Semen Modulates Cell Proliferation and Differentiation-Related Transcripts in the Pig Peri-Ovulatory Endometrium
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Permit
2.2. Animal Handling and Tissue Collection
2.3. Collection of Semen and Seminal Plasma
2.4. Transcriptome Analysis and Bioinformatics
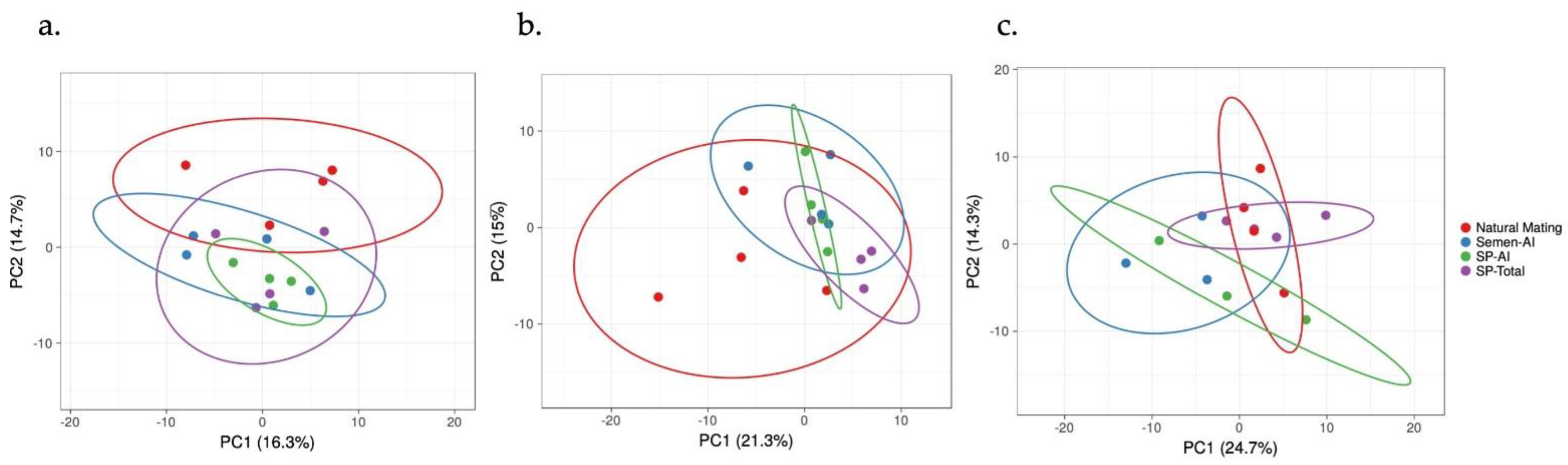
3. Results
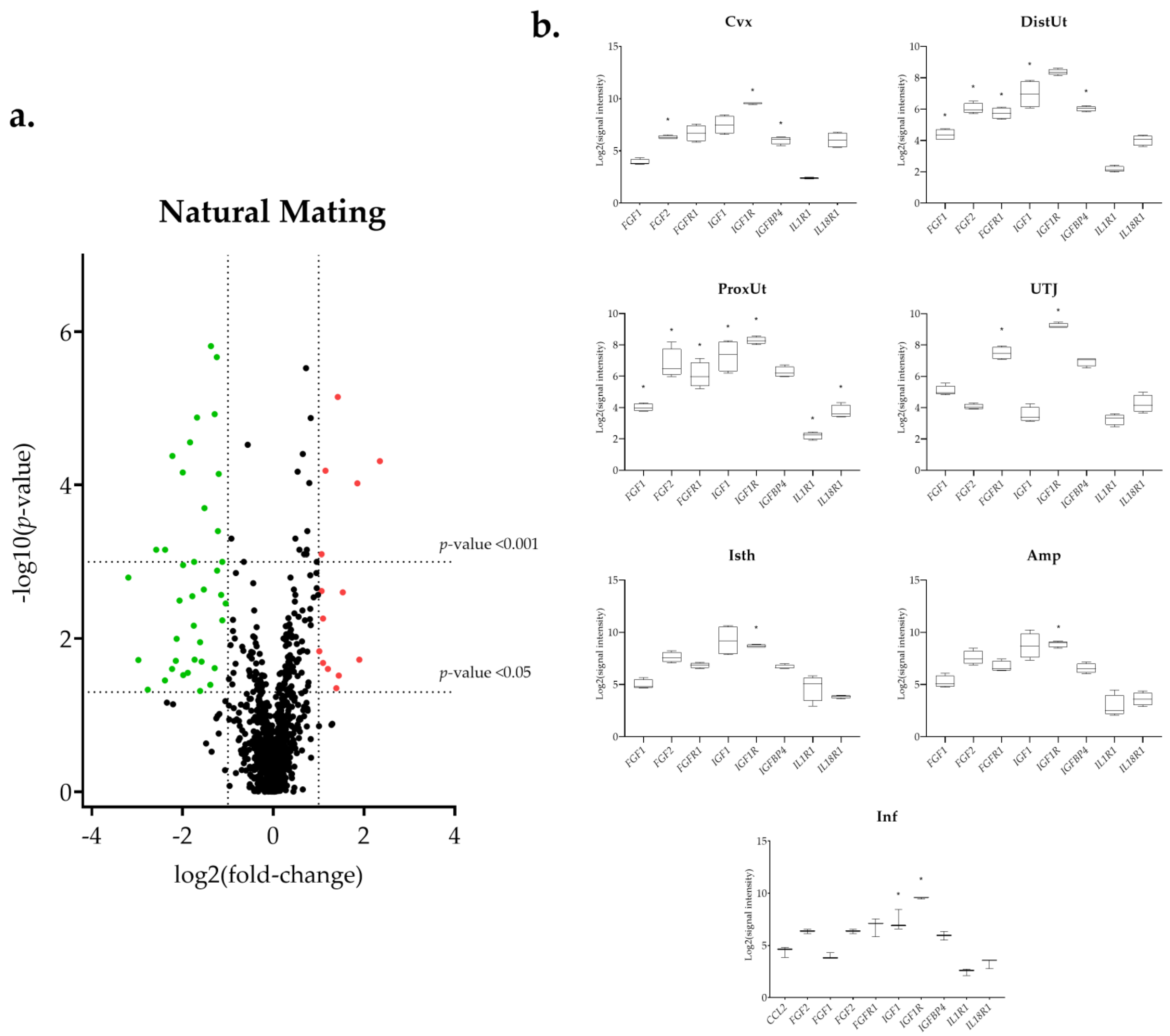
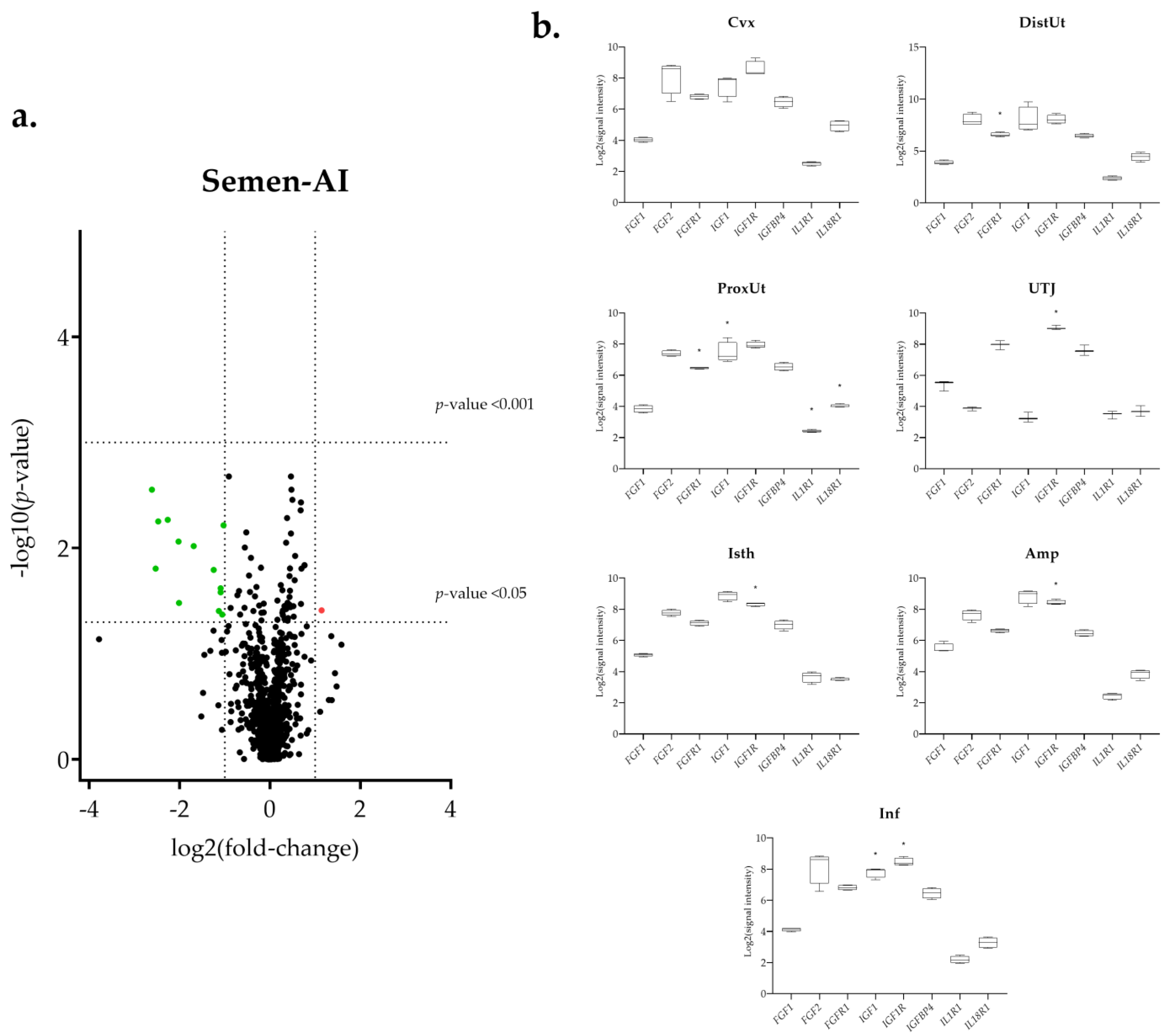
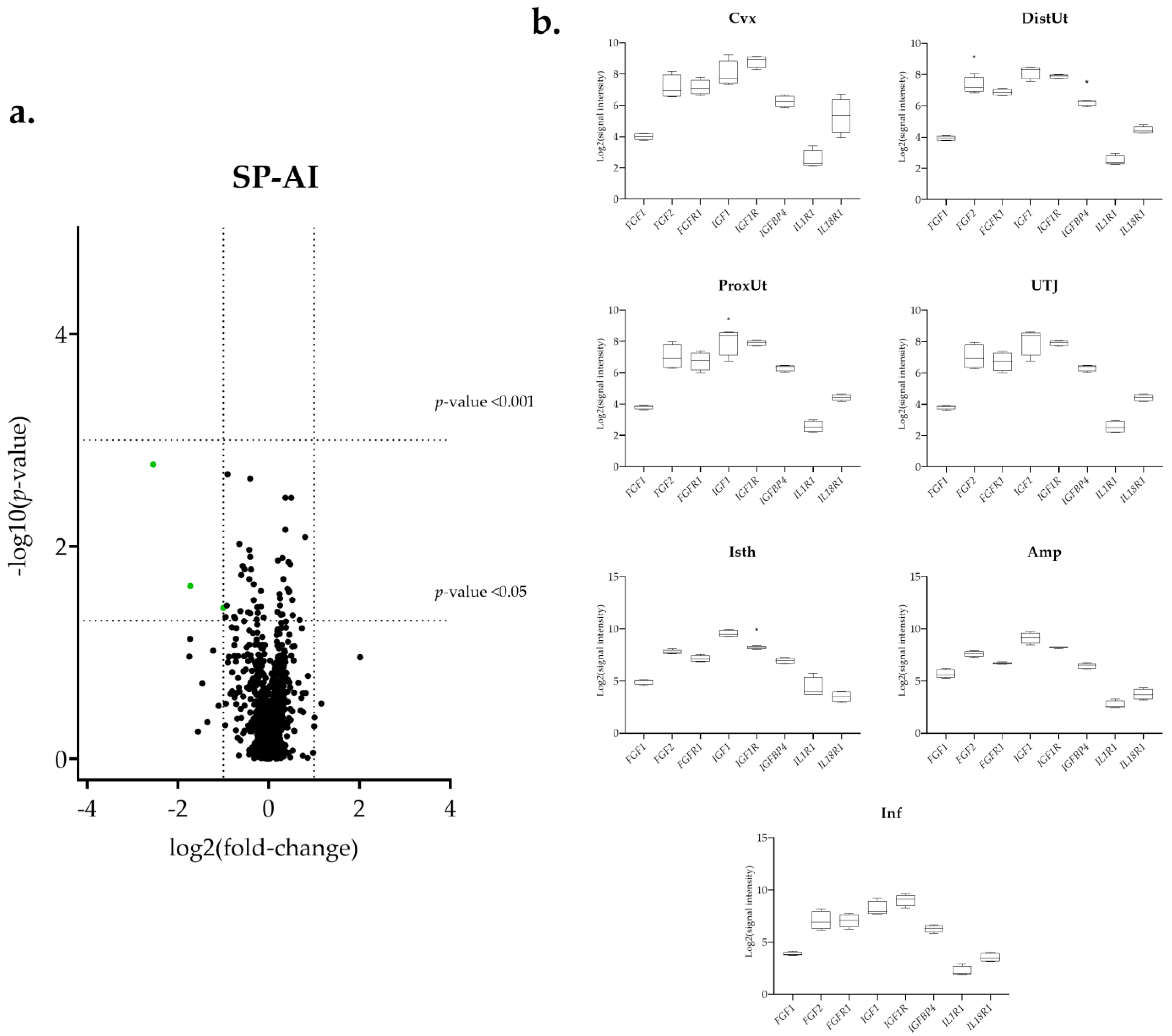
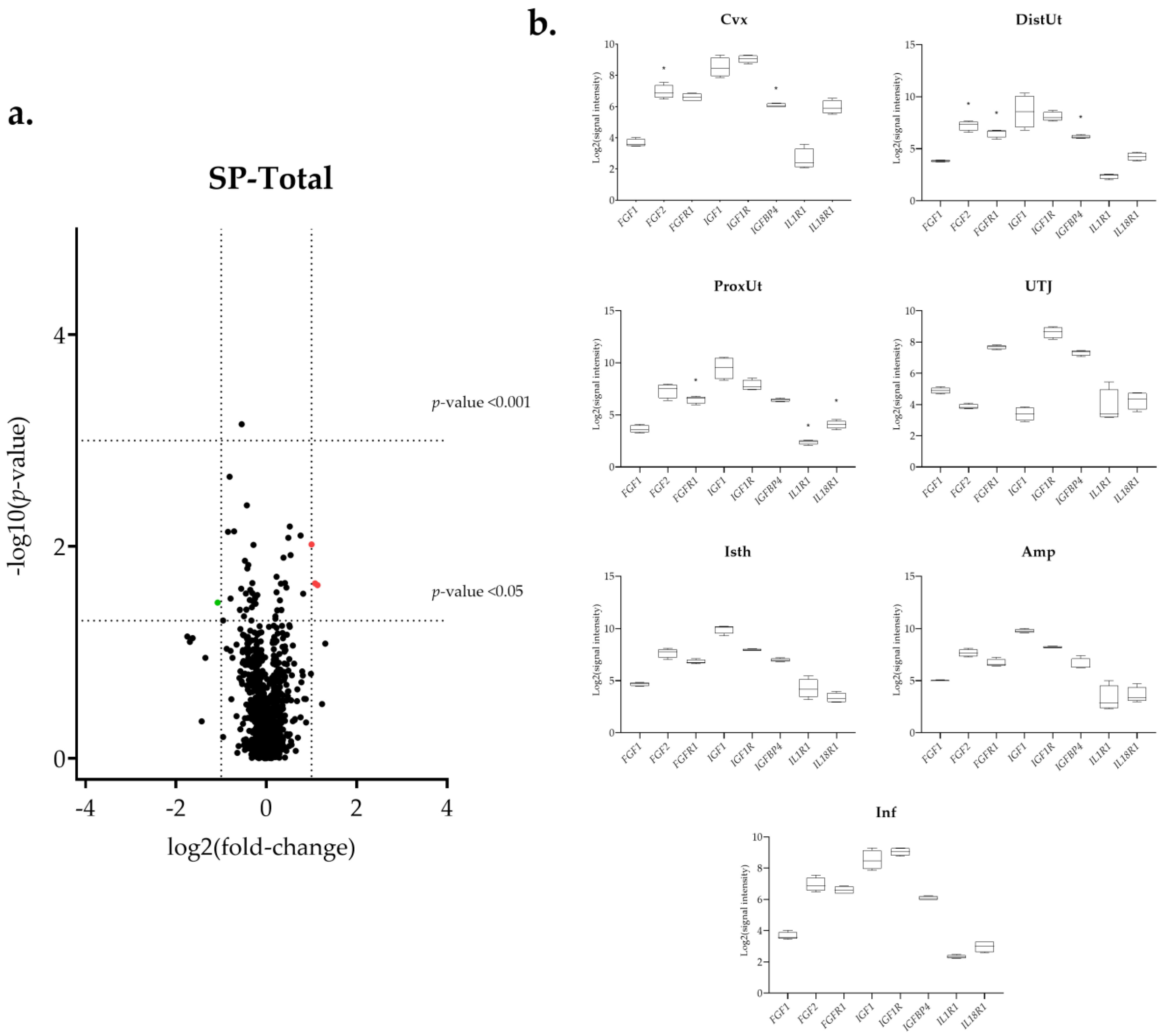
3.1. The IGF1 Transcript Downregulation Was Strongly Marked in Mating Compared to Semen-AI and SP-AI
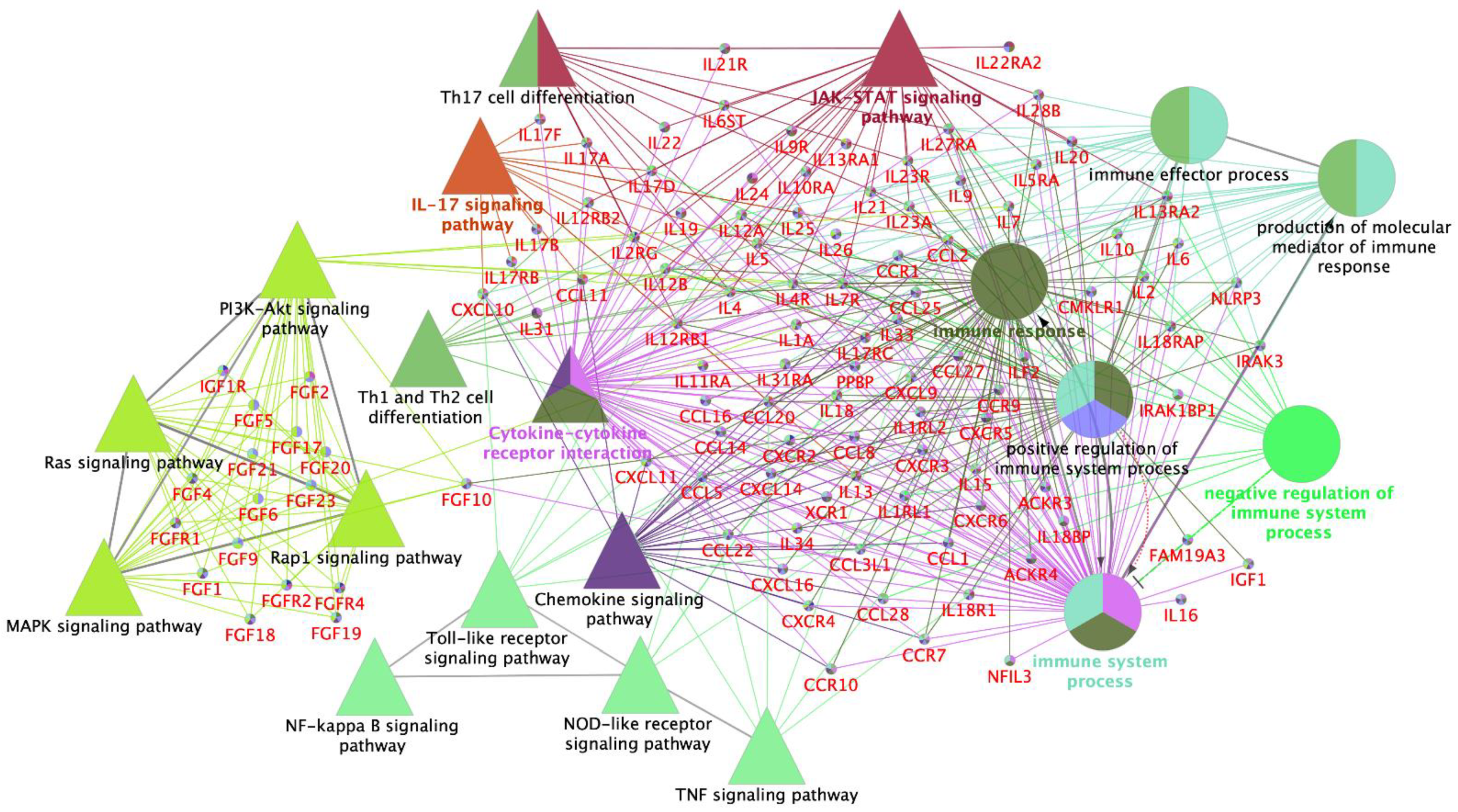
3.2. Interleukin Modulation along the Sow Reproductive Tract in Response to Semen and SP
3.3. Sperm-Free Treatments and Mating Triggered a Common Downregulation of FGF2 Transcript in the Distal Uterus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, W. Mother or nothing: The agony of infertility. Bull. World Health Organ. 2010, 88, 881–882. [Google Scholar] [CrossRef] [PubMed]
- Perleberg, C.; Kind, A.; Schnieke, A. Genetically engineered pigs as models for human disease. DMM Dis. Model. Mech. 2018, 11, dmm030783. [Google Scholar] [CrossRef] [PubMed]
- Mordhorst, B.R.; Prather, R.S. Pig Models of Reproduction. In Animal Models and Human Reproduction; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 213–234. [Google Scholar]
