Aerobic Dance on an Air Dissipation Platform Improves Cardiorespiratory, Muscular and Cellular Fitness in the Overweight and Obese Elderly
Abstract
:Simple Summary
Abstract
1. Introduction
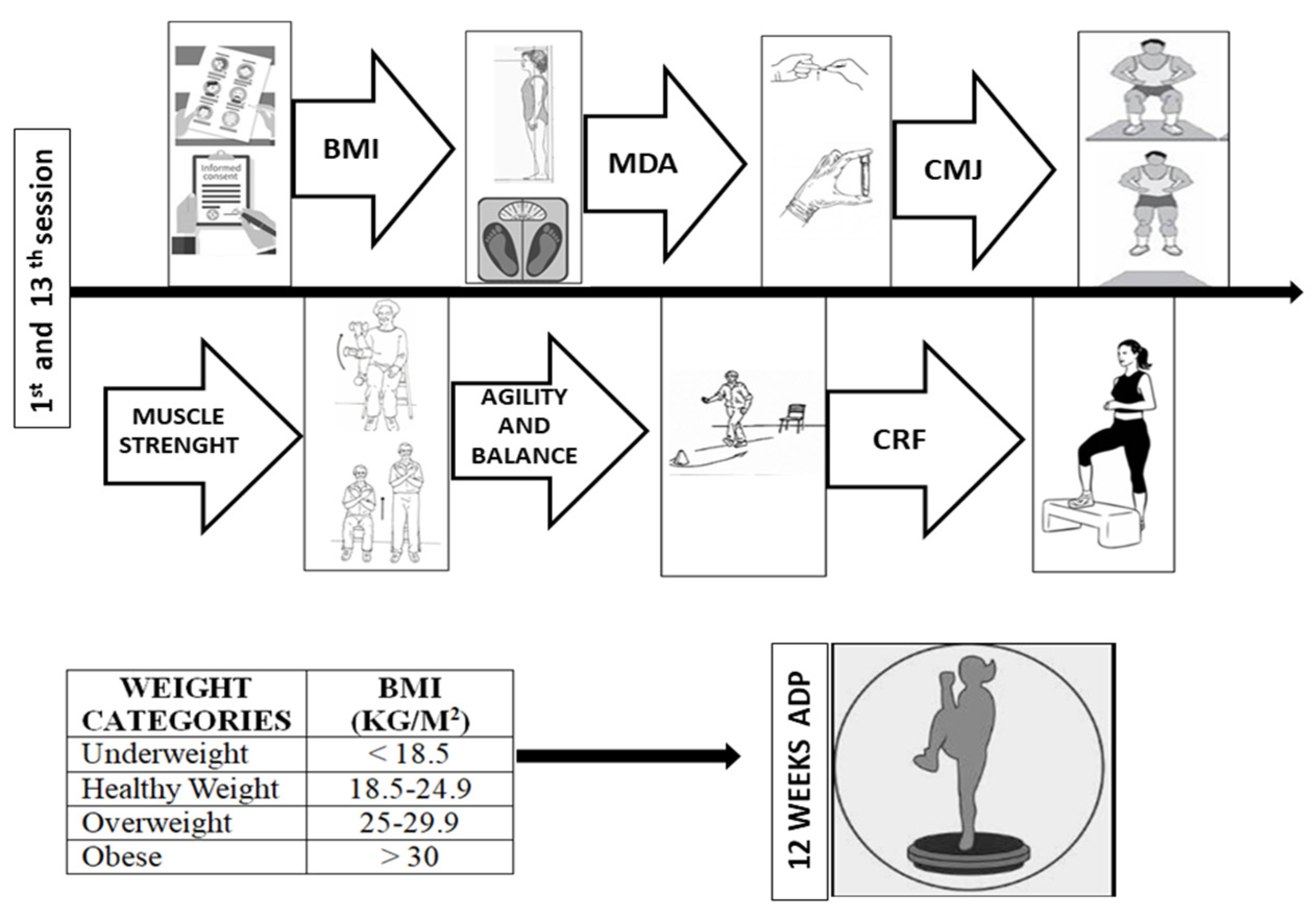
2. Materials and Methods
2.1. Subsection
2.2. Participants
2.3. Body Composition
2.4. Oxidative Stress
2.5. Muscular Fitness
2.5.1. Countermovement Jump
2.5.2. Arm Curl Test
2.5.3. Agility and Dynamic Balance
2.6. Cardiorespiratory Fitness
2.7. Exercise Program
2.8. Statistical Analysis
3. Results
3.1. Body Composition
3.2. Oxidative Stress
3.3. Cardiorespiratory Fitness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Graubard, B.I.; Williamson, D.F.; Gail, M.H. Excess deaths associated with underweight, overweight, and obesity. J. Am. Med. Assoc. 2005, 293, 1861–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Jura, M.; Kozak, L.P. Obesity and related consequences to ageing. Age 2016, 38, 23. [Google Scholar] [CrossRef] [Green Version]
- Freedman, D.M.; Ron, E.; Ballard-Barbash, R.; Linet, M.S. Body mass index and all-cause mortality in a nationwide US cohort. Int. J. Obes. 2006, 30, 822–829. [Google Scholar] [CrossRef] [Green Version]
- Adams, K.F.; Schatzkin, A.; Harris, T.B.; Kipnis, V.; Mouw, T.; Ballard-Barbash, R.; Hollenbeck, A.; Leitzmann, M.F. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N. Engl. J. Med. 2006, 355, 763–778. [Google Scholar] [CrossRef]
- Sánchez-Flores, M.; Marcos-Pérez, D.; Costa, S.; Teixeira, J.P.; Bonassi, S.; Pásaro, E.; Laffon, B.; Valdiglesias, V. Oxidative stress, genomic features and DNA repair in frail elderly: A systematic review. Ageing Res. Rev. 2017, 37, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-induced oxidative stress: Past, present and future. J. Physiol. 2016, 594, 5081–5092. [Google Scholar] [CrossRef] [Green Version]
- Mergener, M.; Martins, M.R.; Antunes, M.V.; da Silva, C.C.; Lazzaretti, C.; Fontanive, T.O.; Suyenaga, E.S.; Ardenghi, P.G.; Maluf, S.W.; Gamaro, D. Oxidative stress and DNA damage in older adults that do exercises regularly. Clin. Biochem. 2009, 42, 1648–1653. [Google Scholar] [CrossRef]
- Stocker, R.; Keaney, J.F. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004, 84, 1381–1478. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Berggren, J.R.; Boyle, K.E.; Chapman, W.H.; Houmard, J.A. Skeletal muscle lipid oxidation and obesity: Influence of weight loss and exercise. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E726–E732. [Google Scholar] [CrossRef] [PubMed]
- Mutlu-Türkoǧlu, Ü.; Öztezcan, S.; Telci, A.; Orhan, Y.; Ayka-Toker, G.; Sivas, A.; Uysal, M. An increase in lipoprotein oxidation and endogenous lipid peroxides in serum of obese women. Clin. Exp. Med. 2003, 2, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Olusi, S.O. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int. J. Obes. 2002, 26, 1159–1164. [Google Scholar] [CrossRef] [Green Version]
