‘Fly to a Safer North’: Distributional Shifts of the Orchid Ophrys insectifera L. Due to Climate Change
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Species Occurrence Dataset and Coordinate Thinning Procedures
2.3. Environmental Data
2.4. Species Distribution Models
2.5. Distribution Shift in Latitudinal and Altitudinal Gradient
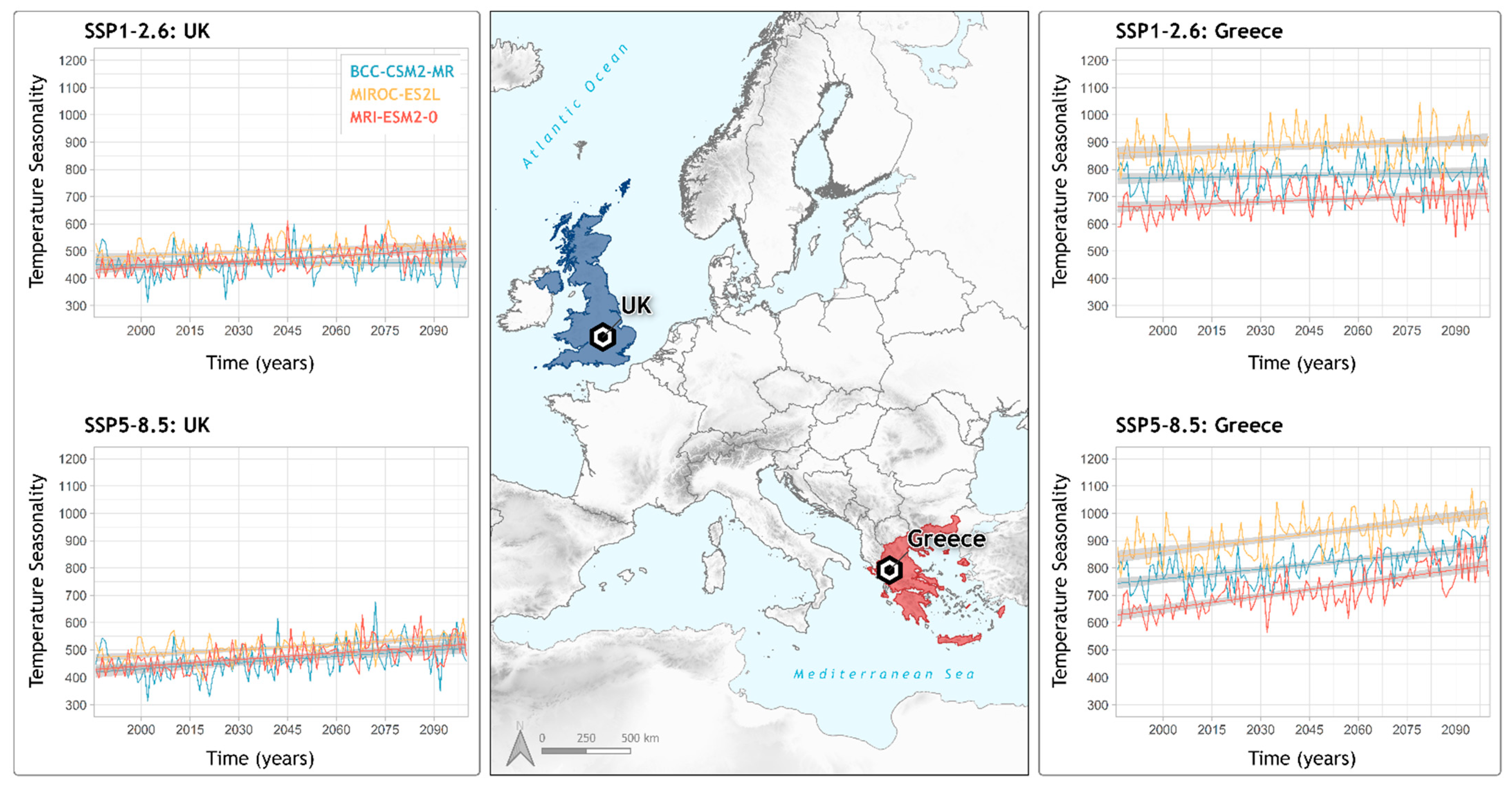
2.6. Response of the Most Important Variable in Locations of Interest
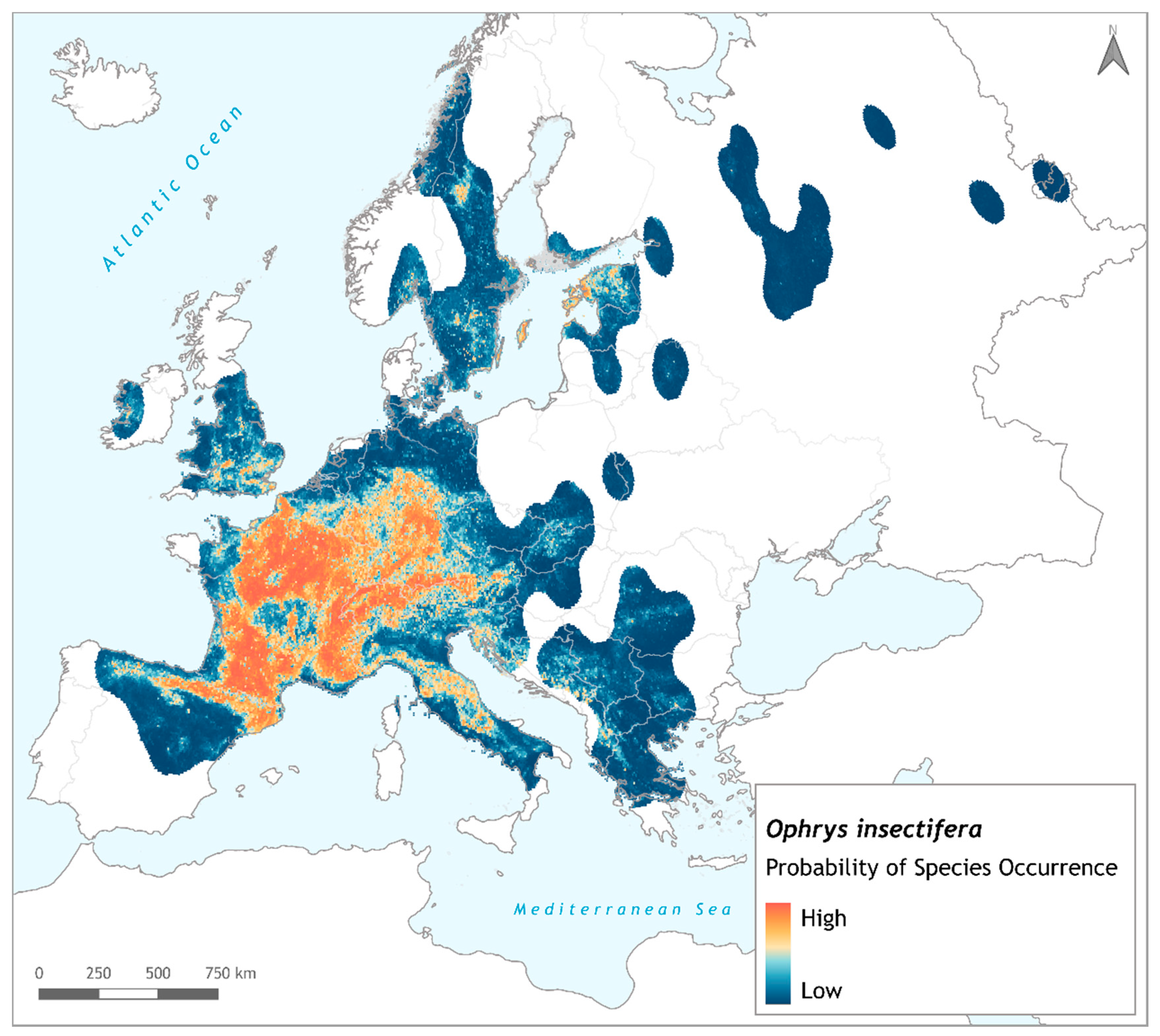
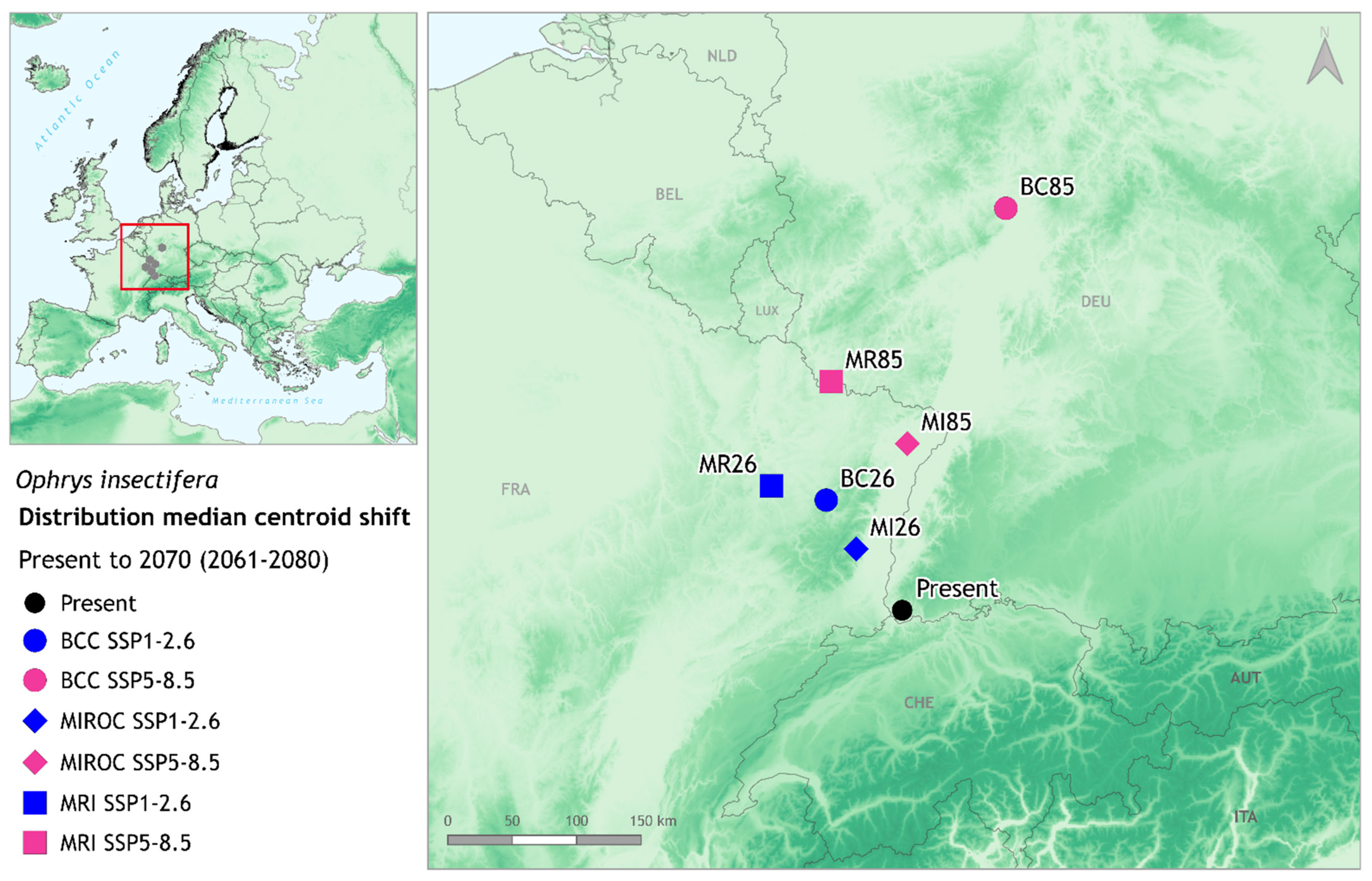
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2022; In Press. [Google Scholar]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; De Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Rymer, P.D.; Byrne, M.; Ruthrof, K.X.; Whinam, J.; McGeoch, M.; Bergstrom, D.M.; Guerin, G.R.; Sparrow, B.; Joseph, L.; et al. Impacts of recent climate change on terrestrial flora and fauna: Some emerging Australian examples. Austral. Ecol. 2019, 44, 3–27. [Google Scholar] [CrossRef]
- Freeman, B.G.; Lee-Yaw, J.A.; Sunday, J.M.; Hargreaves, A.L. Expanding, shifting and shrinking: The impact of global warming on species’ elevational distributions. Glob. Ecol. Biogeogr. 2018, 27, 1268–1276. [Google Scholar] [CrossRef]
- Corlett, R.T.; Westcott, D.A. Will plant movements keep up with climate change? Trends Ecol. Evol. 2013, 28, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Wiens, J.J. Climate-Related Local Extinctions Are Already Widespread among Plant and Animal Species. PLoS Biol. 2016, 14, e2001104. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Hanley, M.E. Plants and climate change: Complexities and surprises. Ann. Bot. 2015, 116, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate Extremes: Observations, Modeling, and Impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aaasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Nic Lughadha, E.; Bachman, S.P.; Leão, T.C.C.; Forest, F.; Halley, J.M.; Moat, J.; Acedo, C.; Bacon, K.L.; Brewer, R.F.A.; Gâteblé, G.; et al. Extinction risk and threats to plants and fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C.; Thuiller, W.; Lavorel, S.; Arau, M.B. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Duchenne, F.; Martin, G.; Porcher, E. European plants lagging behind climate change pay a climatic debt in the North, but are favoured in the South. Ecol. Lett. 2021, 24, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Calinger, K.M.; Queenborough, S.; Curtis, P.S. Herbarium specimens reveal the footprint of climate change on flowering trends across north-central North America. Ecol. Lett. 2013, 16, 1037–1044. [Google Scholar] [CrossRef]
