Significance of Lipid Fatty Acid Composition for Resistance to Winter Conditions in Asplenium scolopendrium
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
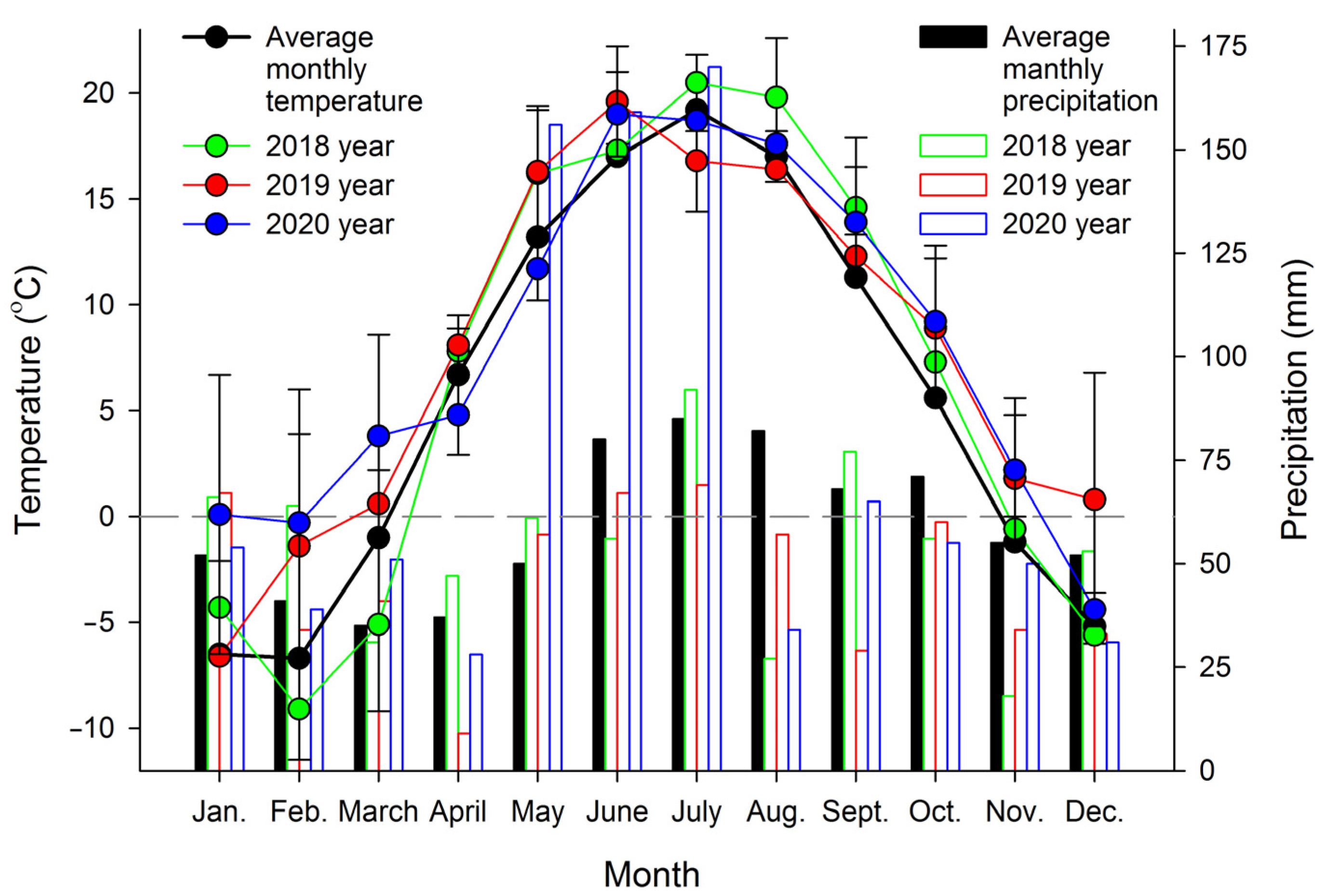
2.1. Plant Materials
2.2. Lipid Extraction
2.3. Preparation of Fatty Acid Methyl Esters
2.4. Data and Statistical Analyses
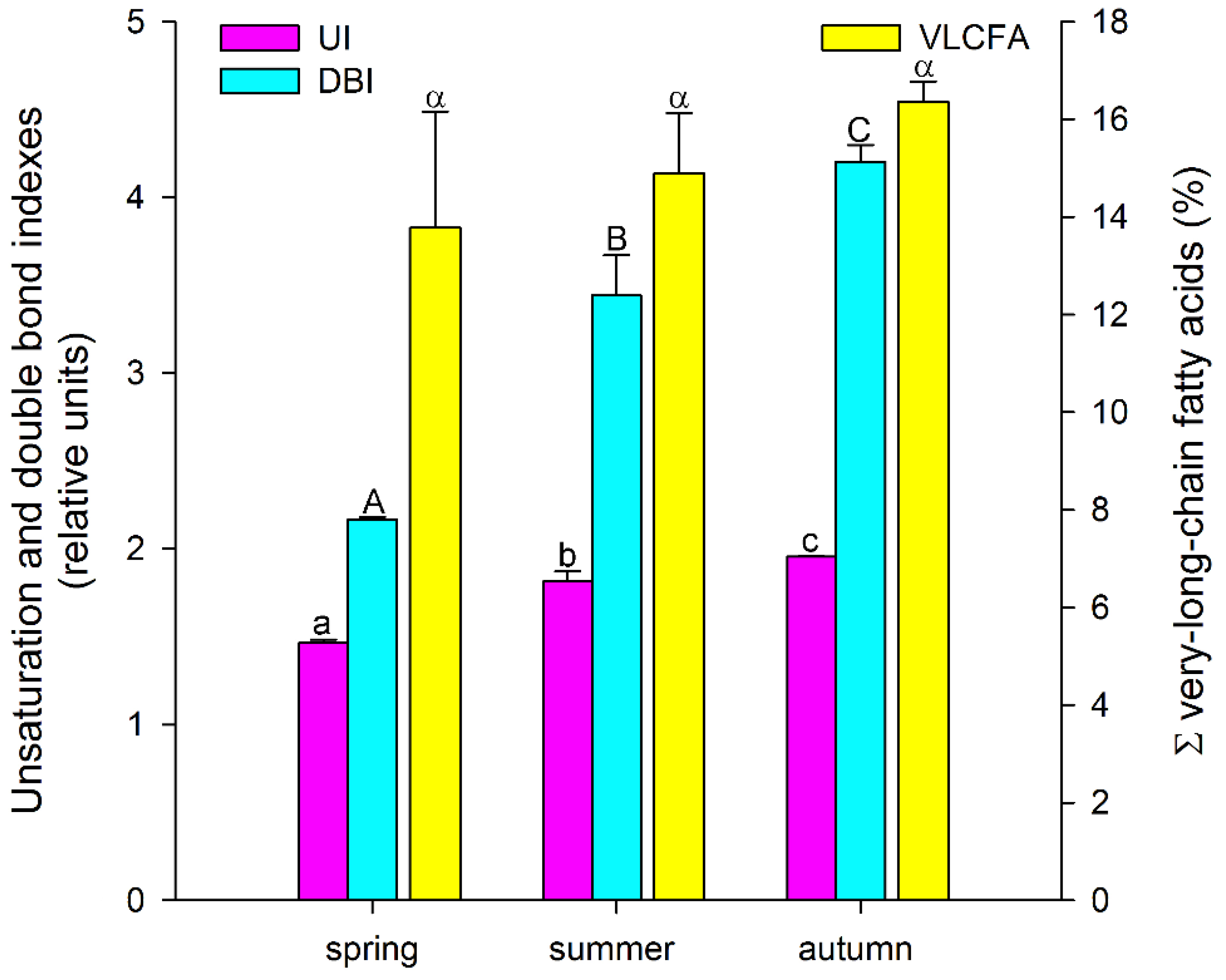
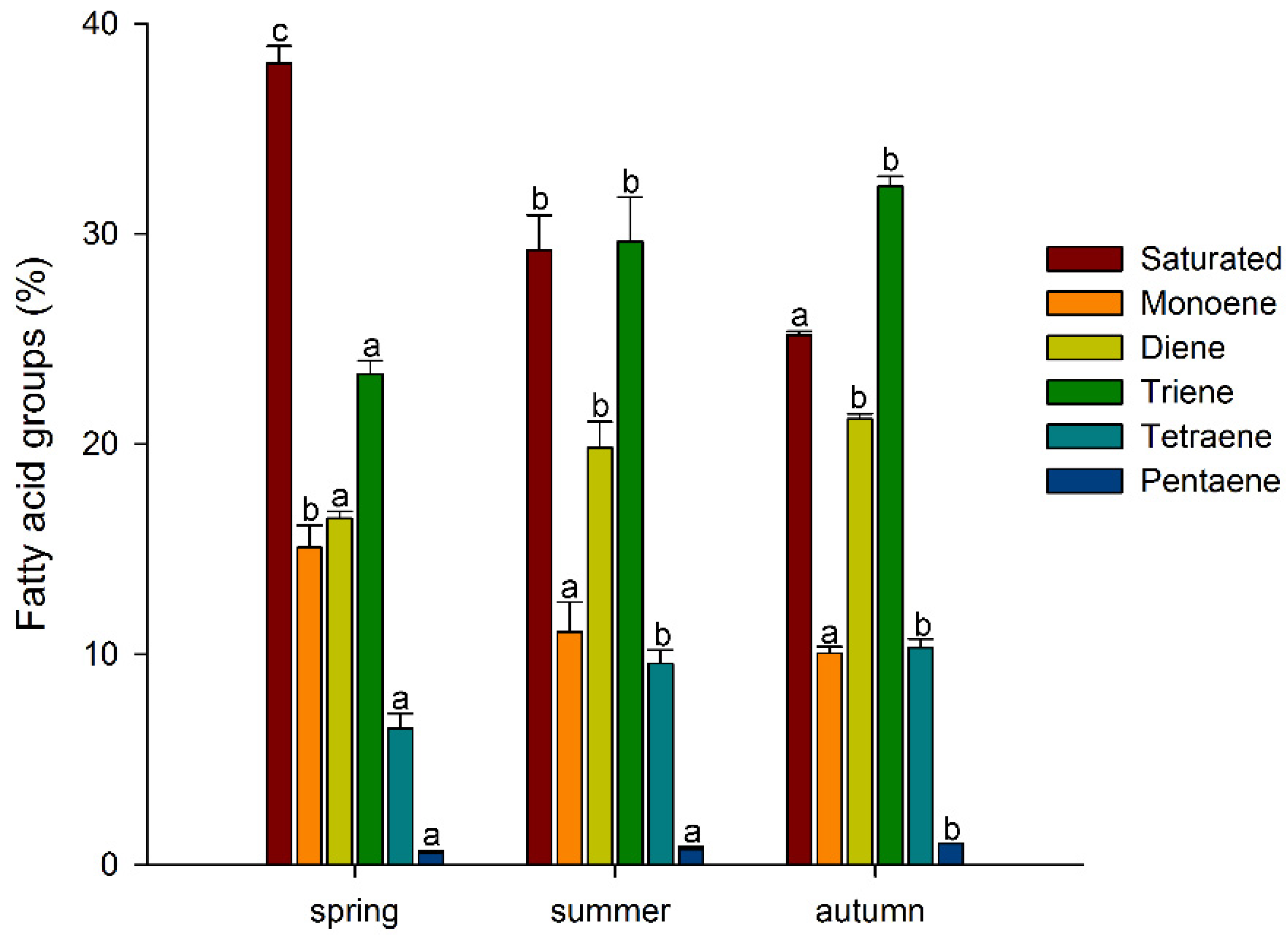
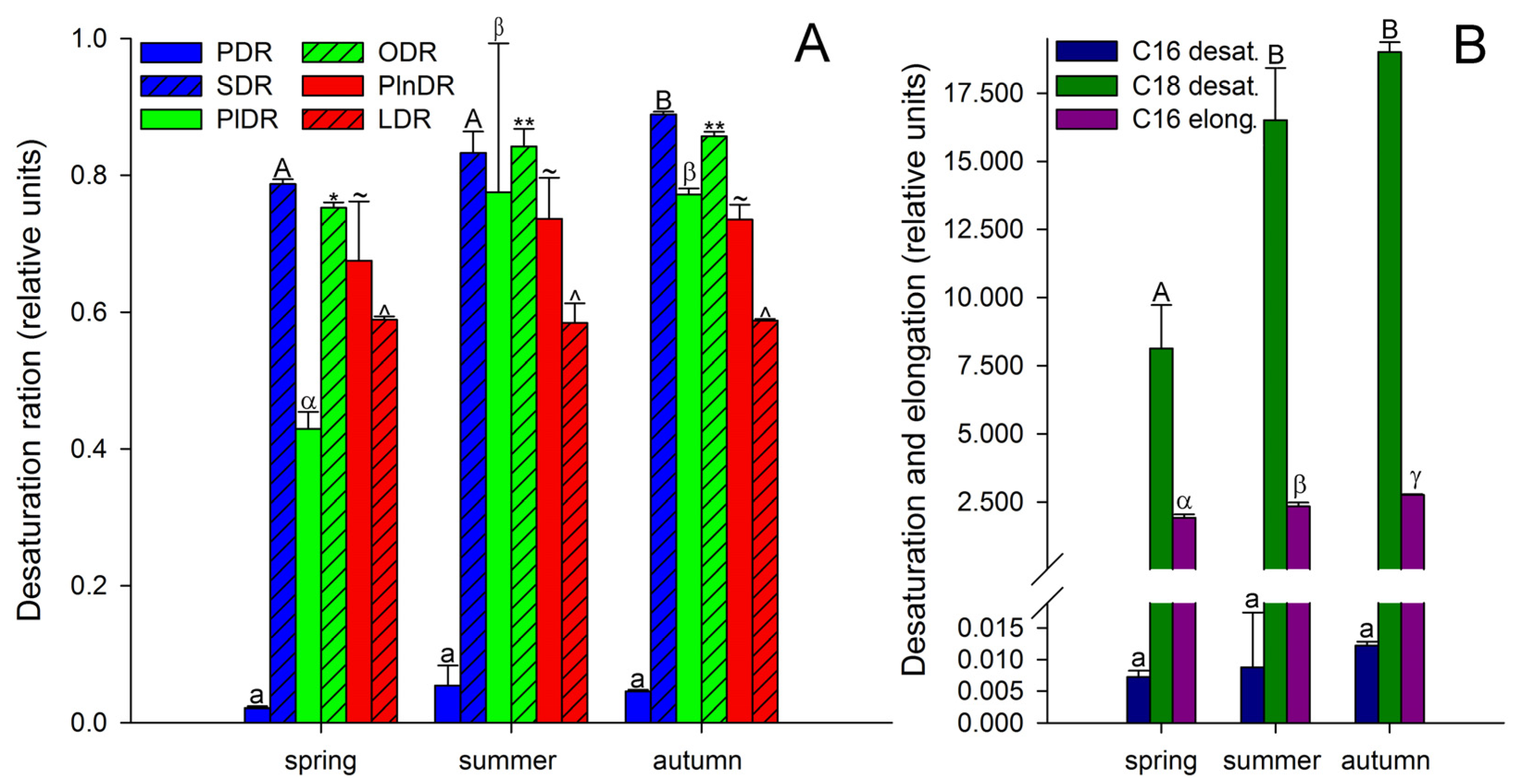
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DiMichele, W.A.; Phillips, T.L. The ecology of Paleozoic ferns. Rev. Palaeobot. Palynol. 2002, 119, 143–159. [Google Scholar] [CrossRef]
- Pryer, K.M.; Schuettpelz, E.; Wolf, P.G.; Schneider, H.; Smith, A.R.; Cranfill, R. Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. Am. J. Bot. 2004, 91, 1582–1598. [Google Scholar] [CrossRef] [PubMed]
- Lidgard, S.; Crane, P.R. Angiosperm diversification and Cretaceous floristic trends: A comparison of palynofloras and leaf macrofloras. Paleobiology 1990, 16, 77–93. [Google Scholar] [CrossRef]
- Crane, P.R.; Friis, E.M.; Pedersen, K.R. The origin and early diversification of angiosperms. Nature 1995, 374, 27–33. [Google Scholar] [CrossRef]
- Nagalingum, N.; Drinnan, A.; Lupia, R.; McLoughlin, S. Fern spore diversity and abundance in Australia during the Cretaceous. Rev. Palaeobot. Palynol. 2002, 119, 69–92. [Google Scholar] [CrossRef]
- Schneider, H.; Schuettpelz, E.; Pryer, K.M.; Cranfill, R.; Magallón, S.; Lupia, R. Ferns diversified in the shadow of angiosperms. Nature 2004, 428, 553–557. [Google Scholar] [CrossRef]
- Schuettpelz, E.; Pryer, K.M. Evidence for a Cenozoic radiation of ferns in an angiosperm-dominated canopy. Proc. Natl. Acad. Sci. USA 2009, 106, 11200–11205. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Ivanova, T.V. Fatty Acids Composition of the Epiphytic Ferns, Platycerium bifurcatum and Asplenium nidus, and the Terrestrial Fern, Asplenium trichomanes. Am. Fern J. 2021, 111, 117–128. [Google Scholar] [CrossRef]
- Rickard, M. Ferns for a Cool Temperate Climate; Pemberley Books: Iver, UK, 2021; ISBN 9781785008917. [Google Scholar]
- Peterson, K.M. Plants in Arctic Environments. In Ecology and the Environment; Springer: New York, NY, USA, 2014; pp. 363–388. [Google Scholar]
- McKenzie, E.H.C. Rust fungi in the subantarctic islands of New Zealand. Mycoscience 2008, 49, 1–10. [Google Scholar] [CrossRef]
- Bannister, P. A note on some observations on frost damage in the field, with particular reference to various ferns. Trans. Bot. Soc. Edinb. 1973, 42, 111–113. [Google Scholar] [CrossRef]
- Tessier, J.T. Reduced winter snowfall damages the structure and function of wintergreen ferns. Am. J. Bot. 2014, 101, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Sakai, A. Cold tolerance of gametophytes and sporophytes of some cool temperate ferns native to Hokkaido. Can. J. Bot. 1981, 59, 604–608. [Google Scholar] [CrossRef]
