Spatiotemporal Variations of Plague Risk in the Tibetan Plateau from 1954–2016
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plague Data Compilation
2.2. Environmental Variables of Plague Foci
2.3. Modelling the Potential Areas of Animal Plague
3. Results
3.1. Model Performance and Changes of Plague Areas
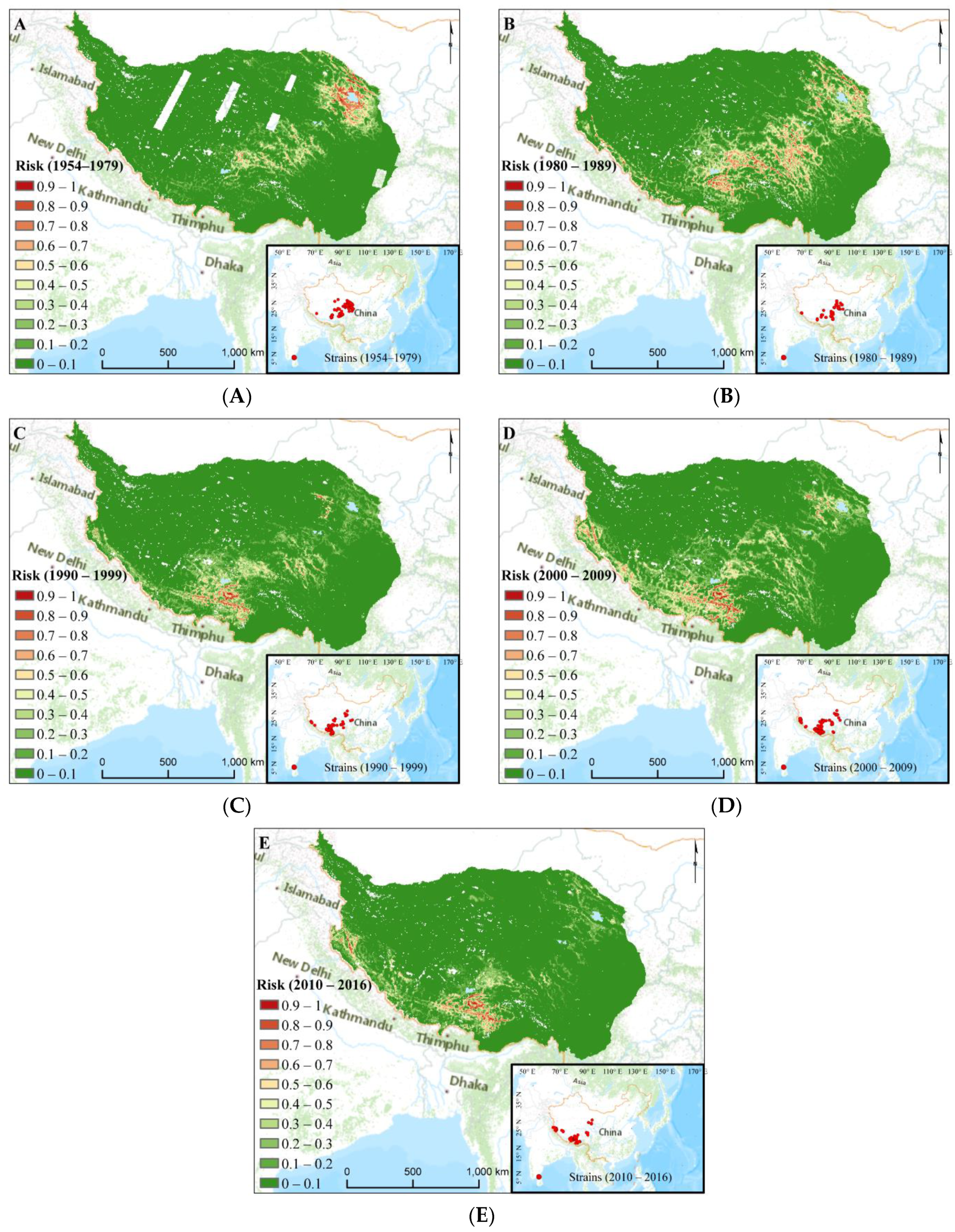
3.2. Plague Risk Areas at Different Time
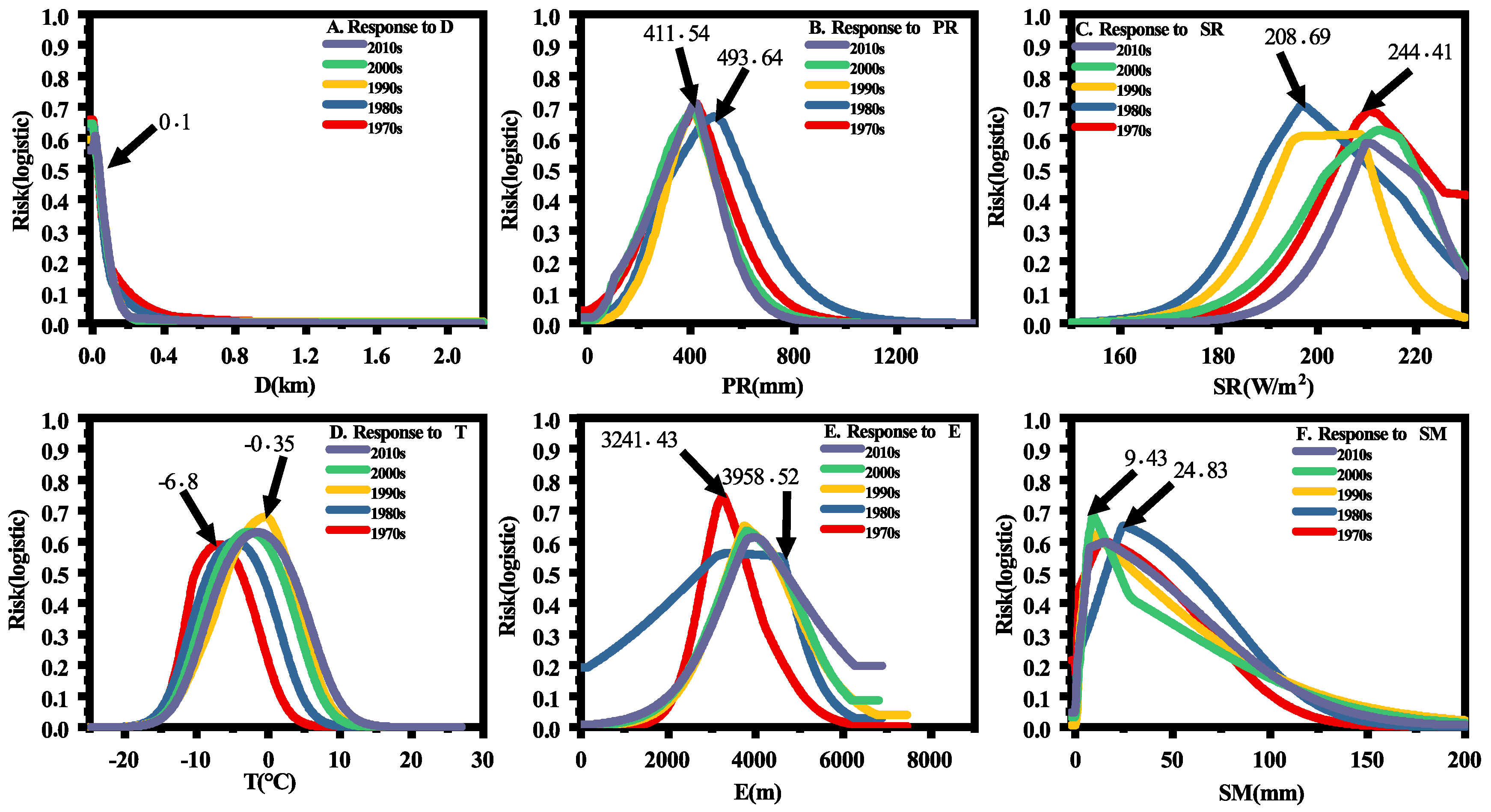
3.3. Impacts of Environmental Variables in Different Time
3.4. Human Disturbance Evaluation on the Different Distributions
4. Discussion
4.1. Basic Factors for Plague Distributions
4.2. Major Factors That Affect Plague Spatiotemporal Distributions
4.3. Human Disturbance for Plague Distributions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, B.A.; Grace, D.; Kock, R.; Alonso, S.; Rushton, J.; Said, M.Y.; McKeever, D.; Mutua, F.; Young, J.; McDermott, J.; et al. Zoonosis emergence linked to agricultural intensificationand environmental change. Proc. Natl. Acad. Sci. USA 2013, 110, 8399–8404. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Allentoft, M.; Nielsen, K.; Orlando, L.; Sikora, M.; Sjögren, K.-G.; Pedersen, A.G.; Schubert, M.; Van Dam, A.; Kapel, C.; et al. Early divergent strains of Yersinia pestis in Eurasia 5,000 years ago. Cell 2015, 163, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Reijniers, J.; Davis, S.; Begon, M.; Heesterbeek, J.A.; Ageyev, V.S.; Leirs, H. A curve of thresholds governs plague epizootics in Central Asia. Ecol. Lett. 2012, 15, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Wimsatt, J.; Biggins, D.E. A review of plague persistence with special emphasis on fleas. J. Vector. Borne. Dis. 2009, 46, 85–99. [Google Scholar]