- Van Gaal, L.F.; Vertommen, J.; De Leeuw, I.H. The in vitro oxidizability of lipoprotein particles in obese and non-obese subjects. Atherosclerosis 1998, 137, S39–S44. [Google Scholar] [CrossRef]
- Bellanti, F.; Romano, A.D.; Lo Buglio, A.; Castriotta, V.; Guglielmi, G.; Greco, A.; Serviddio, G.; Vendemiale, G. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas 2018, 109, 6–12. [Google Scholar] [CrossRef]
- Mezzetti, A.; Zuliani, G.; Romano, F.; Costantini, F.; Pierdomenico, S.D.; Cuccurullo, F.; Fellin, R. Vitamin E and Lipid Peroxide Plasma Levels Predict the Risk of Cardiovascular Events in a Group of Healthy Very Old People. J. Am. Geriatr. Soc. 2001, 49, 533–537. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults. Med. Sci. Sport Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Posch, M.; Schranz, A.; Lener, M.; Tecklenburg, K.; Burtscher, M.; Ruedl, G.; Niedermeier, M.; Wlaschek, W. Effectiveness of a mini-trampoline training program on balance and functional mobility, gait performance, strength, fear of falling and bone mineral density in older women with osteopenia. Clin. Interv. Aging 2019, 14, 2281–2293. [Google Scholar] [CrossRef] [Green Version]
- Mathus-Vliegen, E.M. Obesity Management Task Force of the European Association for the Study of Obesity. Prevalence, pathophysiology, health consequences and treatment options of obesity in the elderly: A guideline. Obes. Facts 2012, 5, 460–483. [Google Scholar] [CrossRef]
- Fan, J.X.; Kowaleski-Jones, L.; Wen, M. Walking or dancing: Patterns of physical activity by cross-sectional age among U.S. women. J. Aging Health 2013, 25, 1182–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, D.R.; Murrah, B.; Hoeger, W.W.; Rhodes, R.C. Effect of low-impact aerobic dance on the functional fitness of elderly women. Gerontologist 1990, 30, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.W.L.; Kilding, A.; Pidgeon, P.; Ashley, L.; Gillis, D. Physical benefits of dancing for healthy older adults: A review. J. Aging Phys. Act. 2009, 17, 479–500. [Google Scholar] [CrossRef] [Green Version]
- Holmerová, I.; Machácová, K.; Vanková, H.; Veleta, P.; Jurasková, B.; Hrnciariková, D.; Volicer, L.; Andel, R. Effect of the Exercise Dance for Seniors (EXDASE) program on lower-body functioning among institutionalized older adults. J. Aging Health 2010, 22, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, R.; Chang, M.; Yabushita, N.; Sakai, T.; Nakagaichi, M.; Nho, H.; Tanaka, K. Dance-based aerobic exercise may improve indices of falling risk in older women. Age Ageing 2002, 31, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Said, M.; Lamya, N.; Olfa, N.; Hamda, M. Effects of high-impact aerobics vs. low-impact aerobics and strength training in overweight and obese women. J. Sports Med. Phys. Fit. 2017, 57, 278–288. [Google Scholar] [CrossRef]
