Number of Teeth Is Related to Craniofacial Morphology in Humans
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Sample
- Permanent tooth agenesis (congenitally missing teeth), without considering the third molars.
- No craniofacial malformations, syndromes, systemic diseases, or any other anomalies affecting craniofacial morphology, as reported in the subjects’ medical record.
- European (White) ancestry.
- Lateral cephalometric radiograph in maximal intercuspation of adequate clinical diagnostic quality and with a reference ruler at the mid-sagittal level.
- Panoramic radiographs of adequate diagnostic quality.
- No history of interventions known to influence craniofacial morphology, such as orthodontic treatment.
- Absence of any other severe dental anomaly regarding tooth number, size, or form in any tooth except for third molars.
- Individuals where the reason of absence of any tooth was definite.
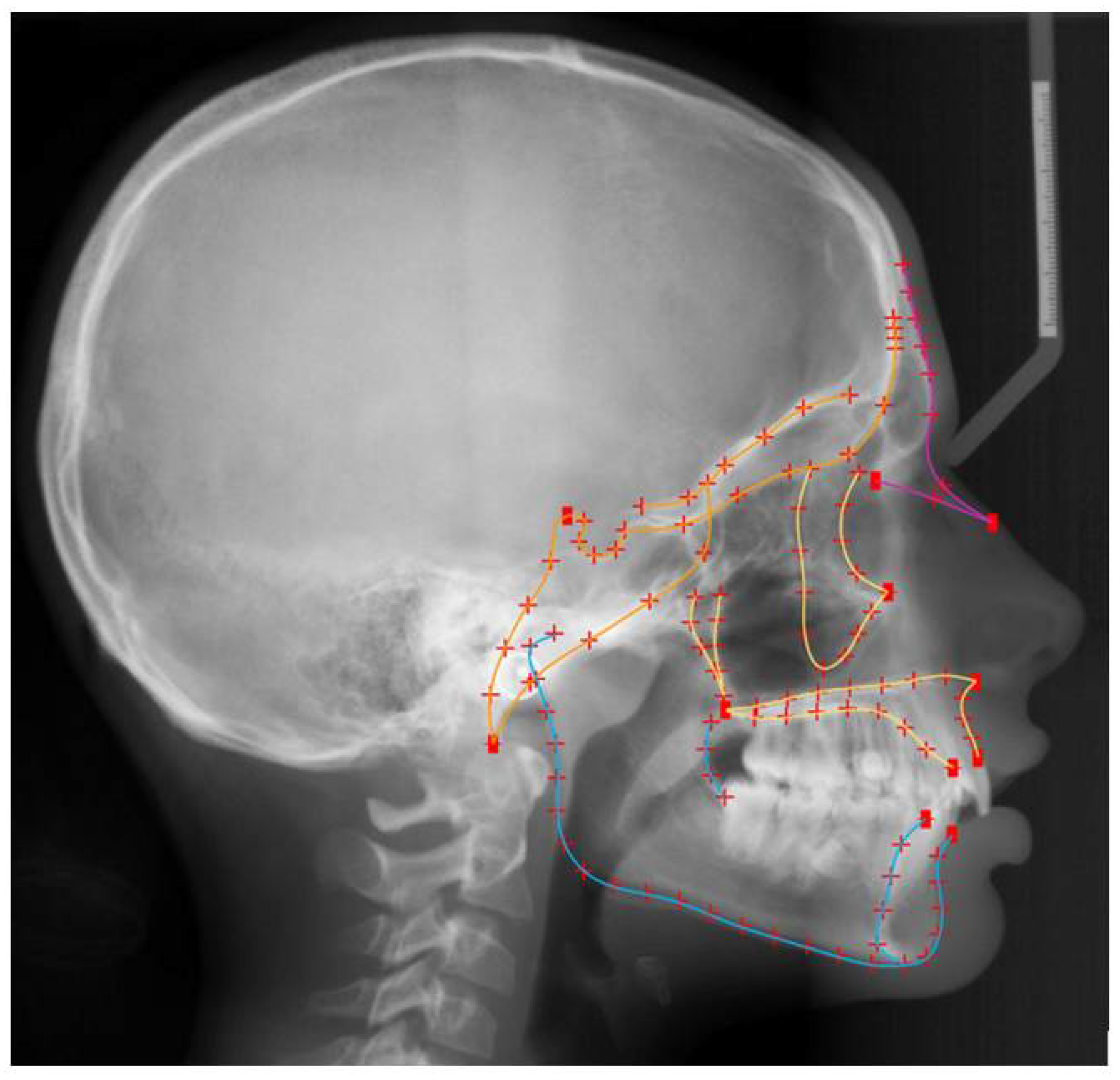
2.3. Shape Assessment
2.4. Statistical Analysis
2.5. Method Error
3. Results
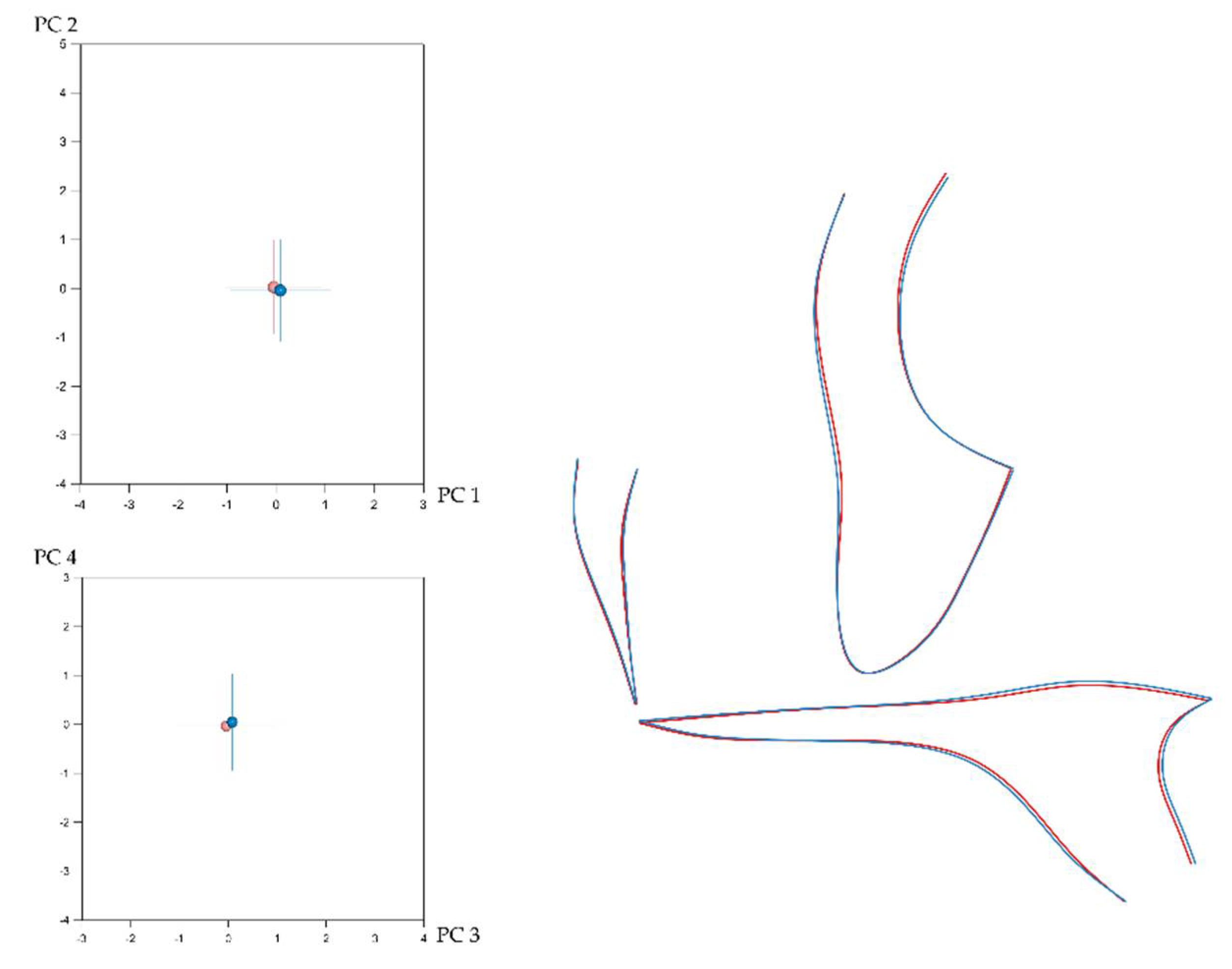
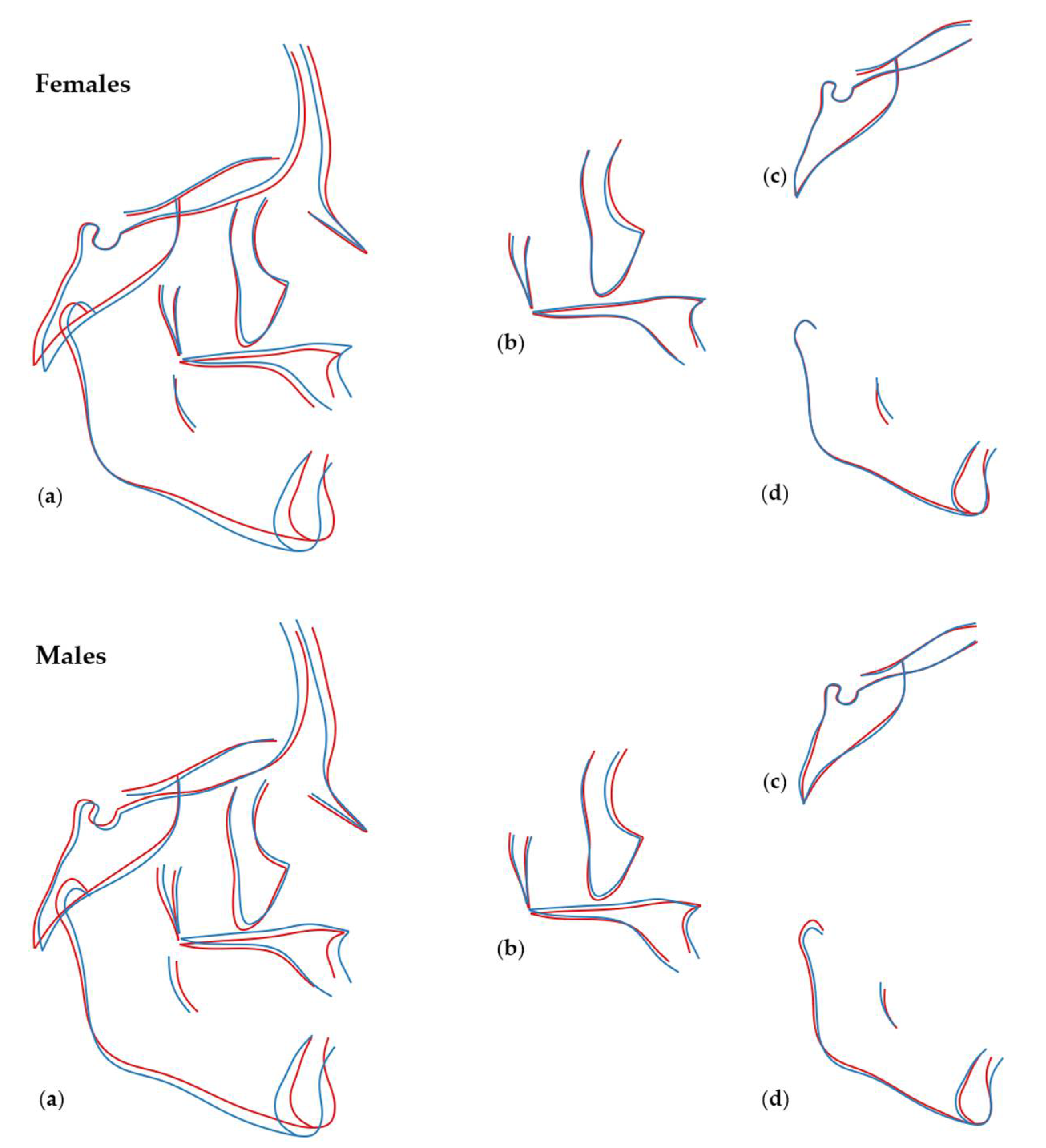
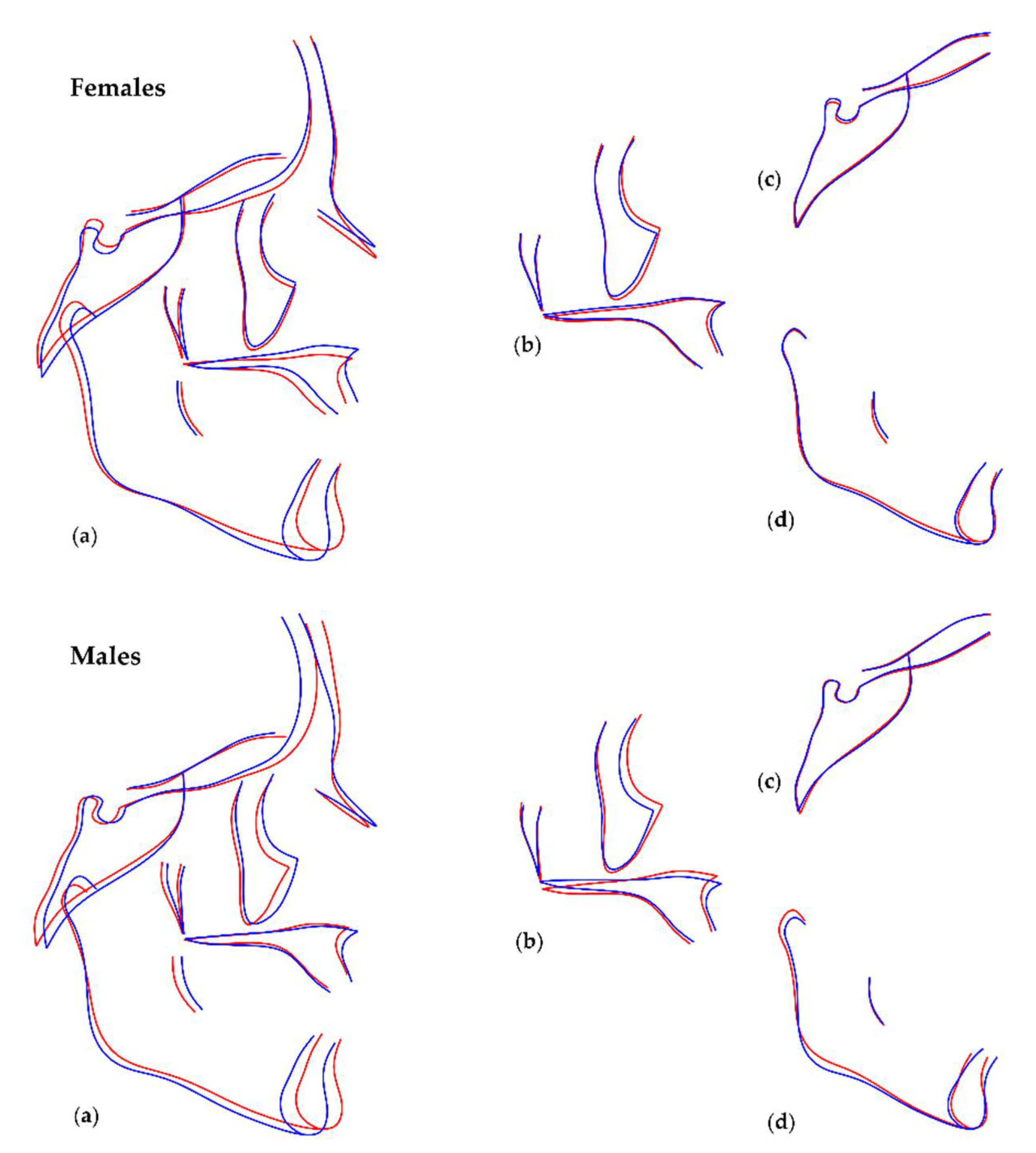
3.1. Sexual Dimorphism and Effect of Size (Allometry) in Craniofacial Shape
3.2. Number of Teeth and Craniofacial Shape
3.3. Local Versus Generalised Effects of Tooth Agenesis on Maxillomandibular Structures
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalaf, K.; Miskelly, J.; Voge, E.; Macfarlane, T. Prevalence of hypodontia and associated factors: A systematic review and meta-analysis. J. Orthod. 2014, 41, 299–316. [Google Scholar] [CrossRef]
- Gkantidis, N.; Katib, H.; Oeschger, E.; Karamolegkou, M.; Topouzelis, N.; Kanavakis, G. Patterns of non-syndromic permanent tooth agenesis in a large orthodontic population. Arch. Oral Biol. 2017, 79, 42–47. [Google Scholar] [CrossRef]
- Yin, W.; Bian, Z. The Gene Network Underlying Hypodontia. J. Dent. Res. 2015, 94, 878–885. [Google Scholar] [CrossRef]
- Brook, A. Multilevel complex interactions between genetic, epigenetic and environmental factors in the aetiology of anomalies of dental development. Arch. Oral Biol. 2009, 54 (Suppl. S1), S3–S17. [Google Scholar] [CrossRef]