- Robbirt, K.M.; Davy, A.J.; Hutchings, M.J.; Roberts, D.L. Validation of biological collections as a source of phenological data for use in climate change studies: A case study with the orchid Ophrys sphegodes. J. Ecol. 2011, 99, 235–241. [Google Scholar] [CrossRef]
- Robbirt, K.M.; Roberts, D.L.; Hutchings, M.J.; Davy, A.J. Potential disruption of pollination in a sexually deceptive orchid by climatic change. Curr. Biol. 2014, 24, 2845–2849. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.J.; Robbirt, K.M.; Roberts, D.L.; Davy, A.J. Vulnerability of a specialized pollination mechanism to climate change revealed by a 356-year analysis. Bot. J. Linn. Soc. 2018, 186, 498–509. [Google Scholar] [CrossRef]
- Pfeifer, M.; Heinrich, W.; Jetschke, G. Climate, size and flowering history determine flowering pattern of an orchid. Bot. J. Linn. Soc. 2006, 151, 511–526. [Google Scholar] [CrossRef]
- Evans, A.; Janssens, S.; Jacquemyn, H. Impact of climate change on the distribution of four closely related Orchis (Orchidaceae) species. Diversity 2020, 12, 312. [Google Scholar] [CrossRef]
- Geppert, C.; Perazza, G.; Wilson, R.J.; Bertolli, A.; Prosser, F.; Melchiori, G.; Marini, L. Consistent population declines but idiosyncratic range shifts in Alpine orchids under global change. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Konowalik, K.; Kolanowska, M. Climatic niche shift and possible future spread of the invasive South African Orchid Disa bracteata in Australia and adjacent areas. PeerJ 2018, 6, e6107. [Google Scholar] [CrossRef]
- Kolanowska, M. The future of a montane orchid species and the impact of climate change on the distribution of its pollinators and magnet species. Glob. Ecol. Conserv. 2021, 32, e01939. [Google Scholar] [CrossRef]
- Foster, E.A.; Ackerman, J.D. Future changes in the distribution of two non-indigenous orchids and their acquired enemy in Puerto Rico. Biol. Invasions 2021, 23, 3545–3563. [Google Scholar] [CrossRef]
- van der Meer, S.; Jacquemyn, H.; Carey, P.D.; Jongejans, E. Recent range expansion of a terrestrial orchid corresponds with climate-driven variation in its population dynamics. Oecologia 2016, 181, 435–448. [Google Scholar] [CrossRef]
- Kolanowska, M.; Jakubska-Busse, A. Is the lady’s-slipper orchid (Cypripedium calceolus) likely to shortly become extinct in Europe?—Insights based on ecological niche modelling. PLoS ONE 2020, 15, e0228420. [Google Scholar] [CrossRef]
