicocele, premature ejaculation and prostatitis symptoms: Possible mechanisms. J. Sex Med. 2009, 6, 2878–2887. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Abdel-Aziz, A.S.; Maarouf, A.M.; Ali, M.; Emara, A.A.; Gomaa, A. Impact of varicocelectomy on premature ejaculation in varicocele patients. Andrologia 2015, 47, 276–281. [Google Scholar] [CrossRef]
- Sakamoto, H.; Ogawa, Y. Is varicocele associated with underlying venous abnormalities? varicocele and the prostatic venous plexus. J. Urol. 2008, 180, 1427–1431. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, R.; Agarwal, A.; Parekh, N.; Finelli, R.; Shah, R.; Kandil, H.; Saleh, R.; Arafa, M.; Ko, E.; et al. A comprehensive guide to sperm recovery in infertile men with retrograde ejaculation. World J. Mens Health 2021, 40, 208–216. [Google Scholar] [CrossRef]
- Yavetz, H.; Yogev, L.; Hauser, R.; Lessing, J.B.; Paz, G.; Homonnai, Z.T. Retrograde ejaculation. Hum. Reprod. 1994, 9, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Jefferys, A.; Siassakos, D.; Wardle, P. The management of retrograde ejaculation: A systematic review and update. Fertil. Steril. 2012, 97, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Parnham, A.; Serefoglu, E.C. Retrograde ejaculation, painful ejaculation and hematospermia. Transl. Androl. Urol. 2016, 5, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Hisasue, S.; Furuya, R.; Itoh, N.; Kobayashi, K.; Furuya, S.; Tsukamoto, T. Ejaculatory disorder caused by alpha-1 adrenoceptor antagonists is not retrograde ejaculation but a loss of seminal emission. Int. J. Urol. 2006, 13, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.D. Targeting recovery: Priorities of the spinal cord-injured population. J. Neurotrauma 2004, 21, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Steadman, C.J.; Vangoor, S.S.; Hubscher, C.H. Telemetric monitoring of penile pressure during mating in rats after chronic spinal cord injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R673–R683. [Google Scholar] [CrossRef]
- Mehta, A.; Sigman, M. Management of the dry ejaculate: A systematic review of aspermia and retrograde ejaculation. Fertil. Steril. 2015, 104, 1074–1081. [Google Scholar] [CrossRef]
- Ibragimov, A.Z.; Aliev, T.A.; Abdullaev, K.I.; Mirza-Zade, V.A. The function of the closure apparatus of the bladder in retrograde ejaculation in diabetics. Urol. Nefrol. 1990, 3, 65–68. [Google Scholar]
- Couteau, N.; Duquesne, I.; Frederic, P.; Thiounn, N.; Timsit, M.O.; Mejean, A.; Pinar, U.; Audenet, F. Ejaculations and benign prostatic hyperplasia: An impossible compromise? a comprehensive review. J. Clin. Med. 2021, 10, 5788. [Google Scholar] [CrossRef]
- Chen, J. The pathophysiology of delayed ejaculation. Transl. Androl. Urol. 2016, 5, 549–562. [Google Scholar] [CrossRef]
- Perelman, M.A. Patient highlights. Delayed ejaculation. J. Sex Med. 2013, 10, 1189–1190. [Google Scholar] [CrossRef]
- Rowland, D.L.; Keeney, C.; Slob, A.K. Sexual response in men with inhibited or retarded ejaculation. Int. J. Impot. Res. 2004, 16, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Perelman, M.A.; Rowland, D.L. Retarded ejaculation. World J. Urol. 2006, 24, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Jern, P.; Santtila, P.; Johansson, A.; Alanko, K.; Salo, B.; Sandnabba, N.K. Is there an association between same-sex sexual experience and ejaculatory dysfunction? J. Sex Marital. Ther. 2010, 36, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.; Carnes, L.; Janssen, E.; Goodrich, D.; Long, J.S. Erectile and ejaculatory problems in gay and heterosexual men. Arch. Sex Behav. 2005, 34, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.E.; Husted, J.R. Penile sensitivity, age, and sexual behavior. J. Clin. Psychol. 1976, 32, 697–700. [Google Scholar] [CrossRef]
- Rowland, D.L. Penile sensitivity in men: A composite of recent findings. Urology 1998, 52, 1101–1105. [Google Scholar] [CrossRef]
- Chiga, M.; Ohmori, T.; Ohba, T.; Katabuchi, H.; Nishinakamura, R. Preformed wolffian duct regulates mullerian duct elongation independently of canonical wnt signaling or lhx1 expression. Int. J. Dev. Biol. 2014, 58, 663–668. [Google Scholar] [CrossRef]