- Al-Shahrani, I.; Dirks, W.; Jepson, N.; Khalaf, K. 3D-Geomorphometrics tooth shape analysis in hypodontia. Front. Physiol. 2014, 5, 154. [Google Scholar] [CrossRef]
- Gorlin, R.J.; Cohen, M.M.; Levin, L.S. Syndromes of the Head and Neck, 3rd ed.; Oxford University Press: New York, NY, USA, 1990; 977p. [Google Scholar]
- Nassif, A.; Senussi, I.; Meary, F.; Loiodice, S.; Hotton, D.; Robert, B.; Bensidhoum, M.; Berdal, A.; Babajko, S. Msx1 role in craniofacial bone morphogenesis. Bone 2014, 66, 96–104. [Google Scholar] [CrossRef]
- Williams, M.A.; Letra, A. The Changing Landscape in the Genetic Etiology of Human Tooth Agenesis. Genes 2018, 9, 255. [Google Scholar] [CrossRef]
- Cocos, A.; Halazonetis, D. Craniofacial shape differs in patients with tooth agenesis: Geometric morphometric analysis. Eur. J. Orthod. 2016, 39, 345–351. [Google Scholar] [CrossRef]
- Créton, M.; Cune, M.S.; De Putter, C.; Ruijter, J.M.; Kuijpers-Jagtman, A.M. Dentofacial characteristics of patients with hypodontia. Clin. Oral Investig. 2009, 14, 467–477. [Google Scholar] [CrossRef]
- Oeschger, E.S.; Kanavakis, G.; Halazonetis, D.J.; Gkantidis, N. Number of teeth is associated with facial size in humans. Sci. Rep. 2020, 10, 1820. [Google Scholar] [CrossRef]
- Gkantidis, N.; Tacchi, M.; Oeschger, E.; Halazonetis, D.; Kanavakis, G. Third Molar Agenesis Is Associated with Facial Size. Biology 2021, 10, 650. [Google Scholar] [CrossRef]
- Øgaard, B.; Krogstad, O. Craniofacial structure and soft tissue profile in patients with severe hypodontia. Am. J. Orthod. Dentofac. Orthop. 1995, 108, 472–477. [Google Scholar] [CrossRef]
- Fernandez, C.C.A.; Pereira, C.V.C.A.; Luiz, R.R.; Faraco, I.M.; Marazita, M.L.; Arnaudo, M.; de Carvalho, F.M.; Poletta, F.E.; Mereb, J.C.; Castilla, E.E.; et al. Third molar agenesis as a potential marker for craniofacial deformities. Arch. Oral Biol. 2018, 88, 19–23. [Google Scholar] [CrossRef]
