Effects of Chronic High-Frequency rTMS Protocol on Respiratory Neuroplasticity Following C2 Spinal Cord Hemisection in Rats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Chronic C2 Hemisection
2.2.1. Intrapleural CTB Injection and Surgery
2.2.2. Brainstem Neuronal Retrograde Labeling with Hydroxystilbamidine
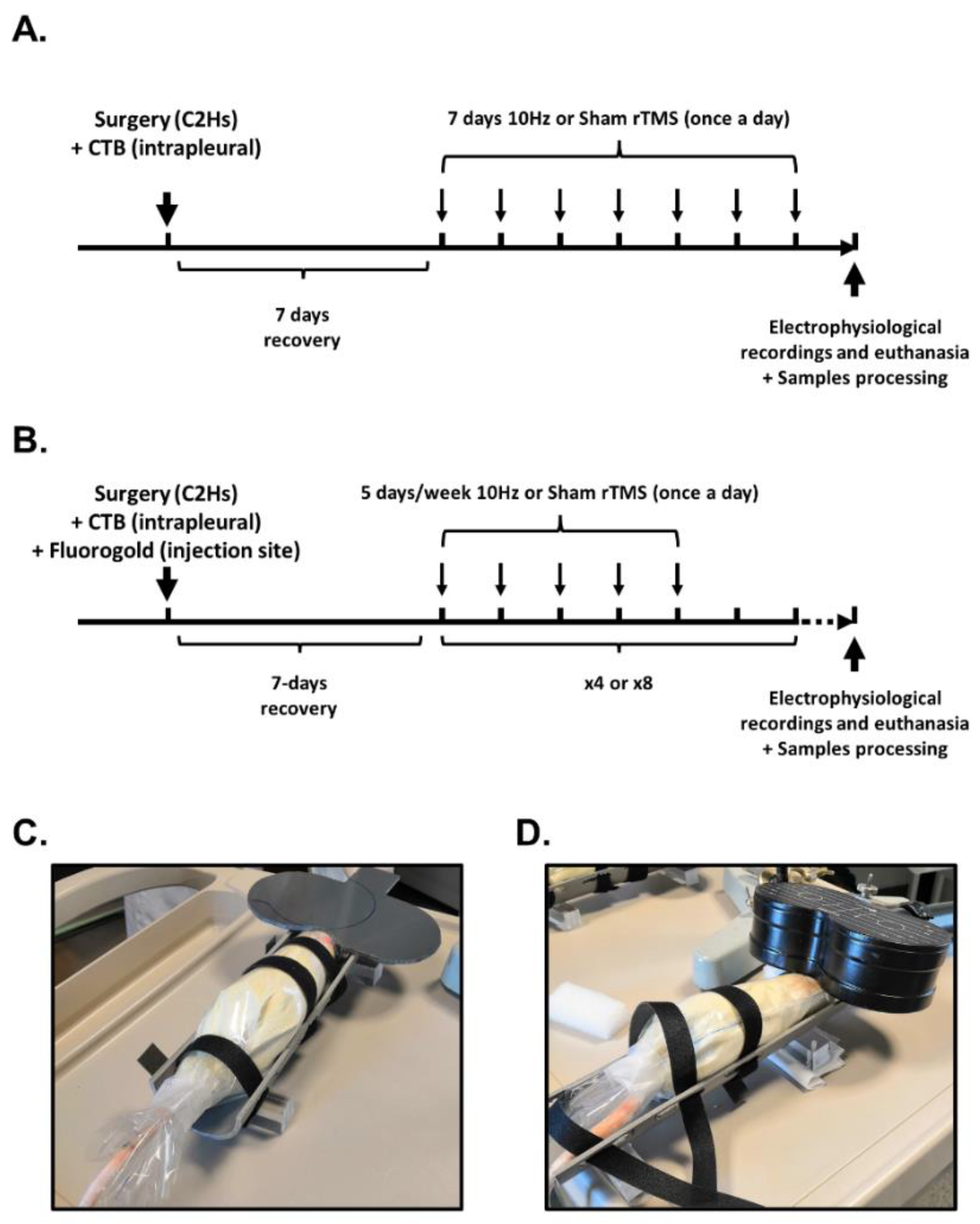
2.3. Repetitive TMS (rTMS) Protocol
2.4. Electrophysiological Recordings
2.4.1. Animal Preparation
2.4.2. Diaphragmatic EMG Recordings
2.4.3. Diaphragmatic MEP Recordings
2.5. Tissue Processing
2.6. Histological Reconstruction of the Extent of C2 Injury
2.7. Immunofluorescence
2.8. Data Processing and Statistical Analyses
3. Results
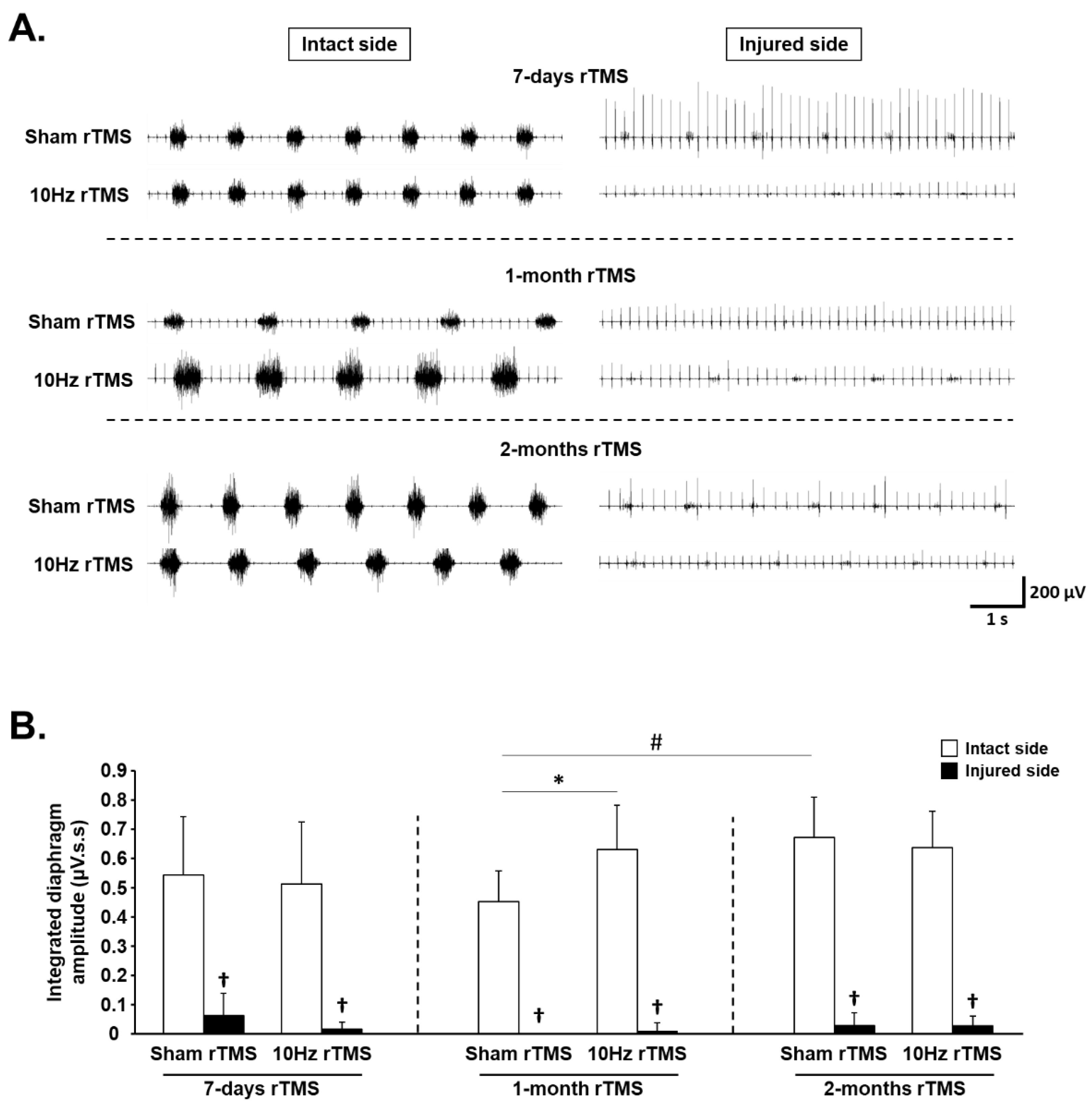
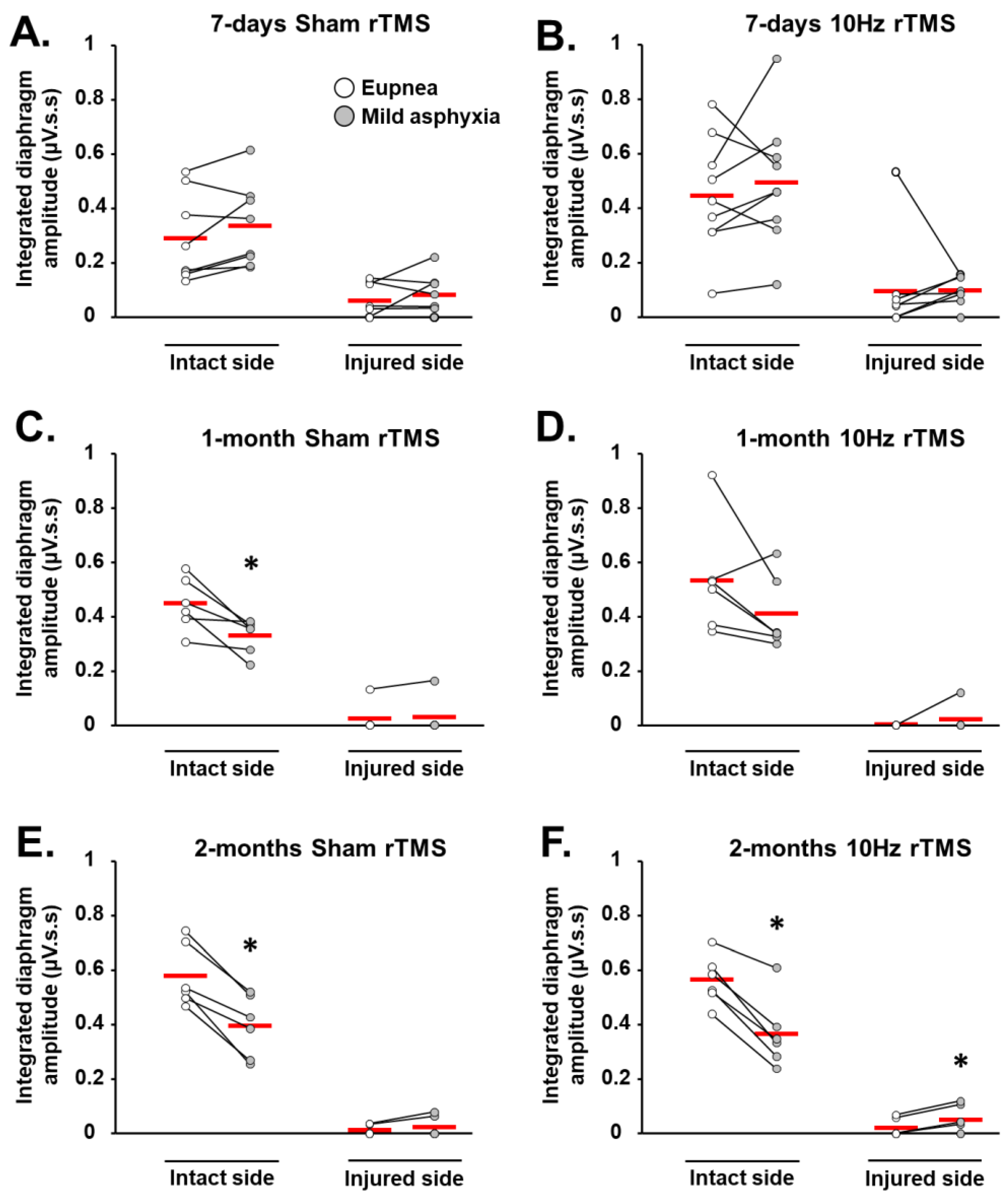
3.1. rTMS-Induced Effects on Diaphragm Activity during Eupnea
3.2. rTMS-Induced Effects on Diaphragm Muscle Response to Respiratory Challenge
3.3. rTMS-Induced Effects on Phrenic System Excitability
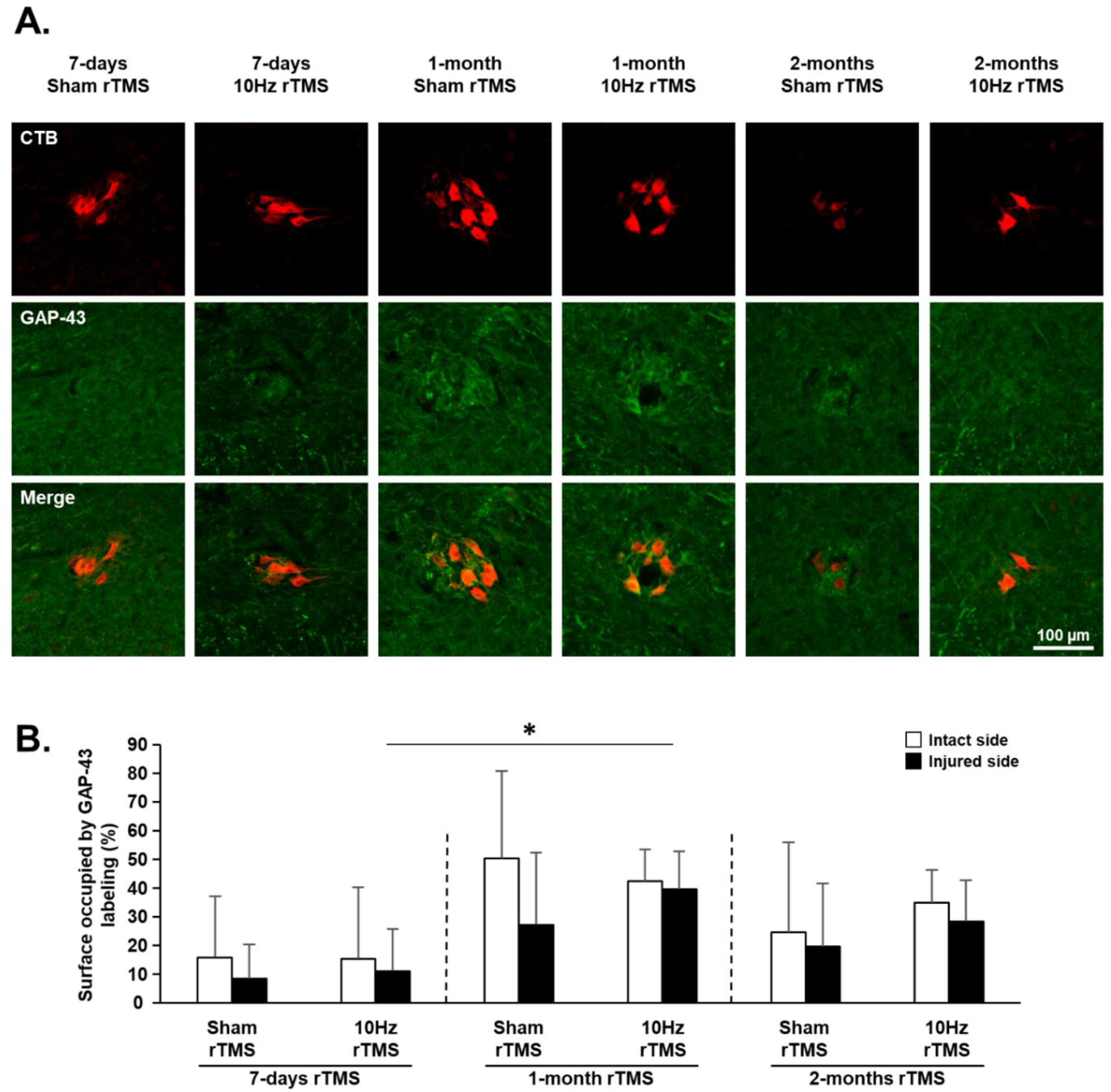
3.4. rTMS-Induced Effects on Plasticity Markers in C3–C6 Spinal Cord
3.5. rTMS-Induced Effects on Neuroinflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winslow, C.; Rozovsky, J. Effect of spinal cord injury on the respiratory system. Am. J. Phys. Med. Rehabil. 2003, 82, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Golder, F.J.; Reier, P.J.; Bolser, D.C. Altered respiratory motor drive after spinal cord injury: Supraspinal and bilateral effects of a unilateral lesion. J. Neurosci. 2001, 21, 8680–8689. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.A.; Lee, K.-Z.; Fuller, D.D.; Reier, P.J. Spinal circuitry and respiratory recovery following spinal cord injury. Respir. Physiol. Neurobiol. 2009, 169, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinit, S.; Gauthier, P.; Stamegna, J.C.; Kastner, A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J. Neurotrauma 2006, 23, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Vinit, S.; Kastner, A. Descending bulbospinal pathways and recovery of respiratory motor function following spinal cord injury. Respir. Physiol. Neurobiol. 2009, 169, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Alilain, W.J.; Horn, K.P.; Hu, H.; Dick, T.E.; Silver, J. Functional regeneration of respiratory pathways after spinal cord injury. Nature 2011, 475, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Keomani, E.; Deramaudt, T.B.; Petitjean, M.; Bonay, M.; Lofaso, F.; Vinit, S. A murine model of cervical spinal cord injury to study post-lesional respiratory neuroplasticity. J. Vis. Exp. 2014, 87, e51235. [Google Scholar] [CrossRef] [Green Version]