- Weinhold, B. Plague linked to precipitation. Environ. Health Perspect. 2000, 108, A210. [Google Scholar] [CrossRef]
- Liu, X.D.; Chen, B.D. Climatic warming in the Tibetan Plateau during recent decades. Int. J. Climatol. 2000, 20, 1729–1742. [Google Scholar] [CrossRef]
- Wang, X.; Wei, X.; Song, Z.; Wang, M.; Xi, J.; Liang, J.; Liang, Y.; Duan, R.; Tian, K.; Zhao, Y.; et al. Mechanism study on a plague outbreak driven by the construction of a large reservoir in southwest china (surveillance from 2000–2015). PLoS Negl. Trop. Dis. 2017, 11, e0005425. [Google Scholar] [CrossRef]
- Xu, X.; Cui, Y.; Xin, Y.; Yang, X.; Zhang, Q.; Jin, Y.; Zhao, H.; He, J.; Jin, X.; Li, C.; et al. Genetic diversity and spatial-temporal distribution of Yersinia pestis in Qinghai Plateau, China. PLoS Negl. Trop. Dis. 2018, 12, e0006579. [Google Scholar] [CrossRef]
- Dai, R.; Qi, M.; Xiong, H.; Yang, X.; He, J.; Zhang, Z.; Yang, H.; Jin, J.; Li, X.; Xin, Y.; et al. Serological Epidemiological Investigation of Tibetan Sheep (Ovis aries) Plague in Qinghai, China. Vector. Borne. Zoonotic. Dis. 2019, 19, 3–7. [Google Scholar] [CrossRef]
- Walsh, M.; Haseeb, M.A. Modeling the ecologic niche of plague in sylvan and domestic animal hosts to delineate sources of human exposure in the western United States. PeerJ 2015, 3, e1493. [Google Scholar] [CrossRef][Green Version]
- Aguilar, M.; Lado, C. Ecological niche models reveal the importance of climate variability for the biogeography of protosteloid amoebae. ISME J. 2012, 6, 1506–1514. [Google Scholar] [CrossRef]