- Halazonetis, D.J. Morphometrics for cephalometric diagnosis. Am. J. Orthod. Dentofac. Orthop. 2004, 125, 571–581. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Antunes, L.S.; Pinheiro, L.H.M.; Guimarães, L.S.; Calansans-Maia, J.D.A.; Küchler, E.C.; Antunes, L.A.A. Is dental agenesis associated with craniofacial morphology pattern? A systematic review and meta-analysis. Eur. J. Orthod. 2020, 42, 534–543. [Google Scholar] [CrossRef]
- Bookstein, F.L. Reconsidering “The inappropriateness of conventional cephalometrics&rdquo”. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 784–797. [Google Scholar] [CrossRef]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. A field comes of age: Geometric morphometrics in the 21st century. Hystrix 2013, 24, 7–14. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Gunz, P. Advances in Geometric Morphometrics. Evol. Biol. 2009, 36, 235–247. [Google Scholar] [CrossRef]
- Scheiwiller, M.; Oeschger, E.S.; Gkantidis, N. Third molar agenesis in modern humans with and without agenesis of other teeth. PeerJ 2020, 8, e10367. [Google Scholar] [CrossRef]
- Soares, C.B.; Figueiroa, J.N.; Dantas, R.M.; Kurita, L.M.; dos Anjos Pontual, A.; de Moraes Ramos-Perez, F.M.; da Cruz Perez, D.E.; dos Anjos Pontual, M.L. Evaluation of third molar development in the estimation of chronological age. Forensic Sci. Int. 2015, 254, 13–17. [Google Scholar] [CrossRef]
- de Oliveira, F.T.; Capelozza, A.L.Á.; Lauris, J.R.P.; de Bullen, I.R.F.R. Mineralization of mandibular third molars can estimate chronological age—Brazilian indices. Forensic Sci. Int. 2012, 219, 147–150. [Google Scholar] [CrossRef]
- Van Wijk, A.J.; Tan, S.P.K. A numeric code for identifying patterns of human tooth agenesis: A new approach. Eur. J. Oral Sci. 2006, 114, 97–101. [Google Scholar] [CrossRef]
- Van Der Weijden, F.; Dell’Acqua, F.; Slot, D.E. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J. Clin. Periodontol. 2009, 36, 1048–1058. [Google Scholar] [CrossRef]
- Bookstein, F.L. Landmark methods for forms without landmarks: Morphometrics of group differences in outline shape. Med. Image Anal. 1997, 1, 225–243. [Google Scholar] [CrossRef]
- Gunz, P.; Mitteroecker, P. Semilandmarks: A method for quantifying curves and surfaces. Hystrix 2013, 24, 103–109. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Slice, D. Extensions of the Procrustes Method for the Optimal Superimposition of Landmarks. Syst. Biol. 1990, 39, 40–59. [Google Scholar] [CrossRef]
