Changes in Watering Frequency Stimulate Differentiated Adaptive Responses among Seedlings of Different Beech Populations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
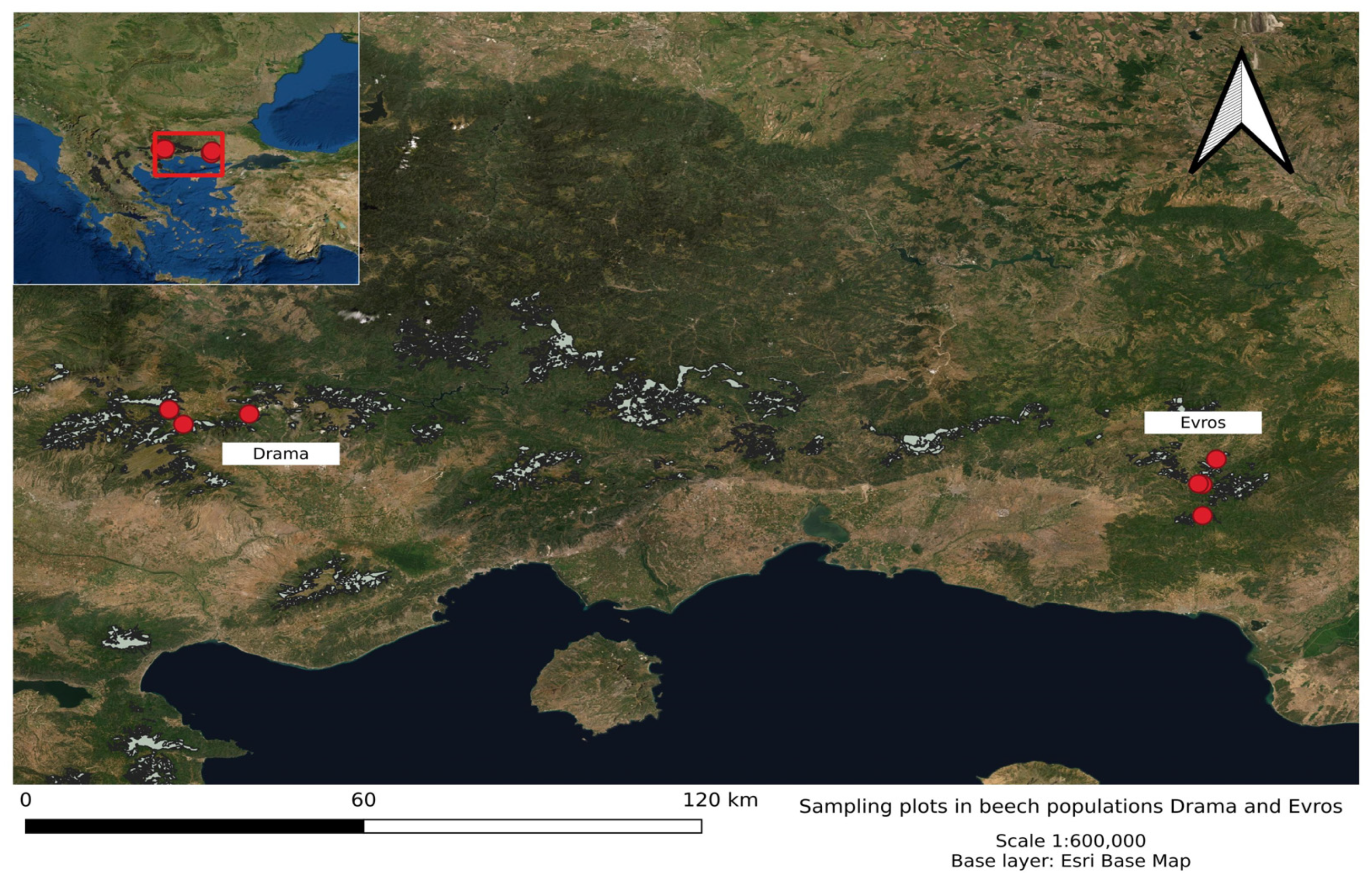
2.1. Selected Populations and Plant Material
2.2. Experimental Design and Simulated Climate Change Precipitation Schemes
2.3. Morphological and Anatomical Traits
2.4. Statistical Analyses
3. Results
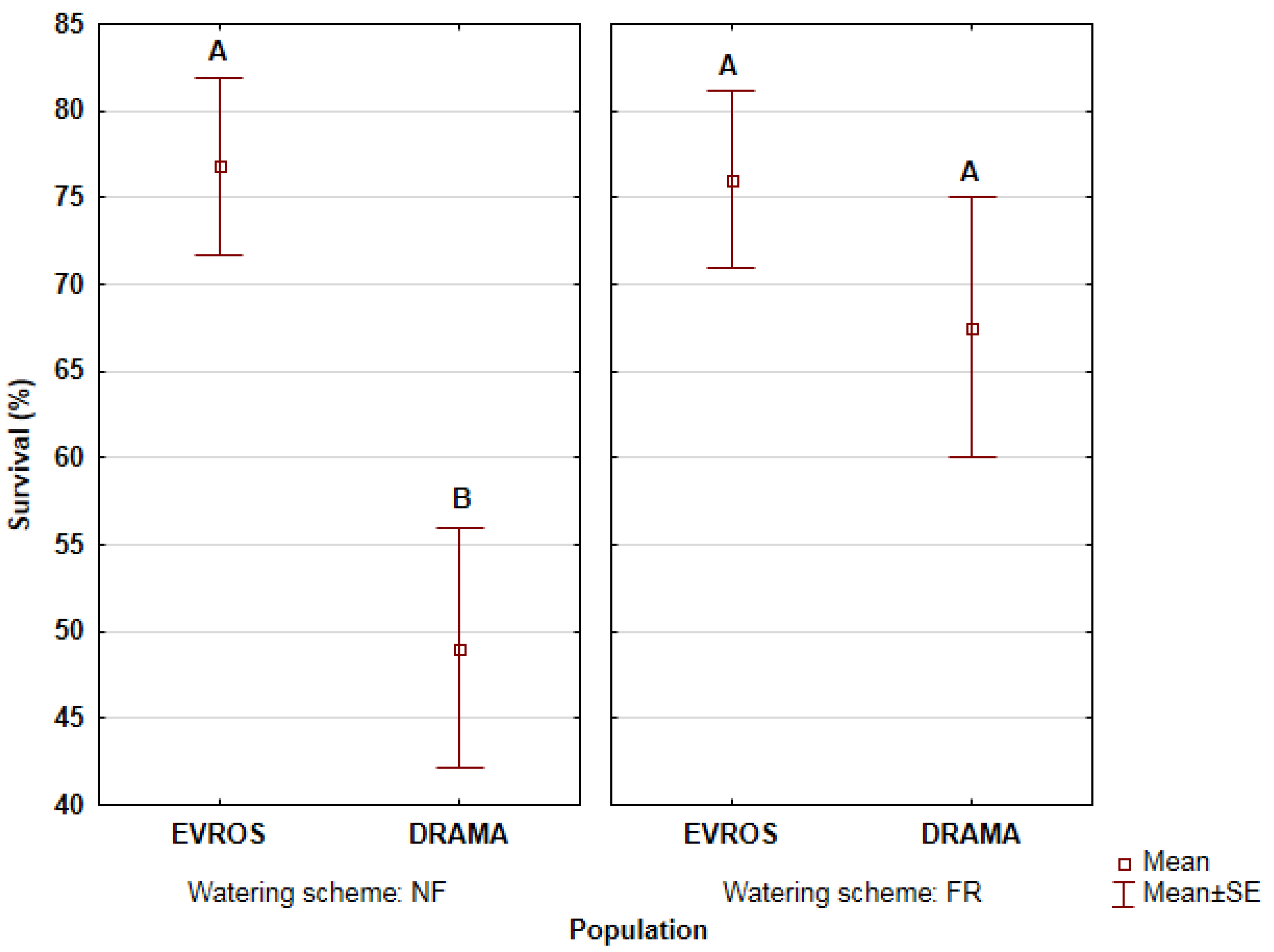
3.1. Seedling Survival
3.2. Differences between Watering Treatments
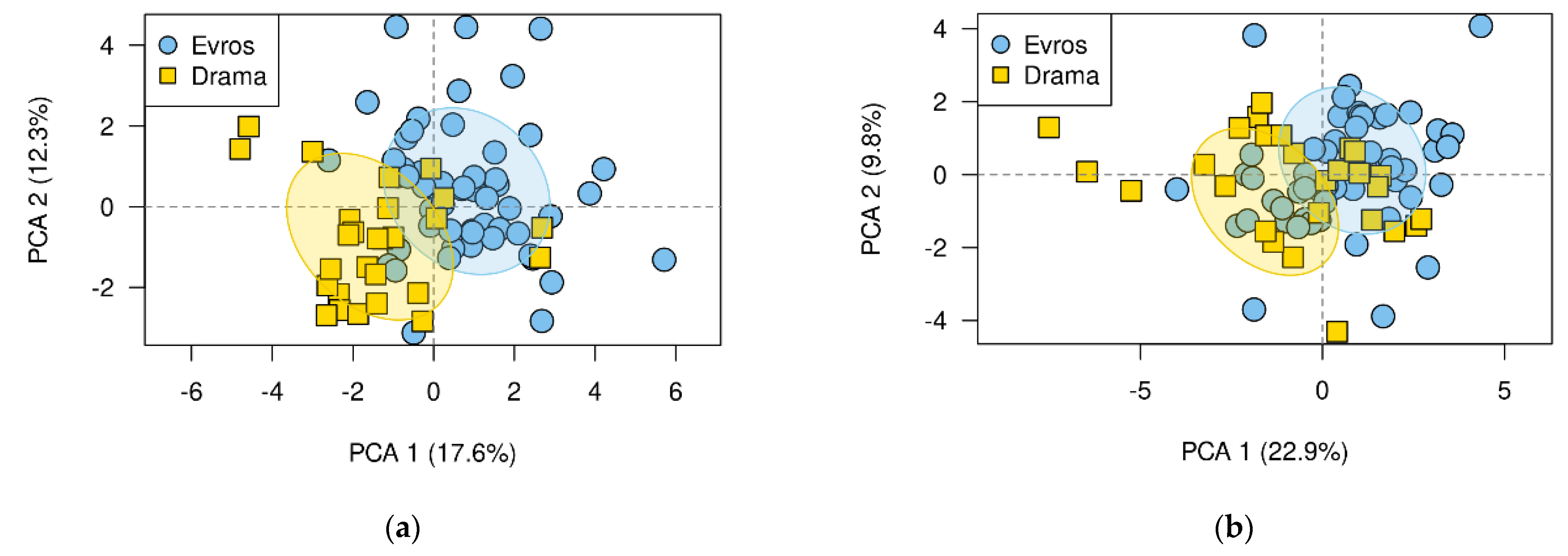
3.3. Differences between Populations
4. Discussion
4.1. Seedling Response to Watering Frequency
4.2. Adaptive Differences between Populations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geßler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential Risks for European Beech (Fagus Sylvatica L.) in a Changing Climate. Trees 2007, 21, 1–11. [Google Scholar] [CrossRef]
- Michelot, A.; Simard, S.; Rathgeber, C.; Dufrêne, E.; Damesin, C. Comparing the Intra-Annual Wood Formation of Three European Species (Fagus Sylvatica, Quercus Petraea and Pinus Sylvestris) as Related to Leaf Phenology and Non-Structural Carbohydrate Dynamics. Tree Physiol. 2012, 32, 1033–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-Wide Reduction in Primary Productivity Caused by the Heat and Drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Fotelli, M.N.; Geßler, A.; Peuke, A.D.; Rennenberg, H. Drought Affects the Competitive Interactions between Fagus Sylvatica Seedlings and an Early Successional Species, Rubus Fruticosus: Responses of Growth, Water Status and δ 13 C Composition. New Phytol. 2001, 151, 427–435. [Google Scholar] [CrossRef]
- Leuschner, C.; Backes, K.; Hertel, D.; Schipka, F.; Schmitt, U.; Terborg, O.; Runge, M. Drought Responses at Leaf, Stem and Fine Root Levels of Competitive Fagussylvatica L. and Quercuspetraea (Matt.) Liebl. Trees in Dry and Wet Years. For. Ecol. Manag. 2001, 149, 33–46. [Google Scholar] [CrossRef]
- Robson, T.M.; Calcerrada, J.R.; Sanchez-Gomez, D.; Aranda, I. Summer Drought Impedes Beech Seedling Performance more in a Sub-Mediterranean Forest Understory than in Small Gaps. Tree Physiol. 2008, 29, 249–259. [Google Scholar] [CrossRef]
- Granier, A.; Reichstein, M.; Bréda, N.; Janssens, I.; Falge, E.; Ciais, P.; Grünwald, T.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; et al. Evidence for Soil Water Control on Carbon and Water Dynamics in European Forests during the Extremely Dry Year. Agric. For. Meteorol. 2007, 143, 123–145. [Google Scholar] [CrossRef]
- Bolte, A.; Czajkowski, T.; Kompa, T. The North-Eastern Distribution Range of European Beech—A Review. Forestry 2007, 80, 413–429. [Google Scholar] [CrossRef]
- Pšidová, E.; Ditmarová, L.; Jamnická, G.; Kurjak, D.; Majerová, J.; Czajkowski, T.; Bolte, A. Photosynthetic Response of Beech Seedlings of Different Origin to Water Deficit. Photosynthetica 2015, 53, 187–194. [Google Scholar] [CrossRef]
- Leuschner, C. Drought Response of European Beech (Fagus Sylvatica L.)—A Review. Perspect. Plant Ecol. Evol. Syst. 2020, 47, 125576. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate Forest Trees and Stands under Severe Drought: A Review of Ecophysiological Responses, Adaptation Processes and Long-Term Consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Gharun, M.; Grams, T.E.E.; Hauck, M.; Hajek, P.; et al. A First Assessment of the Impact of the Extreme 2018 Summer Drought on Central European Forests. Basic Appl. Ecol. 2020, 45, 86–103. [Google Scholar] [CrossRef]