- Qian, Q.; Zhao, J.; Fang, L.; Zhou, H.; Zhang, W.; Wei, L.; Yang, H.; Yin, W.; Cao, W.; Li, Q. Mapping risk of plague in Qinghai-Tibetan Plateau, China. BMC Infect. Dis. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Neerinckx, S.; Peterson, A.T.; Gulinck, H.; Deckers, J.; Kimaro, D.; Leirs, H. Predicting Potential Risk Areas of Human Plague for the Western Usambara Mountains, Lushoto District, Tanzania. Am. J. Trop. Med. Hyg. 2010, 82, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.; Peterson, A.T.; Almeida, A. Ecology and Geography of Plague Transmission Areas in Northeastern Brazil. PLoS Negl. Trop. Dis. 2011, 5, e925. [Google Scholar] [CrossRef] [PubMed]
- Tian, L. Relationship between environmental factors and the spatial distribution of Spermophilus dauricus during 2000–2015 in China. Int. J. Biometeorol. 2018, 62, 1781–1789. [Google Scholar] [CrossRef]
- Lu, L.; Ren, Z.; Yue, Y.; Yu, X.; Lu, S.; Li, G.; Li, H.; Wei, J.; Liu, J.; Mu, Y.; et al. Niche modeling predictions of the potential distribution of Marmota himalayana, the host animal of plague in Yushu County of Qinghai. BMC Public Health 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Stenseth, N.C.; Samia, N.I.; Viljugrein, H.; Kausrud, K.L.; Begon, M.; Davis, S.; Leirs, H.; Dubyanskiy, V.M.; Esper, J.; Ageyev, V.S.; et al. Plague dynamics are driven by climate variation. Proc. Natl. Acad. Sci. USA 2006, 103, 13110–13115. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Q.; Stige, L.C.; Ben Ari, T.; Fang, X.; Chan, K.-S.; Wang, S.; Stenseth, N.C.; Zhang, Z. Nonlinear effect of climate on plague during the third pandemic in China. Proc. Natl. Acad. Sci. USA 2011, 108, 10214–10219. [Google Scholar] [CrossRef]