- Slice, D.E. Geometric Morphometrics. Annu. Rev. Anthr. 2007, 36, 261–281. [Google Scholar] [CrossRef]
- Kanavakis, G.; Halazonetis, D.; Katsaros, C.; Gkantidis, N. Facial shape affects self-perceived facial attractiveness. PLoS ONE 2021, 16, e0245557. [Google Scholar] [CrossRef]
- Kanavakis, G.; Silvola, A.-S.; Halazonetis, D.; Lähdesmäki, R.; Pirttiniemi, P. Profile shape variation and sexual dimorphism amongst middle-aged Northern Europeans. Eur. J. Orthod. 2021, 44, 30–36. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Size, shape, and form: Concepts of allometry in geometric morphometrics. Dev. Genes Evol. 2016, 226, 113–137. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Gunz, P.; Windhager, S.; Schaefer, K. A brief review of shape, form, and allometry in geometric morphometrics, with applications to human facial morphology. Hystrix Ital. J. Mammal. 2013, 24, 59–66. [Google Scholar]
- Peres-Neto, P.R.; Jackson, D.A.; Somers, K.M. How many principal components? Stopping rules for determining the number of non-trivial axes revisited. Comput. Stat. Data Anal. 2005, 49, 974–997. [Google Scholar] [CrossRef]
- Kanavakis, G.; Silvola, A.-S.; Halazonetis, D.; Lähdesmäki, R.; Pirttiniemi, P. Incisor Occlusion Affects Profile Shape Variation in Middle-Aged Adults. J. Clin. Med. 2021, 10, 800. [Google Scholar] [CrossRef]
- Yüksel, S.; Üçem, T. The effect of tooth agenesis on dentofacial structures. Eur. J. Orthod. 1997, 19, 71–78. [Google Scholar] [CrossRef]
- Gungor, A.Y.; Türkkahraman, H. Effects of severity and location of nonsyndromic hypodontia on craniofacial morphology. Angle Orthod. 2013, 83, 584–590. [Google Scholar] [CrossRef]
- Acharya, P.N.; Jones, S.P.; Moles, D.; Gill, D.; Hunt, N.P. A cephalometric study to investigate the skeletal relationships in patients with increasing severity of hypodontia. Angle Orthod. 2010, 80, 699–706. [Google Scholar] [CrossRef]