- Fotelli, M.N.; Nahm, M.; Radoglou, K.; Rennenberg, H.; Halyvopoulos, G.; Matzarakis, A. Seasonal and Interannual Ecophysiological Responses of Beech (Fagus sylvatica) at Its South-Eastern Distribution Limit in Europe. For. Ecol. Manag. 2009, 257, 1157–1164. [Google Scholar] [CrossRef]
- Skrøppa, T.; Tollefsrud, M.M.; Sperisen, C.; Johnsen, Ø. Rapid Change in Adaptive Performance from One Generation to the Next in Picea Abies—Central European Trees in a Nordic Environment. Tree Genet. Genomes 2009, 6, 93–99. [Google Scholar] [CrossRef]
- Sánchez-Gómez, D.; Robson, T.M.; Gascó, A.; Gil-Pelegrín, E.; Aranda, I. Differences in the Leaf Functional Traits of Six Beech (Fagus sylvatica L.) Populations Are Reflected in Their Response to Water Limitation. Environ. Exp. Bot. 2013, 87, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Pardos, M.; Calama, R. Responses of Pinus Pinea Seedlings to Moderate Drought and Shade: Is the Provenance a Differential Factor? Photosynthetica 2017, 56, 786–798. [Google Scholar] [CrossRef]
- Varsamis, G.; Papageorgiou, A.C.; Merou, T.; Takos, I.; Malesios, C.; Manolis, A.; Tsiripidis, I.; Gailing, O. Adaptive Diversity of Beech Seedlings Under Climate Change Scenarios. Front. Plant Sci. 2019, 9, 1918. [Google Scholar] [CrossRef]
- Varsamis, G.; Merou, T.; Takos, I.; Malesios, C.; Manolis, A.; Papageorgiou, A.C. Seed Adaptive Traits of Fagus Sylvatica Populations in Northeastern Greece. For. Sci. 2020, 66, 403–415. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M.; Holland, V.; Brüggemann, W. Functional Traits and Adaptive Capacity of European Forests to Climate Change. Environ. Exp. Bot. 2015, 111, 91–113. [Google Scholar] [CrossRef]
- De Villemereuil, P.; Gaggiotti, O.E.; Mouterde, M.; Till-Bottraud, I. Common Garden Experiments in the Genomic Era: New Perspectives and Opportunities. Heredity 2015, 116, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Fonti, P.; Larsen, J.B.; Ræbild, A.; Callesen, I.; Pedersen, N.B.; Hansen, J. Projecting Tree-Growth Responses into Future Climate: A Study Case from a Danish-Wide Common Garden. Agric. For. Meteorol. 2017, 247, 240–251. [Google Scholar] [CrossRef]
- Rose, L.; Leuschner, C.; Köckemann, B.; Buschmann, H. Are Marginal Beech (Fagus Sylvatica L.) Provenances a Source for Drought Tolerant Ecotypes? Forstwiss. Cent. 2009, 128, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Knutzen, F.; Meier, I.C.; Leuschner, C. Does Reduced Precipitation Trigger Physiological and Morphological Drought Adaptations in European Beech (Fagus sylvatica L.)? Comparing Provenances across a Precipitation Gradient. Tree Physiol. 2015, 35, 949–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojnic, S.; Orlovic, S.; Miljkovic, D.; Von, W.G. Intra- and Interprovenance Variations in Leaf Morphometric Traits in European Beech (Fagus sylvatica L.). Arch. Biol. Sci. 2016, 68, 781–788. [Google Scholar] [CrossRef]
- Pflug, E.E.; Buchmann, N.; Siegwolf, R.T.W.; Schaub, M.; Rigling, A.; Arend, M. Resilient Leaf Physiological Response of European Beech (Fagus sylvatica L.) to Summer Drought and Drought Release. Front. Plant Sci. 2018, 9, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dannoura, M.; Epron, D.; Desalme, D.; Massonnet, C.; Tsuji, S.; Plain, C.; Priault, P.; Gérant, D. The Impact of Prolonged Drought on Phloem Anatomy and Phloem Transport in Young Beech Trees. Tree Physiol. 2018, 39, 201–210. [Google Scholar] [CrossRef]
