Effect of Replacing Sorghum Stubble with Tillandsia recurvata (L.) on Liveweight Change, Blood Metabolites, and Hematic Biometry of Goats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Tillandsia recurvata
2.4. Experimental Diets
2.5. Blood Sampling and Metabolic Profile
2.6. Data Analyses
3. Results
3.1. Mycotoxins Analyses of Tillandsia recurvata
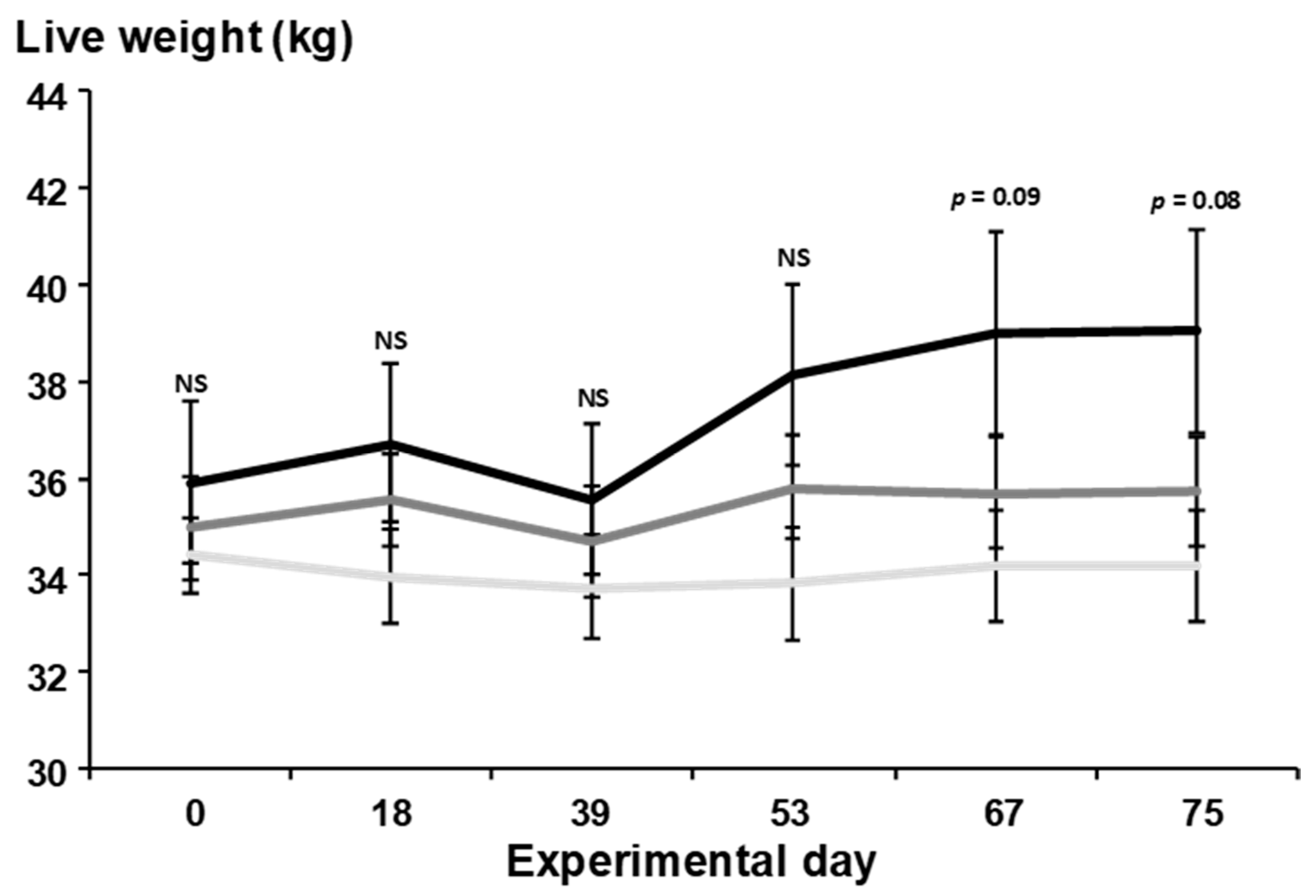
3.2. Daily Live Weight Change
3.3. Metabolic Profile
3.4. Complete Blood Count
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahi, A.K.; Wasike, C.B. Dairy goat production in sub-saharan Africa: Current status, constraints and prospects for research and development. Asian-Australas. J. Anim. Sci. 2019, 32, 1266–1274. [Google Scholar]
- Huenneke, L.F.; Anderson, J.P.; Remmenga, M.; Schlesinger, W.H. Desertification alters patterns of aboveground net primary production in Chihuahuan ecosystems. Glob. Chang. Biol. 2002, 8, 247–264. [Google Scholar]
- Cuevas-Reyes, V.; Rosales-Nieto, C.A. Characterization of the dual-purpose bovine system in northwest Mexico: Producers, resources and problematic. Rev. MVZ Córdoba 2018, 23, 6448–6460. [Google Scholar]
- Panorama-Agroalimentario. Secretaria de Agricultura y Desarrollo Rural y Servicio de Información Agroalimentaria y Pes-quera (SIAP). 2020. Available online: https://nube.siap.gob.mx/gobmx_publicaciones_siap/ (accessed on 18 January 2021).