- Buchner, O.; Neuner, G. Freezing cytorrhysis and critical temperature thresholds for photosystem II in the peat moss Sphagnum capillifolium. Protoplasma 2010, 243, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Schott, R.T.; Voigt, D.; Roth-Nebelsick, A. Extracellular ice management in the frost hardy horsetail Equisetum hyemale L. Flora 2017, 234, 207–214. [Google Scholar] [CrossRef]
- Arora, R. Mechanism of freeze-thaw injury and recovery: A cool retrospective and warming up to new ideas. Plant Sci. 2018, 270, 301–313. [Google Scholar] [CrossRef]
- Konrad, W.; Schott, R.; Roth-Nebelsick, A. A model for extracellular freezing based on observations on Equisetum hyemale. J. Theor. Biol. 2019, 478, 161–168. [Google Scholar] [CrossRef]
- Loesch, R.; Biron, U.; Patrias, T.; Hoeptner, B. Gas exchange and water relations of Asplenium ferns growing on limestone rocks. Nov. Hedwig. 2007, 131, 221–236. [Google Scholar]
- Rascio, N.; Rocca, N. La Resurrection Plants: The Puzzle of Surviving Extreme Vegetative Desiccation. CRC Crit. Rev. Plant Sci. 2005, 24, 209–225. [Google Scholar] [CrossRef]
- Van der Vyver, C.; Peters, S. How Do Plants Deal with Dry Days? Front. Young Minds 2017, 5, 58. [Google Scholar] [CrossRef]
- Kranner, I.; Beckett, R.P.; Wornik, S.; Zorn, M.; Pfeifhofer, H.W. Revival of a resurrection plant correlates with its antioxidant status. Plant J. 2002, 31, 13–24. [Google Scholar] [CrossRef]
- Gaff, D.F.; Oliver, M. The evolution of desiccation tolerance in angiosperm plants: A rare yet common phenomenon. Funct. Plant Biol. 2013, 40, 315. [Google Scholar] [CrossRef] [PubMed]
- López-Pozo, M.; Ballesteros, D.; Laza, J.M.; García-Plazaola, J.I.; Fernández-Marín, B. Desiccation Tolerance in Chlorophyllous Fern Spores: Are Ecophysiological Features Related to Environmental Conditions? Front. Plant Sci. 2019, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Kappen, L. Der Einfluß des Wassergehaltes auf die Widerstandsfähigkeit von Pflanzen gegenüber hohen und tiefen Temperaturen, untersucht an Blättern einiger Farne und von Ramonda myconi. Flora Allg. Bot. Ztg. Abt. A Physiol. Biochem. 1966, 156, 427–445. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Arzac, M.I.; López-Pozo, M.; Laza, J.M.; Roach, T.; Stegner, M.; Neuner, G.; García-Plazaola, J.I. Frozen in the dark: Interplay of night-time activity of xanthophyll cycle, xylem attributes, and desiccation tolerance in fern resistance to winter. J. Exp. Bot. 2021, 72, 3168–3184. [Google Scholar] [CrossRef] [PubMed]
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef]
