High-Fructose Diet Increases Renal ChREBPβ Expression, Leading to Intrarenal Fat Accumulation in a Rat Model with Metabolic Syndrome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Diets
2.3. Metabolic Syndrome Features
2.4. Real-Time Quantitative PCR
2.5. Oil Red O Staining (ORO)
2.6. Cell Culture
2.7. Statistical Analysis
3. Results
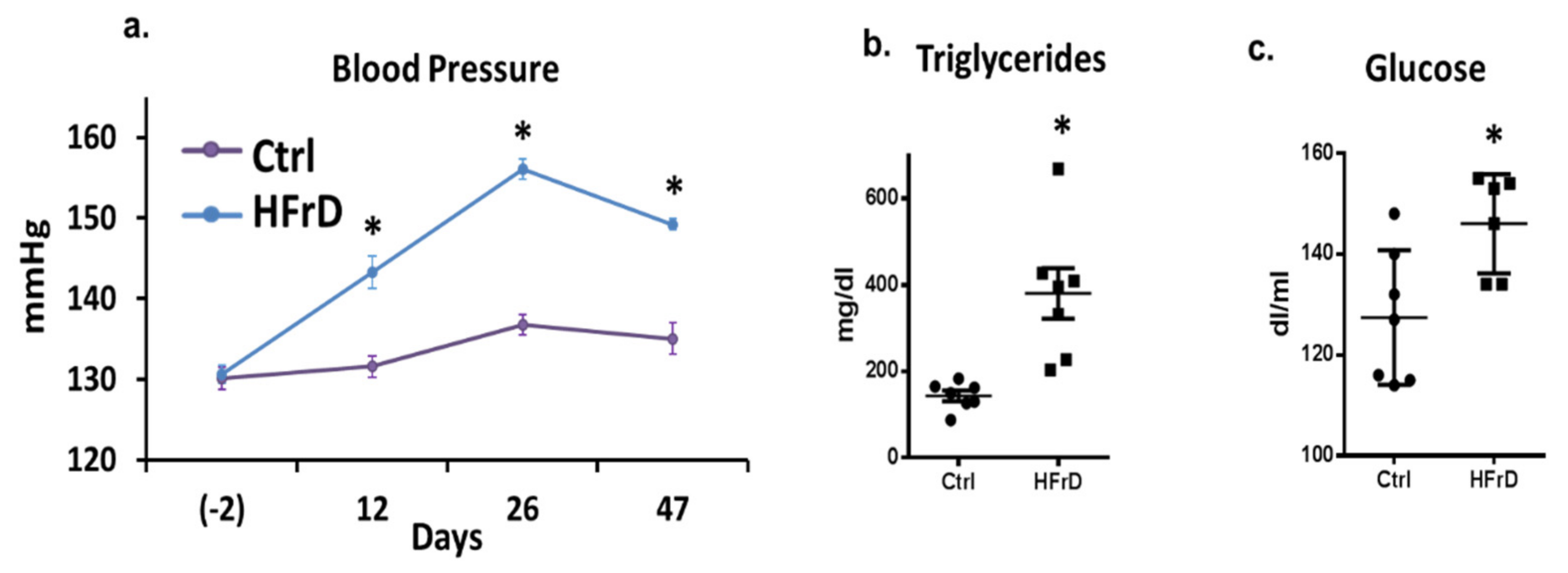
3.1. The Effects of HFrD on Metabolic Featured
3.2. HFrD Is Associated with Ectopic Lipid Accumulation in the Kidney
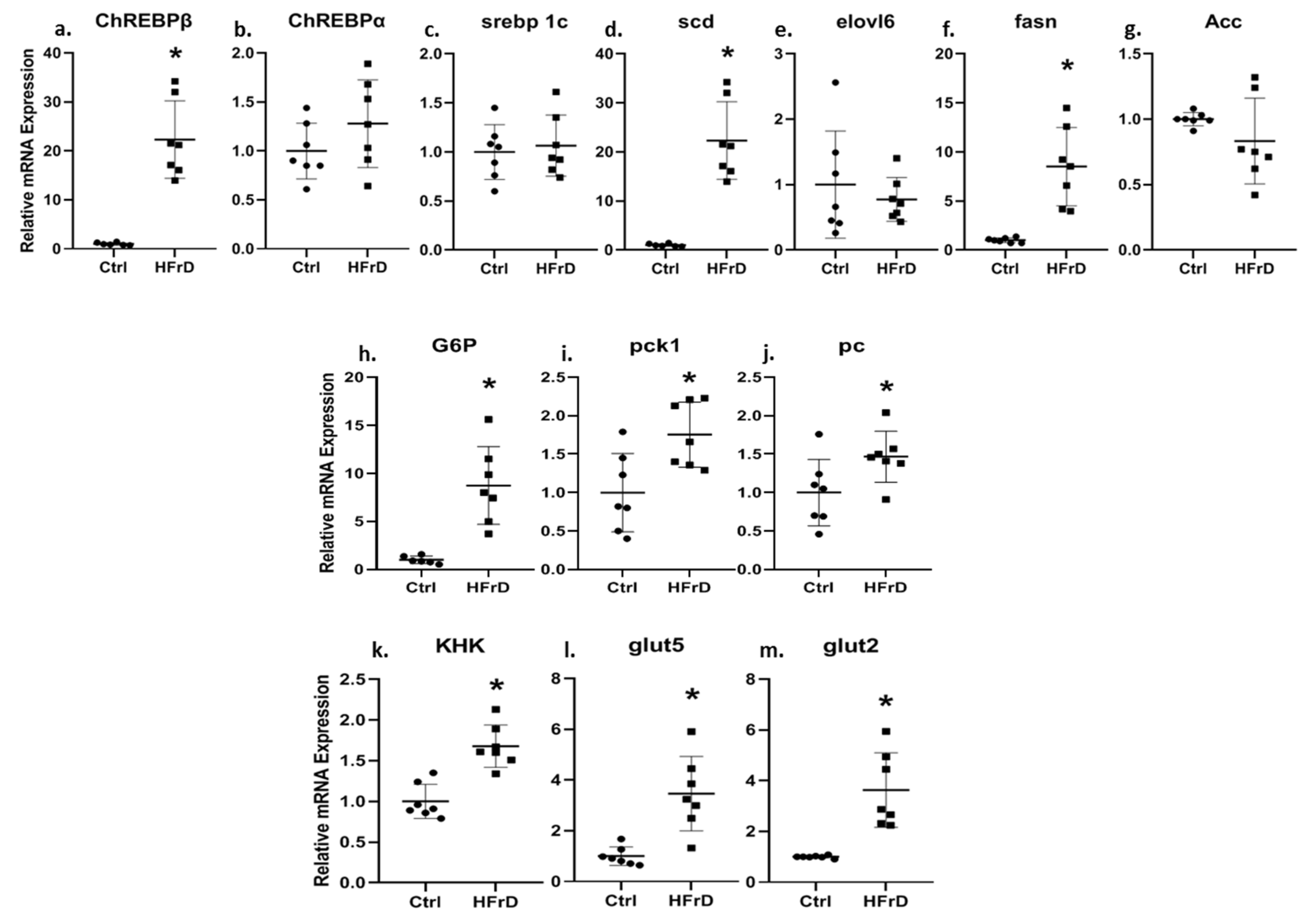
3.3. HFrD Increases Kidney ChREBPβ Expression and Other Genes Involved in Carbohydrate and Fat Metabolism
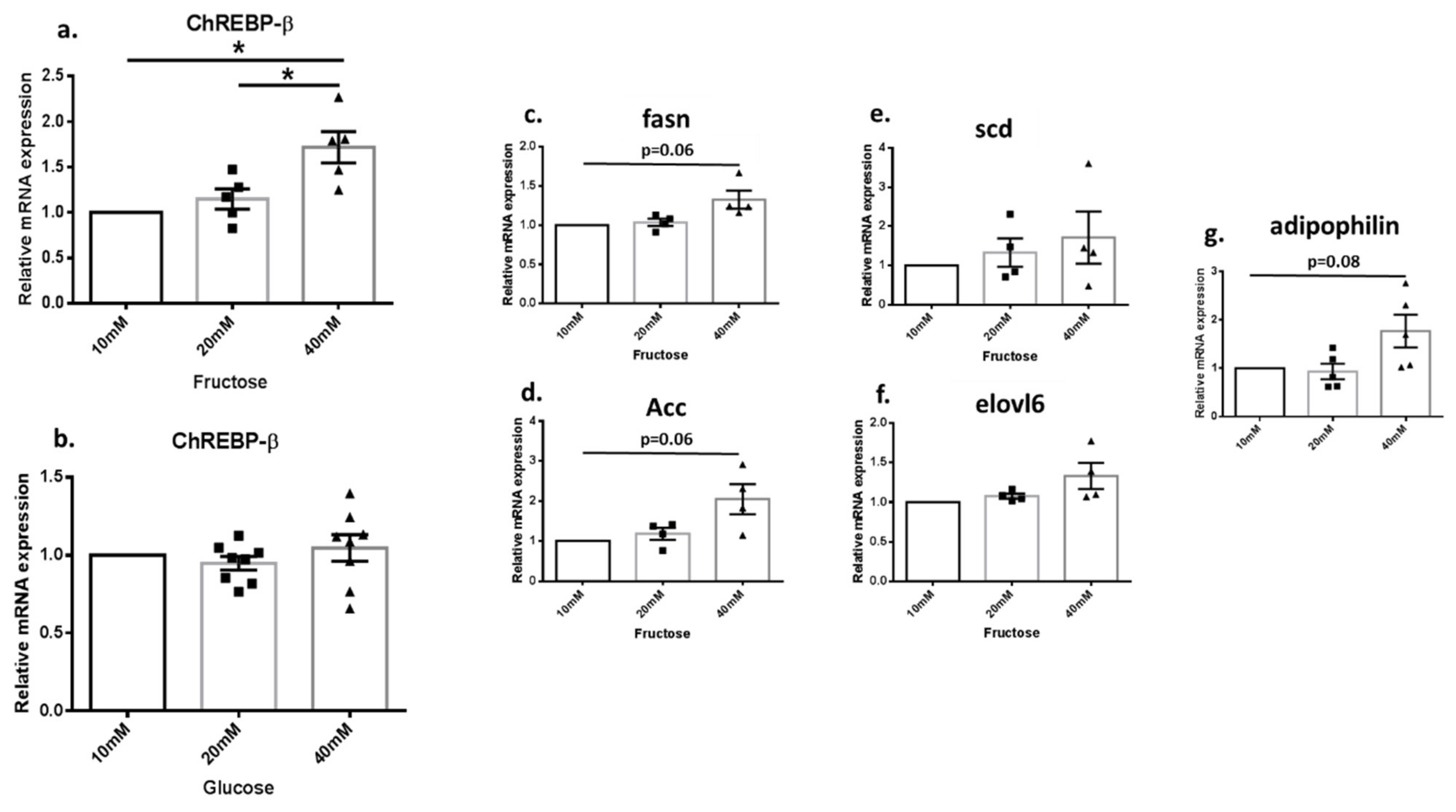
3.4. Fructose Increased the Expression of DNL Genes in Renal Cell Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsu, C.; McCulloch, C.E.; Iribarren, C.; Darbinian, J.; Go, A.S. Body mass index and risk for end-stage renal disease. Ann. Intern. Med. 2006, 144, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D. Non-alcoholic fatty liver disease: An emerging driving force in chronic kidney disease. Nat. Rev. Nephrol. 2017, 13, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Fliser, D.; Pacini, G.; Engelleiter, R.; Kautzky-Willer, A.; Prager, R.; Franek, E.; Ritz, E. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998, 53, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Guebre-Egziabher, F.; Alix, P.M.; Koppe, L.; Pelletier, C.C.; Kalbacher, E.; Fouque, D.; Soulage, C.O. Ectopic lipid accumulation: A potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie 2013, 95, 1971–1979. [Google Scholar] [CrossRef]
- Mandal, R.; Loeffler, A.G.; Salamat, S.; Fritsch, M.K. Organ weight changes associated with body mass index determined from a medical autopsy population. Am. J. Forensic. Med. Pathol. 2012, 33, 382–389. [Google Scholar] [CrossRef]
- Naeye, R.L.; Roode, P. The sizes and numbers of cells in visceral organs in human obesity. Am. J. Clin. Pathol. 1970, 54, 251–253. [Google Scholar] [CrossRef]