- Mellado, M.; Foote, R.H.; Rodriguez, A.; Zarate, P. Botanical composition and nutrient content of diets selected by goats grazing on desert grassland in northern Mexico. Small Rumin. Res. 1991, 6, 141–150. [Google Scholar]
- García-Monjaras, S.; Santos-Díaz, R.; Flores-Najera, M.; Cuevas-Reyes, V.; Meza-Herrera, C.; Mellado, M.; Chay-Canul, A.; Rosales-Nieto, C. Diet selected by goats on xerophytic shrubland with different milk yield potential. J. Arid Environ. 2021, 186, 104429. [Google Scholar]
- Mellado, M. Dietary selection by goats and the implications for range management in the Chihuahuan Desert: A review. Rangel. J. 2016, 38, 331. [Google Scholar]
- Goel, G.; Puniya, A.K.; Aguilar, C.N.; Singh, K. Interaction of gut microflora with tannins in feeds. Naturwissenschaften 2005, 92, 497–503. [Google Scholar]
- Animut, G.; Puchala, R.; Goetsch, A.; Patra, A.; Sahlu, T.; Varel, V.; Wells, J. Methane emission by goats consuming different sources of condensed tannins. Anim. Feed. Sci. Technol. 2008, 144, 228–241. [Google Scholar]
- Rosales-Nieto, C.A.; Gamez-Vazquez, H.G.; Gudino-Reyes, J.; Reyes-Ramirez, E.A.; Eaton, M.; Stanko, R.L.; Meza-Herrera, C.A.; Gonzalez-Bulnes, A. Nutritional and metabolic modulation of the male effect on the resumption of ovulatory activity in goats. Anim. Prod. Sci. 2011, 51, 115–122. [Google Scholar]
- Urrutia Morales, J.; Rosales Nieto, C.A.; Vera Ávila, H.R.; Villagomez Amezcua Manjarres, E. Resumption of ovarian activity is modified by non-photoperiodic environmental cues in Criollo goats in tropical latitudes. Small Rum. Res. 2016, 137, 9–16. [Google Scholar]
- Flores-Najera, M.J.; Vélez-Monroy, L.I.; Sánchez-Duarte, J.I.; Cuevas-Reyes, V.; Mellado, M.; Rosales-Nieto, C.A. Milk yield and composition and body weight of offsprings of mixed-breed goats on semiarid rangelands with different rainfall. Trop. Anim. Health Prod. 2020, 52, 3799–3808. [Google Scholar] [PubMed]
- Lopez-Flores, N.M.; Meza-Herrera, C.A.; Perez-Marin, C.; Blache, D.; Arellano-Rodríguez, G.; Zuñiga-Garcia, S.; Navarrete-Molina, C.; García De la Peña, C.; Rosales-Nieto, C.A.; Veliz-Deras, F.G. Precision betacarotene supplementation enhanced ovarian function and the LH release pattern in yearling crossbred anestrous goats. Animals 2020, 10, 659. [Google Scholar]
- Cuevas Reyes, V.; Santiago Hernandez, F.; Flores Najera, M.d.J.; Vazquez Garcia, J.M.; Urrutia Morales, J.; Hosseini-Ghaffari, M.; Chay-Canul, A.; Meza-Herrera, C.A.; Gonzalez-Bulnes, A.; Martin, G.B.; et al. Intake of spineless cladodes of Opuntia ficus-indica during late pregnancy improves progeny performance in underfed sheep. Animals 2020, 10, 995. [Google Scholar]
- Rosales-Nieto, C.A.; Rodríguez-Aguilar, M.; Santiago-Hernandez, F.; Cuevas-Reyes, V.; Flores-Najera, M.J.; Vázquez-García, J.M.; Urrutia-Morales, J.; Ghaffari, M.H.; Meza-Herrera, C.A.; González-Bulnes, A.; et al. Periconceptional nutrition with spineless cactus (Opuntia ficus-indica) improves metabolomic profiles and pregnancy outcomes in sheep. Sci. Rep. 2021, 11, 7214. [Google Scholar]
- Smith, L.B.; Downs, R.J. Flora Neotropica, Monograph, 14, Part 1, (Pitcairnioideae, Bromeliaceae); Hafner Press: New York, NY, USA, 1974. [Google Scholar]