- Porter, W.T. The Path of the Respiratory Impulse from the Bulb to the Phrenic Nuclei. J. Physiol. 1895, 17, 455–485. [Google Scholar] [CrossRef]
- Nantwi, K.D.; El-Bohy, A.A.; Schrimsher, G.W.; Reier, P.J.; Goshgarian, H.G. Spontaneous Functional Recovery in a Paralyzed Hemidiaphragm Following Upper Cervical Spinal Cord Injury in Adult Rats. Neurorehabilit. Neural Repair 1999, 13, 225–234. [Google Scholar] [CrossRef]
- Lee, K.Z.; Huang, Y.J.; Tsai, I.L. Respiratory motor outputs following unilateral midcervical spinal cord injury in the adult rat. J. Appl. Physiol. 2014, 116, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Goshgarian, H.G. Invited Review: The crossed phrenic phenomenon: A model for plasticity in the respiratory pathways following spinal cord injury. J. Appl. Physiol. 2003, 94, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Ghali, M.G.Z. The crossed phrenic phenomenon. Neural Regen. Res. 2017, 12, 845–864. [Google Scholar] [CrossRef]
- Goshgarian, H.G. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir. Physiol. Neurobiol. 2009, 169, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, D.D.; Golder, F.J.; Olson, E.B., Jr.; Mitchell, G.S. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J. Appl. Physiol. 2006, 100, 800–806. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, B.J.; Lee, K.Z.; Lane, M.A.; Reier, P.J.; Fuller, D.D. Contribution of the spontaneous crossed-phrenic phenomenon to inspiratory tidal volume in spontaneously breathing rats. J. Appl. Physiol. 2012, 112, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.M.; McClintock, S.M.; Forster, J.J.; Lo, T.Y.; Loo, C.K. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: A systematic review and meta-analysis of individual task effects. Depress. Anxiety 2017, 34, 1029–1039. [Google Scholar] [CrossRef]
- Jassova, K.; Albrecht, J.; Ceresnakova, S.; Papezova, H.; Anders, M. Repetitive transcranial magnetic stimulation significantly influences the eating behavior in depressive patients. Neuropsychiatr. Dis. Treat. 2019, 15, 2579–2586. [Google Scholar] [CrossRef] [Green Version]
- McClintock, S.M.; Reti, I.M.; Carpenter, L.L.; McDonald, W.M.; Dubin, M.; Taylor, S.F.; Cook, I.A.; O’Reardon, J.; Husain, M.M.; Wall, C.; et al. Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression. J. Clin. Psychiatry 2018, 79, 3651. [Google Scholar] [CrossRef]