- Leibowitz, A.; Rehman, A.; Paradis, P.; Schiffrin, E.L. Role of T regulatory lymphocytes in the pathogenesis of high-fructose diet-induced metabolic syndrome. Hypertens 2013, 61, 1316–1321. [Google Scholar] [CrossRef]
- Tsuboi, N.; Okabayashi, Y.; Shimizu, A.; Yokoo, T. The Renal Pathology of Obesity. Kidney Int. Rep. 2017, 2, 251–260. [Google Scholar] [CrossRef]
- De Vries, A.P.J.; Ruggenenti, P.; Ruan, X.Z.; Praga, M.; Cruzado, J.M.; Bajema, I.M.; D’Agati, V.D.; Lamb, H.J.; Pongrac Barlovic, D.; Hojs, R.; et al. Fatty kidney: Emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014, 2, 417–426. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, J.-E.; Kim, Y.-W.; Kim, J.-Y.; Park, S.-Y. Nutritional regulation of renal lipogenic factor expression in mice: Comparison to regulation in the liver and skeletal muscle. Am. J. Physiol. Renal Physiol. 2017, 313, F887–F898. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Woollard, J.R.; Wang, S.; Korsmo, M.J.; Ebrahimi, B.; Grande, J.P.; Textor, S.C.; Lerman, A.; Lerman, L.O. Increased glomerular filtration rate in early metabolic syndrome is associated with renal adiposity and microvascular proliferation. Am. J. Physiol. Renal Physiol. 2011, 301, F1078–F1087. [Google Scholar] [CrossRef]
- Kume, S.; Uzu, T.; Araki, S.; Sugimoto, T.; Isshiki, K.; Chin-Kanasaki, M.; Sakaguchi, M.; Kubota, N.; Terauchi, Y.; Kadowaki, T.; et al. Role of altered renal lipid metabolism in the development of renal injury induced by a high-fat diet. J. Am. Soc. Nephrol. 2007, 18, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yuan, J.; Norris, K.; Vaziri, N.D. High-calorie diet partially ameliorates dysregulation of intrarenal lipid metabolism in remnant kidney. J. Nutr. Biochem. 2010, 21, 999–1007. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lustig, R.H.; Schmidt, L.A.; Brindis, C.D. Public health: The toxic truth about sugar. Nature 2012, 482, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Björkman, O.; Felig, P. Role of the kidney in the metabolism of fructose in 60-hour fasted humans. Diabetes 1982, 31, 516–520. [Google Scholar] [CrossRef]
