Biological Response of Irisin Induced by Different Types of Exercise in Obese Subjects: A Non-Inferiority Controlled Randomized Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Biological Samples
2.3. Statistical Analysis
3. Results
3.1. Clinical, Physical and Antropometric Features of the Study Population
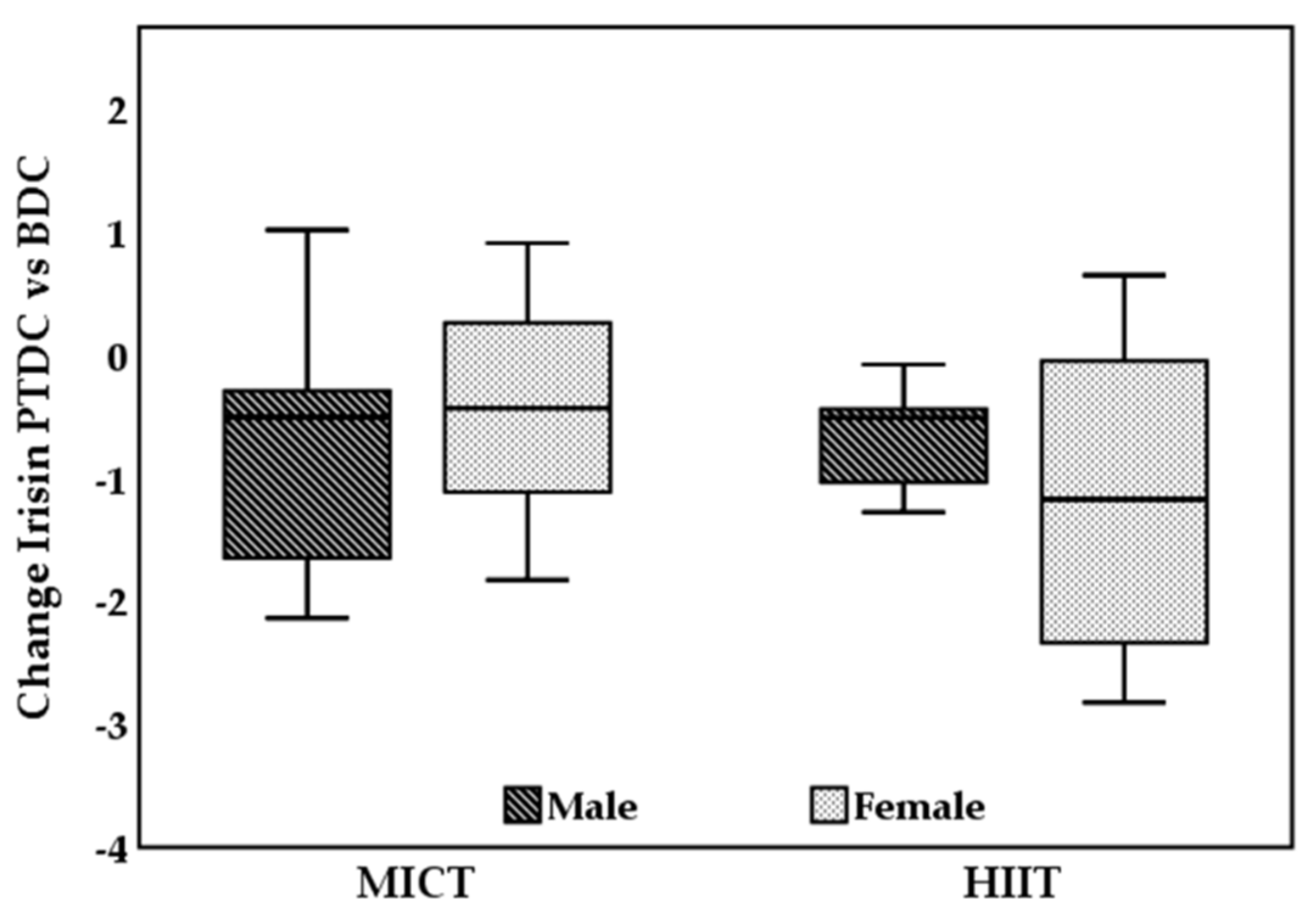
3.2. Irisin Levels in HIIT and MICT
3.3. Factors Associated with Plasma Levels of Irisin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with Health.—Overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H.; American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar]
- NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K.; American College of Sports Medicine. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Petridou, A.; Siopi, A.; Mougios, V. Exercise in the management of obesity. Metabolism 2019, 92, 163–169. [Google Scholar] [CrossRef]
- Davidson, L.E.; Hunt, S.C.; Adams, T.D. Fitness versus adiposity in cardiovascular disease risk. Eur. J. Clin. Nutr. 2019, 73, 225–230. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Silva, L.R.B.; Gentil, P.; Seguro, C.S.; de Oliveira, J.C.M.; Silva, M.S.; Marques, V.A.; Beltrame, T.; Rebelo, A.C.S. High-Intensity Interval Training Improves Cardiac Autonomic Function in Patients with Type 2 Diabetes: A Randomized Controlled Trial. Biology 2022, 11, 66. [Google Scholar] [CrossRef]
- Vaccari, F.; Passaro, A.; D’Amuri, A.; Sanz, J.M.; Di Vece, F.; Capatti, E.; Magnesa, B.; Comelli, M.; Mavelli, I.; Grassi, B.; et al. Effects of 3-month high-intensity interval training vs. moderate endurance training and 4-month follow-up on fat metabolism, cardiorespiratory function and mitochondrial respiration in obese adults. Eur. J. Appl. Physiol. 2020, 120, 1787–1803. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef]
- Shirvani, H.; Arabzadeh, H. Metabolic cross-talk between skeletal muscle and adipose tissue in high-intensity interval training vs. moderate-intensity continuous training by regulation of PGC-1α. Eat Weight Disord. 2020, 25, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Mohebbi, H.; Symonds, M.E.; Karimi, P.; Akbari, A.; Tabari, E.; Faridnia, M.; Moghaddami, K. The impact of moderate-intensity continuous or high-intensity interval training on adipogenesis and browning of subcutaneous adipose tissue in obese male rats. Nutrients 2020, 12, 925. [Google Scholar] [CrossRef] [PubMed]
- Amri, J.; Parastesh, M.; Sadegh, M.; Latifi, S.A.; Alaee, M. High-intensity interval training improved fasting blood glucose and lipid profiles in type 2 diabetic rats more than endurance training; possible involvement of irisin and betatrophin. Physiol. Int. 2019, 106, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Hecksteden, A.; Wegmann, M.; Steffen, A.; Kraushaar, J.; Morsch, A.; Ruppenthal, S.; Kaestner, L.; Meyer, T. Irisin and exercise training in humans–results from a randomized controlled training trial. BMC Med. 2013, 11, 235. [Google Scholar] [CrossRef]
- Pekkala, S.; Wiklund, P.K.; Hulmi, J.J.; Ahtiainen, J.P.; Horttanainen, M.; Pöllänen, E.; Mäkelä, K.A.; Kainulainen, H.; Häkkinen, K.; Nyman, K.; et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 2013, 591, 5393–5400. [Google Scholar] [CrossRef]