- He, M.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- He, M.; Qin, C.-X.; Wang, X.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Biosynthesis and Regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef]
- Kachroo, P.; Shanklin, J.; Shah, J.; Whittle, E.J.; Klessig, D.F. A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc. Natl. Acad. Sci. USA 2001, 98, 9448–9453. [Google Scholar] [CrossRef]
- Lim, G.-H.; Singhal, R.; Kachroo, A.; Kachroo, P. Fatty Acid– and Lipid-Mediated Signaling in Plant Defense. Annu. Rev. Phytopathol. 2017, 55, 505–536. [Google Scholar] [CrossRef]
- Mironov, K.S.; Sidorov, R.A.; Trofimova, M.S.; Bedbenov, V.S.; Tsydendambaev, V.D.; Allakhverdiev, S.I.; Los, D.A. Light-dependent cold-induced fatty acid unsaturation, changes in membrane fluidity, and alterations in gene expression in Synechocystis. Biochim. Biophys. Acta—Bioenerg. 2012, 1817, 1352–1359. [Google Scholar] [CrossRef]
- Da Cruz, R.P.; Golombieski, J.I.; Bazana, M.T.; Cabreira, C.; Silveira, T.F.; da Silva, L.P. Alterations in fatty acid composition due to cold exposure at the vegetative stage in rice. Braz. J. Plant Physiol. 2010, 22, 199–207. [Google Scholar] [CrossRef]
- Willemot, C.; Hope, H.J.; Williams, R.J.; Michaud, R. Changes in fatty acid composition of winter wheat during frost hardening. Cryobiology 1977, 14, 87–93. [Google Scholar] [CrossRef]
- Skoczowski, A.; Filek, M.; Dubert, F. The long-term effect of cold on the metabolism of winter wheat seedlings. II. composition of fatty acids of phospholipids. J. Therm. Biol. 1994, 19, 171–176. [Google Scholar] [CrossRef]
- Nejadsadeghi, L.; Maali-Amiri, R.; Zeinali, H.; Ramezanpour, S.; Sadeghzade, B. Membrane fatty acid compositions and cold-induced responses in tetraploid and hexaploid wheats. Mol. Biol. Rep. 2015, 42, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Nokhsorov, V.V.; Dudareva, L.V.; Senik, S.V.; Chirikova, N.K.; Petrov, K.A. Influence of Extremely Low Temperatures of the Pole of Cold on the Lipid and Fatty-Acid Composition of Aerial Parts of the Horsetail Family (Equisetaceae). Plants 2021, 10, 996. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Structure and expression of fatty acid desaturases. Biochim. Biophys. Acta—Lipids Lipid Metab. 1998, 1394, 3–15. [Google Scholar] [CrossRef]
- Dong, C.-J.; Cao, N.; Zhang, Z.-G.; Shang, Q.-M. Characterization of the Fatty Acid Desaturase Genes in Cucumber: Structure, Phylogeny, and Expression Patterns. PLoS ONE 2016, 11, e0149917. [Google Scholar] [CrossRef]
- Gupta, D.B.; Rai, Y.; Gayali, S.; Chakraborty, S.; Chakraborty, N. Plant Organellar Proteomics in Response to Dehydration: Turning Protein Repertoire into Insights. Front. Plant Sci. 2016, 7, 460. [Google Scholar] [CrossRef]