- Nakagawa, T.; Johnson, R.J.; Andres-Hernando, A.; Roncal-Jimenez, C.; Sanchez-Lozada, L.G.; Tolan, D.R.; Lanaspa, M.A. Fructose production and metabolism in the kidney. J. Am. Soc. Nephrol. 2020, 31, 898–906. [Google Scholar] [CrossRef]
- Milutinović, D.V.; Brkljačić, J.; Teofilović, A.; Bursać, B.; Nikolić, M.; Gligorovska, L.; Kovačević, S.; Djordjevic, A.; Preitner, F.; Tappy, L.; et al. Chronic Stress Potentiates High Fructose–Induced Lipogenesis in Rat Liver and Kidney. Mol. Nutr. Food Res. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- De Castro, U.G.M.; Dos Santos, R.A.S.; Silva, M.E.; De Lima, W.G.; Campagnole-Santos, M.J.; Alzamora, A.C. Age-dependent effect of high-fructose and high-fat diets on lipid metabolism and lipid accumulation in liver and kidney of rats. Lipids Health Dis. 2013, 12, 136. [Google Scholar] [CrossRef]
- Abdul-Wahed, A.; Guilmeau, S.; Postic, C. Sweet Sixteenth for ChREBP: Established Roles and Future Goals. Cell Metab. 2017, 26, 324–341. [Google Scholar] [CrossRef]
- Herman, M.A.; Peroni, O.D.; Villoria, J.; Schon, M.R.; Abumrad, N.A.; Bluher, M.; Klein, S.; Kahn, B.B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012, 484, 333–338. [Google Scholar] [CrossRef]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Krawczyk, S.A.; Doridot, L.; Fowler, A.J.; Wang, J.X.; Trauger, S.A.; Noh, H.-L.; Kang, H.J.; Meissen, J.K.; Blatnik, M.; et al. ChREBP regulates fructose-induced glucose production independently of insulin signaling. J. Clin. Investig. 2016, 126, 4372–4386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Kumar, A.; Katz, L.S.; Li, L.; Paulynice, M.; Herman, M.A.; Scott, D.K. Induction of the ChREBPβ isoform is essential for glucose-stimulated β-cell proliferation. Diabetes 2015, 64, 4158–4170. [Google Scholar] [CrossRef]
- Mehlem, A.; Hagberg, C.E.; Muhl, L.; Eriksson, U.; Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 2013, 8, 1149–1154. [Google Scholar] [CrossRef]