- Abedal-Majed, M.A.; Cupp, A.S. Livestock animals to study infertility in women. Anim. Front. 2019, 9, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Dahm-Kähler, P.; Ghahremani, M.; Lind, A.K.; Sundfeldt, K.; Brännström, M. Monocyte chemotactic protein-1 (MCP-1), its receptor, and macrophages in the perifollicular stroma during the human ovulatory process. Fertil. Steril. 2009, 91, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef]
- Jabbour, H.N.; Sales, K.J.; Catalano, R.D.; Norman, J.E. Inflammatory pathways in female reproductive health and disease. Reproduction 2009, 138, 903–919. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Robertson, S.A. The female response to seminal fluid. Physiol. Rev. 2020. [Google Scholar] [CrossRef]
- Bromfield, J.J. Seminal fluid and reproduction: Much more than previously thought. J. Assist. Reprod. Genet. 2014, 31, 627–636. [Google Scholar] [CrossRef]
- Song, Z.H.; Li, Z.Y.; Li, D.D.; Fang, W.N.; Liu, H.Y.; Yang, D.D.; Meng, C.Y.; Yang, Y.; Peng, J.P. Seminal plasma induces inflammation in the uterus through the γδT/IL-17 pathway. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Ibrahim, L.A.; Rizo, J.A.; Fontes, P.L.P.; Lamb, G.C.; Bromfield, J.J. Seminal plasma modulates expression of endometrial inflammatory meditators in the bovine. Biol. Reprod. 2019, 100, 660–671. [Google Scholar] [CrossRef]