- De Barajas-Lopez, J.D.; Tiwari, A.; Zarza, X.; Shaw, M.W.; Pascual, J.; Punkkinen, M.; Bakowska, J.C.; Munnik, T.; Fujii, H. Early Response to Dehydration 7 Remodels Cell Membrane Lipid Composition during Cold Stress in Arabidopsis. Plant Cell Physiol. 2021, 62, 80–91. [Google Scholar] [CrossRef]
- Quartacci, M.F. Plasma membrane lipids in the resurrection plant Ramonda serbica following dehydration and rehydration. J. Exp. Bot. 2002, 53, 2159–2166. [Google Scholar] [CrossRef]
- Gigon, A.; Matos, A.-R.; Laffray, D.; Zuily-Fodil, Y.; Pham-Thi, A.-T. Effect of Drought Stress on Lipid Metabolism in the Leaves of Arabidopsis thaliana (Ecotype Columbia). Ann. Bot. 2004, 94, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Vasheka, O.; Puglielli, G.; Crescente, M.F.; Varone, L.; Gratani, L. Anatomical and Morphological Leaf Traits of Three Evergreen Ferns (Polystichum setiferum, Polypodium interjectum and Asplenium scolopendrium). Am. Fern J. 2016, 106, 258–268. [Google Scholar] [CrossRef]
- Schäfer, H.; Rasbach, H. Asplenium × rouyi Viane (A. onopteris L. × A. scolopendrium L.) in the Azores (Aspleniaceae, Pteridophyta). Willdenowia 2000, 30, 219–227. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Ivanova, T.V.; Kumachova, T.K. The features of the fatty acid composition of Pyrus L. total lipids are determined by mountain ecosystem conditions. Plant Physiol. Biochem. 2022, 170, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, T.V.; Voronkov, A.S.; Kumakhova, T.K.; Tsydendambaev, V.D. Distinguishing Features of Fatty Acid Content and Composition in Total Lipids of Malus orientalis Uglitzk. Pericarp. Russ. J. Plant Physiol. 2020, 67, 463–471. [Google Scholar] [CrossRef]
- Voronkov, A.; Ivanova, T.; Kumachova, T. Micromorphological and biochemical features of Malus fruit: Malus domestica Borkh. and its parent species—Malus orientalis Uglitzk. Braz. J. Bot. 2020, 43, 21–28. [Google Scholar] [CrossRef]
- Voronkov, A.S.; Ivanova, T.V.; Kumachova, T.K.; Kozhevnikova, A.D.; Tsydendambaev, V.D. Polyunsaturated and Very-Long-Chain Fatty Acids Are Involved in the Adaptation of Maloideae (Rosaceae) to Combined Stress in the Mountains. Chem. Biodivers. 2020, 17, e1900588. [Google Scholar] [CrossRef] [PubMed]
- Voronkov, A.S.; Ivanova, T.V.; Kuznetsova, E.I.; Kumachova, T.K. Adaptations of Malus domestica Borkh. (Rosaceae) Fruits Grown at Different Altitudes. Russ. J. Plant Physiol. 2019, 66, 922–931. [Google Scholar] [CrossRef]
- Lyons, J.M.; Wheaton, T.A.; Pratt, H.K. Relationship between the Physical Nature of Mitochondrial Membranes and Chilling Sensitivity in Plants. Plant Physiol. 1964, 39, 262–268. [Google Scholar] [CrossRef]
- Wismer, W.V.; Worthing, W.M.; Yada, R.Y.; Marangoni, A.G. Membrane lipid dynamics and lipid peroxidation in the early stages of low-temperature sweetening in tubers of Solanum tuberosum. Physiol. Plant. 1998, 102, 396–410. [Google Scholar] [CrossRef]