- Mondragón, D.; Durán, R.; Ramírez, I.; Valverde, T. Temporal variation in the demography of the clonal epiphyte Tillandsia brachycaulos (Bromeliaceae) in the Yucatán Peninsula, Mexico. J. Trop. Ecol. 2004, 20, 189–200. [Google Scholar]
- Vergara-Torres, C.A.; Pacheco-Álvarez, M.C.; Flores-Palacios, A. Host preference and host limitation of vascular epiphytes in a tropical dry forest of central Mexico. J. Trop. Ecol. 2010, 26, 563–570. [Google Scholar]
- Flores-Palacios, A.; Barbosa-Duchateau, C.L.; Valencia-Díaz, S.; Capistrán-Barradas, A.; García-Franco, J.G. Direct and indirect effects of Tillandsia recurvata on Prosopis laevigata in the Chihuahua desert scrubland of San Luis Potosi, Mexico. J. Arid Environ. 2014, 104, 88–95. [Google Scholar]
- Flores-Palacios, A.; Bustamante-Molina, A.B.; Corona-López, A.M.; Valencia-Díaz, S. Seed number, germination and longevity in wild dry forest Tillandsia species of horticultural value. Sci. Hort. 2015, 187, 72–79. [Google Scholar]
- Soria, N.F.; Torres, C.; Galetto, L. Experimental evidence of an increased leaf production in Prosopis after removal of epiphytes (Tillandsia). Flora Morphol. Distrib. Func. Ecol. Plants 2014, 209, 580–586. [Google Scholar]
- Flores-Palacios, A.; García-Franco, J.G.; Capistrán-Barradas, A. Biomass, phorophyte specificity and distribution of Tillandsia recurvata in a tropical semi-desert environment (Chihuahuan Desert, Mexico). Plant Ecol. Evol. 2015, 148, 68–75. [Google Scholar]
- Gámez Vázquez, H.G.; Urrutia Morales, J.; Rosales Nieto, C.A.; Meza-Herrera, C.A.; Echavarría Chaires, F.G.; Beltrán López, S. Tillandsia recurvata and its chemical value as an alternative use for feeding ruminants in northern Mexico. J. Appl. Anim. Res. 2018, 46, 295–300. [Google Scholar]
- Rosales Nieto, C.A.; Ferguson, M.; MacLeay, C.; Briegel, J.; Wood, D.; Martin, G.; Thompson, A. Ewe lambs with higher breeding values for growth achieve higher reproductive performance when mated at age 8 months. Theriogenology 2013, 80, 427–435. [Google Scholar]
- Rosales Nieto, C.A.; Ferguson, M.B.; Thompson, H.; Briegel, J.R.; Macleay, C.A.; Martin, G.B.; Thompson, A.N. Relationships among puberty, muscle and fat, and liveweight gain during mating in young female sheep. Reprod. Dom. Anim. 2015, 50, 637–642. [Google Scholar]
- Rosales-Nieto, C.A.; Ehrhardt, R.; Mantey, A.; Makela, B.; Byrem, T.; Veiga-Lopez, A. Preconceptional diet manipulation and fetus number can influence placenta endocrine function in sheep. Dom. Anim. Endocrinol. 2021, 74, 106577. [Google Scholar]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen; Universidad Nacional Autónoma de México: Mexico City, México, 1973. [Google Scholar]
- National Research Council (US). Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids and New World Camelids; National Academies Press: Washington, DC, USA, 2007; 292p. [Google Scholar]
- Treseler, K.M. Laboratorio Clínico y Pruebas de Diagnóstico, 3rd ed.; Manual Moderno: Mexico City, México, 2008; 616p. [Google Scholar]
- Velázquez, S.R. Manual de Prácticas Bioquímica Clínica; Facultad de Química, Universidad Nacional Autónoma de México: Mexico City, México, 2009; 160p, Available online: http://depa.fquim.unam.mx/amyd/archivero/MANUALBIOQUIMICACLINICA_10817.pdf (accessed on 1 September 2021).