- Charitonidou, M.; Kougioumoutzis, K.; Halley, J.M. An Orchid in Retrograde: Climate-Driven Range Shift Patterns of Ophrys helenae in Greece. Plants 2021, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Tsiftsis, S.; Djordjević, V. Modelling sexually deceptive orchid species distributions under future climates: The importance of plant–pollinator interactions. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Kolanowska, M. Niche Conservatism and the Future Potential Range of Epipactis helleborine (Orchidaceae). PLoS ONE 2013, 8, e77352. [Google Scholar] [CrossRef][Green Version]
- Kolanowska, M.; Kras, M.; Lipińska, M.; Mystkowska, K.; Szlachetko, D.L.; Naczk, A.M. Global warming not so harmful for all plants-response of holomycotrophic orchid species for the future climate change. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, S.; Martellos, S.; Bacaro, G.; De Agostini, A.; Cogoni, A.; Cortis, P. Distributional pattern of sardinian orchids under a climate change scenario. Community Ecol. 2018, 19, 223–232. [Google Scholar] [CrossRef]
- Cos, J.; Doblas-Reyes, F.; Jury, M.; Marcos, R.; Bretonnière, P.; Samsó, M. The Mediterranean climate change hotspot in the CMIP5 and CMIP6 projections. Earth Syst. Dyn. 2022, 13, 321–340. [Google Scholar] [CrossRef]
- Tebaldi, C.; Debeire, K.; Eyring, V.; Fischer, E.; Fyfe, J.; Friedlingstein, P.; Knutti, R.; Lowe, J.; Neill, B.O.; Sanderson, B. Climate model projections from the Scenario Model Intercomparison Project (ScenarioMIP) of CMIP6. Earth Syst. Dyn. 2021, 12, 253–293. [Google Scholar] [CrossRef]
- Bachman, S.P.; Nic Lughadha, E.M.; Rivers, M.C. Quantifying progress toward a conservation assessment for all plants. Conserv. Biol. 2018, 32, 516–524. [Google Scholar] [CrossRef]
- Seaton, P.T.; Hu, H.; Perner, H.; Pritchard, H.W. Ex Situ Conservation of Orchids in a Warming World. Bot. Rev. 2010, 76, 193–203. [Google Scholar] [CrossRef]
- Kull, T.; Selgis, U.; Peciña, M.V.; Metsare, M.; Ilves, A.; Tali, K.; Sepp, K.; Kull, K.; Shefferson, R.P. Factors influencing IUCN threat levels to orchids across Europe on the basis of national red lists. Ecol. Evol. 2016, 6, 6245–6265. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.F.; Pailler, T.; Dixon, K.W. Orchid conservation: Making the links. Ann. Bot. 2015, 116, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.F. Orchid conservation: How can we meet the challenges in the twenty-first century? Bot. Stud. 2018, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Delforge, P. Orchids of Europe, North Africa and the Middle East, 3rd ed.; A&C Black: London, UK, 2006; ISBN 071367525X. [Google Scholar]