- Endo, T.; Ozoe, R.; Yoshino, S.; Shimooka, S. Hypodontia Patterns and Variations in Craniofacial Morphology in Japanese Orthodontic Patients. Angle Orthod. 2006, 76, 996–1003. [Google Scholar] [CrossRef]
- Moyers, R.E.; Bookstein, F.L. The inappropriateness of conventional cephalometrics. Am. J. Orthod. 1979, 75, 599–617. [Google Scholar] [CrossRef]
- Carter, K.; Worthington, S. Morphologic and Demographic Predictors of Third Molar Agenesis. J. Dent. Res. 2015, 94, 886–894. [Google Scholar] [CrossRef]
- Evans, A.R.; Daly, E.S.; Catlett, K.K.; Paul, K.S.; King, S.J.; Skinner, M.M.; Nesse, H.P.; Hublin, J.J.; Townsend, G.C.; Schwartz, G.T.; et al. A simple rule governs the evolution and development of hominin tooth size. Nature 2016, 530, 477–480. [Google Scholar] [CrossRef]
- Roseman, C.C. Detecting interregionally diversifying natural selection on modern human cranial form by using matched molecular and morphometric data. Proc. Natl. Acad. Sci. USA 2004, 101, 12824–12829. [Google Scholar] [CrossRef]
- Kavanagh, K.D.; Evans, A.R.; Jernvall, J. Predicting evolutionary patterns of mammalian teeth from development. Nature 2007, 449, 427–432. [Google Scholar] [CrossRef]
- Trinkaus, E. Neandertal faces were not long; modern human faces are short. Proc. Natl. Acad. Sci. USA 2003, 100, 8142–8145. [Google Scholar] [CrossRef]
- Bastir, M.; Rosas, A.; Stringer, C.; Cuétara, J.M.; Kruszynski, R.; Weber, G.W.; Ross, C.F.; Ravosa, M.J. Effects of brain and facial size on basicranial form in human and primate evolution. J. Hum. Evol. 2010, 58, 424–431. [Google Scholar] [CrossRef]
- Ramiro-Verdugo, J.; De Vicente-Corominas, E.; Montiel-Company, J.M.; Gandía-Franco, J.L.; Bellot-Arcís, C. Association between third molar agenesis and craniofacial structure development. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 799–804. [Google Scholar] [CrossRef]
- Enlow, D.H.; Hans, M.G. Essentials of Facial Growth, 2nd ed.; Distributed by Needham Press: Ann Arbor, MI, USA, 2008; 507p. [Google Scholar]
- Katsadouris, A.; Halazonetis, D.J. Geometric morphometric analysis of craniofacial growth between the ages of 12 and 14 in normal humans. Eur. J. Orthod. 2016, 39, 386–394. [Google Scholar] [CrossRef]