- Rincón-Delgado, R.M.; Meza-López, C. Manual de Prácticas de Laboratorio de Patología Clínica; Actualización No. 3; Unidad Académica de Medicina Veterinaria: Gral Enrique Estrada, México, 2014; 60p. [Google Scholar]
- Jones, M.L.; Allison, R.W. Evaluation of the ruminant complete blood cell count. Vet. Clin. N. Am. Food Anim. Prac. 2007, 23, 377–402. [Google Scholar]
- SAS Institute 2000. JMP Star Statistics, Ver 4.0.3; SAS Institute: Cary, NC, USA, 2000. [Google Scholar]
- Mavrommatis, A.; Giamouri, E.; Tavrizelou, S.; Zacharioudaki, M.; Danezis, G.; Simitzis, P.E.; Zoidis, E.; Tsiplakou, E.; Pappas, A.C.; Georgiou, C.A.; et al. Impact of mycotoxins on animals’ oxidative status. Antioxidants 2021, 10, 214. [Google Scholar]
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Manyes, L. Toxicity of mycotoxins in vivo on vertebrate organisms: A review. Food Chem. Toxicol. 2020, 137, 111161. [Google Scholar]
- Bani Ismail, Z.A.; Al-Majali, A.M.; Amireh, F.; Al-Rawashdeh, O.F. Metabolic profiles in goat does in late pregnancy with and without subclinical pregnancy toxemia. Vet. Clin. Pathol. 2008, 37, 434–437. [Google Scholar]
- Bennis, A.; de La Farge, F.; Bézille, P.; Valdiguié, P.; Rico, A.G.; Braun, J.P. Effects of age of newborn and delivery by female goats on plasma lipids and lipoproteins. Small Rumin. Res. 1992, 9, 243–253. [Google Scholar]
- Zabaleta, J.; Pérez, M.L.; Riera, M.; Nieves, L.; Vila, V. Concentración de glucosa y triglicéridos en el suero sanguíneo de cabras de la raza canaria durante el período de transición. Rev. Cient. Fac. Cienc. Vet. 2012, 22, 225–230. [Google Scholar]
- Mazur, A.; Ozgo, M.; Rayssiguier, Y. Altered plasma triglyceride-rich lipoproteins and triglyceride secretion in feed-restricted pregnant ewes. Vet. Med. 2009, 54, 412–418. [Google Scholar]
- Silanikove, N. The physiological basis of adaptation in goats to harsh environments. Small Rum. Res. 2000, 35, 181–193. [Google Scholar]
- Matheus, N.; Figueiredo, A. Peso corporal: Su relación con la concentración sérica de proteínas, lípidos y glucosa en cabras mestizas criollas. Gac. Cienc. Vet. 2004, 9, 38–43. [Google Scholar]
- Mellado, M.; Meza-Herrera, C.A.; Arévalo, J.R.; García, J.E.; Veliz, F.G. Effect of dietary energy intake and somatotropin administration after weaning on growth rate and semen characteristics of Granadina goat bucks. Turk. J. Vet. Anim. Sci. 2012, 36, 338–345. [Google Scholar]
- Harmeyer, J.; Martens, H. Aspects of urea metabolism in ruminants with reference to the goat. J. Dairy Sci. 1980, 63, 1707–1728. [Google Scholar]
- Benjamin, M.M. Patología Clínica en Veterinaria, 3rd ed.; Noriega-Limusa: Ciudad de México, México, 1984; 458p. [Google Scholar]
- Spears, J.W. Minerals in forages. In Forage Quality, Evaluation, and Utilization; ASA, CSSA: Madison, WI, USA, 1994; pp. 281–317. [Google Scholar]