- Kühn, R.; Pedersen, H.; Cribb, P.J. Field Guide to the Orchids of Europe and the Mediterranean; Royal Botanic Gardens, Kew: Richmond, UK, 2019; ISBN 978-1842466698. [Google Scholar]
- Pedersen, H.; Faurholdt, N. Ophrys: The bee orchids of Europe; Kew Publishing: Richmond, UK, 2007; ISBN 9781842461525. [Google Scholar]
- Stroh, P.A. Ophrys insectifera L. Fly Orchid Species Account; Botanical Society of Britain and Ireland: Durham, UK, 2015. [Google Scholar]
- Tsiftsis, S.; Antonopoulos, Z. Atlas of the Greek Orchids; Mediterraneo Editions: Rethymnon, Greece, 2017; Volume 1–2, ISBN 9789606848933. [Google Scholar]
- Fay, M.F.; Taylor, I.; Sayers, B. 804. Ophrys insectifera. Curtis’s Bot. Mag. 2015, 32, 51–62. [Google Scholar] [CrossRef]
- Borg-Karlson, A.K.; Groth, I.; Ågren, L.; Kullenberg, B. Form-specific fragances from Ophrys insectifera L. (Orchidaceae) attract species of different pollinator genera. Evidence of sympatric speciation? Chemoecology 1993, 4, 39–45. [Google Scholar] [CrossRef]
- Rankou, H. Ophrys insectifera. IUCN Red List Threat. Species 2011, e.T175957A7153465. [Google Scholar] [CrossRef]
- IUCN European Policy Plants 2011. Ophrys insectifera . Available online: https://www.iucnredlist.org/ (accessed on 8 March 2021).
- GBIF.org. Gbif Occurrence Download. Available online: https://doi.org/10.15468/dl.6ykdxh (accessed on 18 January 2021).
- Djordjević, V.; Lakušić, D.; Jovanović, S.; Stevanović, V. Distribution and conservation status of some rare and threatened orchid taxa in the central Balkans and the southern part of the Pannonian plain. Wulfenia 2017, 24, 143–162. [Google Scholar]
- Popatanasov, A. Ophrys insectifera L.—Update of the Status of a Critically Endangered Orchid in Bulgaria. J. Life Sci. 2018, 12, 83–91. [Google Scholar] [CrossRef][Green Version]
- IUCN Standards and Petitions Committee. Guidelines for Using the IUCN Red List Categories and Criteria, Version 14; 2019, Prepared by the Standards and Petitions Committee. Available online: https://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 22 May 2021).
- Joppa, L.N.; Butchart, S.H.M.; Hoffmann, M.; Bachman, S.P.; Akçakaya, H.R.; Moat, J.F.; Böhm, M.; Holland, R.A.; Newton, A.; Polidoro, B.; et al. Impact of Alternative Metrics on Estimates of Extent of Occurrence for Extinction Risk Assessment. Conserv. Biol. 2016, 30, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Dauby, G.; Stévart, T.; Droissart, V.; Cosiaux, A.; Deblauwe, V.; Simo-Droissart, M.; Sosef, M.S.M.; Lowry, P.P.; Schatz, G.E.; Gereau, R.E.; et al. ConR: An R Package to Assist Large-Scale Multispecies Preliminary Conservation Assessments Using Distribution Data. Ecol. Evol. 2017, 7, 11292–11303. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Duarte Ritter, C.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R.; et al. “CoordinateCleaner”: Standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Smith, A.B. Enmsdm: Tools for Modeling Species Niches and Distributions; R Package Version 0.5.1.5; CRAN, R Core Team: Cary, CA, USA, 2020. [Google Scholar]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography Cop. 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Robertson, M.P.; Visser, V.; Hui, C. Biogeo: An R package for assessing and improving data quality of occurrence record datasets. Ecography Cop. 2016, 39, 394–401. [Google Scholar] [CrossRef]
- Varela, S.; Anderson, R.P.; García-Valdés, R.; Fernández-González, F. Environmental filters reduce the effects of sampling bias and improve predictions of ecological niche models. Ecography Cop. 2014, 37, 1084–1091. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L.; et al. The Beijing Climate Center Climate System Model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Hajima, T.; Watanabe, M.; Yamamoto, A.; Tatebe, H.; Noguchi, M.; Abe, M.; Ohgaito, R.; Ito, A.; Yamazaki, D.; Okajima, H.; et al. Description of the MIROC-ES2L Earth system model and evaluation of its climate–biogeochemical processes and feedbacks. Geosci. Model Dev. Discuss. 2019, 5, 1–73. [Google Scholar] [CrossRef]
- Yukimoto, S.; Kawai, H.; Koshiro, T.; Oshima, N.; Yoshida, K.; Urakawa, S.; Tsujino, H.; Deushi, M.; Tanaka, T.; Hosaka, M.; et al. The meteorological research institute Earth system model version 2.0, MRI-ESM2.0: Description and basic evaluation of the physical component. J. Meteorol. Soc. Jpn. 2019, 97, 931–965. [Google Scholar] [CrossRef]
- Riahi, K.; van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Chang. 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Title, P.O.; Bemmels, J.B. ENVIREM: An expanded set of bioclimatic and topographic variables increases flexibility and improves performance of ecological niche modeling. Ecography Cop. 2018, 41, 291–307. [Google Scholar] [CrossRef]
- Jarvis, A.; Reuter, H.I.; Nelson, A.; Guevara, E. Hole-Filled SRTM for the Globe; Version 4: Data Grid. Web Publication/Site; CGIAR Consortium for Spatial Information: 2008. Available online: https://research.utwente.nl/en/publications/hole-filled-srtm-for-the-globe-version-4-data-grid (accessed on 1 March 2022).