- Yan, T.; Xie, Q.; Zheng, Z.; Zou, K.; Wang, L. Different frequency repetitive transcranial magnetic stimulation (rTMS) for posttraumatic stress disorder (PTSD): A systematic review and meta-analysis. J. Psychiatr. Res. 2017, 89, 125–135. [Google Scholar] [CrossRef]
- Kozel, F.A. Clinical Repetitive Transcranial Magnetic Stimulation for Posttraumatic Stress Disorder, Generalized Anxiety Disorder, and Bipolar Disorder. Psychiatr. Clin. N. Am. 2018, 41, 433–446. [Google Scholar] [CrossRef]
- Ma, Q.; Geng, Y.; Wang, H.L.; Han, B.; Wang, Y.Y.; Li, X.L.; Wang, L.; Wang, M.W. High Frequency Repetitive Transcranial Magnetic Stimulation Alleviates Cognitive Impairment and Modulates Hippocampal Synaptic Structural Plasticity in Aged Mice. Front. Aging Neurosci. 2019, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Geng, Y.; Han, B.; Qiang, J.; Li, X.; Sun, M.; Wang, Q.; Wang, M. Repetitive transcranial magnetic stimulation applications normalized prefrontal dysfunctions and cognitive-related metabolic profiling in aged mice. PLoS ONE 2013, 8, e81482. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Pascual-Leone, A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003, 2, 145–156. [Google Scholar] [CrossRef]
- Ziemann, U.; Paulus, W.; Nitsche, M.A.; Pascual-Leone, A.; Byblow, W.D.; Berardelli, A.; Siebner, H.R.; Classen, J.; Cohen, L.G.; Rothwell, J.C. Consensus: Motor cortex plasticity protocols. Brain Stimul. 2008, 1, 164–182. [Google Scholar] [CrossRef]
- Poirrier, A.L.; Nyssen, Y.; Scholtes, F.; Multon, S.; Rinkin, C.; Weber, G.; Bouhy, D.; Brook, G.; Franzen, R.; Schoenen, J. Repetitive Transcranial Magnetic Stimulation Improves Open Field Locomotor Recovery after Low but Not High Thoracic Spinal Cord Compression-Injury in Adult Rats. J. Neurosci. Res. 2004, 75, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Marufa, S.A.; Hsieh, T.H.; Liou, J.C.; Chen, H.Y.; Peng, C.W. Neuromodulatory effects of repetitive transcranial magnetic stimulation on neural plasticity and motor functions in rats with an incomplete spinal cord injury: A preliminary study. PLoS ONE 2021, 16, e0252965. [Google Scholar] [CrossRef]