- Canonne-Hergaux, F.; Zhang, A.-S.; Ponka, P.; Gros, P. Characterization of the iron transporter DMT1 (NRAMP2/DCT1) in red blood cells of normal and anemic mk/mkmice. Blood 2001, 98, 3823–3830. [Google Scholar]
- Stoltzfus, R.J.; Dreyfuss, M.L. Guidelines for the Use of Iron Supplements to Prevent and Treat Iron Deficiency Anemia; INACG/WHO/Unicef; ILSI Press: Washington, DC, USA, 1998. [Google Scholar]
- Rasmussen, K.M. Is there a causal relationship between iron deficiency or iron-deficiency anemia and weight at birth, length of gestation and perinatal mortality? J. Nut. 2001, 131, 590S–603S. [Google Scholar]
- Killip, S.; Bennett, J.M.; Chambers, M.D. Iron deficiency anemia. Am. Fam. Phys. 2007, 75, 671–678. [Google Scholar]
- Flores-Najera, M.J.; Cuevas-Reyes, V.; Vázquez-García, J.M.; Beltrán-López, S.; Meza-Herrera, C.A.; Mellado, M.; Negrete-Sánchez, L.O.; Rivas-Jacobo, M.A.; Rosales-Nieto, C.A. Milk yield and composition of mixed-breed goats on rangeland during the dry season and the effect on the growth of their progeny. Biology 2021, 10, 220. [Google Scholar]
- Antunović, Z.; Novoselec, J.; Klir, Ž. Hematological parameters in ewes during lactation in organic farming. Poljoprivreda 2017, 23, 46–52. [Google Scholar]
- Orden, E.A.; Serra, A.B.; Serra, S.D.; Nakamura, K.; Cruz, L.C.; Fujihara., T. Direct effects of copper and selenium supplementation and its subsequent effects on other plasma minerals, body weight and hematocrit count of grazing Philippine goats. Asian-Australas. J. Anim. Sci. 2000, 13, 323–328. [Google Scholar]
- Aslinia, F.; Mazza, J.J.; Yale, S.H. Megaloblastic anemia and other causes of macrocytosis. Clin. Med. Res. 2006, 4, 236–241. [Google Scholar] [PubMed] [Green Version]
- Adams, R.S.; Kephart, K.B.; Ishler, V.A.; Hutchinson, L.J.; Roth, G.W. Mold and Mycotoxin Problems in Livestock Feeding; Penn State University, College of Agricultural Sciences: University Park, PA, USA, 1993; Volume 289027. [Google Scholar]
- Swamy, H.V.L.N.; Smith, T.K.; MacDonald, E.J.; Karrow, N.A.; Woodward, B.; Boermans, H.J. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on growth and immunological measurements of starter pigs, and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J. Anim. Sci. 2003, 81, 2792–2803. [Google Scholar]
- Blache, D.; Maloney, S.K.; Revell, D.K. Use and limitations of alternative feed resources to sustain and improve reproductive performance in sheep and goats. Anim. Feed Sci. Technol. 2008, 147, 140–157. [Google Scholar]
- Valencia-Díaz, S.; Flores-Palacios, A.; Rodríguez-López, V.; Jiménez-Aparicio, A.R. Effects of Tillandsia recurvata extracts on the seed germination of Tillandsia spp. Allelop. J. 2012, 29, 125–135. [Google Scholar]