- Redding, D.W.; Atkinson, P.M.; Cunningham, A.A.; Lo Iacono, G.; Moses, L.M.; Wood, J.L.; Jones, K.E. Impacts of environmental and socio-economic factors on emergence and epidemic potential of Ebola in Africa. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Ben Ari, T.; Neerinckx, S.; Gage, K.L.; Kreppel, K.; Laudisoit, A.; Leirs, H.; Stenseth, N.C. Plague and climate: Scales matter. PLoS Pathog. 2011, 7, e1002160. [Google Scholar] [CrossRef]
- Seaborn, T.; Goldberg, C.S.; Crespi, E.J. Drivers of distributions and niches of North American cold-adapted amphibians: Evaluating both climate and land use. Ecol. Appl. 2021, 31, e2236. [Google Scholar] [CrossRef] [PubMed]
- Holt, A.C.; Salkeld, D.J.; Fritz, C.L.; Tucker, J.R.; Gong, P. Spatial analysis of plague in California: Niche modeling predictions of the current distribution and potential response to climate change. Int. J. Health Geogr. 2009, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, T.; Neerinckx, S.; Agier, L.; Cazelles, B.; Xu, L.; Zhang, Z.; Fang, X.; Wang, S.; Liu, Q.; Stenseth, N.C. Identification of Chinese plague foci from long-term epidemiological data. Proc. Natl. Acad. Sci. USA 2012, 109, 8196–8201. [Google Scholar] [CrossRef] [PubMed]
- Global Multi-resolution Terrain Elevation Data 2010 courtesy of the U.S. Geological Survey. Available online: https://topotools.cr.usgs.gov/gmted_viewer/viewer.htm (accessed on 9 March 2021).
- Xu, x. Dataset of China River Basin and River Network Based on DEM Extraction. Available online: https://www.resdc.cn/data.aspx?DATAID=226 (accessed on 9 March 2021).