- Lee, S.H.; Ahn, S.J.; Im, Y.J.; Cho, K.; Chung, G.-C.; Cho, B.-H.; Han, O. Differential impact of low temperature on fatty acid unsaturation and lipoxygenase activity in figleaf gourd and cucumber roots. Biochem. Biophys. Res. Commun. 2005, 330, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, T.J.; Trieu, H.H.; Topp, B.; Russell, D.; Pun, S.; Torrisi, C.; Liu, D. Assessing Fatty Acid Profiles of Macadamia Nuts. HortScience 2019, 54, 633–637. [Google Scholar] [CrossRef]
- Bettaieb, I.; Zakhama, N.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Water deficit effects on Salvia officinalis fatty acids and essential oils composition. Sci. Hortic. 2009, 120, 271–275. [Google Scholar] [CrossRef]
- Hamrouni, I.; Salah, H.B.; Marzouk, B. Effects of water-deficit on lipids of safflower aerial parts. Phytochemistry 2001, 58, 277–280. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Blée, E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 2002, 7, 315–322. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Schenk, P.M.; Dennis, P.G. Jasmonic acid signalling and the plant holobiont. Curr. Opin. Microbiol. 2017, 37, 42–47. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Qin, C.; Wood, T.; Olafsdottir, G.; Welti, R.; Wang, X. The oleate-stimulated phospholipase D, PLD, and phosphatidic acid decrease H2O2-induced cell death in Arabidopsis. Plant Cell 2003, 15, 2285–2295. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X. A Novel Phospholipase D of Arabidopsis That Is Activated by Oleic Acid and Associated with the Plasma Membrane. Plant Physiol. 2001, 127, 1102–1112. [Google Scholar] [CrossRef]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. BioEssays 2006, 28, 1091–1101. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Maiorova, O.V.; Orlova, Y.V.; Kuznetsova, E.I.; Khalilova, L.A.; Myasoedov, N.A.; Balnokin, Y.V.; Tsydendambaev, V.D. Cell ultrastructure and fatty acid composition of lipids in vegetative organs of Chenopodium album L. under salt stress conditions. Russ. J. Plant Physiol. 2016, 63, 763–775. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Voronkov, A.S.; Kuznetsova, E.I.; Kumachova, T.K.; Zhirov, V.K.; Tsydendambaev, V.D. Lipid Fatty Acids from the Pericarp of Cydonia oblonga Mill. and Mespilus germanica L. are Involved in Plant Adaptation to Altitudinal Zonality. Dokl. Biochem. Biophys. 2019, 486, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Sinetova, M.A.; Sidorov, R.A.; Starikov, A.Y.; Voronkov, A.S.; Medvedeva, A.S.; Krivova, Z.V.; Pakholkova, M.S.; Bachin, D.V.; Bedbenov, V.S.; Gabrielyan, D.A.; et al. Assessment of the Biotechnological Potential of Cyanobacterial and Microalgal Strains from IPPAS Culture Collection. Appl. Biochem. Microbiol. 2020, 56, 794–808. [Google Scholar] [CrossRef]