- Phillips, E.; Carpenter, C.; Oates, R.D. Ejaculatory dysfunction. Urol. Clin. North Am. 2014, 41, 115–128. [Google Scholar] [CrossRef]
- Corona, G.; Ricca, V.; Bandini, E.; Mannucci, E.; Lotti, F.; Boddi, V.; Rastrelli, G.; Sforza, A.; Faravelli, C.; Forti, G.; et al. Selective serotonin reuptake inhibitor-induced sexual dysfunction. J. Sex Med. 2009, 6, 1259–1269. [Google Scholar] [CrossRef]
- Bishop, J.R.; Moline, J.; Ellingrod, V.L.; Schultz, S.K.; Clayton, A.H. Serotonin 2A-1438 G/A and G-protein Beta3 subunit C825T polymorphisms in patients with depression and SSRI-associated sexual side-effects. Neuropsychopharmacology 2006, 31, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Redelman, M.J. Sexual difficulties for persons with multiple sclerosis in New South Wales, Australia. Int. J. Rehabil. Res. 2009, 32, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Kamischke, A.; Nieschlag, E. Update on medical treatment of ejaculatory disorders. Int. J. Androl. 2002, 25, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, A.; Akbulut, Z.; Atan, A.; Basar, M.M. Common symptoms in men with prostatic inflammation. Int. Urol. Nephrol. 2006, 38, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Giona, S. The epidemiology of prostate cancer. In Prostate Cancer; Bott, S.R.J., Ng, K.L., Eds.; Exon Publications: Brisbane, QLD, Australia, 2021; pp. 1–15. [Google Scholar]
- Green, T.P.; Saavedra-Belaunde, J.; Wang, R. Ejaculatory and orgasmic dysfunction following prostate cancer therapy: Clinical management. Med. Sci. 2019, 7, 109. [Google Scholar] [CrossRef]
- Benson, C.R.; Serefoglu, E.C.; Hellstrom, W.J. Sexual dysfunction following radical prostatectomy. J. Androl. 2012, 33, 1143–1154. [Google Scholar] [CrossRef]
- Uta, D.; Oti, T.; Sakamoto, T.; Sakamoto, H. In vivo electrophysiology of peptidergic neurons in deep layers of the lumbar spinal cord after optogenetic stimulation of hypothalamic paraventricular oxytocin neurons in rats. Int. J. Mol. Sci. 2021, 22, 3400. [Google Scholar] [CrossRef]
- Quadri, S.A.; Farooqui, M.; Ikram, A.; Zafar, A.; Khan, M.A.; Suriya, S.S.; Claus, C.F.; Fiani, B.; Rahman, M.; Ramachandran, A.; et al. Recent update on basic mechanisms of spinal cord injury. Neurosurg. Rev. 2020, 43, 425–441. [Google Scholar] [CrossRef]
- Kathiresan, A.S.; Ibrahim, E.; Modh, R.; Aballa, T.C.; Lynne, C.M.; Brackett, N.L. Semen quality in ejaculates produced by masturbation in men with spinal cord injury. Spinal Cord 2012, 50, 891–894. [Google Scholar] [CrossRef][Green Version]
- Brackett, N.L.; Ibrahim, E.; Iremashvili, V.; Aballa, T.C.; Lynne, C.M. Treatment for ejaculatory dysfunction in men with spinal cord injury: An 18-year single center experience. J. Urol. 2010, 183, 2304–2308. [Google Scholar] [CrossRef]
- Chehensse, C.; Bahrami, S.; Denys, P.; Clement, P.; Bernabe, J.; Giuliano, F. The spinal control of ejaculation revisited: A systematic review and meta-analysis of anejaculation in spinal cord injured patients. Hum. Reprod. Update 2013, 19, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Kozyrev, N.; Staudt, M.D.; Brown, A.; Coolen, L.M. Chronic contusion spinal cord injury impairs ejaculatory reflexes in male rats: Partial recovery by systemic infusions of dopamine D3 receptor agonist 7OHDPAT. J. Neurotrauma 2016, 33, 943–953. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soni, K.K.; Jeong, H.-S.; Jang, S. Neurons for Ejaculation and Factors Affecting Ejaculation. Biology 2022, 11, 686. https://doi.org/10.3390/biology11050686
Soni KK, Jeong H-S, Jang S. Neurons for Ejaculation and Factors Affecting Ejaculation. Biology. 2022; 11(5):686. https://doi.org/10.3390/biology11050686
Chicago/Turabian StyleSoni, Kiran Kumar, Han-Seong Jeong, and Sujeong Jang. 2022. "Neurons for Ejaculation and Factors Affecting Ejaculation" Biology 11, no. 5: 686. https://doi.org/10.3390/biology11050686
APA StyleSoni, K. K., Jeong, H.-S., & Jang, S. (2022). Neurons for Ejaculation and Factors Affecting Ejaculation. Biology, 11(5), 686. https://doi.org/10.3390/biology11050686