- Gkantidis, N.; Halazonetis, D.J. Morphological integration between the cranial base and the face in children and adults. J. Anat. 2011, 218, 426–438. [Google Scholar] [CrossRef]
- Lieberman, D.E.; Pearson, O.M.; Mowbray, K.M. Basicranial influence on overall cranial shape. J. Hum. Evol. 2000, 38, 291–315. [Google Scholar] [CrossRef]
- Nikopensius, T.; Saag, M.; Jagomägi, T.; Annilo, T.; Kals, M.; Kivistik, P.; Milani, L.; Metspalu, A. A Missense Mutation in DUSP6 is Associated with Class III Malocclusion. J. Dent. Res. 2013, 92, 893–898. [Google Scholar] [CrossRef]
- Chen, F.; Li, Q.; Gu, M.; Li, X.; Yu, J.; Zhang, Y.-B. Identification of a Mutation in FGF23 Involved in Mandibular Prognathism. Sci. Rep. 2015, 5, 11250. [Google Scholar] [CrossRef]
- Guan, X.; Song, Y.; Ott, J.; Zhang, Y.; Li, C.; Xin, T.; Li, Z.; Gan, Y.; Li, J.; Zhou, S.; et al. The ADAMTS1 Gene Is Associated with Familial Mandibular Prognathism. J. Dent. Res. 2015, 94, 1196–1201. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Teixeira, E.C.; Antunes, L.S.; Nelson-Filho, P.; Cunha, A.S.; Levy, S.C.; Araújo, M.T.D.S.; Ramos, A.G.D.C.; Cruz, G.V.; Omori, M.A.; et al. Association between craniofacial morphological patterns and tooth agenesis-related genes. Prog. Orthod. 2020, 21, 9. [Google Scholar] [CrossRef]
- Marañón-Vásquez, G.A.; Dantas, B.; Kirschneck, C.; Arid, J.; Cunha, A.; Ramos, A.G.D.C.; Omori, M.A.; Rodrigues, A.S.; Teixeira, E.C.; Levy, S.C.; et al. Tooth agenesis-related GLI2 and GLI3 genes may contribute to craniofacial skeletal morphology in humans. Arch. Oral Biol. 2019, 103, 12–18. [Google Scholar] [CrossRef]
- DE Stefani, A.; Bruno, G.; Frezza, A.; Conte, E.; Balasso, P.; Gracco, A. Association between teeth agenesis and Angle’s classes in an Italian population. Minerva Dent. Oral Sci. 2021, 70, 21–25. [Google Scholar] [CrossRef]
- Fernandez, C.C.A.; Pereira, C.V.C.A.; Luiz, R.R.; Vieira, A.R.; De Castro Costa, M. Dental anomalies in different growth and skeletal malocclusion patterns. Angle Orthod. 2018, 88, 195–201. [Google Scholar] [CrossRef]
- Pinho, T.; Pollmann, C.; Calheiros-Lobo, M.J.; Sousa, A.; Lemos, C. Craniofacial repercussions in maxillary lateral incisors agenesis. Int. Orthod. 2011, 9, 274–285. [Google Scholar] [CrossRef]