- Beike, A.K.; Jaeger, C.; Zink, F.; Decker, E.L.; Reski, R. High contents of very long-chain polyunsaturated fatty acids in different moss species. Plant Cell Rep. 2014, 33, 245–254. [Google Scholar] [CrossRef]
- Pejin, B.; Vujisic, L.; Sabovljevic, A.; Sabovljevic, M.; Tesevic, V.; Vajs, V. Fatty acids of some moss species from Germany. Asian J. Chem. 2011, 23, 5187–5188. [Google Scholar]
- Nekrasov, E.V.; Shelikhan, L.A.; Svetashev, V.I. Fatty acid composition of gametophytes of Matteuccia struthiopteris (L.) Tod. (Onocleaceae, Polypodiophyta). Bot. Pac. 2019, 8, 63–66. [Google Scholar] [CrossRef][Green Version]
- Nekrasov, E.V.; Svetashev, V.I.; Khrapko, O.V.; Vyssotski, M.V. Variability of fatty acid profiles in ferns: Relation to fern taxonomy and seasonal development. Phytochemistry 2019, 162, 47–55. [Google Scholar] [CrossRef]
- Barrero-Sicilia, C.; Silvestre, S.; Haslam, R.P.; Michaelson, L.V. Lipid remodelling: Unravelling the response to cold stress in Arabidopsis and its extremophile relative Eutrema salsugineum. Plant Sci. 2017, 263, 194–200. [Google Scholar] [CrossRef]
- Hayward, S.A.L.; Murray, P.A.; Gracey, A.Y.; Cossins, A.R. Beyond the Lipid Hypothesis. In Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks; Springer: New York, NY, USA, 2007; pp. 132–142. [Google Scholar]
- Ferrante, G.; Ohno, Y.; Kates, M. Influence of temperature and growth phase on desaturase activity of the mesophilic yeast Candida lipolytica. Can. J. Biochem. Cell Biol. 1983, 61, 171–177. [Google Scholar] [CrossRef]
- Jones, A.L.; Harwood, J.L.; Lloyd, D. Induction of Δ12-desaturase activity during temperature adaptation in Acanthamoeba castellanii. Biochem. Soc. Trans. 1992, 20, 170S. [Google Scholar] [CrossRef]
- Sayanova, O.V.; Napier, J.A. Eicosapentaenoic acid: Biosynthetic routes and the potential for synthesis in transgenic plants. Phytochemistry 2004, 65, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Shanab, S.M.M.; Hafez, R.M.; Fouad, A.S. A review on algae and plants as potential source of arachidonic acid. J. Adv. Res. 2018, 11, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Zhukov, A.V.; Shumskaya, M. Very-long-chain fatty acids (VLCFAs) in plant response to stress. Funct. Plant Biol. 2020, 47, 695. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Reynolds, K.B.; Taylor, M.C.; Zhou, X.; Vanhercke, T.; Wood, C.C.; Blanchard, C.L.; Singh, S.P.; Petrie, J.R. Metabolic engineering of medium-chain fatty acid biosynthesis in Nicotiana benthamiana plant leaf lipids. Front. Plant Sci. 2015, 6, 164. [Google Scholar] [CrossRef]
- Gu, H.; Jinkerson, R.E.; Davies, F.K.; Sisson, L.A.; Schneider, P.E.; Posewitz, M.C. Modulation of Medium-Chain Fatty Acid Synthesis in Synechococcus sp. PCC 7002 by Replacing FabH with a Chaetoceros Ketoacyl-ACP Synthase. Front. Plant Sci. 2016, 7, 690. [Google Scholar] [CrossRef]