- Timmons, J.A.; Baar, K.; Davidsen, P.K.; Atherton, P.J. Is irisin a human exercise gene? Nature 2012, 488, E9–E10. [Google Scholar] [CrossRef]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In vivo and in vitro studies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef]
- Kraemer, R.R.; Shockett, P.; Webb, N.D.; Shah, U.; Castracane, V.D. A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm. Metab. Res. 2014, 46, 150–154. [Google Scholar] [CrossRef]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef]
- Huh, J.Y.; Mougios, V.; Skraparlis, A.; Kabasakalis, A.; Mantzoros, C.S. Irisin in response to acute and chronic whole-body vibration exercise in humans. Metabolism 2014, 63, 918–921. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef] [PubMed]
- Safarimosavi, S.; Mohebbi, H.; Rohani, H. High-intensity interval vs. continuous endurance training: Preventive effects on hormonal changes and physiological adaptations in prediabetes patients. J. Strength Cond. Res. 2021, 35, 731–738. [Google Scholar] [CrossRef]
- Dünnwald, T.; Melmer, A.; Gatterer, H.; Salzmann, K.; Ebenbichler, C.; Burtscher, M.; Schobersberger, W.; Grander, W. Supervised short-term high-intensity training on plasma irisin concentrations in type 2 diabetic patients. Int. J. Sports Med. 2019, 40, 158–164. [Google Scholar] [CrossRef]
- D’Amuri, A.; Sanz, J.M.; Capatti, E.; Di Vece, F.; Vaccari, F.; Lazzer, S.; Zuliani, G.; Dalla Nora, E.; Passaro, A. Effectiveness of high-intensity interval training for weight loss in adults with obesity: A randomised controlled non-inferiority trial. BMJ Open Sport Exerc. Med. 2021, 7, e001021. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle. Sports Med. 2013, 43, 313–338. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in metabolic diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef]
- Daskalopoulou, S.S.; Cooke, A.B.; Gomez, Y.; Mutter, A.F.; Filippaios, A.; Mesfum, E.T.; Mantzoros, C.S. Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects. Eur. J. Endocrinol. 2014, 171, 343–352. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Sun, Z.; Schumann, U.; Zügel, M.; Steinacker, J.M. Chronic exercise training and circulating irisin in adults: A meta-analysis. Sports Med. 2015, 45, 1577–1588. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Jameela, F.; Thotaa, R.N.; Woodb, L.G.; Plunketta, B.; Garga, M.L. Sex-dependent association between circulating irisin levels and insulin resistance in healthy adults. JNIM 2015, 2, 86–92. [Google Scholar] [CrossRef][Green Version]
- Zügel, M.; Qiu, S.; Laszlo, R.; Bosnyák, E.; Weigt, C.; Müller, D.; Diel, P.; Steinacker, J.M.; Schumann, U. The role of sex, adiposity, and gonadectomy in the regulation of irisin secretion. Endocrine 2016, 54, 101–110. [Google Scholar] [CrossRef]
- Cooke, A.B.; Gomez, Y.; Daskalopoulou, S.S. 5 years later: Irisin detection still an issue. Eur. J. Endocrinol. 2017, 177, C1–C4. [Google Scholar] [CrossRef]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Pardo, M.; Arturo, R.R.; Navas-Carretero, S.; Zulet, M.A.; Martínez, J.A.; Casanueva, F.F. Longitudinal variation of circulating irisin after an energy restriction-induced weight loss and following weight regain in obese men and women. Am. J. Hum. Biol. 2014, 26, 198–207. [Google Scholar] [CrossRef]