- Krishnan, V.S.; Shin, S.S.; Belegu, V.; Celnik, P.; Reimers, M.; Smith, K.R.; Pelled, G. Multimodal Evaluation of TMS—Induced Somatosensory Plasticity and Behavioral Recovery in Rats with Contusion Spinal Cord Injury. Front. Neurosci. 2019, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Ellaway, P.H.; Vásquez, N.; Craggs, M. Induction of central nervous system plasticity by repetitive transcranial magnetic stimulation to promote sensorimotor recovery in incomplete spinal cord injury. Front. Integr. Neurosci. 2014, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Petrosyan, H.A.; Alessi, V.; Sisto, S.A.; Kaufman, M.; Arvanian, V.L. Transcranial magnetic stimulation (TMS) responses elicited in hindlimb muscles as an assessment of synaptic plasticity in spino-muscular circuitry after chronic spinal cord injury. Neurosci. Lett. 2017, 642, 37–42. [Google Scholar] [CrossRef]
- Michel-Flutot, P.; Zholudeva, L.V.; Randelman, M.L.; Deramaudt, T.B.; Mansart, A.; Alvarez, J.-C.; Lee, K.-Z.; Petitjean, M.; Bonay, M.; Lane, M.A.; et al. High frequency repetitive Transcranial Magnetic Stimulation promotes long lasting phrenic motoneuron excitability via GABAergic networks. Respir. Physiol. Neurobiol. 2021, 292, 103704. [Google Scholar] [CrossRef]
- Vinit, S.; Keomani, E.; Deramaudt, T.B.; Spruance, V.M.; Bezdudnaya, T.; Lane, M.A.; Bonay, M.; Petitjean, M. Interdisciplinary approaches of transcranial magnetic stimulation applied to a respiratory neuronal circuitry model. PLoS ONE 2014, 9, e113251. [Google Scholar] [CrossRef]
- Vinit, S.; Keomani, E.; Deramaudt, T.B.; Bonay, M.; Petitjean, M. Reorganization of Respiratory Descending Pathways following Cervical Spinal Partial Section Investigated by Transcranial Magnetic Stimulation in the Rat. PLoS ONE 2016, 11, e0148180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Z.; Liou, L.M.; Vinit, S.; Ren, M.Y. Rostral-Caudal Effect of Cervical Magnetic Stimulation on the Diaphragm Motor Evoked Potential after Cervical Spinal Cord Contusion in the Rat. J. Neurotrauma 2022. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Z.; Liou, L.M.; Vinit, S. Diaphragm Motor-Evoked Potential Induced by Cervical Magnetic Stimulation following Cervical Spinal Cord Contusion in the Rat. J. Neurotrauma 2021, 38, 2122–2140. [Google Scholar] [CrossRef] [PubMed]
- Gersner, R.; Kravetz, E.; Feil, J.; Pell, G.; Zangen, A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: Differential outcomes in anesthetized and awake animals. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 7521–7526. [Google Scholar] [CrossRef] [PubMed]
- Benali, A.; Trippe, J.; Weiler, E.; Mix, A.; Petrasch-Parwez, E.; Girzalsky, W.; Eysel, U.T.; Erdmann, R.; Funke, K. Theta-burst transcranial magnetic stimulation alters cortical inhibition. J. Neurosci. 2011, 31, 1193–1203. [Google Scholar] [CrossRef]