- Fedorka, C.E.; Scoggin, K.E.; Woodward, E.M.; Squires, E.L.; Ball, B.A.; Troedsson, M.H.T. The effect of select seminal plasma proteins on endometrial mRNA cytokine expression in mares susceptible to persistent mating-induced endometritis. Reprod. Domest. Anim. 2017, 52, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodriguez, M.; Atikuzzaman, M.; Venhoranta, H.; Wright, D.; Rodriguez-Martinez, H. Expression of Immune Regulatory Genes in the Porcine Internal Genital Tract Is Differentially Triggered by Spermatozoa and Seminal Plasma. Int. J. Mol. Sci. 2019, 20, 513. [Google Scholar] [CrossRef] [PubMed]
- Gardela, J.; Jauregi-Miguel, A.; Martinez, C.A.; Rodriguez-Martinez, H.; Lopez-Bejar, M.; Alvarez-Rodriguez, M. Semen Modulates Inflammation and Angiogenesis in the Reproductive Tract of Female Rabbits. Animals 2020, 10, 2207. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Nicander, L.; Viring, S.; Einarsson, S.; Larsson, K. Ultrastructure of the Uterotubal Junction in Preovulatory Pigs. Anat. Histol. Embryol. 1990, 19, 16–36. [Google Scholar] [CrossRef]
- Sternberg, A.K.; Buck, V.U.; Classen-Linke, I.; Leube, R.E. How mechanical forces change the human endometrium during the menstrual cycle in preparation for embryo implantation. Cells 2021, 10, 2008. [Google Scholar] [CrossRef] [PubMed]
- Krzymowski, T.; Stefańczyk-Krzymowska, S. The oestrous cycle and early pregnancy—A new concept of local endocrine regulation. Vet. J. 2004, 168, 285–296. [Google Scholar] [CrossRef]