- Leelarungrayub, D.; Saidee, K.; Pothongsunun, P.; Pratanaphon, S.; Yankai, A.; Bloomer, R.J. Six weeks of aerobic dance exercise improves blood oxidative stress status and increases interleukin-2 in previously sedentary women. J. Bodyw. Mov. Ther. 2011, 15, 355–362. [Google Scholar] [CrossRef]
- Kimura, K.; Hozumi, N. Investigating the acute effect of an aerobic dance exercise program on neuro-cognitive function in the elderly. Psychol. Sport Exerc. 2012, 13, 623–629. [Google Scholar] [CrossRef]
- Eckardt, N. Lower-extremity resistance training on unstable surfaces improves proxies of muscle strength, power and balance in healthy older adults: A randomised control trial. BMC Geriatr. 2016, 16, 191. [Google Scholar] [CrossRef] [Green Version]
- Amat, A.M.; Contreras, F.H.; Vega, R.L.; Martínez, I.C.; Alvarez, P.J.; López, E.M. Effects of 12-week proprioception training program on postural stability, gait, and balance in older adults. J. Strength Cond. Res. 2013, 27, 2180–2188. [Google Scholar] [CrossRef]
- Sukkeaw, W.; Kritpet, T.; Bunyaratavej, N. A Comparison between the effects of aerobic dance training on mini-trampoline and hard wooden surface on bone resorption, health-related physical fitness, balance, and foot plantar pressure in Thai working women. J. Med. Assoc. Thail. 2015, 98, S58–S64. [Google Scholar]
- Cugusi, L.; Manca, A.; Serpe, R.; Romita, G.; Bergamin, M.; Cadeddu, C.; Solla, P.; Mercuro, G. Effects of a mini-trampoline rebounding exercise program on functional parameters, body composition and quality of life in overweight women. J. Sports Med. Phys. Fit. 2018, 58, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Maté-Muñoz, J.L.; Moreira-Reis, A.; de Subijana, C.L.; Rodríguez-Rodríguez, B.; Sacristán-Rubio, A.; Ruiz-Solano, P.; Garnacho-Castaño, M.V. Respuestas cardiorrespiratorias y metabólicas al ejercicio realizado sobre una plataforma de disipación de aire. Apunt Med. Esport 2014, 48, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, G.A.A.; Rodrigues, P.C.; da Silva, F.F.; Nakamura, P.M.; Higino, W.P.; de Souza, R.A. Mini-trampoline enhances cardiovascular responses during a stationary running exergame in adults. Biol. Sport 2018, 35, 335–342. [Google Scholar] [CrossRef]
- Moreira-Reis, A.; Maté-Muñoz, J.L.; Lougedo, J.H.; García-Fernández, P.; Pleguezuelos-Cobo, E.; Carbonell, T.; Alva, N.; Garnacho-Castaño, M.V. Cardiorespiratory, metabolic and muscular responses during a video-recorded aerobic dance session on an air dissipation platform. Int. J. Environ. Res. Public Health 2020, 17, 9511. [Google Scholar] [CrossRef]
- Castañeda, F.J.R.; Aznar, C.T.A.; Baquero, C.M. Medición de la actividad física en personas mayores de 65 años mediantes el IPAQ-E: Validez de contenido, fiabilidad y factores asociados. Rev. Esp. Salud Pública 2017, 91, e1–e12. [Google Scholar]