- Global Gravity Field Model. Available online: https://www.space.dtu.dk/english/Research/Scientific_data_and_models/Global_Marine_Gravity_Field (accessed on 9 March 2021).
- Landsat Collection Courtesy of the, U.S. Geological Survey. Available online: https://earthexplorer.usgs.gov (accessed on 9 March 2021).
- Map of Chemico-Geographic Landscape of China. The Atlas of Plague and Its Environment in the People’s Republic of China. Science Press: Beijing, China, 2000; pp. 32–33. [Google Scholar]
- Soil pH in H2O at 6 Standard Depths (0, 10, 30, 60, 100 and 200 cm) at 250 m Resolution. Available online: https://zenodo.org/record/2525664#.YgX_zN9BySk (accessed on 9 March 2021).
- FAO/IIASA/ISRIC/ISSCAS/JRC. Harmonized World Soil Database (version 1.2). FAO, Rome, Italy and IIASA, Laxenburg, Austria. 2012. Available online: https://previous.iiasa.ac.at/web/home/research/researchPrograms/water/HWSD.html (accessed on 9 March 2021).
- Abatzoglou, J.T.; Dobrowski, S.Z.; Parks, S.A.; Hegewisch, K.C. TerraClimate, a high-resolution global dataset of monthly climate and climatic water balance from 1958–2015. Sci. Data. 2018, 5, 170191. [Google Scholar] [CrossRef]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M.; et al. Global terrestrial Human Footprint maps for 1993 and 2009. Sci. Data. 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Gao, M.; Li, X.; Cao, C.; Zhang, H.; Li, Q.; Zhou, H.; He, Q.; Xu, M.; Zhao, J.; Zheng, S.; et al. Spatial prediction and analysis of Himalayan marmot plague natural epidemic foci in China based on HJ-1 satellite data. Sci. China-Earth Sci. 2010, 53, 8–15. [Google Scholar] [CrossRef]
- Zaporozhan, V.; Ponomarenko, A. Mechanisms of Geomagnetic Field Influence on Gene Expression Using Influenza as a Model System: Basics of Physical Epidemiology. Int. J. Env. Res. Public Health 2010, 7, 938–965. [Google Scholar] [CrossRef]
- Mendoza, H.; Rubio, A.V.; Garcia-Pena, G.E.; Suzan, G.; Simonetti, J.A. Does land-use change increase the abundance of zoonotic reservoirs? Rodents say yes. Eur. J. Wildl. Res. 2020, 66, 1–5. [Google Scholar] [CrossRef]
- Barbieri, R.; Texier, G.; Keller, C.; Drancourt, M. Soil salinity and aridity specify plague foci in the United States of America. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Malek, M.A.; Bitam, I.; Levasseur, A.; Terras, J.; Gaudart, J.; Azza, S.; Flaudrops, C.; Robert, C.; Raoult, D.; Drancourt, M. Yersinia pestis halotolerance illuminates plague reservoirs. Sci. Rep. 2017, 7, 40022. [Google Scholar] [CrossRef]
- Shen, M.; Piao, S.; Cong, N.; Zhang, G.; Jassens, I.A. Precipitation impacts on vegetation spring phenology on the Tibetan Plateau. Glob. Chang. Biol. 2015, 21, 3647–3656. [Google Scholar] [CrossRef] [PubMed]
- Naud, C.M.; Rangwala, I.; Xu, M.; Miller, J.R. A Satellite View of the Radiative Impact of Clouds on Surface Downward Fluxes in the Tibetan Plateau. J. Appl. Meteorol. Clim. 2015, 54, 479–493. [Google Scholar] [CrossRef]
- Munyenyiwa, A.; Zimba, M.; Nhiwatiwa, T.; Barson, M. Plague in Zimbabwe from 1974 to 2018: A review article. PLoS Negl. Trop. Dis. 2019, 13, e0007761. [Google Scholar] [CrossRef] [PubMed]