- Salmon, Y.; Dietrich, L.; Sevanto, S.; Hölttä, T.; Dannoura, M.; Epron, D. Drought Impacts on Tree Phloem: From Cell-Level Responses to Ecological Significance. Tree Physiol. 2019, 39, 173–191. [Google Scholar] [CrossRef] [Green Version]
- Massonnet, C.; Chuste, P.-A.; Levillain, J.; Gérémia, F.; Silva, D.E.; Maillard, P.; Dreyer, E.; Dupouey, J.-L.; Bréda, N. Leafy Season Length Is Reduced by a Prolonged Soil Water Deficit but not by Repeated Defoliation in Beech Trees (Fagus sylvatica L.): Comparison of Response among Regional Populations Grown in a Common Garden. Agric. For. Meteorol. 2020, 297, 108228. [Google Scholar] [CrossRef]
- Stojnić, S.; Orlović, S.; Miljković, D.; Galic, Z.; Kebert, M.; von Wuehlisch, G. Provenance Plasticity of European Beech Leaf Traits under Differing Environmental Conditions at Two Serbian Common Garden Sites. Forstwiss. Cent. 2015, 134, 1109–1125. [Google Scholar] [CrossRef]
- Kucerová, J.; Konôpková, A.; Pšidová, E.; Kurjak, D.; Jamnická, G.; Slugenová, K.; Gömöry, D.; Ditmarová, L. Adaptive Variation in Physiological Traits of Beech Provenances in Central Europe. iFor. Biogeosci. For. 2018, 11, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Petrík, P.; Petek, A.; Konôpková, A.; Bosela, M.; Fleischer, P.; Frýdl, J.; Kurjak, D. Stomatal and Leaf Morphology Response of European Beech (Fagus Sylvatica L.) Provenances Transferred to Contrasting Climatic Conditions. Forests 2020, 11, 1359. [Google Scholar] [CrossRef]
- Magnani, F.; Grace, J.; Borghetti, M. Adjustment of Tree Structure in Response to the Environment under Hydraulic Constraints. Funct. Ecol. 2002, 16, 385–393. [Google Scholar] [CrossRef]
- Mokotedi, M.E. Physiological Responses of Eucalyptus Nitens × Nitensunder Experimentally Imposed Water Stress. South. For. J. For. Sci. 2010, 72, 63–68. [Google Scholar] [CrossRef]
- Granda, V.; Delatorre, C.; Cuesta, C.; Centeno, M.L.; Fernández, B.; Rodríguez, A.; Feito, I. Physiological and Biochemical Responses to Severe Drought Stress of Nine Eucalyptus Globulus Clones: A Multivariate Approach. Tree Physiol. 2014, 34, 778–786. [Google Scholar] [CrossRef] [Green Version]
- Ledo, A.; Paul, K.I.; Burslem, D.; Ewel, J.J.; Barton, C.V.M.; Battaglia, M.; Brooksbank, K.; Carter, J.; Eid, T.H.; England, J.R.; et al. Tree Size and Climatic Water Deficit Control Root to Shoot Ratio in Individual Trees Globally. New Phytol. 2017, 217, 8–11. [Google Scholar] [CrossRef]
- Mrak, T.; Štraus, I.; Grebenc, T.; Gričar, J.; Hoshika, Y.; Carriero, G.; Paoletti, E.; Kraigher, H. Different Belowground Responses to Elevated Ozone and Soil Water Deficit in Three European Oak Species (Quercus ilex, Q. Pubescens and Q. Robur). Sci. Total Environ. 2018, 651, 1310–1320. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Corcuera, L.; Camarero, J.J.; Gil-Pelegrín, E. Effects of a Severe Drought on Quercus ilex Radial Growth and Xylem Anatomy. Trees 2003, 18, 83–92. [Google Scholar] [CrossRef]
- Lens, F.; Sperry, J.S.; Christman, M.A.; Choat, B.; Rabaey, D.; Jansen, S. Testing Hypotheses That Link Wood Anatomy to Cavitation Resistance and Hydraulic Conductivity in the Genus Acer. New Phytol. 2010, 190, 709–723. [Google Scholar] [CrossRef] [Green Version]