- Weir, C.B.; Jan, A. BMI classification percentile and cut off points. In StatPearls Publishing; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bedogni, G.; Malavolti, M.; Severi, S.; Poli, M.; Mussi, C.; Fantuzzi, A.L.; Battistini, N. Accuracy of an eight-point tactile-electrode impedance method in the assessment of total body water. Eur. J. Clin. Nutr. 2002, 56, 1143–1148. [Google Scholar] [CrossRef] [Green Version]
- Yagi, K. Assay for blood plasma or serum. Methods Enzymol. 1984, 105, 328–331. [Google Scholar] [PubMed]
- Cabral, R.M.C.; Silva, I.O.; Medeiros, A.R.; Claudino, J.G.; Reyes, P.J.; Boullosa, D.A. The validity and reliability of the “my Jump App” for measuring jump height of the elderly. PeerJ 2018, 6, e5804. [Google Scholar] [CrossRef] [Green Version]
- Pedrero-Chamizo, R.; Albers, U.; Palacios, G.; Pietrzik, K.; Meléndez, A.; González-Gross, M. Health Risk, Functional Markers and Cognitive Status in Institutionalized Older Adults: A Longitudinal Study. Int. J. Environ. Res. Public Health 2020, 17, 7303. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist 2013, 53, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Golding, L. YMCA Fitness Testing and Assessment Manual, 4th ed.; Human Kinetics: Champaign, IL, USA, 2000; pp. 155–157. [Google Scholar]
- Beutner, F.; Ubrich, R.; Zachariae, S.; Engel, C.; Sandri, M.; Teren, A.; Gielen, S. Validation of a brief step-test protocol for estimation of peak oxygen uptake. Eur. J. Prev. Cardiol. 2015, 22, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Perceived exertion: A note on “history” and methods. Med. Sci. Sports 1973, 5, 90–93. [Google Scholar] [CrossRef]
- Garnacho-Castaño, M.V.; Domínguez, R.; Muñoz-González, A.; Ruano, R.F.; Serra-Payá, N.S.; Maté-Muñoz, J.L. Exercise Prescription Using the Borg Rating of Perceived Exertion to Improve Fitness. Int. J. Sports Med. 2018, 39, 115–123. [Google Scholar] [CrossRef]
- Colleluori, G.; Aguirre, L.; Phadnis, U.; Fowler, K.; Armamento-Villareal, R.; Sun, Z.; Brunetti, L.; Park, J.H.; Kaipparettu, B.A.; Putluri, N.; et al. Aerobic plus resistance exercise in obese older adults improves muscle protein synthesis and preserves myocellular quality despite weight loss. Cell Metab. 2019, 30, 261–273.e6. [Google Scholar] [CrossRef]
- Beavers, K.M.; Ambrosius, W.T.; Rejeski, W.J.; Burdette, J.H.; Walkup, M.P.; Sheedy, J.L.; Nesbit, B.A.; Gaukstern, J.E.; Nicklas, B.J.; Marsh, A.P. Effect of exercise type during intentional weight loss on body composition in older adults with obesity. Obesity 2017, 25, 1823–1829. [Google Scholar] [CrossRef]
- Morgan, P.T.; Smeuninx, B.; Breen, L. Exploring the impact of obesity on skeletal muscle function in older age. Front. Nutr. 2020, 7, 569904. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, Q.; Xia, W.; Zhang, L.; Hu, Z.; Wu, X.; Jia, X. Association between physical exercise and biomarkers of oxidative stress among middle-aged and elderly community residents with essential hypertension in China. Biomed Res. Int. 2018, 2018, 4135104. [Google Scholar] [CrossRef]