- Bazer, F.W.; Johnson, G.A. Pig blastocyst-uterine interactions. Differentiation 2014, 87, 52–65. [Google Scholar] [CrossRef]
- Jung, W.; Yoo, I.; Han, J.; Kim, M.; Lee, S.; Cheon, Y.; Hong, M.; Jeon, B.Y.; Ka, H. Expression of Caspases in the Pig Endometrium Throughout the Estrous Cycle and at the Maternal-Conceptus Interface During Pregnancy and Regulation by Steroid Hormones and Cytokines. Front. Vet. Sci. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 1–22. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Wang, L.; Hauenstein, A.V. The NLRP3 inflammasome: Mechanism of action, role in disease and therapies. Mol. Aspects Med. 2020, 76, 100889. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- de Rivero Vaccari, J.P. The Inflammasome in Reproductive Biology: A Promising Target for Novel Therapies. Front. Endocrinol. 2020, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.E.H.; Rotwein, P. Growth, differentiation, and survival: Multiple physiological functions for insulin-like growth factors. Physiol. Rev. 1996, 76, 1005–1026. [Google Scholar] [CrossRef]
- Duan, C.; Xu, Q. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen. Comp. Endocrinol. 2005, 142, 44–52. [Google Scholar] [CrossRef]
- Bach, L.A. 40 years of IGF1: IGF-binding proteins. J. Mol. Endocrinol. 2018, 61, T11–T28. [Google Scholar] [CrossRef]