- Lens, F.; Tixier, A.; Cochard, H.; Sperry, J.S.; Jansen, S.; Herbette, S. Embolism Resistance as a Key Mechanism to Understand Adaptive Plant Strategies. Curr. Opin. Plant Biol. 2013, 16, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Sevanto, S. Phloem Transport and Drought. J. Exp. Bot. 2014, 65, 1751–1759. [Google Scholar] [CrossRef] [Green Version]
- Nardini, A.; Savi, T.; Trifilò, P.; Gullo, M.A.L. Drought Stress and the Recovery from Xylem Embolism in Woody Plants. In Progress in Botany; Springer: Cham, Switzerland, 2017; pp. 197–231. [Google Scholar] [CrossRef]
- Klein, T.; Zeppel, M.J.B.; Anderegg, W.R.L.; Bloemen, J.; De Kauwe, M.G.; Hudson, P.; Ruehr, N.K.; Powell, T.L.; von Arx, G.; Nardini, A. Xylem Embolism Refilling and Resilience against Drought-Induced Mortality in Woody Plants: Processes and Trade-Offs. Ecol. Res. 2018, 1–17. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Schwarz, D.; Franken, P.; Colla, G. Effects of Drought on Nutrient Uptake and Assimilation in Vegetable Crops. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-32653-0. [Google Scholar]
- Rosell, J.A.; Olson, M.E. The Evolution of Bark Mechanics and Storage across Habitats in a Clade of Tropical Trees. Am. J. Bot. 2014, 101, 764–777. [Google Scholar] [CrossRef] [Green Version]
- Rosell, J.A. Bark Thickness across the Angiosperms: More than Just Fire. New Phytol. 2016, 211, 90–102. [Google Scholar] [CrossRef]
- Nilsen, E.T.; Bao, Y. The Influence of Water Stress on Stem and Leaf Photosynthesis in Glycine Max and Sparteum junceum (Leguminosae). Am. J. Bot. 1990, 77, 1007–1015. [Google Scholar] [CrossRef]
- Barbaroux, C.; Breda, N. Contrasting Distribution and Seasonal Dynamics of Carbohydrate Reserves in Stem Wood of Adult Ring-Porous Sessile Oak and Diffuse-Porous Beech Trees. Tree Physiol. 2002, 22, 1201–1210. [Google Scholar] [CrossRef]
- Pfanz, H. Bark Photosynthesis. Trees 2008, 22, 137–138. [Google Scholar] [CrossRef]
- Ávila, E.; Herrera, A.; Tezara, W. Contribution of Stem CO2 Fixation to Whole-Plant Carbon Balance in Nonsucculent Species. Photosynthetica 2013, 52, 3–15. [Google Scholar] [CrossRef]
- Paul, S.; Wildhagen, H.; Janz, D.; Polle, A. Drought Effects on the Tissue- and Cell-Specific Cytokinin Activity in Poplar. AoB Plants 2017, 10, plx067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijnsbrugge, K.V.; Turcsán, A.; Erdélyi, É.; Beeckman, H. Drought Treated Seedlings of Quercus Petraea (Matt.) Liebl., Q. Robur L. and Their Morphological Intermediates Show Differential Radial Growth and Wood Anatomical Traits. Forests 2020, 11, 250. [Google Scholar] [CrossRef] [Green Version]
- Tognetti, R.; Michelozzi, M.; Borghetti, M. Response to Light of Shade-Grown Beech Seedlings Subjected to Different Watering Regimes. Tree Physiol. 1994, 14, 751–758. [Google Scholar] [CrossRef]
- Tognetti, R.; Johnson, J.D.; Michelozzi, M. The Response of European Beech (Fagus sylvatica L.) Seedlings from Two Italian Populations to Drought and Recovery. Trees 1995, 9, 348–354. [Google Scholar] [CrossRef]
- Gallé, A.; Feller, U. Changes of Photosynthetic Traits in Beech Saplings (Fagus Sylvatica) under Severe Drought Stress and during Recovery. Physiol. Plant. 2007, 131, 412–421. [Google Scholar] [CrossRef]
- Zang, U.; Goisser, M.; Häberle, K.-H.; Matyssek, R.; Matzner, E.; Borken, W. Effects of Drought Stress on Photosynthesis, Rhizosphere Respiration, and Fine-Root Characteristics of Beech Saplings: A Rhizotron Field Study. Z. Pflanz. Bodenk. 2014, 177, 168–177. [Google Scholar] [CrossRef]