- Zhang, D.-M.; Jiao, R.-Q.; Kong, L.-D. High Dietary Fructose: Direct or Indirect Dangerous Factors Disturbing Tissue and Organ Functions. Nutrients 2017, 9, 335. [Google Scholar] [CrossRef]
- Fan, C.-Y.; Wang, M.-X.; Ge, C.-X.; Wang, X.; Li, J.-M.; Kong, L.-D. Betaine supplementation protects against high-fructose-induced renal injury in rats. J. Nutr. Biochem. 2014, 25, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.; Jiang, T.; Iwahashi, M.; Wang, Z.; Li, J.; Levi, M. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes 2006, 55, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Herman-Edelstein, M.; Scherzer, P.; Tobar, A.; Levi, M.; Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 2014, 55, 561–572. [Google Scholar] [CrossRef]
- Andres-Hernando, A.; Li, N.; Cicerchi, C.; Inaba, S.; Chen, W.; Roncal-Jimenez, C.; Le, M.T.; Wempe, M.F.; Milagres, T.; Ishimoto, T.; et al. Protective role of fructokinase blockade in the pathogenesis of acute kidney injury in mice. Nat. Commun. 2017, 8, 14181. [Google Scholar] [CrossRef]
- Le, K.A.; Tappy, L. Metabolic Effects of Fructose and the Worldwide Increase in Obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef]
- Park, M.-J.; Kim, D.-I.; Lim, S.-K.; Choi, J.-H.; Han, H.-J.; Yoon, K.-C.; Park, S.-H. High glucose-induced O-GlcNAcylated carbohydrate response element-binding protein (ChREBP) mediates mesangial cell lipogenesis and fibrosis: The possible role in the development of diabetic nephropathy. J. Biol. Chem. 2014, 289, 13519–13530. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Luo, Y.; Myakala, K.; Orlicky, D.J.; Dobrinskikh, E.; Wang, X.; Levi, M. Serelaxin improves cardiac and renal function in DOCA-salt hypertensive rats. Sci. Rep. 2017, 7, 9793. [Google Scholar] [CrossRef] [PubMed]
- Trinh, K.Y.; O’Doherty, R.M.; Anderson, P.; Lange, A.J.; Newgard, C.B. Perturbation of fuel homeostasis caused by overexpression of the glucose-6-phosphatase catalytic subunit in liver of normal rats. J. Biol. Chem. 1998, 273, 31615–31620. [Google Scholar] [CrossRef] [PubMed]