- Vlachos, A.; Muller-Dahlhaus, F.; Rosskopp, J.; Lenz, M.; Ziemann, U.; Deller, T. Repetitive magnetic stimulation induces functional and structural plasticity of excitatory postsynapses in mouse organotypic hippocampal slice cultures. J. Neurosci. 2012, 32, 17514–17523. [Google Scholar] [CrossRef]
- Volz, L.J.; Benali, A.; Mix, A.; Neubacher, U.; Funke, K. Dose-dependence of changes in cortical protein expression induced with repeated transcranial magnetic theta-burst stimulation in the rat. Brain Stimul. 2013, 6, 598–606. [Google Scholar] [CrossRef]
- Hunanyan, A.S.; Petrosyan, H.A.; Alessi, V.; Arvanian, V.L. Repetitive spinal electromagnetic stimulation opens a window of synaptic plasticity in damaged spinal cord: Role of NMDA receptors. J. Neurophysiol. 2012, 107, 3027–3039. [Google Scholar] [CrossRef]
- Petrosyan, H.A.; Alessi, V.; Hunanyan, A.S.; Sisto, S.A.; Arvanian, V.L. Spinal electro-magnetic stimulation combined with transgene delivery of neurotrophin NT-3 and exercise: Novel combination therapy for spinal contusion injury. J. Neurophysiol. 2015, 114, 2923–2940. [Google Scholar] [CrossRef] [Green Version]
- Trippe, J.; Mix, A.; Aydin-Abidin, S.; Funke, K.; Benali, A. theta burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp. Brain Res. 2009, 199, 411–421. [Google Scholar] [CrossRef]
- Lenz, M.; Galanis, C.; Muller-Dahlhaus, F.; Opitz, A.; Wierenga, C.J.; Szabo, G.; Ziemann, U.; Deller, T.; Funke, K.; Vlachos, A. Repetitive magnetic stimulation induces plasticity of inhibitory synapses. Nat. Commun. 2016, 7, 10020. [Google Scholar] [CrossRef] [Green Version]
- Hausmann, A.; Marksteiner, J.; Hinterhuber, H.; Humpel, C. Magnetic stimulation induces neuronal c-fos via tetrodotoxin-sensitive sodium channels in organotypic cortex brain slices of the rat. Neurosci. Lett. 2001, 310, 105–108. [Google Scholar] [CrossRef]
- Hellmann, J.; Jüttner, R.; Roth, C.; Bajbouj, M.; Kirste, I.; Heuser, I.; Gertz, K.; Endres, M.; Kronenberg, G. Repetitive magnetic stimulation of human-derived neuron-like cells activates cAMP-CREB pathway. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 87–91. [Google Scholar] [CrossRef]
- Chalfouh, C.; Guillou, C.; Hardouin, J.; Delarue, Q.; Li, X.; Duclos, C.; Schapman, D.; Marie, J.P.; Cosette, P.; Guérout, N. The Regenerative Effect of Trans-spinal Magnetic Stimulation After Spinal Cord Injury: Mechanisms and Pathways Underlying the Effect. Neurotherapeutics 2020, 17, 2069–2088. [Google Scholar] [CrossRef] [PubMed]