- Tanser, F.C.; Sharp, B.; Le Sueur, D. Potential effect of climate change on malaria transmission in Africa. Lancet 2003, 362, 1792–1798. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, P.; Lin, F.; Yang, W.; Gaisberger, H.; Christopher, K.; Zheng, Y. MaxEnt modelling for predicting the potential distribution of a near threatened rosewood species (Dalbergia cultrata Graham ex Benth). Ecol. Eng. 2019, 141, 105612. [Google Scholar] [CrossRef]
- Wan, J.; Qi, G.J.; Ma, J.; Ren, Y.; Wang, R.; McKirdy, S. Predicting the potential geographic distribution of Bactrocera bryoniae and Bactrocera neohumeralis (Diptera: Tephritidae) in China using MaxEnt ecological niche modeling. J. Integr. Agric. 2020, 19, 2072–2082. [Google Scholar] [CrossRef]
- Potter, K.A.; Arthur Woods, H.; Pincebourde, S. Microclimatic challenges in global change biology. Glob. Chang. Biol. 2013, 19, 2932–2939. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudik, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Wang, G.; Wang, C.; Guo, Z.; Dai, L.; Wu, Y.; Liu, H.; Li, Y.; Chen, H.; Zhang, Y.; Zhao, Y.; et al. Integrating Maxent model and landscape ecology theory for studying spatiotemporal dynamics of habitat: Suggestions for conservation of endangered Red-crowned crane. Ecol. Indic. 2020, 116, 106472. [Google Scholar] [CrossRef]
- Xu, N.; Meng, F.; Zhou, G.; Li, Y.; Wang, B.; Lu, H. Assessing the suitable cultivation areas for Scutellaria baicalensis in China using the Maxent model and multiple linear regression. Biochem. Syst. Ecol. 2020, 90, 104052. [Google Scholar] [CrossRef]
- Nikol–skii, A.A.; Ulak, A. Key factors determining the ecological niche of the Himalayan marmot, Marmota himalayana Hodgson (1841). Russ. J. Ecol. 2006, 37, 46–52. [Google Scholar] [CrossRef]
- Li, D. Preliminary Observation of Marmot Habitat Movement. J. Med. Pest. Control. 1995, 11, 190. (In Chinese) [Google Scholar]
- Han, Y.; Ma, W.; Wang, B.; Ma, Y.; Tian, R. Climatic characteristics of rainfall change over the Qinghai- Tibetan Plateau from 1980 to 2013. Plateau. Meteor. 2017, 36, 1477–1486. (In Chinese) [Google Scholar]
- Ben Ari, T.; Gershunov, A.; Gage, K.L.; Snäll, T.; Ettestad, P.; Kausrud, K.L.; Stenseth, N.C. Human plague in the USA: The importance of regional and local climate. Biol. Lett. 2008, 4, 737–740. [Google Scholar] [CrossRef]
- Davis, S.; Begon, M.; De Bruyn, L.; Ageyev, V.S.; Klassovskiy, N.L.; Pole, S.B.; Viljugrein, H.; Stenseth, N.C.; Leirs, H. Predictive thresholds for plague in Kazakhstan. Science 2004, 304, 736–738. [Google Scholar] [CrossRef]
- Russell, R.E.; Abbott, R.C.; Tripp, D.W.; Rocke, T.E. Local factors associated with on-host flea distributions on prairie dog colonies. Ecol. Evol. 2018, 8, 8951–8972. [Google Scholar] [CrossRef]
- Eisen, R.J.; Gage, K.L. Adaptive strategies of Yersinia pestis to persist during inter-epizootic and epizootic periods. Vet. Res. 2009, 40, 1. [Google Scholar] [CrossRef]
- Eads, D.A.; Abbott, R.C.; Biggins, D.E.; Rocke, T.E. Flea Parasitism and Host Survival in a Plague-Relevant System: Theoretical and Conservation Implications. J. Wildl. Dis. 2020, 56, 378–387. [Google Scholar] [CrossRef]