- Koeberl, D.D.; Kishnani, P.S.; Chen, Y.T. Glycogen storage disease types I and II: Treatment updates. J. Inherit Metab. Dis. 2007, 30, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Bali, D.S.; El-Gharbawy, A.; Austin, S.; Pendyal, S.; Kishnani, P.S. Glycogen Storage Disease Type I. In GeneReviews®; 2006 Apr 19 [updated 2021 Oct, 14]; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Mirzaa, G.M., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2021. [Google Scholar]

| Control (TD.170308) (g/Kg) | HFrD (TD.89247) (g/Kg) | |
|---|---|---|
| Casein | 207 | 207 |
| DL-Methionine | 3 | 3 |
| Corn starch | 400.15 | --- |
| Maltodextrin | 200 | --- |
| Fructose | --- | 600 |
| Lard | 50 | 50 |
| Cellulose | 79.81 | 79.81 |
| Mineralmixrogers-harper (170760) | 50 | 50 |
| Zinc carbonate | 0.04 | 0.04 |
| Vitamin mix Teklad (40060) | 10 | 10 |
| % by weight %kcal from | % by weight %kcal from | |
| Protein | 18.3 21.4 | 18.3 20.2 |
| Carbohydrate | 55.4 64.9 | 60.4 66.8 |
| fat | 5.2 13.7 | 5.2 13 |
| Kcal/g | 3.4 | 3.6 |
| Gene Name (GeneID) | Forward | Reverse | Predicted Amplicon Size (bp) |
|---|---|---|---|
| Human | |||
| Rplp0-B (6175) | CCTTCTCCTTTGGGCTGGTCATCCA | CAGACACTGGCAACATTGCGGACAC | 133 |
| ChREBPβ (51085) | AGCGGATTCCAGGTGAGG | TTGTTCAGGCGGATCTTGTC | Primer design in [20] |
| Scd (6319) | GCAAACACCCAGCTGTCAAA | GCCAGGTTTGTAGTACCTCC | 101 |
| elovl6 (79071) | AATGGATGCAGGAAAACTGGAAG | ACTGACTGCTTCAGGCCTTT | 223 |
| Fasn (2194) | CTTCAAGGAGCAAGGCGTGA | ACTGGTACAACGAGCGGATG | 210 |
| Acc (31) | AATCTTGAGGGCTAGGTCTTTT | GTCCAACTTCACCAGGTTGC | 208 |
| Adipophilin (123) | GCTGCAGTCCGTCGATTTCT | CCACACTCGGTTGTGGATCA | 73 |
| Rat | |||
| Rplp0-B (64205) | GAACATCTCCCCCTTCTCCTTC | ATTGCGGACACCCTCTAGGAA | 130 |
| Adipophilin (25629) | GTCCATCTGATTGAATTCGCCA | CTCAGCACAATGGGACTCGT | 135 |
| ChREBPβ (171078) | TCTGCAGATCGCGCGGAG | CTTGTCCCGGCATAGCAAC | Primer design in [23] |