- Cullen, C.L.; Young, K.M. How Does Transcranial Magnetic Stimulation Influence Glial Cells in the Central Nervous System? Front. Neural Circuits 2016, 10, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebetanz, D.; Fauser, S.; Michaelis, T.; Czéh, B.; Watanabe, T.; Paulus, W.; Frahm, J.; Fuchs, E. Safety aspects of chronic low-frequency transcranial magnetic stimulation based on localized proton magnetic resonance spectroscopy and histology of the rat brain. J. Psychiatr. Res. 2003, 37, 277–286. [Google Scholar] [CrossRef]
- Rauš, S.; Selaković, V.; Manojlović-Stojanoski, M.; Radenović, L.; Prolić, Z.; Janać, B. Response of hippocampal neurons and glial cells to alternating magnetic field in gerbils submitted to global cerebral ischemia. Neurotox. Res. 2013, 23, 79–91. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, G.S.; Cho, Y.W.; Cho, H.; Hwang, S.J.; Ahn, S.H. Attenuation of spinal cord injury-induced astroglial and microglial activation by repetitive transcranial magnetic stimulation in rats. J. Korean Med. Sci. 2013, 28, 295–299. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Song, L.; Liu, Z. The effect of repetitive transcranial magnetic stimulation on a model rat of Parkinson’s disease. Neuroreport 2010, 21, 268–272. [Google Scholar] [CrossRef]
- Aftanas, L.I.; Gevorgyan, M.M.; Zhanaeva, S.Y.; Dzemidovich, S.S.; Kulikova, K.I.; Al’perina, E.L.; Danilenko, K.V.; Idova, G.V. Therapeutic Effects of Repetitive Transcranial Magnetic Stimulation (rTMS) on Neuroinflammation and Neuroplasticity in Patients with Parkinson’s Disease: A Placebo-Controlled Study. Bull. Exp. Biol. Med. 2018, 165, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Lenz, M.; Platschek, S.; Priesemann, V.; Becker, D.; Willems, L.M.; Ziemann, U.; Deller, T.; Muller-Dahlhaus, F.; Jedlicka, P.; Vlachos, A. Repetitive magnetic stimulation induces plasticity of excitatory postsynapses on proximal dendrites of cultured mouse CA1 pyramidal neurons. Brain Struct. Funct. 2015, 220, 3323–3337. [Google Scholar] [CrossRef] [PubMed]
- Doperalski, N.J.; Fuller, D.D. Long-term facilitation of ipsilateral but not contralateral phrenic output after cervical spinal cord hemisection. Exp. Neurol. 2006, 200, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Opazo, A.; Vinit, S.; Dougherty, B.J.; Mitchell, G.S. Daily acute intermittent hypoxia elicits functional recovery of diaphragm and inspiratory intercostal muscle activity after acute cervical spinal injury. Exp. Neurol. 