- Bassiouny, D.S.; Afify, A.R.; Baeshen, H.A.; Birkhed, D.; Zawawi, K.H. Prevalence of maxillary lateral incisor agenesis and associated skeletal characteristics in an orthodontic patient population. Acta Odontol. Scand. 2016, 74, 456–459. [Google Scholar] [CrossRef]
- Bertl, M.H.; Bertl, K.; Wagner, M.; Gahleitner, A.; Stavropoulos, A.; Ulm, C.; Mitteroecker, P. Second premolar agenesis is associated with mandibular form: A geometric morphometric analysis of mandibular cross-sections. Int. J. Oral Sci. 2016, 8, 254–260. [Google Scholar] [CrossRef]
- Zelditch, M.L.; Wood, A.R.; Bonett, R.M.; Swiderski, D.L. Modularity of the rodent mandible: Integrating bones, muscles, and teeth. Evol. Dev. 2008, 10, 756–768. [Google Scholar] [CrossRef]
- Heuberer, S.; Ulm, C.; Zechner, W.; Laky, B.; Watzak, G. Patterns of congenitally missing teeth of non-syndromic and syndromic patients treated at a single-center over the past thirty years. Arch. Oral Biol. 2019, 98, 140–147. [Google Scholar] [CrossRef]
- Wen, Y.F.; Wong, H.M.; Lin, R.; Yin, G.; McGrath, C. Inter-Ethnic/Racial Facial Variations: A Systematic Review and Bayesian Meta-Analysis of Photogrammetric Studies. PLoS ONE 2015, 10, e0134525. [Google Scholar] [CrossRef] [PubMed]
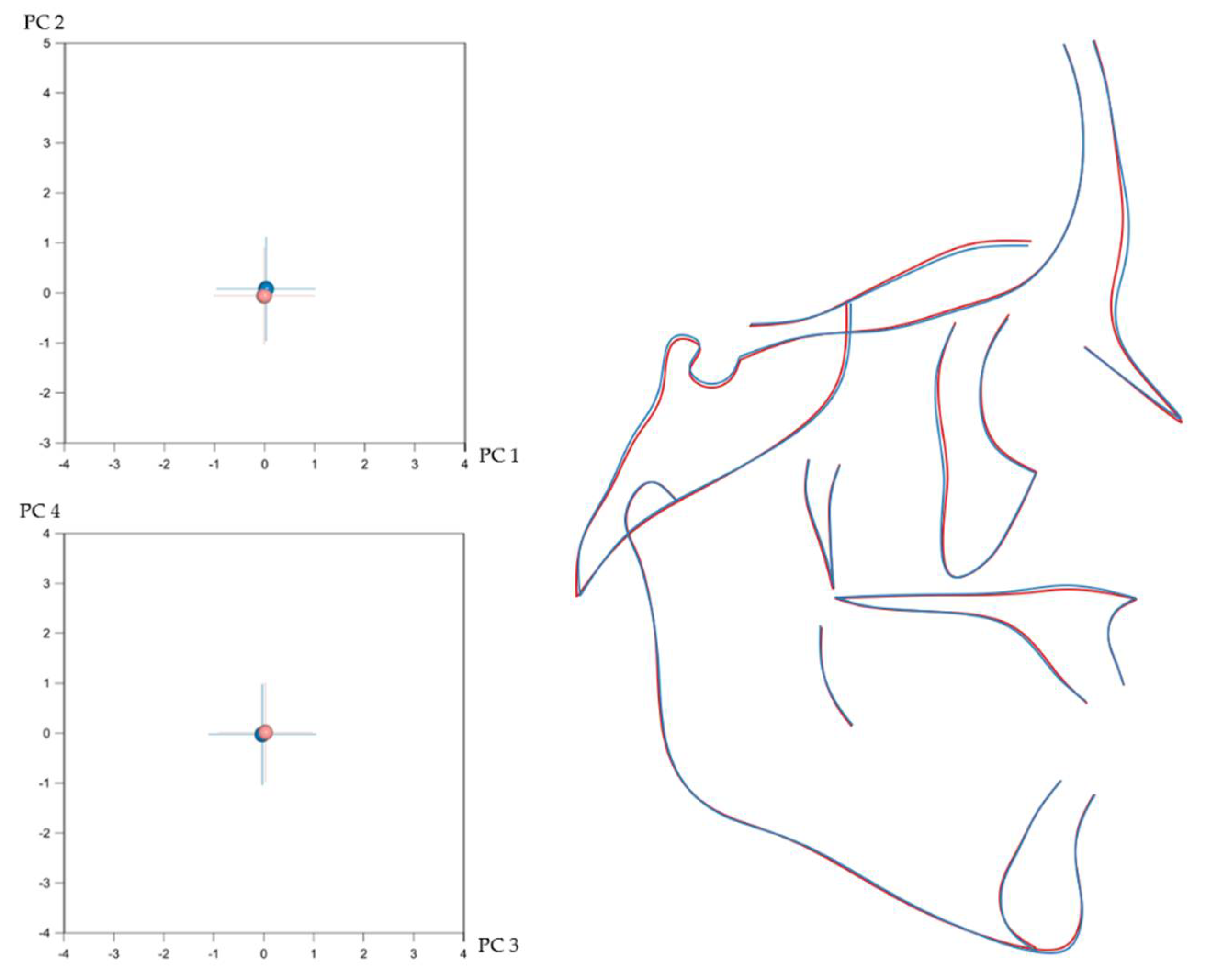
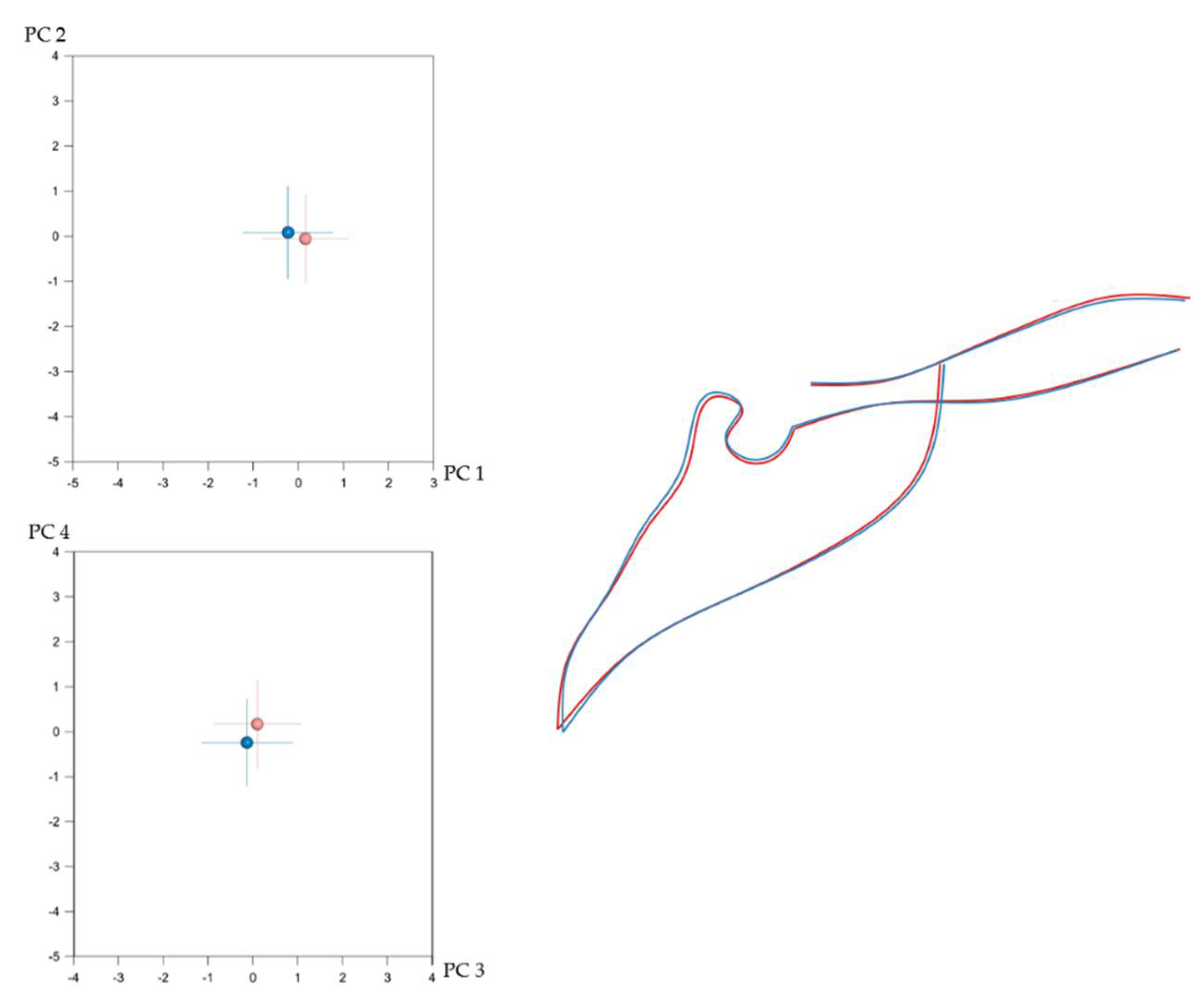
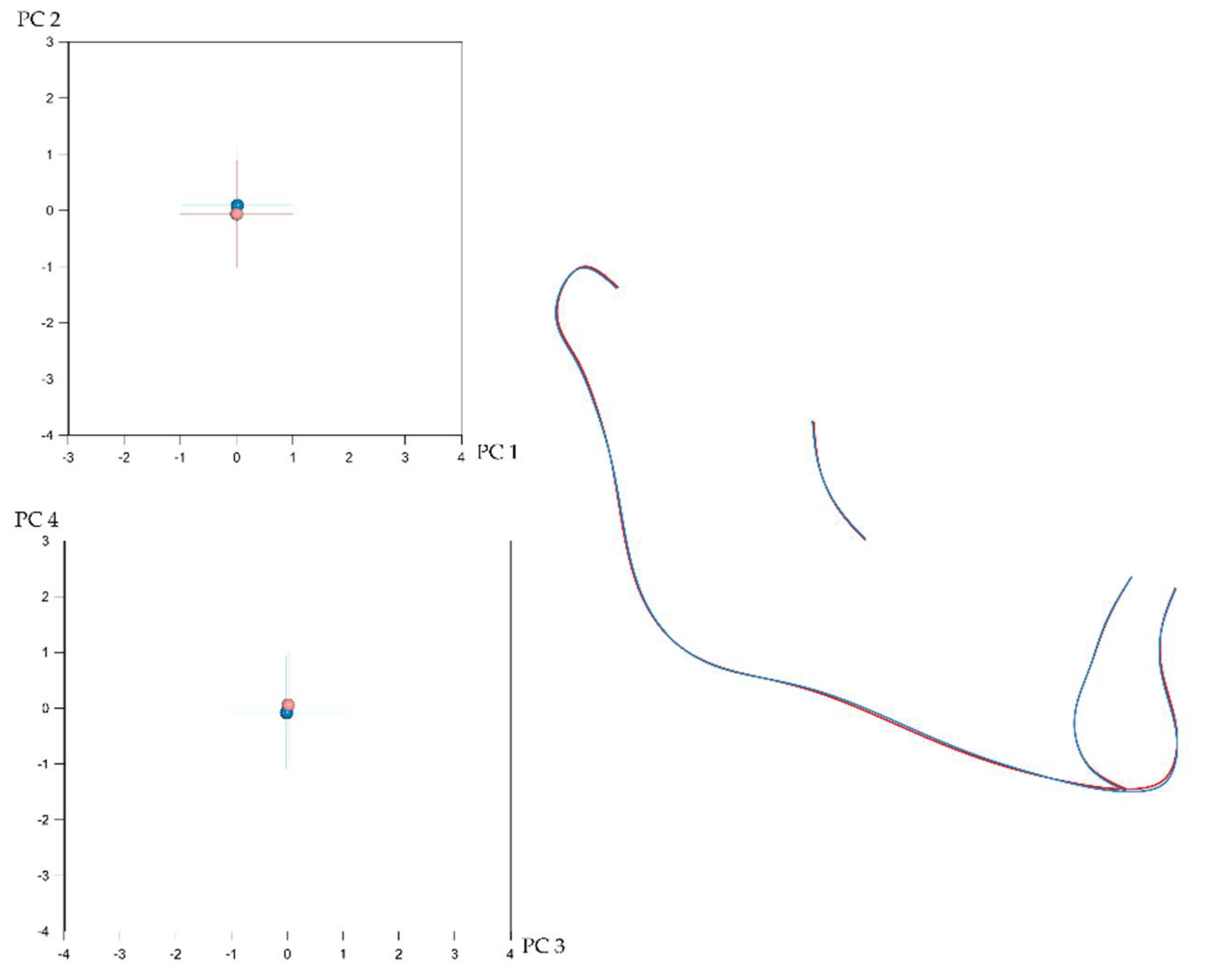
| Shape | Mean Procrustes Distance | p-Value |
|---|---|---|
| Cranial base | 0.01859 | <0.001 * |
| Maxilla | 0.01053 | 0.001 * |
| Mandible | 0.00649 | 0.048 *,1 |
| Entire craniofacial configuration | 0.00954 | <0.001 * |
| Shape Configurations | η2 | p-Value | ||
|---|---|---|---|---|
| Cranial Base (PC1–PC9) | Females | Age | 0.120 | <0.001 * |
| Number of missing teeth | 0.030 | 0.110 | ||
| Males | Age | 0.122 | <0.001 * | |
| Number of missing teeth | 0.033 | 0.290 | ||
| Maxilla (PC1–PC10) | Females | Age | 0.142 | <0.001 * |
| Number of missing teeth | 0.057 | 0.002 * | ||
| Males | Age | 0.199 | <0.001 * | |
| Number of missing teeth | 0.142 | <0.001 * | ||