- Campbell, P.T.; Gross, M.D.; Potter, J.D.; Schmitz, K.H.; Duggan, C.; McTiernan, A.; Ulrich, C.M. Effect of exercise on oxidative stress: A 12-month randomized, controlled trial. Med. Sci. Sports Exerc. 2010, 42, 1448–1453. [Google Scholar] [CrossRef] [Green Version]
- Distefano, G.; Standley, R.A.; Zhang, X.; Carnero, E.A.; Yi, F.; Cornnell, H.H.; Coen, P.M. Physical activity unveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. J. Cachexia Sarcopenia Muscle 2018, 9, 279–294. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Ferrel, R.E.; Park, J.J.; Hagberg, J.M.; Phares, D.A.; Jones, J.M.; Brown, M.D. NADPH oxidase p22phox gene variants are associated with systemic oxidative stress biomarker responses to exercise training. J. Appl. Physiol. 2005, 99, 1905–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, H.K.; Bourguignon, C.; Vincent, K.R. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity 2006, 14, 1921–1930. [Google Scholar] [CrossRef]
- Vicent, H.K.; Vincent, K.R.; Bourguignon, C.; Braith, R.W. Obesity and postexercise oxidative stress in older women. Med. Sci. Sport Exerc. 2005, 37, 213–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, H.K.; Bourguignon, C.; Vincent, K.R.; Weltman, A.L.; Bryant, M.; Taylor, A.G. Antioxidant supplementation lowers exercise-induced oxidative stress in young overweight adults. Obesity 2006, 14, 2224–2235. [Google Scholar] [CrossRef] [Green Version]
- Saiki, S.; Sato, T.; Kohzuki, M.; Kamimoto, M.; Yosida, T. Changes in serum hypoxanthine levels by exercise in obese subjects. Metabolism 2001, 50, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, Y.B.; Vélez, R.R.; Amat, A.M.; González, E.V. Effect of two choreographed fitness group-workouts on the body composition, cardiovascular and metabolic health of sedentary female workers. Int. J. Environ. Res. Public Health 2019, 16, 4986. [Google Scholar] [CrossRef] [Green Version]
- Kokkinos, P.; Manolis, A.; Pittaras, A.; Doumas, M.; Giannelou, A.; Panagiotakos, D.B.; Faselis, C.; Narayan, P.; Singh, S.; Myers, J. Exercise capacity and mortality in hypertensive men with and without additional risk factors. Hypertension 2009, 53, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef] [Green Version]
- van Schoor, N.M.; van der Veen, A.J.; Schaap, L.A.; Smit, T.H.; Lips, P. Biomechanical comparison of hard and soft hip protectors, and the influence of soft tissue. Bone 2006, 39, 401–407. [Google Scholar] [CrossRef]
- Ignasiak, Z.; Sebastjan, A.; Kaczorowska, A.; Skrzek, A. Estimation of the risk of the frailty syndrome in the independent-living population of older people. Aging Clin. Exp. Res. 2020, 32, 2233–2240. [Google Scholar] [CrossRef]
- Moran, J.; Ramírez-Campillo, R.; Granacher, U. Effects of jumping exercise on muscular power in older adults: A meta-analysis. Sport Med. 2018, 48, 2843–2857. [Google Scholar] [CrossRef] [PubMed]
- Laurin, J.L.; Reid, J.J.; Lawrence, M.M.; Miller, B.F. Long-term aerobic exercise preserves muscle mass and function with age. Curr. Opin. Physiol. 2019, 10, 70–74. [Google Scholar] [CrossRef]