2015, 266, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robac, A.; Neveu, P.; Hugede, A.; Garrido, E.; Nicol, L.; Delarue, Q.; Guérout, N. Repetitive Trans Spinal Magnetic Stimulation Improves Functional Recovery and Tissue Repair in Contusive and Penetrating Spinal Cord Injury Models in Rats. Biomedicines 2021, 9, 1827. [Google Scholar] [CrossRef] [PubMed]
- Mahar, M.; Cavalli, V. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 2018, 19, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Zholudeva, L.V.; Karliner, J.S.; Dougherty, K.J.; Lane, M.A. Anatomical Recruitment of Spinal V2a Interneurons into Phrenic Motor Circuitry after High Cervical Spinal Cord Injury. J. Neurotrauma 2017, 34, 3058–3065. [Google Scholar] [CrossRef]
- Lane, M.A.; Lee, K.Z.; Salazar, K.; O’Steen, B.E.; Bloom, D.C.; Fuller, D.D.; Reier, P.J. Respiratory function following bilateral mid-cervical contusion injury in the adult rat. Exp. Neurol. 2012, 235, 197–210. [Google Scholar] [CrossRef] [Green Version]
- Satkunendrarajah, K.; Karadimas, S.K.; Laliberte, A.M.; Montandon, G.; Fehlings, M.G. Cervical excitatory neurons sustain breathing after spinal cord injury. Nature 2018, 562, 419–422. [Google Scholar] [CrossRef]
- Streeter, K.A.; Sunshine, M.D.; Patel, S.R.; Gonzalez-Rothi, E.J.; Reier, P.J.; Baekey, D.M.; Fuller, D.D. Mid-cervical interneuron networks following high cervical spinal cord injury. Respir. Physiol. Neurobiol. 2020, 271, 103305. [Google Scholar] [CrossRef]
- Leydeker, M.; Delva, S.; Tserlyuk, I.; Yau, J.; Wagdy, M.; Hawash, A.; Bendaoud, S.; Mohamed, S.; Wieraszko, A.; Ahmed, Z. The effects of 15 Hz trans-spinal magnetic stimulation on locomotor control in mice with chronic contusive spinal cord injury. Electromagn. Biol. Med. 2013, 32, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, V.R.; Harkema, S. Epidural stimulation of the spinal cord in spinal cord injury: Current status and future challenges. Expert Rev. Neurother. 2011, 11, 1351–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonizzato, M.; James, N.D.; Pidpruzhnykova, G.; Pavlova, N.; Shkorbatova, P.; Baud, L.; Martinez-Gonzalez, C.; Squair, J.W.; DiGiovanna, J.; Barraud, Q.; et al. Multi-pronged neuromodulation intervention engages the residual motor circuitry to facilitate walking in a rat model of spinal cord injury. Nat. Commun. 2021, 12, 1925. [Google Scholar] [CrossRef]