- Hijmans, R.J.; van Etten, J. Raster: Geographic Analysis and Modeling with Raster Data; R Package Version 3.3.13. Available online: http://CRAN.R-project.org/package=raster (accessed on 23 February 2022).
- Evans, J.S.; Murphy, M.A. SpatialEco; R Package Version 1.2-0; 2021. Available online: https://github.com/jeffreyevans/spatialEco (accessed on 23 February 2022).
- Poggio, L.; De Sousa, L.M.; Batjes, N.H.; Heuvelink, G.B.M.; Kempen, B.; Ribeiro, E.; Rossiter, D. SoilGrids 2.0: Producing soil information for the globe with quantified spatial uncertainty. Soil 2021, 7, 217–240. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System; Open Source Geospatial Foundation Project; Open Source Geospatial Foundation: Chicago, IL, USA, 2021. [Google Scholar]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography Cop. 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography Cop. 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Barbosa, A.M.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef] [PubMed]
- Vignali, S.; Barras, A.G.; Arlettaz, R.; Braunisch, V. SDMtune: An R package to tune and evaluate species distribution models. Ecol. Evol. 2020, 10, 11488–11506. [Google Scholar] [CrossRef]
- Carlson, C.J. embarcadero: Species distribution modelling with Bayesian additive regression trees in r. Methods Ecol. Evol. 2020, 11, 850–858. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Roberts, D.R.; Bahn, V.; Ciuti, S.; Boyce, M.S.; Elith, J.; Guillera-Arroita, G.; Hauenstein, S.; Lahoz-Monfort, J.J.; Schröder, B.; Thuiller, W.; et al. Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography Cop. 2017, 40, 913–929. [Google Scholar] [CrossRef]
- Carlson, C.J.; Bevins, S.N.; Schmid, B. V Plague risk in the western United States over seven decades of environmental change. Glob. Chang. Biol. 2022, 28, 753–769. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography Cop. 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Velasco, J.A.; González-Salazar, C. Akaike information criterion should not be a “test” of geographical prediction accuracy in ecological niche modelling. Ecol. Inform. 2019, 51, 25–32. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. The effect of sample size on the accuracy of species distribution models: Considering both presences and pseudo-absences or background sites. Ecography Cop. 2019, 42, 535–548. [Google Scholar] [CrossRef]
- Guevara, L.; Gerstner, B.E.; Kass, J.M.; Anderson, R.P. Toward ecologically realistic predictions of species distributions: A cross-time example from tropical montane cloud forests. Glob. Chang. Biol. 2018, 24, 1511–1522. [Google Scholar] [CrossRef]
- Konowalik, K.; Nosol, A. Evaluation metrics and validation of presence-only species distribution models based on distributional maps with varying coverage. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Somodi, I.; Lepesi, N.; Botta-Dukát, Z. Prevalence dependence in model goodness measures with special emphasis on true skill statistics. Ecol. Evol. 2017, 7, 863–872. [Google Scholar] [CrossRef]
- Leroy, B.; Delsol, R.; Hugueny, B.; Meynard, C.N.; Barhoumi, C.; Barbet-Massin, M.; Bellard, C. Without quality presence-absence data, discrimination metrics such as TSS can be misleading measures of model performance. J. Biogeogr. 2018, 45, 1994–2002. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Modell. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; White, M.; Newell, G. Measuring and comparing the accuracy of species distribution models with presence-absence data. Ecography Cop. 2011, 34, 232–243. [Google Scholar] [CrossRef]
- Sofaer, H.R.; Jarnevich, C.S.; Pearse, I.S.; Smyth, R.L.; Auer, S.; Cook, G.L.; Edwards, T.C.; Guala, G.F.; Howard, T.G.; Morisette, J.T.; et al. Development and Delivery of Species Distribution Models to Inform Decision-Making. Bioscience 2019, 69, 544–557. [Google Scholar] [CrossRef]