- Gebauer, R.; Plichta, R.; Urban, J.; Volařík, D.; Hájíčková, M. The Resistance and Resilience of European Beech Seedlings to Drought Stress during the Period of Leaf Development. Tree Physiol. 2020, 40, 1147–1164. [Google Scholar] [CrossRef]
- Kunz, J.; Räder, A.; Bauhus, J. Effects of Drought and Rewetting on Growth and Gas Exchange of Minor European Broadleaved Tree Species. Forests 2016, 7, 239. [Google Scholar] [CrossRef]
- Barigah, T.S.; Charrier, O.; Douris, M.; Bonhomme, M.; Herbette, S.; Ameglio, T.; Fichot, R.; Brignolas, F.; Cochard, H. Water Stress-Induced Xylem Hydraulic Failure Is a Causal Factor of Tree Mortality in Beech and Poplar. Ann. Bot. 2013, 112, 1431–1437. [Google Scholar] [CrossRef]
- Bolte, A.; Czajkowski, T.; Cocozza, C.; Tognetti, R.; De Miguel, M.; Pšidová, E.; Ditmarová, Ĺ.; Dinca, L.; Delzon, S.; Cochard, H.; et al. Desiccation and Mortality Dynamics in Seedlings of Different European Beech (Fagus sylvatica L.) Populations under Extreme Drought Conditions. Front. Plant Sci. 2016, 7, 751. [Google Scholar] [CrossRef] [Green Version]
- Cocozza, C.; De Miguel, M.; Pšidová, E.; Ditmarová, L.; Marino, S.; Maiuro, L.; Alvino, A.; Czajkowski, T.; Bolte, A.; Tognetti, R. Variation in Ecophysiological Traits and Drought Tolerance of Beech (Fagus sylvatica L.) Seedlings from Different Populations. Front. Plant Sci. 2016, 7, 886. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Israel, D.; Ramírez-Valiente, J.-A.; Sánchez-Gómez, D.; Aranda, I.; Aphalo, P.J.; Robson, T.M. Seedlings from Marginal and Core Populations of European Beech (Fagus sylvatica L.) Respond Differently to Imposed Drought and Shade. Trees 2020, 35, 53–67. [Google Scholar] [CrossRef]
- Brooks, H. Severe Thunderstorms and Climate Change. Atmos. Res. 2013, 123, 129–138. [Google Scholar] [CrossRef]
- Papageorgiou, A.C.; Vidalis, A.; Gailing, O.; Tsiripidis, I.; Hatziskakis, S.; Boutsios, S.; Galatsidas, S.; Finkeldey, R. Genetic Variation of Beech (Fagus sylvatica L.) in Rodopi (N.E. Greece). Forstwiss. Cent. 2007, 127, 81–88. [Google Scholar] [CrossRef]
- Müller, M.; Lopez, P.A.; Papageorgiou, A.C.; Tsiripidis, I.; Gailing, O. Indications of Genetic Admixture in the Transition Zone between Fagus sylvatica L. and Fagus Sylvatica ssp. Orientalis Greut. & Burd. Diversity 2019, 11, 90. [Google Scholar] [CrossRef] [Green Version]
- ISTA. Chapter 5: The Germination Test. In Rules Proposal for the International Rules for Seed Testing; International Rules Seed Testing: Bassersdorf, Switzerland, 2017; pp. 1–62. [Google Scholar] [CrossRef]
- Syktus, J.; Jeffrey, S.; Rotstayn, L.; Wong, K.; Toombs, N.; Dravitzki, S.; Collier, M.; Hamalainen, C.; Moeseneder, C. The CSIRO-QCCCE Contribution to CMIP5 Using the CSIRO Mk3.6 Climate Model. In Proceedings of the MODSIM 2011–19th International Congress on Modelling and Simulation-Sustaining Our Future: Understanding and Living with Uncertainty; The Modelling and Simulation Society of Australia and New Zealand: Perth, Australia, 2011; pp. 2782–2788. [Google Scholar]
- Kriticos, D.J.; Webber, B.L.; Leriche, A.; Ota, N.; Macadam, I.; Bathols, J.; Scott, J.K. CliMond: Global High-Resolution Historical and Future Scenario Climate Surfaces for Bioclimatic Modelling. Methods Ecol. Evol. 2012, 3, 53–64. [Google Scholar] [CrossRef]
- Radoglou, K.; Jarvis, P.G. Effects of CO2 Enrichment on Four Poplar Clones. II. Leaf Surface Properties. Ann. Bot. 1990, 65, 627–632. [Google Scholar] [CrossRef]