- Mandsager, K.; Harb, S.; Cremer, P.; Phelan, D.; Nissen, S.E.; Jaber, W. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA 2018, 1, e183605. [Google Scholar] [CrossRef] [PubMed]
- Melton, L.J., 3rd; Khosla, S.; Crowson, C.S.; O’Connor, M.K.; O’Fallon, W.M.; Riggs, B.L. Epidemiology of sarcopenia. J. Am. Geriatr. Soc. 2000, 48, 625–630. [Google Scholar] [CrossRef]
- Holloszy, J.O. The biology of aging. Mayo. Clin. Proc. 2000, 75, S3–S9. [Google Scholar] [CrossRef]
- Curcio, F.; Ferro, G.; Basile, C.; Liguori, I.; Parrella, P.; Pirozzi, F.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Tocchetti, C.G.; et al. Biomarkers in sarcopenia: A multi-factorial approach. Exp. Gerontol. 2016, 85, 1–8. [Google Scholar] [CrossRef]
- Clausen, J.S.R.; Marott, J.L.; Holtermann, A.; Gyntelberg, F.; Jensen, M.T. Midlife Cardiorespiratory fitness and the long-term risk of mortality: 46 years of follow-up. J. Am. Coll. Cardiol. 2018, 72, 987–995. [Google Scholar] [CrossRef]
- Blair, S.N. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996, 276, 205. [Google Scholar] [CrossRef]

| Variable/Weeks | 1–3 | 4–8 | 9–12 |
|---|---|---|---|
| Sessions for week | 2 | 2 | 2 |
| Exercise intensity | moderate | intensity | vigorous |
| Expected RPE (1–10) | 5–6 | 6–7 | 7–8 |
| Assessment | HG | OWG | OG | P1 for interaction/ES/SP | P2 for time/ES/SP | P3 for group/ES/SP | |
|---|---|---|---|---|---|---|---|
| Participants (n) | 10 | 10 | 12 | ||||
| Weight (kg) | Pre † | 58.20 (4.21) | 68.29 (5.70) | 80.16 (9.94) | 0.028 | 0.001 | <0.001 |
| Post † | 58.22 (3.57) | 67.51 (5.61) * | 78.98 (9.54) * | 0.23/0.68 | 0.33/0.95 | 0.62/1.00 | |
| Body mass index (kg·m−2) | Pre † | 24.23 (1.24) | 28.83 (1.13) | 32.17 (1.93) | 0.121 | 0.002 | <0.001 |
| Post † | 24.16 (0.99) | 28.59 (0.87) | 31.71 (1.95) * | 0.14/0.42 | 0.30/0.92 | 0.84/1.00 | |
| Fat Mass (kg) | Pre ¥ | 22.02 (3.28) | 26.22 (2.51) | 31.65 (4.54) | 0.732 | 0.078 | <0.001 |
| Post ¥ | 21.72 (2.69) | 25.46 (2.17) | 31.30 (4.22) | 0.02/0.09 | 0.11/0.42 | 0.61/1.00 | |
| Body Fat (%) | Pre | 36.79 (4.29) | 38.56 (3.43) | 40.78 (6.32) | 0.901 | 0.038 | 0.180 |
| Post | 36.23 (3.78) | 37.74 (3.56) | 40.25 (5.98) | 0.01/0.06 | 0.15/0.56 | 0.12/0.35 | |
| Fat-Free Mass (kg) | Pre | 37.91 (3.91) β | 41.78 (5.09) | 46.72 (9.99) | 0.971 | 0.095 | 0.030 |
| Post | 38.20 (3.83) β | 42.15 (5.31) | 47.13 (9.76) | 0.00/0.05 | 0.10/0.39 | 0.23/0.67 | |
| Lean Mass (kg) | Pre | 20.91 (2.70) β | 22.92 (3.01) | 25.81 (6.16) | 0.401 | 0.960 | 0.041 |
| Post | 20.57 (2.33) β | 23.02 (3.19) | 26.02 (6.08) | 0.07/0.20 | 0.00/0.05 | 0.21/0.62 | |
| Basal Metabolic Rate (kcal·day−1) | Pre | 1182.11 (82.28) β | 1276.20 (108.51) | 1383.64 (224.23) | 0.775 | 0.155 | 0.027 |
| Post | 1195.89 (82.62) β | 1281.20 (114.61) | 1389.00 (211.44) | 0.02/0.09 | 0.07/0.292 | 0.24/0.68 |
| Assessment | HG | OWG | OG | P1 for interaction/ES/SP | P2 for time/ES/SP | P3 for group/ES/SP | |