| ChREBPα (171078) | TGCATCGATCACAGGTCATT | AGGCTCAAGCATTCGAAGAG | 163 |
| Srebp1c (78968) | AGTTCCAGCATGGCTACCAC | GGGGTCTCTCAGTTTCCTGC | 166 |
| Scd (246074) | TGCTCTGGGGGATATTTTACTACC | GAGAAGAAAAAGCCACGGCG | 238 |
| elovl6 (171402) | GAGGCGCAGAGAACACGTAG | CGCTTGTTCATCAGATGCCG | 202 |
| Fasn (50671) | AGCCTGAGCTTGTCCCTAGA | CACTGGTACACTTTCCCGCT | 179 |
| Acc (60581) | CTTGGGGTGATGCTCCCATT | GCTGGGCTTAAACCCCTCAT | 116 |
| G6P (25634) | CGTCACCTGTGAGACTGGAC | ACGACATTCAAGCACCGGAA | 144 |
| Pck1 (362282) | GGATGTGGCCAGGATCGAAA | ATACATGGTGCGGCCTTTCA | 172 |
| Pc (25104) | CCAAGCAGGTTGGCTATGAGAA | GATGTTTTCCTGCCGCAGCC | 206 |
| KHK (25659) | ATGGCCATGTTGCCGACTT | TCTGGCAGGTTCGTGTCGTA | 202 |
| Glut5 (65197) | CATGGTCACGGTTTTTGTGG | AGACGATGCTGACATAGGGC | 149 |
| Glut2 (25351) | CGCACGCAACATGTCAGAAG | TTATTACCTCTTGAGGTGCATTGA | 125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bier, A.; Shapira, E.; Khasbab, R.; Sharabi, Y.; Grossman, E.; Leibowitz, A. High-Fructose Diet Increases Renal ChREBPβ Expression, Leading to Intrarenal Fat Accumulation in a Rat Model with Metabolic Syndrome. Biology 2022, 11, 618. https://doi.org/10.3390/biology11040618
Bier A, Shapira E, Khasbab R, Sharabi Y, Grossman E, Leibowitz A. High-Fructose Diet Increases Renal ChREBPβ Expression, Leading to Intrarenal Fat Accumulation in a Rat Model with Metabolic Syndrome. Biology. 2022; 11(4):618. https://doi.org/10.3390/biology11040618
Chicago/Turabian StyleBier, Ariel, Eliyahu Shapira, Rawan Khasbab, Yehonatan Sharabi, Ehud Grossman, and Avshalom Leibowitz. 2022. "High-Fructose Diet Increases Renal ChREBPβ Expression, Leading to Intrarenal Fat Accumulation in a Rat Model with Metabolic Syndrome" Biology 11, no. 4: 618. https://doi.org/10.3390/biology11040618
APA StyleBier, A., Shapira, E., Khasbab, R., Sharabi, Y., Grossman, E., & Leibowitz, A. (2022). High-Fructose Diet Increases Renal ChREBPβ Expression, Leading to Intrarenal Fat Accumulation in a Rat Model with Metabolic Syndrome. Biology, 11(4), 618. https://doi.org/10.3390/biology11040618