- Bussotti, F.; Prancrazi, M.; Matteucci, G.; Gerosa, G. Leaf Morphology and Chemistry in Fagus Sylvatica (Beech) Trees as Affected by Site Factors and Ozone: Results from CONECOFOR Permanent Monitoring Plots in Italy. Tree Physiol. 2005, 25, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A Handbook of Protocols for Standardised and Easy Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef] [Green Version]
- Vile, D.; Garnier, É.; Shipley, B.; Laurent, G.; Navas, M.-L.; Roumet, C.; Lavorel, S.; Díaz, S.; Hodgson, J.G.; Lloret, F.; et al. Specific Leaf Area and Dry Matter Content Estimate Thickness in Laminar Leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef]
- Jupa, R.; Plavcová, L.; Gloser, V.; Jansen, S. Linking Xylem Water Storage with Anatomical Parameters in Five Temperate Tree Species. Tree Physiol. 2016, 36, 756–769. [Google Scholar] [CrossRef] [Green Version]
- Bissing, D.R. Haupt’s Gelatin Adhesive Mixed with Formalin for Affixing Paraffin Sections to Slides. Stain Technol. 1974, 49, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Edward, C.; Yeung, A. Beginner’s Guide to the Study of Plant Structure. In Proceedings of the Tested Studies for Laboratory Teaching; Karcher, J.S., Ed.; Association for Biology Laboratory Education: Irvine, CA, USA, 1998; pp. 125–142. [Google Scholar]
- Crang, R.; Lyons-Sobaski, S.; Wise, R. The Nature of Plants. In Plant Anatomy; Springer International Publishing: Cham, Switzerland, 2018; pp. 3–44. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: AnRPackage for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Guo, W.; Xu, W.; Wang, R. Habitat Effects on Leaf Morphological Plasticity in Quercus Acutissima. Acta Biol. Crac. Ser. Bot. 2008, 50, 19–26. [Google Scholar]
- Peltzer, D. Anpassung Antioxidativer Systeme an Licht und Temperatur: Holzige und Krautige Pflanzen im Vergleich; Georg-August-Universität Göttingen: Göttingen, Germany, 2001. [Google Scholar]
- Haldimann, P.; Feller, U. Inhibition of Photosynthesis by High Temperature in Oak (Quercus pubescens L.) Leaves Grown under Natural Conditions Closely Correlates with a Reversible Heat-Dependent Reduction of the Activation State of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase. Plant Cell Environ. 2004, 27, 1169–1183. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How Do Environmental Stresses Accelerate Photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Hüve, K.; Bichele, I.; Rasulov, B.; Niinemets, Ü.L.O. When It Is Too Hot for Photosynthesis: Heat-Induced Instability of Photosynthesis in Relation to Respiratory Burst, Cell Permeability Changes and H2O2 Formation. Plant Cell Environ. 2010, 34, 113–126. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of Photosynthesis during Abiotic Stress-Induced Photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [Green Version]
- Leigh, A.; Sevanto, S.; Close, J.; Nicotra, A. The Influence of Leaf Size and Shape on Leaf Thermal Dynamics: Does Theory Hold Up under Natural Conditions? Plant Cell Environ. 2016, 40, 237–248. [Google Scholar] [CrossRef]
- Pfautsch, S.; Holtta, T.; Mencuccini, M. Hydraulic Functioning of Tree Stems—Fusing Ray Anatomy, Radial Transfer and Capacitance. Tree Physiol. 2015, 35, 706–722. [Google Scholar] [CrossRef] [Green Version]
- Schall, P.; Lödige, C.; Beck, M.; Ammer, C. Biomass Allocation to Roots and Shoots is More Sensitive to Shade and Drought in European Beech than in Norway Spruce Seedlings. For. Ecol. Manag. 2012, 266, 246–253. [Google Scholar] [CrossRef]