|---|---|---|---|---|---|---|---|
| Participants (n) | 10 | 10 | 12 | ||||
| VO2peak (mL·kg−1·min−1) | Pre | 30.38 (3.62) | 28.66 (2.41) | 29.45 (2.89) | 0.008 | 0.005 | 0.763 |
| Post | 29.87 (2.39) | 30.13 (3.09) * | 30.93 (2.46) * | 0.29/0.83 | 0.24/0.83 | 0.02/0.09 | |
| Strength DA (repetitions) | Pre | 21.00 (2.21) | 21.60 (3.17) | 18.55 (2.54) | 0.696 | 0.003 | 0.031 |
| Post | 23.20 (2.04) | 22.80 (3.91) | 20.91 (2.77) | 0.03/0.10 | 0.27/0.87 | 0.22/0.66 | |
| Strength NDA (repetitions) | Pre | 21.20 (1.23) | 21.80 (3.43) | 18.09 (3.11) | 0.754 | 0.001 | 0.01 |
| Post | 23.00 (2.11) | 23.40 (3.95) | 20.64 (2.46) | 0.02/0.09 | 0.32/0.94 | 0.28/0.81 | |
| 8 foot UP & Go (seconds) | Pre | 5.93 (0.49) | 6.00 (0.59) | 6.12 (0.94) | 0.481 | 0.008 | 0.786 |
| Post | 5.34 (0.31) | 5.72 (0.62) | 5.24 (1.77) | 0.05/0.17 | 0.22/0.79 | 0.02/0.08 | |
| Jump height (cm) | Pre | 9.21 (2.05) | 9.84 (1.79) | 11.05 (4.23) | 0.001 | 0.001 | 0.226 |
| Post | 10.66 (2.10) * | 9.93 (2.10) | 11.17 (4.53) | 0.17/0.95 | 0.13/0.92 | 0.04/0.31 | |
| Power output (watts) | Pre † | 390.64 (66.35) | 466.28 (60.33) | 554.76 (173.51) | 0.044 | 0.005 | <0.001 |
| Post | 418.25 (65.31) * | 462.99 (64.41) | 577.03 (168.11) *‡ | 0.08/0.60 | 0.09/0.82 | 0.29/0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira-Reis, A.; Maté-Muñoz, J.L.; Hernández-Lougedo, J.; Vilches-Sáez, S.; Benet, M.; García-Fernández, P.; Pleguezuelos, E.; Carbonell, T.; Alva, N.; Garnacho-Castaño, M.V. Aerobic Dance on an Air Dissipation Platform Improves Cardiorespiratory, Muscular and Cellular Fitness in the Overweight and Obese Elderly. Biology 2022, 11, 579. https://doi.org/10.3390/biology11040579
Moreira-Reis A, Maté-Muñoz JL, Hernández-Lougedo J, Vilches-Sáez S, Benet M, García-Fernández P, Pleguezuelos E, Carbonell T, Alva N, Garnacho-Castaño MV. Aerobic Dance on an Air Dissipation Platform Improves Cardiorespiratory, Muscular and Cellular Fitness in the Overweight and Obese Elderly. Biology. 2022; 11(4):579. https://doi.org/10.3390/biology11040579
Chicago/Turabian StyleMoreira-Reis, Alessandra, José Luis Maté-Muñoz, Juan Hernández-Lougedo, Sergio Vilches-Sáez, Marta Benet, Pablo García-Fernández, Eulogio Pleguezuelos, Teresa Carbonell, Norma Alva, and Manuel Vicente Garnacho-Castaño. 2022. "Aerobic Dance on an Air Dissipation Platform Improves Cardiorespiratory, Muscular and Cellular Fitness in the Overweight and Obese Elderly" Biology 11, no. 4: 579. https://doi.org/10.3390/biology11040579
APA StyleMoreira-Reis, A., Maté-Muñoz, J. L., Hernández-Lougedo, J., Vilches-Sáez, S., Benet, M., García-Fernández, P., Pleguezuelos, E., Carbonell, T., Alva, N., & Garnacho-Castaño, M. V. (2022). Aerobic Dance on an Air Dissipation Platform Improves Cardiorespiratory, Muscular and Cellular Fitness in the Overweight and Obese Elderly. Biology, 11(4), 579. https://doi.org/10.3390/biology11040579