- Wang, S.; Alseekh, S.; Fernie, A.R.; Luo, J. The structure and function of major plant metabolite modifications. Mol. Plant 2019, 12, 899–919. [Google Scholar] [PubMed]
- Federation Animal Science Society. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, 3rd ed.; Federation Animal Science Society: Champaing, IL, USA, 2010; p. 177. [Google Scholar]
- NAM-National Academy of Medicine. Guide for the Care and Use of Laboratory Animals. Co-Produced by the National Academy of Medicine–Mexico and the Association for Assessment and Accreditation of Laboratory Animal Care International, 1st ed.; Harlan: Mexico City, Mexico, 2010. [Google Scholar]
| Ingredient Composition (% in Diet) | Treatment | DM (%) | CP (%) | ME (Mcal) | ||
|---|---|---|---|---|---|---|
| T0 | T30 | T60 | ||||
| Ground sorghum grain | 32 | 32 | 32 | 88 | 7.7 | 3.07 |
| Soybean meal | 8 | 8 | 8 | 89 | 30.4 | 4.99 |
| Tillandsia recurvata | 0 | 30 | 60 | 53 | 6.1 | 0.58 |
| Sorghum stubble | 60 | 30 | 0 | 88 | 5.2 | 1.95 |
| Nutrient composition experimental diet | ||||||
| Crude protein, % | 6.58 | 6.91 | 7.25 | |||
| Metabolizable energy (Mcal kg−1 DM) | 1.49 | 1.29 | 1.09 | |||
| Variable | Dietary Treatment | ||
|---|---|---|---|
| T0 | T30 | T60 | |
| Live weight (kg) | 34.4 ± 0.6 | 34.2 ± 0.5 | 34.5 ± 0.5 |
| Metabolic Profile | |||
| Total proteins (g dL−1) | 6.01 ± 0.42 | 6.03 ± 0.01 | 6.08 ± 0.57 |
| Glucose (mg dL−1) | 62.5 ± 8.2 | 59.2 ± 3.5 | 56.3 ± 2.2 |
| Triglycerides (mg dL−1) | 57.7 ± 11.7 | 53.9 ± 5.9 | 55.9 ± 10.6 |
| Calcium (mg dL−1) | 8.4 ± 0.1 | 8.4 ± 1.5 | 8 ± 0.6 |
| Phosphorus (mg dL−1) | 5.8 ± 0.7 | 5.9 ± 0.6 | 6.1 ± 1.3 |
| Complete Blood Count | |||
| Hematocrit (%) | 33.3 ± 4.9 | 38.7 ± 6.0 | 33.2 ± 4.5 |
| Hemoglobin (g/dL) | 10.1 ± 3.0 | 10.0 ± 2.6 | 10.4 ± 1.7 |
| Red blood cells (106/mL) | 5.1 ± 0.9 | 5.2 ± 1 | 4.7 ± 1.1 |
| White blood cells (103/mL) | 11.6 ± 2.6 | 9.7 ± 4.7 | 10.2 ± 3.9 |
| MCV (fl) | 20.2 ± 2.8 | 19.2 ± 2.9 | 19.5 ± 2.8 |
| MCH (pg/cell) | 21.1 ± 9.3 | 19.8 ± 6.7 | 23.2 ± 6.4 |
| MCHC (%) | 30.4 ± 8.7 | 26.7 ± 8.9 | 31.5 ± 7.4 |
| Secondary Metabolite | As Sampled Basis | Dry Matter Basis |
|---|---|---|
| Dry Matter (%) | 46.3 | |
| Aflatoxins, mg/g (10−9) | 4.5 | 9.7 |
| Zearalenone, mg/g (10−9) | 45.4 | 98.2 |
| Deoxynivalenol, mg/kg | 1.36 | 2.93 |
| Trichothecene, mg/kg (10−9) | 65.4 | 141.3 |
| Variable | TRT | Experimental Day | Significance | |||
|---|---|---|---|---|---|---|
| 28 | 56 | TRT | Time (T) | TRT × T | ||
| Total proteins (g dL−1) | T0 | 6.19 ± 0.13 | 6.11 ± 0.12 | NS | NS | NS |
| T30 | 5.74 ± 0.11 | 5.66 ± 0.11 | ||||
| T60 | 5.86 ± 0.12 | 5.6 ± 0.2 | ||||
| Glucose (mg dL−1) | T0 | 60.3 ± 4.04 | 49.9 ± 2.64 | NS | *** | NS |
| T30 | 55.7 ± 3.42 | 51.9 ± 3.02 | ||||
| T60 | 57.5 ± 3.18 | 44.2 ± 2.42 | ||||
| Triglycerides (mg dL−1) | T0 | 61.9 ± 1.6 | 71.8 ± 2.1 | NS | *** | NS |