- Cuervo-Alarcon, L.; Arend, M.; Müller, M.; Sperisen, C.; Finkeldey, R.; Krutovsky, K.V. A Candidate Gene Association Analysis Identifies SNPs Potentially Involved in Drought Tolerance in European Beech (Fagus sylvatica L.). Sci. Rep. 2021, 11, 2386. [Google Scholar] [CrossRef]
- Robakowski, P.; Wyka, T.; Kowalkowski, W.; Barzdajn, W.; Pers-Kamczyc, E.; Jankowski, A.; Politycka, B. Practical Implications of Different Phenotypic and Molecular Responses of Evergreen Conifer and Broadleaf Deciduous Forest Tree Species to Regulated Water Deficit in a Container Nursery. Forests 2020, 11, 1011. [Google Scholar] [CrossRef]
- Zadworny, M.; Mucha, J.; Jagodziński, A.M.; Kościelniak, P.; Łakomy, P.; Modrzejewski, M.; Ufnalski, K.; Żytkowiak, R.; Comas, L.H.; Rodríguez-Calcerrada, J. Seedling Regeneration Techniques Affect Root Systems and the Response of Quercus Robur Seedlings to Water Shortages. For. Ecol. Manag. 2020, 479, 118552. [Google Scholar] [CrossRef]
- Borchert, R.; Pockman, W. Water Storage Capacitance and Xylem Tension in Isolated Branches of Temperate and Tropical Trees. Tree Physiol. 2005, 25, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How Tree Roots Respond to Drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef] [Green Version]
- del Río, S.; Álvarez-Esteban, R.; Cano, E.; Pinto-Gomes, C.; Penas, Á. Potential Impacts of Climate Change on Habitat Suita-bility of Fagus sylvatica L. Forests in Spain. Plant Biosyst. 2018, 152, 1205–1213. [Google Scholar] [CrossRef]
- Larysch, E.; Stangler, D.F.; Nazari, M.; Seifert, T.; Kahle, H.-P. Xylem Phenology and Growth Response of European Beech, Silver Fir and Scots Pine along an Elevational Gradient during the Extreme Drought Year 2018. Forests 2021, 12, 75. [Google Scholar] [CrossRef]
- Gömöry, D.; Paule, L.; Brus, R.; Zhelev, P.; Tomović, Z.; Gračan, J. Genetic Differentiation and Phylogeny of Beech on the Balkan Peninsula. J. Evol. Biol. 1999, 12, 746–754. [Google Scholar] [CrossRef] [Green Version]
- Hatziskakis, S.; Papageorgiou, A.C.; Gailing, O.; Finkeldey, R. High Chloroplast Haplotype Diversity in Greek Populations of Beech (Fagus sylvatica L.). Plant Biol. 2009, 11, 425–433. [Google Scholar] [CrossRef]
- Mátyás, C.; Bozic, G.; Gömöry, D.; Ivankovic, M.; Rasztovits, E. Juvenile Growth Response of European Beech (Fagus sylvatica L.) to Sudden Change of Climatic Environment in SE European Trials. iFor. Biogeosci. For. 2009, 2, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Lefèvre, F.; Boivin, T.; Bontemps, A.; Courbet, F.; Davi, H.; Durand-Gillmann, M.; Fady, B.; Gauzere, J.; Gidoin, C.; Karam, M.-J.; et al. Considering Evolutionary Processes in Adaptive Forestry. Ann. For. Sci. 2013, 71, 723–739. [Google Scholar] [CrossRef] [Green Version]
- Fady, B.; Cottrell, J.; Ackzell, L.; Alía, R.; Muys, B.; Prada, A.; González-Martínez, S.C. Forests and Global Change: What Can Genetics Contribute to the Major Forest Management and Policy Challenges of the Twenty-First Century? Reg. Environ. Chang. 2015, 16, 927–939. [Google Scholar] [CrossRef]
| Trait | Description |
|---|---|
| Specific leaf area (SLA) | The ratio of leaf area to dry weight. |
| Leaf dry matter content (LDMC) | The ratio of leaf fresh weight to its dry weight. |
| Leaf thickness | The estimated leaf lamina thickness. |
| Leaf length | The length of leaf lamina. |
| Leaf width | The width of leaf lamina at its maximum. |
| Leaf base angle | The angle of leaf lamina base. |