| Mandible (PC1–PC8) | Females | Age | 0.159 | <0.001 * |
| Number of missing teeth | 0.056 | 0.001 * | ||
| Males | Age | 0.231 | <0.001 * | |
| Number of missing teeth | 0.112 | <0.001 * | ||
| Entire craniofacial configuration (PC1–PC18) | Females | Age | 0.369 | <0.001 * |
| Number of missing teeth | 0.143 | <0.001 * | ||
| Males | Age | 0.439 | <0.001 * | |
| Number of missing teeth | 0.192 | <0.001 * |
| Shape Configurations | η2 | p-Value | ||
|---|---|---|---|---|
| Cranial Base (PC1–PC9) | Females | Age | 0.000 | 1.000 |
| Number of missing teeth | 0.031 | 0.095 | ||
| Males | Age | 0.000 | 1.000 | |
| Number of missing teeth | 0.034 | 0.270 | ||
| Maxilla (PC1–PC10) | Females | Age | 0.000 | 1.000 |
| Number of missing teeth | 0.051 | 0.006 * | ||
| Males | Age | 0.001 | 1.000 | |
| Number of missing teeth | 0.134 | <0.001 * | ||
| Mandible (PC1–PC8) | Females | Age | 0.000 | 1.000 |
| Number of missing teeth | 0.060 | <0.001 * | ||
| Males | Age | 0.000 | 1.000 | |
| Number of missing teeth | 0.114 | <0.001 * | ||
| Entire craniofacial configuration (PC1–PC18) | Females | Age | 0.001 | 1.000 |
| Number of missing teeth | 0.138 | <0.001 * | ||
| Males | Age | 0.001 | 1.000 | |
| Number of missing teeth | 0.192 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oeschger, E.S.; Kanavakis, G.; Cocos, A.; Halazonetis, D.J.; Gkantidis, N. Number of Teeth Is Related to Craniofacial Morphology in Humans. Biology 2022, 11, 544. https://doi.org/10.3390/biology11040544
Oeschger ES, Kanavakis G, Cocos A, Halazonetis DJ, Gkantidis N. Number of Teeth Is Related to Craniofacial Morphology in Humans. Biology. 2022; 11(4):544. https://doi.org/10.3390/biology11040544
Chicago/Turabian StyleOeschger, Elias S., Georgios Kanavakis, Alina Cocos, Demetrios J. Halazonetis, and Nikolaos Gkantidis. 2022. "Number of Teeth Is Related to Craniofacial Morphology in Humans" Biology 11, no. 4: 544. https://doi.org/10.3390/biology11040544
APA StyleOeschger, E. S., Kanavakis, G., Cocos, A., Halazonetis, D. J., & Gkantidis, N. (2022). Number of Teeth Is Related to Craniofacial Morphology in Humans. Biology, 11(4), 544. https://doi.org/10.3390/biology11040544