- Watson, A.J.; Westhusin, M.E.; Winger, Q.A. IGF paracrine and autocrine interactions between conceptus and oviduct. J. Reprod. Fertil. Suppl. 1999, 54, 303–315. [Google Scholar] [CrossRef][Green Version]
- Tang, X.-M.; Rossi, M.J.; Masterson, B.J.; Chegini, N. Insulin-Like Growth Factor I (IGF-I), IGF-I Receptors, and IGF Binding Proteins 1–4 in Human Uterine Tissue: Tissue Localization and IGF-I Action in Endometrial Stromal and Myometrial Smooth Muscle Cells in Vitro. Biol. Reprod. 1994, 50, 1113–1125. [Google Scholar] [CrossRef]
- Cerro, J.A.; Pintar, J.E. Insulin-like Growth Factor Binding Protein Gene Expression in the Pregnant Rat Uterus and Placenta. Dev. Biol. 1997, 184, 278–295. [Google Scholar] [CrossRef][Green Version]
- Wathes, D.C.; Reynolds, T.S.; Robinson, R.S.; Stevenson, K.R. Role of the Insulin-Like Growth Factor System in Uterine Function and Placental Development in Ruminants. J. Dairy Sci. 1998, 81, 1778–1789. [Google Scholar] [CrossRef]
- Hofig, A.; Michel, F.; Simmen, F.A.; Simmen, R.C.M. Constitutive expression of uterine receptors for isulin-like growth factor-I during the peri-implantation period in the pig. Biol. Reprod. 1991, 45, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Simmen, F.A.; Simmen, R.C.M.; Geisert, R.D.; Martinat-Botte, F.; Bazer, F.W.; Terqui, M. Differential expression, during the estrous cycle and pre- and postimplantation