- Schwarz, J.; Heider, D. GUESS: Projecting machine learning scores to well-calibrated probability estimates for clinical decision-making. Bioinformatics 2019, 35, 2458–2465. [Google Scholar] [CrossRef] [PubMed]
- Márcia Barbosa, A.; Real, R.; Muñoz, A.R.; Brown, J.A. New measures for assessing model equilibrium and prediction mismatch in species distribution models. Divers. Distrib. 2013, 19, 1333–1338. [Google Scholar] [CrossRef]
- Signorell, A.; Aho, K.; Anderegg, N.; Aragon, T.; Arppe, A.; Baddeley, A.; Bolker, B.; Caeiro, F.; Champely, S.; Chessel, D. DescTools: Tools for Descriptive Statistics; R Package Version 0.99-40; CRAN, R Core Team: Cary, CA, USA, 2021. [Google Scholar]
- Broennimann, O.; Di Cola, V.; Guisan, A. Ecospat: Spatial Ecology Miscellaneous Methods; R Package Version 3.2; CRAN, R Core Team: Cary, CA, USA, 2021. [Google Scholar]
- Hammer, B.; Frasco, M. Metrics: Evaluation Metrics for Machine Learning; R Package Version 0.1.4; CRAN, R Core Team: Cary, CA, USA, 2018. [Google Scholar]
- Yan, Y. MLmetrics: Machine Learning Evaluation Metrics, R package version 1.1.1; CRAN, R Core Team: Cary, CA, USA, 2016. [Google Scholar]
- Zhu, G.; Fan, J.; Peterson, A.T. Cautions in weighting individual ecological niche models in ensemble forecasting. Ecol. Modell. 2021, 448, 109502. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography Cop. 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography Cop. 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Arditti, J.; Ghani, A.K.A. Numerical and physical properties of orchid seeds and their biological implications. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef] [PubMed]
- Pebesma, E. Simple features for R: Standardized support for spatial vector data. R J. 2018, 10, 439–446. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Carey, P.D. Changes in the distribution and abundance of Himantoglossum hircinum (L.) Sprengel (Orchidaceae) over the last 100 years. Flora 1999, 364, 353–364. [Google Scholar]
- Kull, T.; Hutchings, M.J. A comparative analysis of decline in the distribution ranges of orchid species in Estonia and the United Kingdom. Biol. Conserv. 2006, 129, 31–39. [Google Scholar] [CrossRef]
- Vogt-Schilb, H.; Munoz, F.; Richard, F.; Schatz, B. Recent declines and range changes of orchids in Western Europe (France, Belgium and Luxembourg). Biol. Conserv. 2015, 190, 133–141. [Google Scholar] [CrossRef]
- Reina-Rodríguez, G.A.; Rubiano Mejía, J.E.; Castro Llanos, F.A.; Soriano, I. Orchids distribution and bioclimatic niches as a strategy to climate change in areas of tropical dry forest in Colombia. Lankesteriana 2017, 17, 17–47. [Google Scholar] [CrossRef][Green Version]
- Djordjević, V.; Tsiftsis, S. The Role of Ecological Factors in Distribution and Abundance of Terrestrial Orchids. In Orchids Phytochemistry, Biology and Horticulture; Mérillon, J.-M., Kodja, H., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–71. ISBN 9783030112578. [Google Scholar]
- Zhang, S.; Chen, W.; Huang, J.; Bi, Y.; Yang, X. Orchid Species Richness along Elevational and Environmental Gradients in Yunnan, China. PLoS ONE 2015, 10, e0142621. [Google Scholar] [CrossRef]
- Dorland, E.; Willems, J.H. Light climate and plant performance of Ophrys insectifera; a four-year field experiment in The Netherlands (1998–2001). In Trends and Fluctuations and Underlying Mechanisms in Terrestrial Orchid Populations; Kindlmann, P., Willems, J.H., Whigham, D.F., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2002; pp. 225–238. ISBN 9057821230. [Google Scholar]
- Preston, C.D.; Pearman, D.A.; Dines, T.D. New Atlas of the British and Irish Flora; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Jacquemyn, H.; Brys, R.; Hermy, M.; Willems, J.H. Does nectar reward affect rarity and extinction probabilities of orchid species? An assessment using historical records from Belgium and the Netherlands. Biol. Conserv. 2005, 121, 257–263. [Google Scholar] [CrossRef]