- Bezdudnaya, T.; Lane, M.A.; Marchenko, V. Paced breathing and phrenic nerve responses evoked by epidural stimulation following complete high cervical spinal cord injury in rats. J. Appl. Physiol. 2018, 125, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Mercier, L.M.; Gonzalez-Rothi, E.J.; Streeter, K.A.; Posgai, S.S.; Poirier, A.S.; Fuller, D.D.; Reier, P.J.; Baekey, D.M. Intraspinal microstimulation and diaphragm activation after cervical spinal cord injury. J. Neurophysiol. 2017, 117, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Dale, E.A.; Sunshine, M.D.; Kelly, M.N.; Mitchell, G.S.; Fuller, D.D.; Reier, P.J. Chronic, closed-loop, cervical epidural stimulation elicits plasticity in diaphragm motor output and upregulates spinal neurotrophic factor gene expression. FASEB J. 2019, 33, 843.10. [Google Scholar] [CrossRef]
- Jesus, I.; Michel-Flutot, P.; Deramaudt, T.B.; Paucard, A.; Vanhee, V.; Vinit, S.; Bonay, M. Effects of aerobic exercise training on muscle plasticity in a mouse model of cervical spinal cord injury. Sci. Rep. 2021, 11, 112. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel-Flutot, P.; Jesus, I.; Vanhee, V.; Bourcier, C.H.; Emam, L.; Ouguerroudj, A.; Lee, K.-Z.; Zholudeva, L.V.; Lane, M.A.; Mansart, A.; et al. Effects of Chronic High-Frequency rTMS Protocol on Respiratory Neuroplasticity Following C2 Spinal Cord Hemisection in Rats. Biology 2022, 11, 473. https://doi.org/10.3390/biology11030473
Michel-Flutot P, Jesus I, Vanhee V, Bourcier CH, Emam L, Ouguerroudj A, Lee K-Z, Zholudeva LV, Lane MA, Mansart A, et al. Effects of Chronic High-Frequency rTMS Protocol on Respiratory Neuroplasticity Following C2 Spinal Cord Hemisection in Rats. Biology. 2022; 11(3):473. https://doi.org/10.3390/biology11030473
Chicago/Turabian StyleMichel-Flutot, Pauline, Isley Jesus, Valentin Vanhee, Camille H. Bourcier, Laila Emam, Abderrahim Ouguerroudj, Kun-Ze Lee, Lyandysha V. Zholudeva, Michael A. Lane, Arnaud Mansart, and et al. 2022. "Effects of Chronic High-Frequency rTMS Protocol on Respiratory Neuroplasticity Following C2 Spinal Cord Hemisection in Rats" Biology 11, no. 3: 473. https://doi.org/10.3390/biology11030473
APA StyleMichel-Flutot, P., Jesus, I., Vanhee, V., Bourcier, C. H., Emam, L., Ouguerroudj, A., Lee, K.-Z., Zholudeva, L. V., Lane, M. A., Mansart, A., Bonay, M., & Vinit, S. (2022). Effects of Chronic High-Frequency rTMS Protocol on Respiratory Neuroplasticity Following C2 Spinal Cord Hemisection in Rats. Biology, 11(3), 473. https://doi.org/10.3390/biology11030473