- Fukushima, Y.; Kurose, S.; Shinno, H.; Thi Thu, H.C.; Takao, N.; Tsutsumi, H.; Hasegawa, T.; Nakajima, T.; Kimura, Y. Effects of Body Weight Reduction on Serum Irisin and Metabolic Parameters in Obese Subjects. Diabetes Metab. J. 2016, 40, 386–395. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.J.; So, B.; Son, J.S.; Yoon, D.; Song, W. Effect of aerobic training and resistance training on circulating irisin level and their association with change of body composition in overweight/obese adults: A pilot study. Physiol. Res. 2016, 65, 271–279. [Google Scholar] [CrossRef]


| Variables | MICT Group (n = 16) | HIIT Group (n = 16) | ||
| Age, years | 37.2 ± 9.1 | 40.1 ± 7.0 | ||
| Female sex, n (%) | 7 (43.8) | 8 (50.0) | ||
| Prior or current smokers, n (%) | 2 (12.5) | 3 (18.8) | ||
| Prior weight loss attempts, n (%) | 11 (68.8) | 11 (68.8) | ||
| Family history of diabetes, n (%) | 3 (18.8) | 4 (25.0) | ||
| BDC | PTDC | BDC | PTDC | |
| Weight (kg) | 105.7 ± 17.4 | 98.5 ± 17.9 | 103.4 ± 10.9 | 97.6 ± 10.1 |
| BMI (kg/m2) | 35.7 [32.3–35.4] | 33.1 [30.7–35.4] | 34.3 [32.5–37.6] | 32.0 [29.8–36.3] |
| VO2peak (mL) | 2966.5 [2438.0–3520.5] | 3263.5 [2496.5–3571.5] | 2724.5 [2305.0–3541.0] | 3302.5 [2566.0–3992.0] |
| FM (Kg) | 37.7 ± 10.9 | 32.4 ± 9.1 | 38.4 ± 8.2 | 32.9 ± 10.0 |
| FM (%) | 35.8 [28.2–42.7] | 31.8 [24.5–40.9] | 39.1 [31.4–43.1] | 32.6 [26.6–42.2] |
| FFM (Kg) | 69.4 ± 15.5 | 68.6 ± 16.3 | 65.1 ± 11.7 | 64.7 ± 11.0 |
| FFM (%) | 64.2 [57.2–71.8] | 68.2 [59.1–75.3] | 60.9 [57.0–69.0] | 67.4 [58.4–73.4] |
| Creatinine (mg/dL) | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| HOMA Index | 1.8 [1.4–3.1] | 1.4 [1.1–2.1] | 2.0 [1.6–3.0] | 1.4 [1.2–2.8] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amuri, A.; Raparelli, V.; Sanz, J.M.; Capatti, E.; Di Vece, F.; Vaccari, F.; Lazzer, S.; Zuliani, G.; Dalla Nora, E.; Neri, L.M.; et al. Biological Response of Irisin Induced by Different Types of Exercise in Obese Subjects: A Non-Inferiority Controlled Randomized Study. Biology 2022, 11, 392. https://doi.org/10.3390/biology11030392
D’Amuri A, Raparelli V, Sanz JM, Capatti E, Di Vece F, Vaccari F, Lazzer S, Zuliani G, Dalla Nora E, Neri LM, et al. Biological Response of Irisin Induced by Different Types of Exercise in Obese Subjects: A Non-Inferiority Controlled Randomized Study. Biology. 2022; 11(3):392. https://doi.org/10.3390/biology11030392
Chicago/Turabian StyleD’Amuri, Andrea, Valeria Raparelli, Juana Maria Sanz, Eleonora Capatti, Francesca Di Vece, Filippo Vaccari, Stefano Lazzer, Giovanni Zuliani, Edoardo Dalla Nora, Luca Maria Neri, and et al. 2022. "Biological Response of Irisin Induced by Different Types of Exercise in Obese Subjects: A Non-Inferiority Controlled Randomized Study" Biology 11, no. 3: 392. https://doi.org/10.3390/biology11030392
APA StyleD’Amuri, A., Raparelli, V., Sanz, J. M., Capatti, E., Di Vece, F., Vaccari, F., Lazzer, S., Zuliani, G., Dalla Nora, E., Neri, L. M., & Passaro, A. (2022). Biological Response of Irisin Induced by Different Types of Exercise in Obese Subjects: A Non-Inferiority Controlled Randomized Study. Biology, 11(3), 392. https://doi.org/10.3390/biology11030392