conceptus development, of messenger ribonucleic acids encoding components of the pig uterine insulin-like growth factor system. Endocrinology 1992, 130, 1547–1556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Persson, E.; Sahlin, L.; Masironi, B.; Dantzer, V.; Eriksson, H.; Rodriguez-Martinez, H. Insulin-like growth factor-I in the porcine endometrium and placenta: Localization and concentration in relation to steroid influence during early pregnancy. Anim. Reprod. Sci. 1997, 46, 261–281. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. Fibroblast growth factors. Genome Biol. 2001, 2, 1–12. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004, 20, 563–569. [Google Scholar] [CrossRef]
- Gardela, J.; Ruiz-Conca, M.; Martinez, C.A.; Wright, D.; López-Béjar, M.; Rodriguez-Martinez, H.; Alvarez-Rodriguez, M. The Expression of Cold-Inducible RNA-Binding Protein mRNA in Sow Genital Tract Is Modulated by Natural Mating, But Not by Seminal Plasma. Int. J. Mol. Sci. 2020, 21, 5333. [Google Scholar] [CrossRef]
- Ruiz-Conca, M.; Gardela, J.; Martínez, C.A.; Wright, D.; López-Bejar, M.; Rodríguez-Martínez, H.; Álvarez-Rodríguez, M. Natural Mating Differentially Triggers Expression of Glucocorticoid Receptor (NR3C1)-Related Genes in the Preovulatory Porcine Female Reproductive Tract. Int. J. Mol. Sci. 2020, 21, 4437. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 2 March 2022).