- Mashima, T.; Seimiya, H.; Tsuruo, T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br. J. Cancer 2009, 100, 1369–1372. [Google Scholar] [CrossRef]
- Rawsthorne, S. Carbon flux and fatty acid synthesis in plants. Prog. Lipid Res. 2002, 41, 182–196. [Google Scholar] [CrossRef]
- Takami, T.; Shibata, M.; Kobayashi, Y.; Shikanai, T. De Novo Biosynthesis of Fatty Acids Plays Critical Roles in the Response of the Photosynthetic Machinery to Low Temperature in Arabidopsis. Plant Cell Physiol. 2010, 51, 1265–1275. [Google Scholar] [CrossRef]
- Liberato, M.V.; Nascimento, A.S.; Ayers, S.D.; Lin, J.Z.; Cvoro, A.; Silveira, R.L.; Martínez, L.; Souza, P.C.T.; Saidemberg, D.; Deng, T.; et al. Medium Chain Fatty Acids Are Selective Peroxisome Proliferator Activated Receptor (PPAR) γ Activators and Pan-PPAR Partial Agonists. PLoS ONE 2012, 7, e36297. [Google Scholar] [CrossRef]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.-E.; Rajashekar, C.B.; Williams, T.D.; Wang, X. Profiling Membrane Lipids in Plant Stress Responses. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Li, L.; Li, W. Glycerolipidome responses to freezing- and chilling-induced injuries: Examples in Arabidopsis and rice. BMC Plant Biol. 2016, 16, 70. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.-H.; Skinner, D.Z. Production of reactive oxygen species by freezing stress and the protective roles of antioxidant enzymes in plants. J. Agric. Chem. Environ. 2012, 1, 34–40. [Google Scholar] [CrossRef]
- Benson, E.; Bremner, D. Oxidative Stress in the Frozen Plant. In Life in the Frozen State; CRC Press: Boca Raton, FL, USA, 2004; pp. 205–241. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Migdal, C.; Serres, M. Espèces réactives de l’oxygène et stress oxydant. Méd. Sci. 2011, 27, 405–412. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.-Y.; Li, J.; Wang, P.-Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Nobusawa, T.; Okushima, Y.; Nagata, N.; Kojima, M.; Sakakibara, H.; Umeda, M. Synthesis of Very-Long-Chain Fatty Acids in the Epidermis Controls Plant Organ Growth by Restricting Cell Proliferation. PLoS Biol. 2013, 11, e1001531. [Google Scholar] [CrossRef]
- Batsale, M.; Bahammou, D.; Fouillen, L.; Mongrand, S.; Joubès, J.; Domergue, F. Biosynthesis and Functions of Very-Long-Chain Fatty Acids in the Responses of Plants to Abiotic and Biotic Stresses. Cells 2021, 10, 1284. [Google Scholar] [CrossRef]
- Kunst, L. Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 2003, 42, 51–80. [Google Scholar] [CrossRef]
- Nekrasov, E.V.; Svetashev, V.I. Edible Far Eastern Ferns as a Dietary Source of Long-Chain Polyunsaturated Fatty Acids. Foods 2021, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acid | Spring | Summer | Autumn |