- Cohen, J.M.; Sauer, E.L.; Santiago, O.; Spencer, S.; Rohr, J.R. Divergent impacts of warming weather on wildlife disease risk across climates. Science 2020, 370, 6519. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Piao, S.; Li, L.Z.X.; Li, Y.; Huntingford, C.; Ciais, P.; Cescatti, A.; Janssens, I.A.; Peñuelas, J.; Buermann, W.; et al. Summer soil drying exacerbated by earlier spring greening of northern vegetation. Sci. Adv. 2020, 6, eaax0255. [Google Scholar] [CrossRef] [PubMed]
- Eads, D.A.; Biggins, D.E.; Xu, L.; Liu, Q. Plague cycles in two rodent species from China: Dry years might provide context for epizootics in wet years. Ecosphere 2016, 7, e01495. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.; Wang, Z.; Li, C.; Li, M. Retrospection and present state of plague prevention and control in Qinghai province about 50 years. Zhonghua Difangbingxue Zazhi 2004, 23, 610–613. (In Chinese) [Google Scholar]
- Wilschut, L.I.; Addink, E.A.; Heesterbeek, H.; Heier, L.; Laudisoit, A.; Begon, M.; de Jong, S.M. Potential corridors and barriers for plague spread in central Asia. Int. J. Health. Geogr. 2013, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- McCauley, D.J.; Dirzo, R.; Young, H.S.; Salkeld, D.J.; Gaffikin, L.; Barry, M.; Lambin, E.F.; Helgen, K.M.; Eckerlin, R.P.; Makundi, R. Effects of Land Use on Plague (Yersinia pestis) Activity in Rodents in Tanzania. Am. J. Trop. Med. Hyg. 2015, 92, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.E.; Tripp, D.W.; Rocke, T.E. Differential plague susceptibility in species and populations of prairie dogs. Ecol. Evol. 2019, 9, 11962–11971. [Google Scholar] [CrossRef] [PubMed]
- Cabanel, N.; Leclercq, A.; Chenal-Francisque, V.; Annajar, B.; Rajerison, M.; Bekkhoucha, S.; Bertherat, E.; Carniel, E. Plague Outbreak in Libya, 2009, Unrelated to Plague in Algeria. Emerg. Infect. Dis. 2013, 19, 230–236. [Google Scholar] [CrossRef]
- Duplantier, J.M.; Duchemin, J.B.; Chanteau, S.; Carniel, E. From the recent lessons of the Malagasy foci towards a global understanding of the factors involved in plague reemergence. Vet. Res. 2005, 36, 437–453. [Google Scholar] [CrossRef]
- Tang, C.Q.; Matsui, T.; Ohashi, H.; Dong, Y.F.; Momohara, A.; Herrando-Moraira, S.; Qian, S.; Yang, Y.; Ohsawa, M.; Luu, H.T.; et al. Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun. 2018, 9, 4488. [Google Scholar] [CrossRef]
- Soberon, J.; Peterson, A.T. Interpretation of Models of Fundamental Ecological Niches and Species’ Distributional Areas. Biodiv. Inf. 2005. [Google Scholar] [CrossRef]
- Collinge, S.K.; Johnson, W.C.; Ray, C.; Matchett, R.; Grensten, J.; Cully, J.F., Jr.; Martin, A.P. Landscape structure and plague occurrence in black-tailed prairie dogs on grasslands of the western USA. Landsc. Ecol. 2005, 20, 941–955. [Google Scholar] [CrossRef]

| Data Type | Variables | Biological Relevance | Abbreviation | Units |
|---|---|---|---|---|
| Topography | DEM | Habitats of hosts: Number of marmot holes is largest at an altitude between 3200–3500 m [33]; | E | m |