- Damgaard, C.; Moeslund, J.E.; Wind, P. Changes in the abundance of Danish orchids over the past 30 years. Diversity 2020, 12, 244. [Google Scholar] [CrossRef]
- Wraith, J.; Pickering, C. A continental scale analysis of threats to orchids. Biol. Conserv. 2019, 234, 7–17. [Google Scholar] [CrossRef]
- Triponez, Y.; Arrigo, N.; Pellissier, L.; Schatz, B.; Alvarez, N. Morphological, ecological and genetic aspects associated with endemism in the Fly Orchid group. Mol. Ecol. 2013, 22, 1431–1446. [Google Scholar] [CrossRef]
- Waterman, R.J.; Bidartondo, M.I. Deception above, deception below: Linking pollination and mycorrhizal biology of orchids. J. Exp. Bot. 2008, 59, 1085–1096. [Google Scholar] [CrossRef]
- Shefferson, R.P.; Jacquemyn, H.; Kull, T.; Hutchings, M.J. The demography of terrestrial orchids: Life history, population dynamics and conservation. Bot. J. Linn. Soc. 2020, 192, 315–332. [Google Scholar] [CrossRef]
- Schweiger, J.M.-I.; Bidartondo, M.I.; Gebauer, G. Stable isotope signatures of underground seedlings reveal the organic matter gained by adult orchids from mycorrhizal fungi. Funct. Ecol. 2018, 32, 870–881. [Google Scholar] [CrossRef]
- Breitkopf, H.; Onstein, R.E.; Cafasso, D.; Schlüter, P.M.; Cozzolino, S. Multiple shifts to different pollinators fuelled rapid diversification in sexually deceptive Ophrys orchids. New Phytol. 2015, 207, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Charitonidou, M.; Halley, J.M. What goes up must come down—Why high fecundity orchids challenge conservation beliefs. Biol. Conserv. 2020, 252, 108835. [Google Scholar] [CrossRef]


| Time Slice | Transition | GCM | Area Loss (%) | Area Gain (%) | Overall Change (%) | Current Occurrences Lost (%) |
|---|---|---|---|---|---|---|
| 2070 | Present to SSP1-2.6 | BC | 20.58 | 17.45 | −3.14 | 8.27 |
| MI | 16.40 | 21.12 | 4.72 | 10.54 | ||
| MR | 38.29 | 14.36 | −23.93 | 22.03 | ||
| Mean | 25.09 | 17.64 | −7.45 | 13.60 | ||
| Present to SSP5-8.5 | BC | 51.42 | 23.89 | −27.54 | 38.46 | |
| MI | 20.03 | 29.36 | 9.33 | 13.37 | ||
| MR | 44.00 | 15.44 | −28.55 | 31.13 | ||
| Mean | 38.48 | 22.90 | −15.59 | 27.65 | ||
| 2090 | Present to SSP1-2.6 | BC | 20.07 | 15.03 | −5.04 | 8.99 |
| MI | 16.13 | 22.91 | 6.78 | 10.27 | ||
| MR | 31.64 | 13.25 | −18.39 | 16.98 | ||
| Mean | 22.61 | 17.06 | −5.55 | 12.08 | ||
| Present to SSP5-8.5 | BC | 77.03 | 25.65 | −51.38 | 73.20 | |
| MI | 40.51 | 23.41 | −17.09 | 30.91 | ||
| MR | 29.06 | 15.00 | −14.06 | 16.26 | ||
| Mean | 48.87 | 21.35 | −27.51 | 40.12 |
| Time Slice | SSP | Period/GCM | Mean Altitude (m) |
|---|---|---|---|
| Present | Current | 542.6 | |
| 2070 | SSP1-2.6 | BC | 484.7 |
| MI | 520.3 | ||
| MR | 467.5 | ||
| SSP5-8.5 | BC | 428.6 | |
| MI | 447.8 | ||
| MR | 447.0 | ||
| 2090 | SSP1-2.6 | BC | 504.6 |
| MI | 505.0 | ||
| MR | 498.3 | ||
| SSP5-8.5 | BC | 278.4 | |
| MI | 442.9 | ||
| MR | 492.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charitonidou, M.; Kougioumoutzis, K.; Karypidou, M.C.; Halley, J.M. ‘Fly to a Safer North’: Distributional Shifts of the Orchid Ophrys insectifera L. Due to Climate Change. Biology 2022, 11, 497. https://doi.org/10.3390/biology11040497
Charitonidou M, Kougioumoutzis K, Karypidou MC, Halley JM. ‘Fly to a Safer North’: Distributional Shifts of the Orchid Ophrys insectifera L. Due to Climate Change. Biology. 2022; 11(4):497. https://doi.org/10.3390/biology11040497
Chicago/Turabian StyleCharitonidou, Martha, Konstantinos Kougioumoutzis, Maria Chara Karypidou, and John M. Halley. 2022. "‘Fly to a Safer North’: Distributional Shifts of the Orchid Ophrys insectifera L. Due to Climate Change" Biology 11, no. 4: 497. https://doi.org/10.3390/biology11040497
APA StyleCharitonidou, M., Kougioumoutzis, K., Karypidou, M. C., & Halley, J. M. (2022). ‘Fly to a Safer North’: Distributional Shifts of the Orchid Ophrys insectifera L. Due to Climate Change. Biology, 11(4), 497. https://doi.org/10.3390/biology11040497