| T30 | 63.1 ± 3.5 | 69.2 ± 1.65 | ||||
| T60 | 63 ± 2.6 | 71.2 ± 2.05 | ||||
| Calcium (mg dL−1) | T0 | 7.49 ± 0.2 | 8.64 ± 0.34 | NS | ** | NS |
| T30 | 7.91 ± 0.3 | 8.58 ± 0.23 | ||||
| T60 | 7.56 ± 0.2 | 7.92 ± 0.34 | ||||
| Phosphorus (mg dL−1) | T0 | 5.63 ± 0.25 | 5.65 ± 0.23 | NS | *** | NS |
| T30 | 5.64 ± 0.26 | 4.8 ± 0.13 | ||||
| T60 | 5.6 ± 0.37 | 4.62 ± 0.17 | ||||
| Variable | Day of Feeding Trial | Significance | ||
|---|---|---|---|---|
| 56 | ||||
| T0 | T30 | T60 | ||
| Hematocrit (%) | 32.2 ± 1.53 | 35.3 ± 1.52 | 29.9 ± 1.64 | 0.06 |
| Hemoglobin (g/dL) | 11.3 ± 0.89 | 10.3 ± 0.58 | 9.0 ± 0.59 | NS |
| Red blood cells (106/mL) | 6.64 ± 0.75 ab | 5.89 ± 0.37 b | 9.44 ± 1.25 a | ** |
| White blood cells (103/mL) | 9.27 ± 0.79 b | 12.0 ± 0.71 a | 9.34 ± 1.08 b | * |
| MCV (fl) | 45.3 ± 4.98 a | 21.0 ± 0.71 b | 23.4 ± 0.55 b | *** |
| MCH (pg/cell) | 19.6 ± 3.14 a | 18.7 ± 1.61 a | 10.7 ± 1.34 b | * |
| MCHC (%) | 34.4 ± 2.49 | 30.1 ± 2.01 | 30.5 ± 1.89 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gámez-Vázquez, H.G.; Rosales-Nieto, C.A.; Urrutia-Morales, J.; Mellado, M.; Meza-Herrera, C.A.; Vázquez-García, J.M.; Hernández-Arteaga, L.E.S.; Negrete-Sánchez, L.O.; Loredo-Osti, C.; Rivas-Jacobo, M.A.; et al. Effect of Replacing Sorghum Stubble with Tillandsia recurvata (L.) on Liveweight Change, Blood Metabolites, and Hematic Biometry of Goats. Biology 2022, 11, 517. https://doi.org/10.3390/biology11040517
Gámez-Vázquez HG, Rosales-Nieto CA, Urrutia-Morales J, Mellado M, Meza-Herrera CA, Vázquez-García JM, Hernández-Arteaga LES, Negrete-Sánchez LO, Loredo-Osti C, Rivas-Jacobo MA, et al. Effect of Replacing Sorghum Stubble with Tillandsia recurvata (L.) on Liveweight Change, Blood Metabolites, and Hematic Biometry of Goats. Biology. 2022; 11(4):517. https://doi.org/10.3390/biology11040517
Chicago/Turabian StyleGámez-Vázquez, Héctor G., César A. Rosales-Nieto, Jorge Urrutia-Morales, Miguel Mellado, César A. Meza-Herrera, Juan M. Vázquez-García, Luisa E. S. Hernández-Arteaga, Luis O. Negrete-Sánchez, Catarina Loredo-Osti, Marco A. Rivas-Jacobo, and et al. 2022. "Effect of Replacing Sorghum Stubble with Tillandsia recurvata (L.) on Liveweight Change, Blood Metabolites, and Hematic Biometry of Goats" Biology 11, no. 4: 517. https://doi.org/10.3390/biology11040517
APA StyleGámez-Vázquez, H. G., Rosales-Nieto, C. A., Urrutia-Morales, J., Mellado, M., Meza-Herrera, C. A., Vázquez-García, J. M., Hernández-Arteaga, L. E. S., Negrete-Sánchez, L. O., Loredo-Osti, C., Rivas-Jacobo, M. A., & Beltrán-López, S. (2022). Effect of Replacing Sorghum Stubble with Tillandsia recurvata (L.) on Liveweight Change, Blood Metabolites, and Hematic Biometry of Goats. Biology, 11(4), 517. https://doi.org/10.3390/biology11040517