| Leaf tip angle | The angle of leaf lamina tip. |
| Number of leaf secondary veins | Number of first-class veins. |
| Leaf circularity | The ratio of area to perimeter of leaf. |
| Shoot length | The length from the root collar to the apical bud. |
| Shoot dry weight | The shoot weight after drying in an oven. |
| Root area | The projected area of the whole seedling root system. |
| Root dry weight | The root system weight after drying in an oven. |
| Section area | Total projected area of the produced stem section. |
| Phellem length | Length of the outer plant suberized epidermis. |
| Cortex length | Length of the tissue layer between epidermis and phloem tissue. |
| Phloem length | Length of the tissue layer between cortex and xylem. |
| Xylem length | Length of the tissue layer between phloem and pith. |
| Pith length | Maximum length of the pith |
| Stomatal density | Density of stomata number in the middle leaf lamina part. |
| Number of pith rays | The number of the rays that connect the vascular system (xylem, phloem) with the pith. |
| Trait | Factor | ||
|---|---|---|---|
| Population | Watering Treatment | Population × Watering Treatment | |
| Specific leaf area | 0.315 | 0.133 | 0.806 |
| Leaf dry matter content | 0.757 | 0.216 | 0.146 |
| Leaf thickness | 0.811 | 0.449 | 0.375 |
| Leaf length | 0.002 * | 0.262 | 0.518 |
| Leaf width | 0.005 * | 0.333 | 0.552 |
| Leaf base angle | 0.517 | 0.716 | 0.153 |
| Leaf tip angle | 0.083 | 0.801 | 0.454 |
| Number of secondary leaf veins | 0.715 | 0.016 | 0.250 |
| Leaf circularity | 0.025 * | 0.000 *** | 0.001 *** |
| Shoot length | 0.005 ** | 0.200 | 0.601 |
| Shoot dry weight | 0.142 | 0.342 | 0.200 |
| Root area | 0.166 | 0.677 | 0.284 |
| Root dry weight | 0.535 | 0.357 | 0.110 |
| Section area | 0.000 *** | 0.559 | 0.000 *** |
| Phellem length | 0.104 | 0.752 | 0.106 |
| Cortex length | 0.727 | 0.465 | 0.001 *** |
| Phloem length | 0.064 | 0.040 * | 0.000 *** |
| Xylem length | 0.656 | 0.087 | 0.000 *** |
| Pith length | 0.002 ** | 0.214 | 0.000 *** |
| Stomatal number | 0.001 *** | 0.521 | 0.008 ** |
| Number of pith rays | 0.001 | 0.682 | 0.430 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varsamis, G.; Adamidis, G.C.; Merou, T.; Takos, I.; Tseniklidou, K.; Dimitrakopoulos, P.G.; Papageorgiou, A.C. Changes in Watering Frequency Stimulate Differentiated Adaptive Responses among Seedlings of Different Beech Populations. Biology 2022, 11, 306. https://doi.org/10.3390/biology11020306
Varsamis G, Adamidis GC, Merou T, Takos I, Tseniklidou K, Dimitrakopoulos PG, Papageorgiou AC. Changes in Watering Frequency Stimulate Differentiated Adaptive Responses among Seedlings of Different Beech Populations. Biology. 2022; 11(2):306. https://doi.org/10.3390/biology11020306
Chicago/Turabian StyleVarsamis, Georgios, George C. Adamidis, Theodora Merou, Ioannis Takos, Katerina Tseniklidou, Panayiotis G. Dimitrakopoulos, and Aristotelis C. Papageorgiou. 2022. "Changes in Watering Frequency Stimulate Differentiated Adaptive Responses among Seedlings of Different Beech Populations" Biology 11, no. 2: 306. https://doi.org/10.3390/biology11020306
APA StyleVarsamis, G., Adamidis, G. C., Merou, T., Takos, I., Tseniklidou, K., Dimitrakopoulos, P. G., & Papageorgiou, A. C. (2022). Changes in Watering Frequency Stimulate Differentiated Adaptive Responses among Seedlings of Different Beech Populations. Biology, 11(2), 306. https://doi.org/10.3390/biology11020306