- Phipson, B.; Lee, S.; Majewski, I.J.; Alexander, W.S.; Smyth, G.K. Robust hyperparameter estimation protects against hypervariable genes and improves power to detect differential expression. Ann. Appl. Stat. 2016, 10, 946–963. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Casagrande, J.T.; Thomas, P.D. Large-scale gene function analysis with the panther classification system. Nat. Protoc. 2013, 8, 1551–1566. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Mantione, K.J.; Kream, R.M.; Kuzelova, H.; Ptacek, R.; Raboch, J.; Samuel, J.M.; Stefano, G.B. Comparing bioinformatic gene expression profiling methods: Microarray and RNA-Seq. Med. Sci. Monit. Basic Res. 2014, 20, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.L.; Watkins, A.J. The influence of seminal plasma on offspring development and health. Semin. Cell Dev. Biol. 2020, 97, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Salamonsen, L.A. Role of chemokines in the endometrium and in embryo implantation. Curr. Opin. Obstet. Gynecol. 2007, 19, 266–272. [Google Scholar] [CrossRef]
- Du, M.R.; Wang, S.C.; Li, D.J. The integrative roles of chemokines at the maternal-fetal interface in early pregnancy. Cell. Mol. Immunol. 2014, 11, 438–448. [Google Scholar] [CrossRef]
- Choi, Y.; Seo, H.; Han, J.; Yoo, I.; Kim, J.; Ka, H. Chemokine (C-C Motif) ligand 28 and its receptor CCR10: Expression and function at the maternal-conceptus interface in pigs. Biol. Reprod. 2016, 95, 1–10. [Google Scholar] [CrossRef]
- Nibbs, R.J.B.; Graham, G.J. Immune regulation by atypical chemokine receptors. Nat. Rev. Immunol. 2013, 13, 815–829. [Google Scholar] [CrossRef]
- Bonecchi, R.; Graham, G.J. Atypical chemokine receptors and their roles in the resolution of the inflammatory response. Front. Immunol. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Han, J.; Yoo, I.; Lee, S.; Jung, W.; Kim, H.J.; Hyun, S.-H.; Lee, E.; Ka, H. Atypical chemokine receptors 1, 2, 3 and 4: Expression and regulation in the endometrium during the estrous cycle and pregnancy and with somatic cell nucleus transfer–cloned embryos in pigs. Theriogenology 2019, 129, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.M.; Linton, N.F.; Van Den Heuvel, M.J.; Cnossen, S.A.; Edwards, A.K.; Croy, B.A.; Tayade, C. Expression of chemokine decoy receptors and their ligands at the porcine maternal-fetal interface. Immunol. Cell Biol. 2011, 89, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Bae, H.; Bazer, F.W.; Song, G. Cell-Specific Expression and Signal Transduction of C-C Motif Chemokine Ligand 2 and Atypical Chemokine Receptors in the Porcine Endometrium during Early Pregnancy; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 81, ISBN 8223290499. [Google Scholar]
- Infantino, S.; Moepps, B.; Thelen, M. Expression and Regulation of the Orphan Receptor RDC1 and Its Putative Ligand in Human Dendritic and B Cells. J. Immunol. 2006, 176, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E.; Mezzapelle, R. The Chemokine Receptor CXCR4 in Cell Proliferation and Tissue Regeneration. Front. Immunol. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.E.; Mackie, D.I.; Caron, K.M. Emerging roles of atypical chemokine receptor 3 (ACKR3) in normal development and physiology. Cytokine 2018, 109, 17–23. [Google Scholar] [CrossRef]
- Waszkiewicz, E.M.; Kozlowska, W.; Zmijewska, A.; Franczak, A. Expression of insulin-like growth factor 1 (IGF-1) and epidermal growth factor (EGF) receptors and the effect of IGF-1 and EGF on androgen and estrogen release in the myometrium of pigs—In vitro study. Animals 2020, 10, 915. [Google Scholar] [CrossRef]
- Sahlin, L.; Rodriguez-Martinez, H.; Stanchev, P.; Dalin, A.; Norstedt, G.; Eriksson, H. Regulation of the Uterine Expression of Messenger Ribonucleic Acids Encoding the Oestrogen Receptor and IGF–I Peptides in the Pig Uterus. J. Vet. Med. Ser. A 1990, 37, 795–800. [Google Scholar] [CrossRef]
- Simmen, R.C.M.; Simmen, F.A.; Hofig, A.; Farmer, S.J.; Bazer, F.W. Hormonal regulation of insulin-like growth factor gene expression in pig uterus. Endocrinology 1990, 127, 2166–2174. [Google Scholar] [CrossRef]
- Wiseman, D.L.; Henricks, D.M.; Eberhardt, D.M.; Bridges, W.C. Identification and Content of Insulin-like Growth Factors in Porcine Oviductal Fluid. Biol. Reprod. 1992, 47, 126–132. [Google Scholar] [CrossRef][Green Version]