| Distance to river | Field investigation: Almost all marmot holes are around one river; | D | km | |
| Gravity | Effect in astronomy: Geomagnetism may affect the plague cycle [34]; | G | mGal | |
| Vegetation | NDVI | NDVI → Population density: Higher density is often linked to higher prevalence [35]; | NDVI | — |
| Soil | Geochemical landscape | Evolution of Y. pestis: Geochemical evolution and biological evolution are a kind of conjugation process→ Persistence of plague [36,37]; | GL | — |
| Soil type | ST | — | ||
| pH | pH | −log (H+) | ||
| Soil moisture | Vegetation → Population density, migration→ Increased risks [35]; | SM | mm | |
| Climate | PDSI | Aridity is significantly associated with ecological risk factors for relapsing plague [36], and drought can control the synchrony of plague outbreaks [36]; | PDSI | — |
| Precipitation | Phenology [38] → Vegetation → Population density, migration → Increased risks [35]; | PR | mm | |
| Solar Radiation | Governing the surface temperature and hydrologic cycle [39] → Vegetation → Increased risks; | SR | W/m2 | |
| Temperature | Yersinia pestis: survives for a long time under low temperature conditions [40];Fleas: survival and development → plague persistence [4] Hosts: a prolonged active season [41]. | T | °C |
| Phases | The Average Test/Training AUC | Threshold | Average Risk | Areas of Prediction (Thousand km2) | Areas of Published Data (Thousand km2) |
|---|---|---|---|---|---|
| S1 | 0.93/0.95 | 0.169 | 0.041 | 301.9 | 99.79 |
| S2 | 0.90/0.95 | 0.319 | 0.055 | 319.6 | — |
| S3 | 0.94/0.96 | 0.218 | 0.033 | 247.7 | 408.38 |
| S4 | 0.92/0.95 | 0.259 | 0.045 | 303.2 | 634.49 |
| S5 | 0.93/0.96 | 0.180 | 0.032 | 263.7 | 687.04 |
| Change in Human Footprints from 1993 to 2009 | Mean Risk in S2 | Mean Risk in S3 | Mean Risk in S5 | Areas with Risk > 0.5 in S2(%) | Areas with Risk > 0.5 in S3(%) | Areas with Risk > 0.5 in S5(%) |
|---|---|---|---|---|---|---|
| −19–−0.01 | 0.157 | 0.072 | 0.068 | 8.706 | 1.997 | 1.198 |
| 0 | 0.094 | 0.058 | 0.055 | 4.999 | 1.68 | 1.641 |
| 0–2 | 0.091 | 0.063 | 0.052 | 3.778 | 1.866 | 1.588 |
| 2–5 | 0.114 | 0.060 | 0.053 | 6.602 | 2.117 | 2.268 |
| 5–10 | 0.107 | 0.065 | 0.062 | 5.310 | 2.438 | 3.369 |
| 10–20 | 0.149 | 0.082 | 0.077 | 7.5 | 2.5 | 4.167 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Yang, L.; Li, H.; Wang, L. Spatiotemporal Variations of Plague Risk in the Tibetan Plateau from 1954–2016. Biology 2022, 11, 304. https://doi.org/10.3390/biology11020304
Yuan X, Yang L, Li H, Wang L. Spatiotemporal Variations of Plague Risk in the Tibetan Plateau from 1954–2016. Biology. 2022; 11(2):304. https://doi.org/10.3390/biology11020304
Chicago/Turabian StyleYuan, Xing, Linsheng Yang, Hairong Li, and Li Wang. 2022. "Spatiotemporal Variations of Plague Risk in the Tibetan Plateau from 1954–2016" Biology 11, no. 2: 304. https://doi.org/10.3390/biology11020304
APA StyleYuan, X., Yang, L., Li, H., & Wang, L. (2022). Spatiotemporal Variations of Plague Risk in the Tibetan Plateau from 1954–2016. Biology, 11(2), 304. https://doi.org/10.3390/biology11020304