|---|---|---|---|
| 10:0 | 0.1 ± 0.1 b | * a | * a |
| 12:0 | 0.3 ± 0.0 b | * a | * a |
| 13:0 | 0.1 ± 0.0 b | * a | * a |
| 14:0 | 1.4 ± 0.1 c | 0.6 ± 0.2 b | 0.2 ± 0.0 a |
| 15:0 | 0.6 ± 0.1 c | 0.4 ± 0.1 b | 0.2 ± 0.0 a |
| 16:0 | 27.5 ± 1.6 c | 23.4 ± 1.7 b | 20.0 ± 0.3 a |
| 4-16:1 | 0.6 ± 0.3 b | * a | * a |
| 7-16:1 | 0.6 ± 0.1 a | 0.7 ± 0.7 a | 1.0 ± 0.1 a |
| 9-16:1 | 1.4 ± 0.8 a | 1.6 ± 1.0 a | 0.3 ± 0.0 a |
| 14-16:1 | 0.1 ± 0.1 b | * a | * a |
| 7,10-16:2 | 0.2 ± 0.1 a | 0.5 ± 0.1 b | 0.9 ± 0.1 c |
| 7,10,13-16:3 | 0.3 ± 0.0 a | 1.6 ± 0.4 b | 2.4 ± 0.1 c |
| 18:0 | 3.2 ± 0.2 a | 1.7 ± 0.2 c | 1.0 ± 0.0 b |
| 9-18:1 | 11.9 ± 0.2 b | 8.4 ± 1.5 a | 8.0 ± 0.3 a |
| 11-18:1 | * a | 0.4 ± 0.4 ab | 0.6 ± 0.0 b |
| 9,12-18:2 | 14.9 ± 0.3 a | 18.5 ± 1.1 b | 19.8 ± 0.2 b |
| 10,13-18:2 | 0.8 ± 0.1 b | 0.5 ± 0.4 ab | 0.1 ± 0.1 a |
| 6,9,12-18:3 | 0.3 ± 0.1 a | 0.7 ± 0.1 c | 0.5 ± 0.1 b |
| 9,12,15-18:3 | 21.4 ± 0.6 a | 26.1 ± 1.6 b | 28.2 ± 0.4 b |
| 19:0 | 0.2 ± 0.2 a | 0.1 ± 0.1 a | 0.1 ± 0.0 a |
| 20:0 | 0.8 ± 1.0 a | 0.4 ± 0.4 a | 0.7 ± 0.0 a |
| 11-20:1 | 0.1 ± 0.2 a | 0.1 ± 0.1 a | 0.2 ± 0.1 a |
| 11,14-20:2 | 0.6 ± 0.1 b | 0.3 ± 0.1 a | 0.5 ± 0.0 b |
| 8,11,14-20:3 | 1.0 ± 0.3 a | 1.2 ± 0.6 a | 1.1 ± 0.1 a |
| 11,14, 17-20:3 | 0.4 ± 0.1 b | * a | * a |
| 5,8,11,14-20:4 | 6.3 ± 0.5 a | 9.4 ± 0.6 b | 10.3 ± 0.3 b |
| 8,11,14, 17-20:4 | 0.2 ± 0.2 a | 0.1 ± 0.2 a | 0.1 ± 0.1 a |
| 5,8,11,14, 17-20:5 | 0.6 ± 0.1 a | 0.7 ± 0.1 a | 1.0 ± 0.0 b |
| 21:0 | 0.1 ± 0.1 a | * a | 0.1 ± 0.1 a |
| 22:0 | 2.2 ± 0.3 b | 1.1 ± 0.3 a | 1.3 ± 0.1 a |
| 13-22:1 | 0.5 ± 0.1 b | * a | * a |
| 23:0 | 0.2 ± 0.1 a | 0.2 ± 0.1 a | 0.2 ± 0.1 a |
| 24:0 | 1.1 ± 0.3 a | 0.9 ± 0.2 a | 0.8 ± 0.1 a |
| 25:0 | * a | 0.1 ± 0.1 b | 0.1 ± 0.0 b |
| 26:0 | * a | 0.2 ± 0.2 b | 0.2 ± 0.1 ab |
| 28:0 | * a | 0.1 ± 0.1 a | 0.1 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voronkov, A.; Ivanova, T. Significance of Lipid Fatty Acid Composition for Resistance to Winter Conditions in Asplenium scolopendrium. Biology 2022, 11, 507. https://doi.org/10.3390/biology11040507
Voronkov A, Ivanova T. Significance of Lipid Fatty Acid Composition for Resistance to Winter Conditions in Asplenium scolopendrium. Biology. 2022; 11(4):507. https://doi.org/10.3390/biology11040507
Chicago/Turabian StyleVoronkov, Alexander, and Tatiana Ivanova. 2022. "Significance of Lipid Fatty Acid Composition for Resistance to Winter Conditions in Asplenium scolopendrium" Biology 11, no. 4: 507. https://doi.org/10.3390/biology11040507
APA StyleVoronkov, A., & Ivanova, T. (2022). Significance of Lipid Fatty Acid Composition for Resistance to Winter Conditions in Asplenium scolopendrium. Biology, 11(4), 507. https://doi.org/10.3390/biology11040507