- Novak, S.; Treacy, B.K.; Almeida, F.R.C.L.; Mao, J.; Buhi, W.C.; Dixon, W.T.; Foxcroft, G.R. Regulation of IGF-I and porcine oviductal secretory protein (pOSP) secretion into the pig oviduct in the peri-ovulatory period, and effects of previous nutrition. Reprod. Nutr. Dev. 2002, 42, 355–372. [Google Scholar] [CrossRef]
- Wollenhaupt, K.; Welter, H.; Einspanier, R.; Manabe, N.; Brüssow, K.P. Expression of epidermal growth factor receptor (EGF-R), vascular endothelial growth factor receptor (VEGF-R) and fibroblast growth factor receptor (FGF-R) systems in porcine oviduct and endometrium during the time of implantation. J. Reprod. Dev. 2004, 50, 269–278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katsahambas, S.; Hearn, M.T.W. Localization of basic fibroblast growth factor mRNA (FGF-2 mRNA) in the uterus of mated and unmated gilts. J. Histochem. Cytochem. 1996, 44, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Welter, H.; Wollenhaupt, K.; Einspanier, R. Developmental and hormonal regulated gene expression of fibroblast growth factor 2 (FGF-2) and its receptors in porcine endometrium. J. Steroid Biochem. Mol. Biol. 2004, 88, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Okumu, L.A.; Forde, N.; Mamo, S.; McGettigan, P.; Mehta, J.P.; Roche, J.F.; Lonergan, P. Temporal regulation of fibroblast growth factors and their receptors in the endometrium and conceptus during the pre-implantation period of pregnancy in cattle. Reproduction 2014, 147, 825–834. [Google Scholar] [CrossRef]
- Krawczynski, K.; Kaczmarek, M.M. Does seminal plasma affect angiogenesis in the porcine oviduct? Reprod. Biol. 2012, 12, 347–354. [Google Scholar] [CrossRef]
- ZhuGe, D.-L.; Javaid, H.M.A.; Sahar, N.E.; Zhao, Y.-Z.; Huh, J.Y. Fibroblast growth factor 2 exacerbates inflammation in adipocytes through NLRP3 inflammasome activation. Arch. Pharm. Res. 2020, 43, 1311–1324. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Muñoz-Planillo, R.; Nuñez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef]
- Gardela, J.; Ruiz-Conca, M.; Martinez, C.A.; Wright, D.; López-Béjar, M.; Rodriguez-Martinez, H.; Alvarez-Rodriguez, M. Natural mating represses pro-inflammatory responses in the pre-ovulatory porcine endometrium and endosalpinx (ampulla) by down-regulation of caspase-1 (CASP1) and caspase-12 (CASP12). Anim. Reprod. 2020, 17, 15. [Google Scholar]
- Suarez, S.S. Regulation of sperm storage and movement in the mammalian oviduct. Int. J. Dev. Biol. 2008, 52, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, H.; Saravia, F.; Wallgren, M.; Tienthai, P.; Johannisson, A.; Vázquez, J.M.; Martínez, E.; Roca, J.; Sanz, L.; Calvete, J.J. Boar spermatozoa in the oviduct. Theriogenology 2005, 63, 514–535. [Google Scholar] [CrossRef] [PubMed]
- Katila, T. Post-mating Inflammatory Responses of the Uterus. Reprod. Domest. Anim. 2012, 47, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-Y.; Li, X.-L. Pyroptosis and inflammasomes in obstetrical and gynecological diseases. Gynecol. Endocrinol. 2021, 37, 385–391. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardela, J.; Ruiz-Conca, M.; Wright, D.; López-Béjar, M.; Martínez, C.A.; Rodríguez-Martínez, H.; Álvarez-Rodríguez, M. Semen Modulates Cell Proliferation and Differentiation-Related Transcripts in the Pig Peri-Ovulatory Endometrium. Biology 2022, 11, 616. https://doi.org/10.3390/biology11040616
Gardela J, Ruiz-Conca M, Wright D, López-Béjar M, Martínez CA, Rodríguez-Martínez H, Álvarez-Rodríguez M. Semen Modulates Cell Proliferation and Differentiation-Related Transcripts in the Pig Peri-Ovulatory Endometrium. Biology. 2022; 11(4):616. https://doi.org/10.3390/biology11040616
Chicago/Turabian StyleGardela, Jaume, Mateo Ruiz-Conca, Dominic Wright, Manel López-Béjar, Cristina A. Martínez, Heriberto Rodríguez-Martínez, and Manuel Álvarez-Rodríguez. 2022. "Semen Modulates Cell Proliferation and Differentiation-Related Transcripts in the Pig Peri-Ovulatory Endometrium" Biology 11, no. 4: 616. https://doi.org/10.3390/biology11040616
APA StyleGardela, J., Ruiz-Conca, M., Wright, D., López-Béjar, M., Martínez, C. A., Rodríguez-Martínez, H., & Álvarez-Rodríguez, M. (2022). Semen Modulates Cell Proliferation and Differentiation-Related Transcripts in the Pig Peri-Ovulatory Endometrium. Biology, 11(4), 616. https://doi.org/10.3390/biology11040616










